Abstract
The assessment of potential benefits versus harms from mammographic examinations as described in the controversial breast cancer screening recommendations of the U.S. Preventive Task Force included limited consideration of absorbed dose to the fibroglandular tissue of the breast (glandular tissue dose), the tissue at risk for breast cancer. Epidemiological studies on cancer risks associated with diagnostic radiological examinations often lack accurate information on glandular tissue dose, and there is a clear need for better estimates of these doses. Our objective was to develop a quantitative summary of glandular tissue doses from mammography by considering sources of variation over time in key parameters including imaging protocols, x-ray target materials, voltage, filtration, incident air kerma, compressed breast thickness, and breast composition. We estimated the minimum, maximum, and mean values for glandular tissue dose for populations of exposed women within 5-year periods from 1960 to the present, with the minimum to maximum range likely including 90% to 95% of the entirety of the dose range from mammography in North America and Europe. Glandular tissue dose from a single view in mammography is presently about 2 mGy, about one-sixth the dose in the 1960s. The ratio of our estimates of maximum to minimum glandular tissue doses for average-size breasts was about 100 in the 1960s compared to a ratio of about 5 in recent years. Findings from our analysis provide quantitative information on glandular tissue doses from mammographic examinations which can be used in epidemiologic studies of breast cancer.
Keywords: glandular tissue dose, historical dose estimates, breast, mammography, literature review
INTRODUCTION
The November 2009 release of breast cancer screening recommendations by the U.S. Preventive Services Task Force (1) led to a storm of controversy about the task force’s assessment of benefits versus harms. The key goal of mammography used as a diagnostic as well as a screening tool is the early detection of breast cancer in females with improved survival of patients. Among the potential harms, limited attention was given to absorbed dose to the fibroglandular tissue of the breast (glandular tissue dose) for women who have undergone repeated mammograms during the 1960s through much of the 1970s, when glandular tissue doses were substantially higher than more recently. Follow-up studies of patients undergoing radiotherapy for medical conditions and Japanese atomic bomb survivors have demonstrated that exposure to moderate to high doses of ionizing radiation is a risk factor for breast cancer in women, particularly when exposure takes place at young ages (2–4). However, there is little quantitative data on glandular tissue doses from mammography in past decades and few epidemiologic studies of radiation-related breast cancer risks that quantitatively include the contribution of mammography to the total glandular tissue dose received from other medical radiation, environmental or occupational sources. Epidemiologic studies to-date have primarily utilized the type and frequency of radiologic examinations rather than a more precise quantitative measure of glandular tissue dose to estimate radiation-related breast cancer. This is especially true for glandular tissue doses from mammography or other diagnostic or screening procedures in which the breasts were exposed to medical radiation prior to the 1980s. Better estimates of glandular tissue dose from different types of radiographic examinations are needed, particularly for earlier decades, to improve radiation-related breast cancer risk estimates.
The objective of this study was to review literature on radiation exposure of the female breast from mammography and to reconstruct associated glandular tissue doses from 1960 to the present. In this paper, we provide literature- and calculation-based estimates of glandular tissue dose by 5-year periods based on reports from the U.S., Canada and European countries. While we found that it was not possible to develop statistical distributions describing the variation of these doses received in each time period, our comprehensive review and careful examination of many parameters enabled us to estimate, mean, minimum, and maximum glandular tissue doses within each time period. These minimum and maximum values do not attempt to capture unusual exposure circumstances but, rather, routine radiologic examinations provided in medical care. From the data presented here, temporal trends in glandular tissue doses from mammography in North America and Europe can be deduced as well as the variation of these doses among women.
MAMMOGRAPHY AND GLANDULAR TISSUE DOSE ESTIMATION: BASICS
Mammography is a radiographic procedure using relatively low energy x rays (generally below 50 keV) to form an image of the internal structure of the human breast. The images were initially captured on film and more recently on digital media. To achieve high resolution and good contrast in mammographic examinations, it is necessary to optimize the spectrum of x-ray energies to the composition, density, and thickness of the breast. Because ionizing radiation is used to form the image, some energy penetrates breast tissue, resulting in absorbed dose in the breast.
In mammography, the tissue at risk for breast cancer is the fibroglandular tissue. Glandular tissue dose is defined as the mean energy imparted to the mass of fibroglandular tissue of the breast (5). It should be understood that the glandular tissue dose is not measured directly but is calculated as the product of the incident air kerma, determined by measurement and a conversion coefficient (DgN),:
| (1) |
where,
Dg is the glandular tissue dose (mGy),
Ka,i is the incident air kerma (free-in-air) (mGy),
DgN is a conversion coefficient (glandular tissue dose per unit incident air kerma) (mGy per mGy).
The quantity Ka,i is the air kerma from the incident beam on the central x-ray beam axis at the focal spot-to-surface distance (i.e., at the skin-entrance plane) (6). Only the primary radiation incident on the patient or phantom is included; backscattered radiation is excluded.
The incident air kerma is determined primarily by the energy spectrum of the emitted x rays and the intensity (photon fluence) of the x-ray beam that is generated. The intensity, for many years, was under control of the technologist and reflected the machine settings which were either derived from imaging protocols or reflected the technologists experience in obtaining high quality images for a patient of a particular breast size. The total x-ray intensity can be set by adjustments to the beam current (millamperes) (mA), the length of time the x-ray beam remains on (in modern systems, the exposure time is under automatic control), and/or the amount of material through which the x rays are filtered before reaching the breast. In general, mammographic images require a minimum number of x-ray quanta to reach the film or imaging device to achieve an image of acceptable quality. Because the breast tissue absorbs much of the radiation that passes through it, the intensity of the x-ray beam (and hence, the incident air kerma) must be modified for each woman to account for the thickness of the breast that is being imaged.
Also needed for dose computations are conversion coefficients (DgN) that are applicable for the physical attributes of the breast to be imaged as well as the characteristics of the incident radiation field. Each DgN value is for a specific set of exposure-related variables and assumptions about breast characteristics that reflect a particular physical or mathematical phantom, typical women, or even, but more rarely, a specific individual. While there are numerous values of DgN available in the published literature, we found that DgN values are not available for all combinations of x-ray energies and filtration used over the decades as well as the full range of breast characteristics.
The most important exposure-related variables that determine the magnitude of incident air kerma, the DgN values, and ultimately, to the glandular tissue dose from mammography can be described by the following five parameters, moving in concept from x-ray generation to the patient.
Electrical potential (kilovolts) (kV) placed on the x-ray machine target (7): determines the maximum energy of emitted photons; values around 25 kV (peak) are typical for mammographic machines.
Composition of x-ray target (typically tungsten, molybdenum, or rhodium) (8–12): determines to a large degree the shape of the x-ray spectrum. X-ray tubes have been constructed of tungsten (W) for decades because of good heat-load capacities of the metal. Other target materials include molybdenum (Mo) and rhodium (Rh). Molybdenum targets were largely introduced in the 1970s to improve contrast (12) by providing a good compromise between an x-ray energy spectrum that gives high contrast and high dose and a higher-penetrating, lower dose, and lower contrast image. Molybdenum targets are often used in conjunction with an external molybdenum filter which filters the spectrum in such a way as to leave a narrow spectrum that is highly suitable for imaging the breast.
Filtration of the x-ray beam (7,13–16): often under control of the technologist; filtration is the thickness of a specific material that the x-ray beam must pass through before exiting the x-ray tube (typically measured in mm of Al). Historically, filtration was measured by a quantity termed half-value layer (HVL) which is the thickness of material that will reduce the beam intensity by one-half. Filtration is used to provide a good compromise between dose reduction and high contrast by removing quanta that are highly unlikely to successfully pass through the breast to the imaging device. For the purpose of this work, we have derived, from the literature, the amount of added filtration typically used over the decades in order to select conversion coefficients in the most realistic way possible for specific years of interest to this work.
Compressed breast thickness (CBT) (12,17–21): thickness of breast (cm) when compressed for imaging; breasts with a thicker CBT generally receive higher glandular tissue doses than those with thinner CBT for equal image quality, primarily because the incident air kerma must be greater to penetrate the thicker tissue.
Breast composition (22) quoting (23), (18,20,24–28): refers to the proportions of fibroglandular and adipose tissue and density. Typical assumptions are 50% each of fibroglandular and adipose tissue, but in actuality, breasts with thinner than average CBT have a high fibroglandular percentage (as much as 70%) while breasts with larger than average CBT have a lower fibroglandular percentage (less than 10%) (18,20,25,28). In addition, breast density decreases with age (29).
MAMMOGRAPHY IN PRACTICE: EVOLUTION WITH TIME
Mammography is recognised today as an important tool for cancer detection, hence, screening programs have been implemented in many countries since the 1990’s (1,20,25,30–42). However, the technology used to form images of the breast tissue has evolved over time, particularly during the 1960s and 1970s. The technologic evolution of mammography is discussed in the Appendix along with some of the information available for glandular tissue dose estimation together with detailed protocols implemented in time periods from 1960 until the present. A brief summary is provided in Table 1 and below.
Table 1.
Historical review of mammography practices and availability of data by time period.
| Period | Main technology improvements / Source of information for the study | References |
|---|---|---|
| 1913 | ➢ First radiographic examinations of the breast on 3000 mastectomy specimens | (43) |
| 1930–1940’s | ➢ Experimental phase - no attempt to estimate radiation dose quantities | (44,88,89) |
| 1950’s | ➢ X-rays machine settings described for the medical community | (45) |
| ➢ Cancer follow-up study with first statistical analysis of the diagnostic value of mammography | (90) | |
| 1960’s | ➢ Description of mammography and cancer detection | (89,91–94) |
| ➢ Egan protocol: 22–28 kV (peak), W-Al target-filter combination, no added filtration (0.9 mm Al inherent filtration), target film distance of 30–40 in.(76–100 cm), x-ray beam intensity between 1500 and 1800 mAs. | (46,75,90,95,96) | |
| ➢ Gershon-Cohen protocol: 25–30 kV (peak), W-Al target-filter combination, 0.5 mm Al filtration, target film distance of 18 in.(46 cm), x-ray beam intensity between 100 and 350 mAs. | (47,75,91) | |
| ➢ Senographe mainly used with Egan protocol: Mo-Mo target-filter combination, 0.4 mm Al inherent filtration | (49,71) | |
| ➢ Senographe also used with Gershon-Cohen protocol and various types of films | (75,95,97,98) | |
| 1970’s | ➢ Implementation of Mo rotating anode allowing lower exposure times | (99) |
| ➢ Introduction of several screen/film combinations and various filters | (10,14,48–50) Bicehouse HJ1 | |
| ➢ Introduction of xeroradiography: film replaced by an aluminium plate coated with a layer of amorphous selenium | (12,23,51,52) Bicehouse HJ1 | |
| 1975 onward | ➢ Main surveys with comparison of radiation dose quantities: | |
| • Industrial film, no screen film, xeroradiography, low-dose technique, high density technique | BicehouseHJ1 | |
| • Senograph with AA film, xero plate, low-dose vacuum cassette, W tube with xero plate | (51) | |
| • No-screen, screen-film (Low-Dose) and xeroradiography | (44,52,100–104) | |
| 1980’s | ➢ Introduction of anti-scatter grid and automatic exposure control | (21) |
| 1985 & 1988 | ➢ NEXT surveys in the US: survey on mammography units | (53–57) |
| ➢ Surveys in Canada: assessment of practices and radiation dose quantities with film- screen with and without grid and xeroradiography | (105) | |
| ➢ Surveys in the Netherlands: survey on mammography units in screening centres | (106) | |
| 1990’s | ➢ Introduction of rhodium as target and filter material | (9,11) |
| ➢ In the U.S. and Canada: | ||
| • Regular surveys on radiation dose quantities and image quality | (9,55–57,107,108) | |
| • Survey with individual measurements of Dg | (18,27,109) | |
| ➢ In Europe: | ||
| • Assessment of radiation dose quantities on women in screening programs | (20,25,30–40) | |
| • Estimation of Dg from specification of mammography units and standard breast | (72,110–115) | |
| 2000’s | ➢ Additional surveys of radiation dose quantities | (8,74,80,116–118) |
| ➢ Introduction of digital mammography | (119–121) |
H. J. Bicehouse. Survey of Mammographic exposure levels and techniques used in Eastern Pennsylvania. Proceedings of the Seventh Annual National Conference on Radiation Control. April 27 - May 2. Hyannis, Massachussetts, 1975
Mammography has evolved since the first examinations conducted by Salomon in 1913 on 3,000 mastectomy specimens (43) and the evolution of the technology can be divided in four periods:
The period of early experimentation in the 1930’s–1940’s (44), during which the technology was tested to improve the quality of images with no concern about the level of dose to the breast.
In the 1950’s, mammography was introduced in clinical practice with different protocols implemented. Leborgne (45) was the first to describe his technique and the x-ray machine settings (often called technical parameters of radiographic technique). During the 1960s, protocols described by Egan (46) and by Gershon-Cohen (47) were widely implemented. Diagnostic protocols included one to three films per breast with the major views being craniocaudal, mediolateral and mediolateral oblique (Fig 1).
During the 1970s and 1980s, the Egan and Gershon-Cohen protocols were modified to incorporate technology developments, such as the introduction of the Senographe, screen-film technology (10,14,48–50) (Bicehouse HJ,19751) and xeroradiography (23,51,52) (Bicehouse HJ, 19751). National surveys were implemented in the late 1970’s (Bicehouse HJ, 19751) (51), and the Nationwide Evaluation of X-ray Trends (NEXT) surveys were initiated in the US in the 1980s (53–57) to assess the overall quality of mammography practices and to estimate various radiation dose quantities. The Mammography Quality Standards Act regulation (MQSA) was introduced by the U.S. FDA in the early 1990’s to regulate the use of mammography and provide accreditations (58). In parallel, user’s practice guides were provided to the medical community (59,60). As screen-film technology improved with the introduction of new screens, new films and grids, this technology became widely accepted, with xeroradiography disappearing from use in the 1990’s (55). From the 1990s onward, digital mammography has become increasingly available and is expected to grow in use since the quality of images is currently being improved. Quality control programs for digital mammography are, therefore, being developed in the U.S. and in Europe (12,58).
Fig. 1.
Typical mammography projections (reproduced with permission from Merrill’s Atlas of Radiographic Positioning & Procedures 11/e, 978-0323033176, Frank et al, 2007).
METHODS
1. Collection of literature data
We conducted a literature review to obtain, where available, direct estimates of glandular tissue dose of women from mammographic examinations, and secondarily, data describing mammography imaging protocols from which glandular tissue doses could be estimated. We also reconstructed glandular tissue doses for a variety of exposure conditions and imaging protocols for which reports described exposure conditions but lacked quantitative measures of dose quantities.
Publications discussing various radiation dose quantities for mammography were sought with an ordered priority as follows: (1) data on individually determined glandular tissue doses for mammography, (2) conversion coefficients to estimate glandular tissue dose from such quantities, as free-in-air exposure, incident air kerma (excludes backscattered radiation), and entrance- surface dose or entrance-surface air kerma (includes backscattered radiation), (3) x-ray machine settings (including peak electrical potential, beam filtration, beam current, exposure time) as well as anatomic data on compressed breast thickness, and breast composition that could be used to estimate glandular tissue doses, and (4) literature-based estimates of glandular tissue dose.
Regarding (1) above, the literature most directly applicable for our purposes reported individually determined glandular tissue doses. However, in most circumstances, absorbed dose in the fibroglandular tissue is rarely measured directly but is inferred from measurements that can be used to estimate glandular tissue dose. Hence, the term individually determined glandular tissue doses refers to derived values of glandular tissue dose based on measurements of other radiation dose quantities made on an individual basis.
To locate publications relevant to our purposes, Pubmed™ (including Medline™) was searched using keywords and phrases including “radiation dose and mammography.” The individual reference lists of the collected publications were also used to obtain additional relevant publications. The number of mammography-related dosimetry publications rose after 1980 as the technique became standardized and clinically accepted for diagnostic purposes. Because there was only a single publication discussing radiation dose data prior to 1960 (46), our evaluation of glandular tissue doses in this paper is limited to 1960 and after.
2. Classification of literature data
The most useful publications for this analysis contained data that could be used to derive a temporal summary of glandular tissues doses received from mammography. We classified the publications into three groups, Tier 1, Tier 2, and Tier 3 to indicate the value of the data they contained (Table 2).
Table 2.
Number of publications by time period and Tier (1, 2, or 3) used in estimating glandular tissue doses received by populations undergoing mammographic examinations.
| Period | Tier 1 publications (Distribution of Dg to individuals) |
Tier 2 publications (Dg for an average breast size) |
Tier 3 publications (Incident air kerma) |
|---|---|---|---|
| 1960–1964 | - | - | 5 |
| 1965–1969 | - | - | 5 |
| 1970–1974 | - | - | 3 |
| 1975–1979 | - | - | 9 |
| 1980–1984 | - | 1 | 1 |
| 1985–1989 | - | 4 | 1 |
| 1990–1994 | 5 | 4 | 3 |
| 1995–1999 | 9 | 3 | 1 |
| 2000–2004 | 5 | 1 | - |
| 2005+ | 1 | - | 1 |
Tier 1 included publications in which results for glandular tissue dose were presented for individuals or for groups of patients with various CBTs. In most of these publications, a statistical summary of the glandular tissue doses (including ranges) was provided by the authors. The data from the publications that we categorized as Tier 1 were given highest priority.
Tier 2 publications were those in which results for glandular tissue dose were calculated for the average breast size reported in the particular publication as simulated by a phantom with CBTs varying from 4 to 6 cm. For each Tier 2 publication, we converted the glandular tissue dose to incident air kerma (Ka,i) assuming a reference CBT of 5 cm [i.e., Ka,i (5 cm)]. We used conversion coefficients appropriate for the imaging protocol noted in the publication, or if not stated, we used the protocol that was most commonly used in that time period. Estimation of conversion coefficients is described in detail below and conversion coefficients are presented by protocol in the Appendix of this paper.
The Ka,i values for thinner compressed breasts were typically lower than Ka,i for thicker compressed breasts. Variation in Ka,i according to breast thickness results from changing the milliampere-seconds (mAs) of the machine and/or the target-to-film distance to produce x-ray images of equal quality for each CBT. We extrapolated from Ka,i (5 cm) to Ka,i (3 cm) and Ka,i (8 cm)] using the data and approach described by Gentry and DeWerd (18) i.e., by decreasing incident air kerma for small CBTs (3 cm) and increasing incident air kerma for larger CBTs (8 cm). The range of Ka,i provided by Gentry and DeWerd (18) is in agreement with findings reported by other authors (37,38,61–63). However, because the data of Gentry and DeWerd (18) came from a large study conducted in 170 facilities in the U.S. it was our preferred source for data to extrapolate Ka,i values for 3 and 8 cm CBTs from the nominal average thickness of 5 cm. Smaller studies published by other authors (37,38,61–63) on the relationship of Ka,i and CBT were likely less representative of the many possible variations in imaging technology and mammography protocols.
While the relationship of Ka,i as a function of CBT in Gentry and DeWerd (see Fig. 3 of (18)) is quantitatively descriptive, the one standard deviation confidence intervals (CI) did not provide adequate information for understanding population variability. The magnitude of the variation was such that extending the CI to two standard deviations so that we could capture 95% variation, would result in negative lower bounds. Hence, we implemented the Approximate Bayesian Computation (ABC) method as described by Beaumont et al. (64) to estimate the 95% CIs while maintaining positive lower bounds. Our application of this method is described further in the Appendix of this paper. We estimated the mean and approximate 95% range for Dg based on mean Ka,i values from Gentry and DeWerd (18) for CBTs ranging from 3 to 8 cm, using the appropriate conversion coefficients for the specific CBT and relevant imaging protocol.
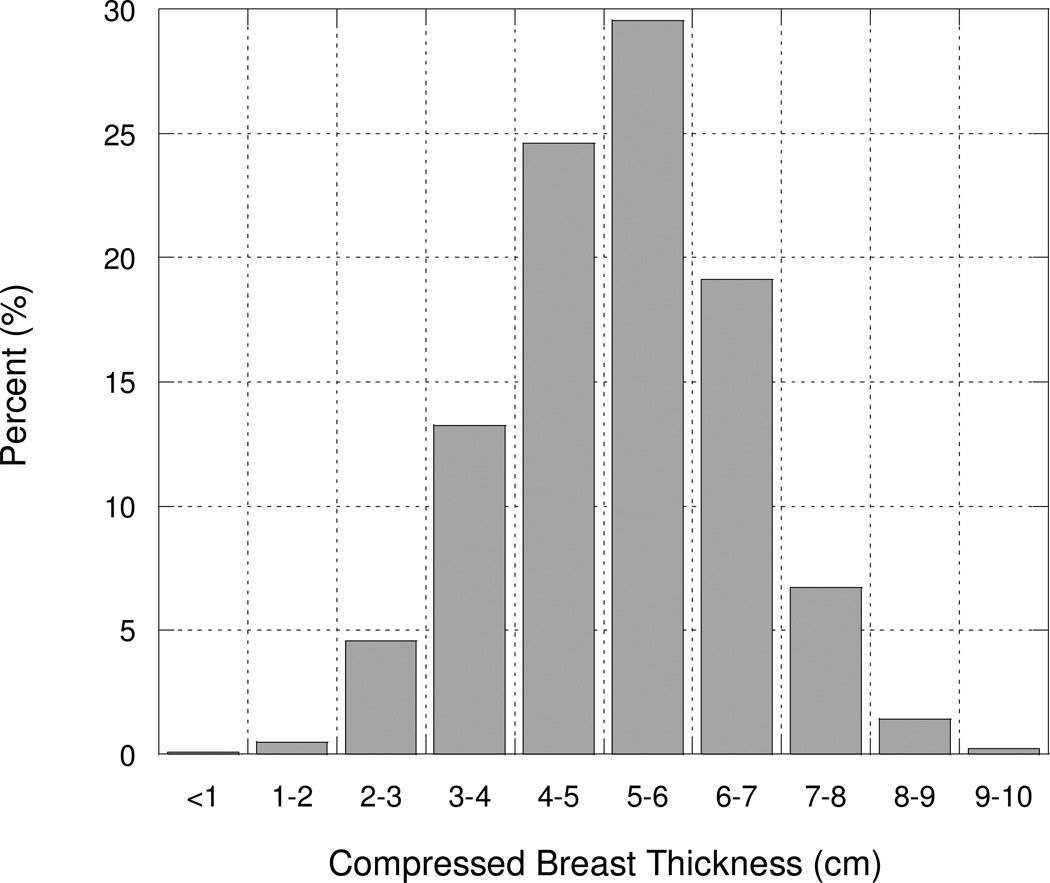
Fig. 3.
Histogram of compressed breast thickness (cm) derived from 11 publications and more than 48,000 mammograms.
Tier 3 publications reported only estimates of exposure (R) or Ka,i (mGy). When values were provided for average women (CBT5_cm), extrapolation to Ka,i (3 cm) and Ka,i (8 cm) was conducted using the data of Gentry and DeWerd (18) as described above. The derived values of Ka,i (5 cm) were then converted to glandular tissue dose, for each publication and each protocol, using the appropriate conversion coefficients. In addition to Ka,i values, relevant information from the imaging protocols was collected on the technical parameters (including target-filter combinations, peak tube potential, filtration, and mAs).
3. Conversion of reported quantities to glandular tissue dose
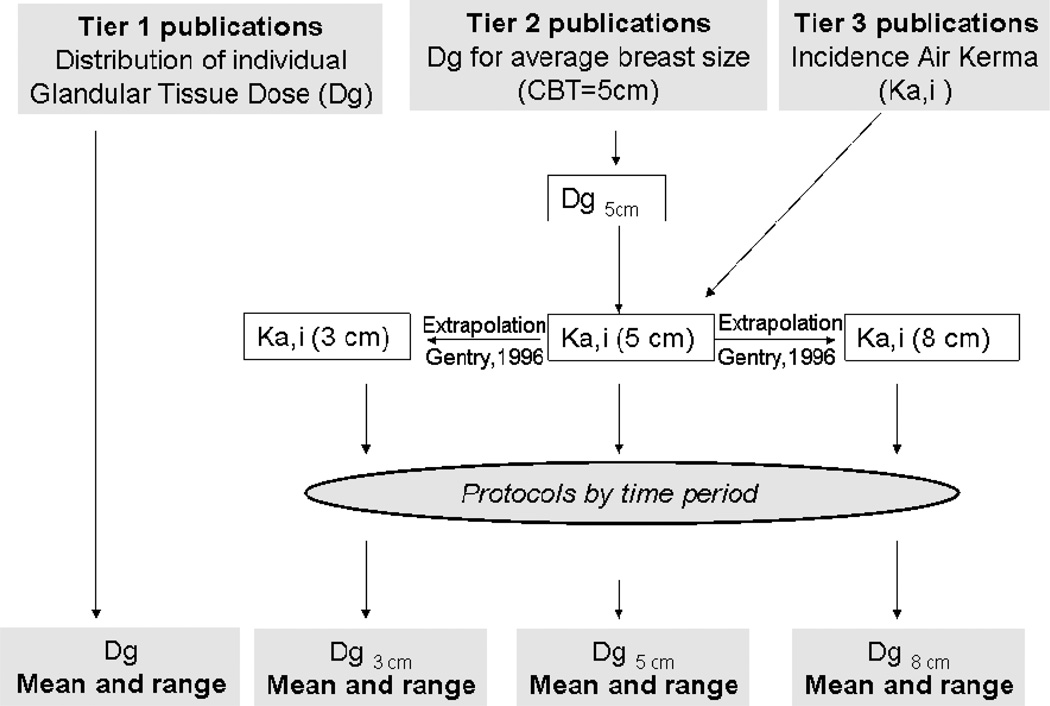
The strategy for calculating glandular tissue doses from Tier 1, 2, and 3 publications, is shown in Fig. 2 and conversion coefficients are described below.
Fig. 2.
Diagram of strategy of using data from publications for deriving the mean values and ranges of Dg for populations of exposed women..
Conversion coefficients (DgN) for mammography have been derived over the years for certain combinations of technical parameters and imaging protocols (7,11,17,65–68). Early on the most common target material used was tungsten (W), while molybdenum (Mo) was introduced in the late 1960’s.
Because conversion coefficients are not available for all imaging protocols used in the past, we had to derive DgN for many combinations of target-filter, peak tube potential and HVL and for three thicknesses of compressed breast tissue (3 cm, 5 cm, and 8 cm). As noted earlier, there are data supporting a correlation between breast size and glandularity (18,20,25,28). Those data together suggest that the glandularity proportion for small compressed breast thickness (CBT=3 cm) is actually about 52% and the glandularity proportion for a large compressed breast thickness (CBT=8 cm) is about 10%. Our estimates of the proportions of fibroglandular tissue utilized reported findings of the rate of change of fibroglandular tissue per cm of CBT (25) with a scale adjustment from 30% at 6 cm CBT to 20% CBT based on recent findings of Yaffe (28) suggesting that glandularity is usually over-estimated.
From the information collected on machine settings for each protocol, we were able to specify each spectrum in 0.5 keV increments, using data either from IPEM (69) or the models and data of Boone et al (70). From each of these spectra, we derived DgN values from calculations using the model formulation described by Boone (7) by assuming the geometry for the compressed breast as described and CBT values of 3, 5, and 8 cm.
To ensure reliability of our computed conversion coefficients, we compared our calculated values to those published by Wu (67). For Mo-Mo target-filter combination, peak tube potentials of 25, 27, and 29 kV, an HVL of 0.3 mm Al, and for CBT thicknesses of 3, 5, and 8 cm, the average agreement of our calculated conversion coefficients with those of Wu (67) was within 5%.
4. Estimation of the glandular tissue dose and the range of dose by time period
Glandular tissue doses were either estimated for full examinations (2 views per breast) or per film (i.e., per view) to achieve comparability. Glandular tissue doses for full examinations were divided by the number of views.
From each publication, we derived an average, minimum and maximum reported glandular tissue dose, but it was not possible in most cases to derive a full statistical distribution of doses from individual publications.
One major goal of our study was to develop a quantitative temporal summary of glandular tissue doses. For our purposes, we chose 5-year time periods since there were not sufficient data to estimate yearly changes in doses nor do changes in technology occur that frequently. If the period when breast examinations were conducted was cited in the report, we assigned the examination to the relevant 5-year period. If the year(s) during which the breast examination was performed was not cited, we assigned the glandular tissue dose to the years when the paper was published.
The glandular tissue dose assigned to each 5-year time period was the average of the mean values derived from each publication applicable to that time period. In general, equal weight was given to all publications as there was no specific evidence that greater relevance could be assigned to any individual publication. The minimum and maximum glandular tissue doses assigned to each 5-year period were obtained directly from the minimum and maximum values derived from the group of publications in that time period.
FINDINGS
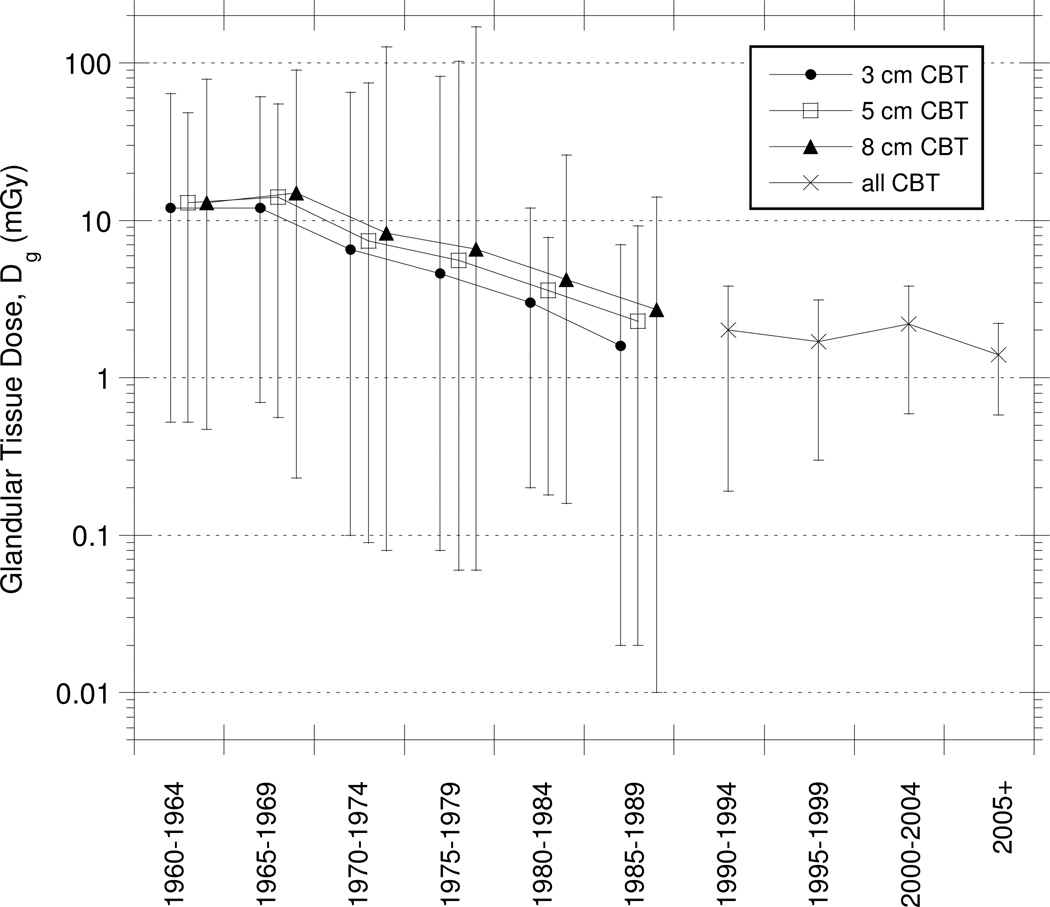
Our primary findings, summarized as means and ranges (approximately 90–95%), are provided for compressed breast thickness (cm), incident air kerma (Ka,i) (mGy) and glandular tissue dose (Dg) (mGy) in Figs. 3, 4 and 5, respectively.
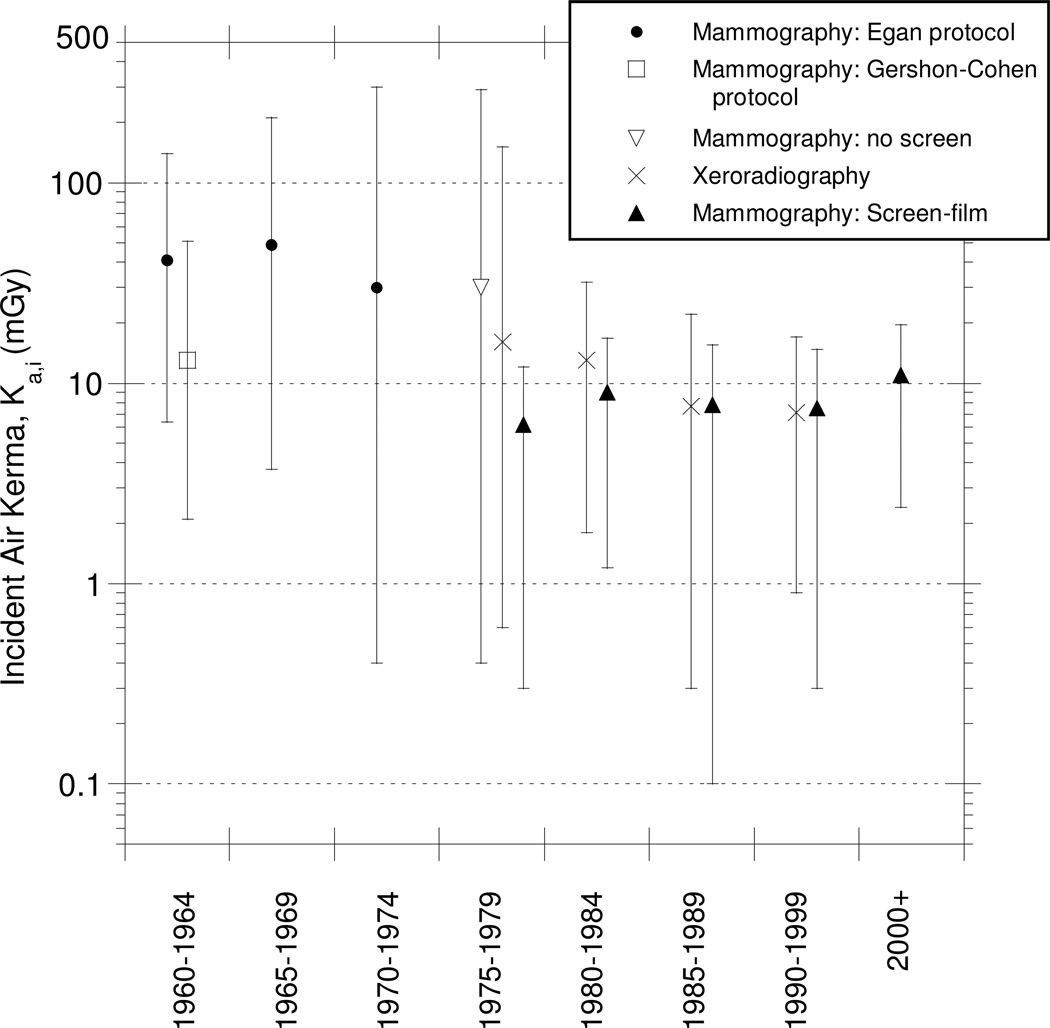
Fig. 4.
Summary of collected data (mean, minimum, maximum) of incident air kerma (Ka,i, mGy) for 5 cm compressed breast thickness (CBT) by time period and mammography protocol.
Fig. 5.
Derived population estimates (mean, minimum, maximum) of glandular tissue dose (mGy) from mammography by time period and compressed breast thickness (CBT).
1. Compressed breast thickness
The normal anatomic structure of the female breast can be characterized, in simplistic terms, by size, composition, and firmness of the tissue. Depending on these characteristics, the female breast can be of various dimensions when there is no physical constraint. It was recognized in the 1960s that a technique was needed to create a uniform thickness of tissue to be imaged though it was not until the 1970s that developments into what is today called “breast compression” were regularly implemented (12,19,21,71). Without uniform compression of the breast during imaging, differences in tissue thickness and composition can lead to difficulties in interpretation of mammograms as well as difficulties in limiting the dose to each woman.
While estimating glandular tissue dose to an individual patient can be done with good accuracy by utilizing the actual thickness of their compressed breast, reconstruction of typical glandular tissue doses, as undertaken in this paper, requires information on the distribution of compressed breast thicknesses of women undergoing mammography during each of the past decades.
From literature reports on variations in CBT (including Whall and Roberts (72), Bulling and Nicoll (30), Gentry and DeWerd (18), Heggie (20), Klein et al (25), Young et al. (31), Dance et al. (22) quoting Thilander (32), Young and Burch (33), Kruger and Schueler (27), Jamal et al. (73), Tsapaki (74)), we derived a composite distribution of CBTs measured in more than 48,000 mammograms for use in estimating the average, minimum, and maximum glandular tissue doses. Here, we define minimum and maximum to be approximately the 5 and 95 % quantiles of the CBT distribution.
Most of the individual publications cite the most common value for CBT to be about 5 cm, and we have confirmed from developing a composite distribution based on data from tens of thousands of mammograms that the mean and median CBT are about 5 to 6 cm. Moreover, from our analysis less than 5% of CBT values are less than 3 cm and less than 5% are greater than 8 cm. The composite distribution we constructed has good symmetry with only a slight suggestion of positive skewness (Fig. 3). In this analysis, we have derived approximate minimum glandular tissue doses for 3 cm CBT, average doses at 5 cm CBT, and approximate maximum doses at 8 cm CBT in order to roughly bound the 5th and 95th percentile of the distribution of doses received in the general population of women undergoing mammography.
2. Incident air kerma
We found that the mean Ka,i in the 1960s was typically about 13 mGy for the Gershon-Cohen imaging protocol and about 40 mGy for the Egan protocol. These values pertain to the average compressed breast thickness and were either significantly less and greater for thinner and thicker CBTs, respectively (Table 3). Estimated incidence air kerma values in this period ranged from a few mGy to more than 300 mGy. One plausible explanation for this wide range is that there were significant differences in the mid-1960s in the way the Gershon-Cohen protocol was implemented (75), leading to significant differences in Ka,i. There were also notable differences in application of the Egan protocol at different medical institutions (Fig. 4).
Table 3.
Derived estimates of mean, minimum and maximum values of incident air kerma (mGy) by time period, imaging protocol and compressed breast thickness (CBT) (all estimates presented to two significant digits).
| Period | Protocol | CBT | Incident Air Kerma (mGy) |
||
|---|---|---|---|---|---|
| (cm) | Mean | minimum | maximum | ||
| 1960–1964 | Egan | 3 | 36 | 4.2 | 120 |
| 5 | 41 | 6.4 | 140 | ||
| 8 | 51 | 4 | 310 | ||
| Gershon-Cohen | 3 | 7.1 | 1.2 | 29 | |
| 5 | 13 | 2.1 | 51 | ||
| 8 | 21 | 2.4 | 120 | ||
| 1965–1969 | Egan | 3 | 29 | 4.4 | 120 |
| 5 | 49 | 3.7 | 210 | ||
| 8 | 74 | 2.4 | 500 | ||
| 1970–1974 | Egan | 3 | 27 | 0.28 | 350 |
| 5 | 30 | 0.43 | 300 | ||
| 8 | 42 | 0.59 | 540 | ||
| 1975–1979 | Typical (non-screen) | 3 | 17 | 0.24 | 160 |
| 5 | 30 | 0.36 | 290 | ||
| 8 | 50 | 0.50 | 700 | ||
| Xeroradiography | 3 | 9.0 | 0.39 | 85 | |
| 5 | 16 | 0.59 | 150 | ||
| 8 | 27 | 0.81 | 370 | ||
| Screen-Film (Low-Dose) |
3 | 3.5 | 0.21 | 48 | |
| 5 | 6.2 | 0.32 | 85 | ||
| 8 | 10 | 0.44 | 210 | ||
| 1980–1984 | Xeroradiography | 3 | 7.4 | 1.2 | 18 |
| 5 | 13 | 1.8 | 32 | ||
| 8 | 23 | 2.5 | 79 | ||
| Screen-Film (Low-Dose) |
3 | 5.0 | 0.78 | 15 | |
| 5 | 9.0 | 1.2 | 27 | ||
| 8 | 15 | 1.6 | 66 | ||
| 1985–1989 | Xeroradiography | 3 | 4.3 | 0.2 | 13 |
| 5 | 7.7 | 0.29 | 22 | ||
| 8 | 13 | 0.41 | 47 | ||
| Screen-Film (Low-Dose) |
3 | 4.3 | 0.080 | 22 | |
| 5 | 7.8 | 0.12 | 40 | ||
| 8 | 13 | 0.17 | 83 | ||
| 1990–1999 | Xeroradiography | 3 | 4 | 0.59 | 8.8 |
| 5 | 7.1 | 0.90 | 17 | ||
| 8 | 12 | 1.23 | 39 | ||
| Screen-Film (Low-Dose) |
3 | 4.1 | 0.22 | 20 | |
| 5 | 7.5 | 0.33 | 35 | ||
| 8 | 13 | 0.46 | 74 | ||
| 2000- | Screen-Film (Low-Dose) | 3 | 5.0 | 1.2 | 14 |
| 5 | 11 | 2.4 | 25 | ||
| 8 | 19 | 3.3 | 58 | ||
The introduction of xeroradiography in the late 1970s and the first screen-film combinations (non-xeroradiographic systems) both resulted in a decrease of the mean Ka,i from earlier types of mammography. Xeroradiography and the first screen-film combinations resulted in decreases in the Ka,i in the late 1970s of about 45% and 80%, respectively. While xeroradiography provided improved levels of detail in the mammographic images, this technology was superseded in the 1990s by better screens and films. In general, thicker CBTs required higher Ka,i values to achieve the same image quality than did thinner CBTs (see Table 3).
The Ka,i values declined substantially beginning in the late 1970s to reach 8 to 10 mGy in the 1980s regardless of the imaging system used. However, screen-film systems subsequently became the accepted technology with xeroradiography disappearing from use altogether, with no major impact on the level of glandular tissue dose received by women.
3. Glandular Tissue Dose
Estimated glandular tissue doses throughout the 1960s were about 12 to 15 mGy on average with wide ranges, extending as high as 90 mGy. Similar to the results for Ka,i, glandular tissue doses for average and thicker CBTs were higher than doses for thinner CBTs (Table 4 and Fig. 5).
Table 4.
Derived estimates of mean, minimum and maximum values of Dg (mGy) by time period for populations of exposed women (all estimates presented to two significant digits).
| Period | CBT = 3 cm |
CBT = 5 cm |
CBT = 8 cm |
All CBT1 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | Mean | Min | Max | |
| 1960–1964 | 12 | 0.52 | 64 | 13 | 0.52 | 48 | 13 | 0.47 | 79 | - | - | - |
| 1965–1969 | 12 | 0.70 | 61 | 14 | 0.56 | 55 | 15 | 0.23 | 90 | - | - | - |
| 1970–1974 | 5.4 | 0.080 | 52 | 6.1 | 0.070 | 60 | 6.7 | 0.060 | 102 | - | - | - |
| 1975–1979 | 3.9 | 0.070 | 82 | 4.8 | 0.050 | 103 | 5.6 | 0.050 | 170 | - | - | - |
| 1980–1984 | 2.7 | 0.20 | 12 | 3.4 | 0.18 | 7.7 | 4 | 0.16 | 26 | - | - | - |
| 1985–1989 | 1.8 | 0.023 | 9.0 | 2.6 | 0.021 | 12 | 3 | 0.019 | 17 | - | - | - |
| 1990–1994 | 1.5 | 0.059 | 6.4 | 1.8 | 0.055 | 6.9 | 2 | 0.049 | 14.6 | 2 | 0.19 | 7.8 |
| 1995–1999 | 0.83 | 0.072 | 5.3 | 1.3 | 0.092 | 5.8 | 1.4 | 0.082 | 9.5 | 1.7 | 0.3 | 7.6 |
| 2000–2004 | 1.4 | 0.34 | 2.1 | 2 | 0.54 | 2 | 2.2 | 0.48 | 6.3 | 2.2 | 0.59 | 7.5 |
| 2005- | - | - | - | - | - | - | - | - | - | 1.4 | 0.58 | 2.9 |
Results from Tier 1 publications only. These are preferred values.
In the 1970’s, estimated glandular tissue doses declined on average about 50% from those in the 1960s. As mentioned in the UNSCEAR report in 1977 (76), the introduction of new technology such as the low-dose technique led to a considerable reduction of glandular tissue dose during the 1970’s. However, the range of doses was extremely wide for the full range of CBTs, up to 3000-fold from 0.05 mGy to 170 mGy. The wide range reflected the progressive introduction of new technology and efforts to optimize images while also reducing doses. The reduction in average glandular tissue dose, for average breast size, continued through the early 1980s to about 2 to 4 mGy. Low-dose screen-film combinations, introduced in the 1980s were a significant factor in lowering the glandular tissue doses.
Average estimated glandular tissue doses, changed little through the 1990s, though the variation in dose became smaller. Since the year 2000, reported glandular tissue doses for a CBT of 5 cm have generally ranged from 1.5 to 2 mGy but can vary from about one-half to about 4 times those estimates when considering the full range of CBTs and the specific techniques employed. In all time periods prior to 1990, when glandular tissue dose could be estimated as a function of CBT, breasts of thinner CBT received lower average doses than did breasts of thicker CBT. While it might be possible to obtain data as a function of CBT for years after 1990, the publications we assessed to be most reliable (Tier 1) emphasized determination of individual values for glandular tissue dose that were pooled into one distribution rather than as a function of CBT.
DISCUSSION
We found that glandular tissue doses from mammographic examinations declined significantly over time since 1960, from about 12 mGy (on average) to about 2 mGy or slightly less (on average).
We noted a large variation of glandular tissue dose in the 1960’s and 1970’s, with substantial differences according to the imaging protocol used. We found that the Egan imaging protocol led to higher glandular tissue doses than the Gershon-Cohen protocol during the 1960s. Modifications to those protocols in the 1970’s, along with the introduction of new technologies (screen-film mammography and xeroradiography) led to significant reductions in glandular tissue dose. This reduction in glandular tissue continued in the 1980s. By 1990, the total reduction from the 1960s was, on average, about 90%, along with substantial reduction in dose variation. The smaller variation in the 1990s suggested that mammographic protocols and practices had become harmonised within and between centres and countries.
In this analysis, it is our intention to captures at least 90% of the actual glandular tissue doses received and possibly closer to 95%, with estimated doses for women with three different compressed breast thicknesses (i.e., CBTs of 3, 5, and 8 cm) and associated glandularity (52%, 30% and 10%, respectively). While percent glandularity depends on breast size, it is also depends on the age of patient. Age-related variation in breast composition is an important determinant of the accuracy of screening mammography (77). Variation in breast cancer density during different phases of adulthood is an important consideration for when and how to carry out screening mammography (78), and the decrease in breast parenchymal density (and a corresponding decrease in breast metabolic activity) has been demonstrated using different imaging modalities (29).
In addition to the possible variation in glandularity according to breast size and age, there are several other potential sources of uncertainty. One component of variation is the force of compression used. Before the mid 1970’s, when compression plates were introduced, the breast was compressed with a cylindrical cone (79) which exerted only moderate force and resulted in only moderate physical compression. We conducted a sensitivity analysis to assess the potential differences in glandular tissue dose with less compression than currently used. If the CBT used in calculations is increased by 20% as a result of assuming less compressive force, glandular tissue dose is predicted to be only modestly higher, by 1 to 7%, depending on the original CBT. If the CBT used in calculations is allowed to increase by 40%, the increase in glandular tissue dose is predicted to be somewhat greater, 2 to 16 %, depending on the original CBT. This analysis suggested that our estimates of glandular tissue dose for the earlier years (pre-1975) when vigorous compression was not regularly used, might be underestimated by a maximum of 15 to 20%, assuming that, at worst case, CBT was 40% greater than today due to the routine use of low compressive force.
Another source of uncertainty in our study is the assumption we made for the estimation of glandular tissue dose for a single view when the publication reported a cumulative dose for a full examination. In that case, we divided the dose for the full examination by 2 or 3 depending on the number of views reported; however, this can slightly overestimate the dose for a single examination when the full examination includes several views. As noted by Burch (34) and Young (33,80), dose received during a two-view examination is less than twice the dose from a single view examination because the craniocaudal and the mediolateral oblique examinations do not result, on average, in equal doses. Glandular tissue doses from lateral views are higher (33,34,80). The overestimation of the glandular tissue dose as a result of dividing the cumulative examination dose by a whole number can be about 10% to 15% for a few cases (14,48). The reader is referred to ICRU (Table E.1) (6) for a discussion of at least seven different sources of uncertainty that can each contribute 3% to 19% variation.
It is also worthwhile to note that the radiologists or radiologic technologists conducting the imaging may adjust technical parameters of the imaging protocol for purposes of dose optimization and/or for image optimization, leading to a significant uncertainty in the actual glandular tissue doses received by a particular patient.
Where possible, we compared our findings for higher-income countries to the few publications from lower- or middle-income countries although the opportunity for a detailed systematic comparison was not possible due to limited data. For example, recent publications from Malaysia (16,73), and Saudi Arabia (81) reported estimated glandular tissue doses that were slightly higher compared to those in Europe and the U.S. This comparison was possible, however, only for recent years when data are relatively stable and consistent between publications.
In recent years, the data we report represent glandular doses from both screen-film and digital mammography. The performance of the two systems has been compared in recent publications (82–84) where it was shown that the use of anode-filter combinations such as Mo/Rh, W/Rh, Rh/Rh and Rh/Al in screen-film mammography provides better dose performance than Mo-Mo combinations in digital mammography (82). A reduction in glandular tissue dose of about 40% can be achieved with digital mammography compared with conventional slow film technique (61,83,58) but, as reported by Moran in 2005, there is little evidence of a consistent reduction in dose with digital mammography for smaller breasts (61). Those findings were recently confirmed by Hendrick et al. (84) who found in a large survey conducted by the American College of Radiology Imaging Network Digital Mammographic Imaging Screening Trial, that the glandular tissue dose is found to be about 22% lower, on average, for digital mammography compared with screen-film, with major differences, if any, only for larger breasts.
Comparison of our estimated values of glandular tissue dose from mammography over time with those described by other investigators for the same time-periods for women with 5 cm CBT demonstrates consistencies for periods following 1970 (12,85,86,87 quoting 58) but several reported glandular tissue dose estimates for periods before 1970 (12,86,87 quoting 58) were higher than our estimates of the average value of mean glandular dose (but were included within our upper bound). It is difficult to determine reasons for this difference, particularly because most publications of earlier doses lacked detailed documentation of the basis of their estimates (12,86) and did not quantitatively derive dose estimates over the range of CBT as done in our analysis.
Because of the limitations on the quality of information, especially in the 1960s, our findings for the earliest time period must be interpreted cautiously. The large variation in glandular tissue doses received among individual women during the 1960s, as well as the uncertainty of the dose received by any single woman, should be recognized. Nevertheless, this summary on glandular tissue doses received from mammography among women in higher-income countries is more comprehensive than earlier assessments. The historical quantitative data on glandular tissue doses for 1960 onward fills an important gap. Application of the dose estimates to epidemiologic studies of breast cancer risk will contribute valuable quantitative data for breast cancer risk estimates and also provide additional information for assessment of potential harms versus benefits from mammographic examinations.
ACKNOWLEDGEMENTS
The authors are grateful to Mrs Monika Moissonnier and Mr Brian Moroz for their assistance in completing calculations to derive conversion coefficients and to Mrs Moissonnier for her assistance in the development and management of the database of literature data. The authors are also appreciative of the assistance of Dr. J.M. Boone of UC Davis in providing some otherwise unavailable x-ray spectra. Dr Marvin Rosenstein is also warmly thanked for his valuable comments on the manuscript, especially on the use of appropriate radiation dose quantities. This work was partly financed by the European Commission under the EURATOM 6th Framework Programme as part of the GENE-RAD-RISK project (project number FP6-012926).
APPENDIX
This appendix presents: (1) details of the application of the Approximate Bayesian Computation (ABC) method to estimate the variation for incident air kerma (Ka,i) based on the data of Gentry and DeWerd (see Table A1) (18), and (2) detailed information derived from the literature on how mammography was conducted from 1960 to the present. Technical parameters (machine settings) for the mammography imaging protocols, by decade, are presented in Table A2. Conversion coefficients from incident air kerma (Ka,i) to glandular tissue dose are presented for thinner, average and thicker CBTs by time period and imaging protocol (Table A3), based on the possible target-filter combinations and values of peak tube voltage and filtration derived from the literature.
1. Application of ABC method to estimation of Ka,i
In reconstructing historical radiation doses, it may be necessary to use regression coefficients, confidence intervals, or prediction intervals for parameters used in dosimetry models. The difficulty in using literature data often arises because the raw dosimetry data are not available, rather, only summary statistics such as means and standard deviations are typically provided in publications. For example, the data of Gentry and DeWerd on Ka,i as a function of CBT is the most complete available though the “error bars” are inadequate to determine the actual variation beyond the one standard deviation (18). In this work, we used the Approximate Bayesian Computation (ABC) approach to estimate the 95% variation at each Ka,i based on the provided one standard deviation data.
In Gentry and DeWerd, the data available were: a histogram of compressed breast thickness (see Fig. 1 of(18)), the total number of individuals, and information on how CBT was rounded (with either a 0.5 cm or 1 cm resolution). We assumed CBT measurements were distributed as a truncated normal distribution with a lower cutoff point of 0.75 cm and an upper cutoff point of 10 cm (see Fig. 2 of this paper). The rounding of the CBT was described with a 0.5 or 1.0 cm resolution and we assumed these errors to occur in equal proportion. Using these assumptions, we estimated the mean and standard deviation for CBT to be 4.46 cm and 1.52 for a truncated normal distribution.
Based on the estimated mean, standard deviation, and proportions of rounding errors, we simulated Ka,i values as a function of compressed breast thickness. Using the ABC method, we used linear regression with log-transformed Ka,i values and simulated the compressed breast thickness values.
The steps in implementing the ABC method were as follows. First, we generated parameters, μ and σ, from prior distributions, π(μ) and π(σ) [step 1]. Then we simulated CBT data points (n=4400) based on a truncated normal distribution with mean=μ and SD=σ [step 2]. Next, we calculated distance ρ(Sd, Sd‘)<ε, where ρ(•) is a distance measure such as Euclidean or Mahalanobis distance measures and SD and Sd’ are summary statistics of data D and D’, respectively. In this case Sd are histogram frequencies of original data and SD’ are histogram frequencies from simulated dataset, D’, then we accepted μ and σ. Otherwise, we rejected μ and σ [step 3] and went back to step 1. We repeated these steps until we got accepted μ and σ values with pre-specified number (e.g., 10,000). Table A1 presents our estimates of the 5th and 95th percentile of Ka,i at 3 cm, 5 cm, and 8 cm CBT based on application of the ABC method.
Table A1.
Estimates of limits of variation of Ka,i based on data of Gentry and DeWerd(16)
| Incident Air Kerma (Ka,i) | ||
|---|---|---|
|
CBT (cm) |
5th percentile (mGy) |
95th percentile (mGy) |
| 3 | 0.29 | 1.8 |
| 5 | 0.43 | 3.1 |
| 8 | 0.60 | 7.6 |
2. Evolution of Mammographic Practices
1. Mammography prior to 1960
The earliest radiographic examination of breast tissue that used a technique similar to present-day mammography was conducted by Salomon in 1913 on 3,000 mastectomy specimens. His purpose was to compare roentgenographic findings with gross and microscopic anatomy evaluations (43). Since then, mammography techniques have evolved to improve image quality with the primary goal of improving the diagnosis of breast cancer.
In the 1930s and 1940s, mammography was still in an experimental and developmental phase (44). At that time, the focus was on the imaging of benign and pathological tumors (88,89). Radiation dosimetry was in its infancy and no attempt was made to estimate radiation dose quantities. In an effort to standardize and improve diagnosis of breast cancer, Leborgne described in 1951 one of the first imaging protocols, i.e., x-ray machine settings, often called technical parameters of radiographic technique and standardized practices (45). An important cancer follow-up study conducted by Egan in the late 1950s (90) reported the first large statistical analysis of the diagnostic value of mammography.
1. Mammography in the 1960’s
Throughout the 1960s, several publications described the use of mammography in detection of breast cancer (89,91–94). In the 1960s, two mammography imaging protocols were used: the Egan and Gershon-Cohen protocols. The primary factors specified in these mammography protocols were the maximum potential (peak kilovolts), beam filtration [given either in terms of added filtration (mm Al) or half-value layer (equivalent mm Al)], and x-ray beam intensity expressed as milliampere-seconds (mAs) [the product of the machine beam current (mA) and the exposure time (seconds)]. The glandular tissue dose received is directly related to x-ray beam intensity. The x-ray beam intensity is determined by x-ray field quantities such as the incident skin exposure (R) or incident air kerma (mGy) (both exclude backscattered radiation),, and entrance-surface exposure (R) or entrance-surface air kerma (mGy) (both include backscattered radiation)., These quantities implicitly account for mAs so a separate numerical value of mAs is usually not required for dose estimation. In some cases, the target-to-film distance (TFD) was also specified.
The Egan protocol was characterized by an electrical peak potential between 22 and 28 kV, with no added filtration (assuming 0.9 mm inherent filtration of the tube), a TFD of 30 to 40 inches and a mAs between 1500 and 1800. Some modifications were reported for different breast characteristics (46,75,90,95,96). The Gershon-Cohen protocol was characterized by an electrical peak potential between 25 and 30 kV, 0.5 mm Al added filtration (assuming 1 mm inherent filtration), a TFD of 18 inches and a mAs from 100 to 350 (47,75,91).
These two protocols were first implemented with a tungsten target and aluminium filter until the end of the 1960s when the Egan protocol was modified to be used with the Senographe which had a Mo-Mo target-filter combination with 0.4 mm Al inherent filtration (49). Several authors investigated potential modifications of these protocols with, for example, the introduction of low tube potential in Gershon-Cohen and a selection of various film types (75,95,97,98).
2. Mammography in the 1970’s
In the early 1970’s, the Senographe was widely introduced and the Egan protocol was adjusted for target filter combinations of W-Al and Mo-Mo. Later in this period, protocols were adapted for optimal practice with other new technology developments: implementation of a molybdenum rotating anode that allowed lower exposure times (99), introduction of several screen/film combinations and various filters (10,14,48–50) (Bicehouse HJ, 19751) and finally, the introduction of xeroradiography (23,51,52) (Bicehouse HJ, 19751). Screen-film technology was more sensitive than the non-screen film technology, allowing for lower exposure. In xeroradiography, the film was replaced by an aluminium plate coated with a layer of amorphous selenium (12). It was introduced to improve quality of the image for dense breasts and to visualize small disparities in density (44). Beginning in 1975, reports compared various radiation dose quantities for mammography from various systems and techniques. Bicehouse (Bicehouse HJ, 19751) in a survey conducted in 70 facilities compared the use of industrial film with no screen film technique, xerography, low-dose technique and high density technique. Rothenberg (51) compared the use of senographe with AA film, Xerox plate or Low-Dose vacuum cassette system and tungsten tube with Xerox plate. Similarly, several authors (44,52,100–104) conducted surveys to assess the overall quality of mammography systems, typically mammography with no screen, film-screen mammography (Low-Dose) and xeroradiography. After protocols were well defined for screen-film mammography and xeroradiography, the various radiation dose levels evaluated were comparable between the two systems.
3. Mammography in the 1980’s
The major improvements of technology during the 1980s included the introduction of anti-scatter grids and of automatic exposure controls (AEC) (21). In practice, regular surveys to estimate various radiation dose quantities for mammography were implemented by the U.S. Food and Drug Administration’s Nationwide Evaluation of X-ray Trends (NEXT) surveys on mammography units that were conducted in 1985 and 1988. Data from those surveys were analysed and published by several authors (53–57). Surveys were also regularly conducted in other countries such as Canada (105) and the Netherlands (106) to assess practices and estimate the various radiation dose quantities from typical protocols (film-screen with grid, film-screen without grid and xerography) and to survey mammography units in screening centres. These nationwide surveys led to adjustments of imaging protocols to optimize practices (e.g., maximize image information and minimize dose).
A 1980 report of the NCRP (122) provided recommendations on mammography practices for a variety of beam qualities in both screen-film technique and xeroradiography and the estimated incident skin exposure (R) associated with optimal images. Since then, several mammography user’s guides were published with recommendations on best practices (59,60).
4. Mammography in the 1990s
Xeroradiography was still in use in the 1990s but screen-film mammography remained the primary technology used. No major changes in imaging protocols were reported in that time period with the exception of the introduction of rhodium as target and filter material (9,11).
Surveys to estimate various radiation dose quantities and image quality were regularly conducted in the U.S. and Canada (9,55–58,107,108), and in some cases, individual glandular tissue doses were assessed (18,27,109).
In Europe, radiation dose investigations were also performed in several countries, with most involving women in screening programs (20,25,30–40,123). In some cases, glandular tissue doses were estimated from the specifications of the mammography units considering the characteristics of a standard breast (72,110–115). Surveys in conjunction with implementation of accreditation programs contributed to reducing glandular tissue doses (65,124).
5. Mammography after 2000
Similar quality assurance surveys to those performed in 1990s were also conducted in recent years (8,74,80,116–118).
Mammography techniques leading to computer storage of images in digital form was developed in the 1990s and was made widely available around 2000 (119). Even though digital mammography has been implemented in some screening programs (120,121), it has not yet widely replaced mammography performed with conventional technology. Current developments of the digital mammography and promising improvements of the quality of images are expected to increase the implementation of this relatively new technology. Quality assurance programs dedicated to digital mammography are therefore being developed by the American College of Radiology, U.S. Food and Drug Administration, the European Commission, and the International Atomic Energy Agency (12).
Table A2.
Details of mammography protocols by time period.
| Period | Mammography technique |
Target-filter materials |
CBTa | kVb | HVL or filtrationc | References | ||
|---|---|---|---|---|---|---|---|---|
| (cm) | min | max | min | max | ||||
| 1960–1964 | Egan protocold | W-Al | 3 | 22e | 24e | 0.9 mm inherent filtrationf | (46,75,90,95–97) | |
| 5 | 26e | 35f | ||||||
| 8 | 26e | 35f | ||||||
| Gershon-Cohen protocol |
W-Al | All | 25f | 30f | 1.5 mm Al total filtration (1 mm inherent) f |
(47,75) | ||
| 1965–1969 | Egan protocol | Mo-Mo | All | 26g | 30h,i | HVL=0.4h | HVL=0.61g | (14,49) |
| 0.78 mm Al filtration | (14,48) | |||||||
| 1970–1974 | Egan Protocol | W-Al | All | 30j | 32j | HVL=0.44k | HVL=0.66l | (10) |
| Mo-Mo | All | 26m | 35j | HVL=0.36j | HVL=0.6j | (10) | ||
| Mo-Mo | All | 26m | 35j | 0.78 mm Al filtration m | (14,48) | |||
| 1975–1979 | Mammography (no screen) |
W-Al | 5 | 25n,o | 40p,o | HVL=0.44kq | HVL=0.66l,q | (10,52,122) Bicehouse, 1975n |
| Mo-Mo | 5 | 25p,o | 40o | HVL=0.35r | HVL=0.6q | (10,51,52,122) | ||
| Xeroradiography | W-Al | 5 | 34r | 60p,q | 0.7 mm Alr | 3.5 mm Alp | (51,52,122) Bicehouse, 1975n |
|
| Mo-Al | 5 | 35p,o | 52p | 0.5 mm Alp | 2.5 mm Alp | |||
| Mo/W | 5 | 35p,o | 55p,o | 0.5 mm Alp,o | 3.5 mm Al p,o | |||
| Screen-Film (Low-Dose) |
Mo-Mo | 5 | 25n | 35n | HVL=0.35r | (52) Bicehouse, 1975n | ||
| Mo/W | 5 | 25p,o | 40p,o | 0.03 mm Mop,o | (52,59,66,122) | |||
| 1980–1984 | Xeroradiography | W-Al | All | 40s | 55s | HVL=1 mm Als | (59,66) | |
| Screen-Film (Low-Dose) |
Mo-Mo | All | 28s | 28s | HVL=0.31 mm Als | |||
| 1985–1989 | Xeroradiography | W | All | 44t | 45t | HVL=1.26t | HVL=1.45t | (53,55) |
| Screen-Film (Low-Dose) |
Mo-Mo | All | 27u | 29u | HVL=0.37t | HVL=0.49t | (53,55,106) | |
| 1990–1999 | Xeroradiography | W-Al | All | 46v | 46v | HVL=1.3v | HVL=0.37v | (53) |
| Screen-Film (Low-Dose) |
Mo-Mo | All | 25w | 28w,x | HVL=0.35y | (35,55,114,123) | ||
| 2000–2005+ | Screen-Film (Low-Dose) |
Mo-Mo | All | 24z | 28w | 0.03 mm Mow | (117) | |
CBT is compressed breast thickness (cm)
kV is peak electrical potential, i.e., peak kilovoltage (volts x 1000)
Half-value layer (HVL in mm Al equivalent) or beam filtration (mm Al)
Axillary views: peak kV= 54 and mAs= 1050, from Egan, 1963.
From Egan, 1964
From Stanton 1964
From Palmer 1970
From Gilbertson 1970, total filtration = 0.4 mm Al (correspond to HVL=0.4 with Mo target and glass window)
Axillary view peak kV=50 to 54
From Palmer, 1971
HVL=0.44 corresponds to 1.5 mm Al total filtration for tungsten target with beryllium window
HVL=0.66 corresponds to 1 mm Al filtration for tungsten target with glass window
From Gilbertson 1970 a&b
From Bicehouse, 1975 (H. J. Bicehouse. Survey of Mammographic exposure levels and techniques used in Eastern Pennsylvania. Proceedings of the Seventh Annual National Conference on Radiation Control. April 27 - May 2. Hyannis, Massachussetts, 1975)
From NCRP, 1980
From Snyder 1977
From Palmer, 1971
From Rothenberg, 1975
From Stanton 1984, NRCP 1986
From Conway 1990, 1994
From Conway 1990, 1994; confirmed by Zoetelief 1992
From Conway 1990
From Eklund 1993
From Young 1993
From Conway 1994
From Warren-Forward, 2004
Table A3.
Conversion coefficients (this work) derived for typical protocols by time period and compressed breast thickness (CBT).
| Period | Technique | Target-filter | HVLaor filtration | CBT (cm) |
kVb | Conversion coefficient (DgN) |
||
|---|---|---|---|---|---|---|---|---|
| min | max | min | max | |||||
| 1960–1964 | Egan | W-Al | 0.9 mm Al inherent | 3 | 22 | 24 | 0.305 | 0.353 |
| 5 | 26 | 35 | 0.258 | 0.377 | ||||
| 8 | 26 | 35 | 0.169 | 0.255 | ||||
| Gershon-Cohen | W-Al | 1.5 mm Al 1 mm inherent |
3 | 25 | 30 | 0.449 | 0.535 | |
| 5 | 25 | 30 | 0.294 | 0.365 | ||||
| 8 | 25 | 30 | 0.192 | 0.243 | ||||
| 1965–1969 | Egan | Mo-Mo | HVL = 0.4 | 3 | 26 | 30 | 0.305 | 0.309 |
| 5 | 26 | 30 | 0.190 | 0.194 | ||||
| 8 | 26 | 30 | 0.123 | 0.126 | ||||
| HVL = 0.61 | 3 | 26 | 30 | 0.449 | 0.453 | |||
| 5 | 26 | 30 | 0.290 | 0.297 | ||||
| 8 | 26 | 30 | 0.189 | 0.195 | ||||
| 0.78 mm Al | 3 | 26 | 30 | 0.347 | 0.375 | |||
| 5 | 26 | 30 | 0.217 | 0.238 | ||||
| 8 | 26 | 30 | 0.141 | 0.156 | ||||
| 1970–1974 | Egan | W-Al | HVL = 0.44 | 3 | 30 | 32 | 0.367 | 0.373 |
| 5 | 30 | 32 | 0.242 | 0.249 | ||||
| 8 | 30 | 32 | 0.160 | 0.166 | ||||
| HVL = 0.66 | 3 | 30 | 32 | 0.493 | 0.496 | |||
| 5 | 30 | 32 | 0.333 | 0.338 | ||||
| 8 | 30 | 32 | 0.222 | 0.226 | ||||
| Mo-Mo | HVL = 0.36 | 3 | 26 | 35 | 0.277 | 0.291 | ||
| 5 | 26 | 35 | 0.172 | 0.186 | ||||
| 8 | 26 | 35 | 0.111 | 0.118 | ||||
| HVL = 0.6 | 3 | 26 | 35 | 0.442 | 0.465 | |||
| 5 | 26 | 35 | 0.285 | 0.309 | ||||
| 8 | 26 | 35 | 0.186 | 0.205 | ||||
| 0.78 mm Al | 3 | 26 | 35 | 0.347 | 0.425 | |||
| 5 | 26 | 35 | 0.217 | 0.278 | ||||
| 8 | 26 | 35 | 0.141 | 0.183 | ||||
| 1975–1979 | Mammography (no screen) |
W-Al | HVL = 0.44 | 3 | 25 | 40 | 0.338 | 0.397 |
| 5 | 25 | 40 | 0.216 | 0.273 | ||||
| 8 | 25 | 40 | 0.141 | 0.185 | ||||
| HVL = 0.66 | 3 | 25 | 40 | 0.454 | 0.507 | |||
| 5 | 25 | 40 | 0.298 | 0.354 | ||||
| 8 | 25 | 40 | 0.195 | 0.241 | ||||
| Mo-Mo | HVL = 0.35 | 3 | 25 | 40 | 0.269 | 0.292 | ||
| 5 | 25 | 40 | 0.166 | 0.188 | ||||
| 8 | 25 | 40 | 0.108 | 0.120 | ||||
| HVL = 0.6 | 3 | 25 | 40 | 0.441 | 0.414 | |||
| 5 | 25 | 40 | 0.283 | 0.270 | ||||
| 8 | 25 | 40 | 0.184 | 0.179 | ||||
| W Al | 0.7 mm Al | 3 | 34 | 60 | 0.446 | 0.612 | ||
| 5 | 34 | 60 | 0.303 | 0.447 | ||||
| 8 | 34 | 60 | 0.203 | 0.313 | ||||
| 3.5 mm Al | 3 | 34 | 60 | 0.732 | 0.886 | |||
| 5 | 34 | 60 | 0.532 | 0.886 | ||||
| 8 | 34 | 60 | 0.366 | 0.501 | ||||
| Mo-Al | 0.5 mm Al | 3 | 35 | 52 | 0.399 | 0.500 | ||
| 5 | 35 | 52 | 0.258 | 0.339 | ||||
| 8 | 35 | 52 | 0.170 | 0.230 | ||||
| 2.5 mm Al | 3 | 35 | 52 | 0.580 | 0.840 | |||
| 5 | 35 | 52 | 0.405 | 0.645 | ||||
| 8 | 35 | 52 | 0.276 | 0.468 | ||||
| Mo/W-Al | 0.5 mm Al | 3 | 35 | 55 | 0.391 | 0.513 | ||
| 5 | 35 | 55 | 0.258 | 0.359 | ||||
| 8 | 35 | 55 | 0.171 | 0.247 | ||||
| 3.5 mm Al | 3 | 35 | 55 | 0.710 | 0.961 | |||
| 5 | 35 | 55 | 0.516 | 0.757 | ||||
| 8 | 35 | 55 | 0.357 | 0.558 | ||||
| Screen Film (Low-Dose) |
Mo-Mo | HVL = 0.35 | 3 | 25 | 35 | 0.269 | 0.284 | |
| 5 | 25 | 35 | 0.166 | 0.181 | ||||
| 8 | 25 | 35 | 0.108 | 0.116 | ||||
| Mo/W-Mo | 0.03 mm Mo | 3 | 25 | 40 | 0.217 | 0.298 | ||
| 5 | 25 | 40 | 0.134 | 0.194 | ||||
| 8 | 25 | 40 | 0.087 | 0.129 | ||||
| 1980–1984 | Xeroradiography | W-Al | HVL = 1 | 3 | 40 | 55 | 0.652 | 0.643 |
| 5 | 40 | 55 | 0.469 | 0.470 | ||||
| 8 | 40 | 55 | 0.323 | 0.329 | ||||
| Screen Film (Low-Dose) |
Mo-Mo | HVL = 0.31 | 3 | 28 | - | 0.252 | - | |
| 5 | 28 | - | 0.156 | - | ||||
| 8 | 28 | - | 0.101 | - | ||||
| 1985–1989 | Xeroradiography | W-Al | HVL = 1.26 | 3 | 44 | 45 | 0.714 | 0.713 |
| 5 | 44 | 45 | 0.524 | 0.524 | ||||
| 8 | 44 | 45 | 0.365 | 0.366 | ||||
| Screen Film (Low-Dose) |
Mo-Mo | HVL = 0.37 | 3 | 27 | 29 | 0.286 | 0.289 | |
| 5 | 27 | 29 | 0.178 | 0.180 | ||||
| 8 | 27 | 29 | 0.115 | 0.117 | ||||
| HVL = 0.49 | 3 | 27 | 29 | 0.368 | 0.370 | |||
| 5 | 27 | 29 | 0.232 | 0.234 | ||||
| 8 | 27 | 29 | 0.151 | 0.152 | ||||
| -1999 | Xeroradiography | W-Al | HVL = 1.3 | 3 | 46 | - | 0.726 | - |
| 5 | 46 | - | 0.536 | - | ||||
| 8 | 46 | - | 0.375 | - | ||||
| Screen Film (Low-Dose) |
Mo-Mo | HVL = 0.35 | 3 | 25 | 28 | 0.269 | 0.274 | |
| 5 | 25 | 28 | 0.166 | 0.170 | ||||
| 8 | 25 | 28 | 0.108 | 0.111 | ||||
| HVL = 0.37 | 3 | 25 | 28 | 0.283 | 0.287 | |||
| 5 | 25 | 28 | 0.175 | 0.179 | ||||
| 8 | 25 | 28 | 0.113 | 0.116 | ||||
| 2000+ | Screen Film (Low-Dose) |
Mo-Mo | 0.03 mm Mo | 3 | 24 | 28 | 0.241 | 0.252 |
| 5 | 24 | 28 | 0.149 | 0.156 | ||||
| 8 | 24 | 28 | 0.097 | 0.101 | ||||
HVL values in mm Al
kV is peak electrical potential, i.e., peak kilovoltage (volts x 1000)
Footnotes
H. J. Bicehouse. Survey of Mammographic exposure levels and techniques used in Eastern Pennsylvania. Proceedings of the Seventh Annual National Conference on Radiation Control. April 27 - May 2. Hyannis, Massachussetts, 1975.
REFERENCES
- 1.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151(10):727–42. doi: 10.1059/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IARC working group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans - Ionizing Radiation, part 1: X- and gamma (g) - radiation, and neutrons. Volume 75. Lyon: IARC Press; 2000. p. 491. [Google Scholar]
- 3.Land CE, Tokunaga M, Koyama K, Soda M, Preston DL, Nishimori I, et al. Incidence of female breast cancer among atomic bomb survivors, Hiroshima and Nagasaki, 1950–1990. Radiat Res. 2003;160:707–717. doi: 10.1667/rr3082. [DOI] [PubMed] [Google Scholar]
- 4.Pukkala E, Kesminiene A, Poliakov S, Ryzhov A, Drozdovitch V, Kovgan L, et al. Breast cancer in Belarus and Ukraine after the Chernobyl accident. Int J Cancer. 2006;119(3):651–658. doi: 10.1002/ijc.21885. [DOI] [PubMed] [Google Scholar]
- 5.International Commission on Radiation Units and Measurements (ICRU) Fundamental Quantities and Units for Ionizing Radiation. Bethesda (MD): ICRU; 1998. p. 16. Report 60. [Google Scholar]
- 6.International Commission on Radiation Units and Measurements (ICRU) Patient dosimetry for x rays used in medical imaging. Bethesda MD: ICRU; 2005. p. 113. Report 74. [Google Scholar]
- 7.Boone JM. Glandular breast dose for monoenergetic and high-energy X-ray beams: Monte Carlo assessment. Radiology. 1999;213(1):23–37. doi: 10.1148/radiology.213.1.r99oc3923. [DOI] [PubMed] [Google Scholar]
- 8.Chevalier M, Moran P, Ten JI, Fernandez JM, Capeda T, Vano E. Patient dose in digital mammography. Med Phys. 2004;31(9):2471–2479. doi: 10.1118/1.1784591. [DOI] [PubMed] [Google Scholar]
- 9.Monticciolo DL, Sprawls P, Kruse BD, Peterson JE. Optimization of radiation dose and image quality in mammography. A clinical evaluation of Rhodium versus molybdenium. South Med J. 1996;89(4):391–394. doi: 10.1097/00007611-199604000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Palmer RC, Egan RL, Tanner BK, Barnette PA. Absorbed dose in mammography using three tungsten and three molybdenum target tubes. Radiology. 1971;101:697–699. doi: 10.1148/101.3.697. [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Gingold EL, Barnes GT, Tucker DM. Normalized average glandular dose in molybdenum target-rhodium filter and rhodium target-rhodium filter mammography. Radiology. 1994;193:83–89. doi: 10.1148/radiology.193.1.8090926. [DOI] [PubMed] [Google Scholar]
- 12.Yaffe MJ, Mainprize JG, Jong RA. Technical developments in mammography. Health Phys. 2008;95(5):599–611. doi: 10.1097/01.HP.0000327648.42431.75. [DOI] [PubMed] [Google Scholar]
- 13.Trout ED. The life history of an x-ray beam. Radiol Technol. 1963;35:161–170. [PubMed] [Google Scholar]
- 14.Gilbertson JD, Randall MG, Fingerhut AG. Evaluation of roentgen exposure in mammography. I. six views. Radiology. 1970;95:383–394. doi: 10.1148/95.2.383. [DOI] [PubMed] [Google Scholar]
- 15.Maudal S. Iron filters as a mean of reducing the dose in roentgen examination of the female breast. Acta Radiol Ther Phys Biol. 1968;7(3):238–240. doi: 10.3109/02841866809133197. [DOI] [PubMed] [Google Scholar]
- 16.Lee KH, Kandaiya S. Estimation of breast radiation dose in a mammographic system. Appl Radiat Isot. 1996;47(3):361–363. doi: 10.1016/0969-8043(95)00294-4. [DOI] [PubMed] [Google Scholar]
- 17.Rosenstein M, Andersen LW, Warner GG. Rockville,MD: US Department of Health and Human Services; 1985. Handbook of glandular tissue doses in mammography; p. 16.FDA Report 85–8239 [Google Scholar]
- 18.Gentry JR, DeWerd LA. TLD measurements of In Vivo mammographic exposures and the calculated mean glandular dose across the United States. Med Phys. 1996;23(6):899–903. doi: 10.1118/1.597824. [DOI] [PubMed] [Google Scholar]
- 19.Hendee WR. History and status of x-ray mammography. Health Phys. 1995;69(5):636–648. doi: 10.1097/00004032-199511000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Heggie JCP. Survey of doses in screnning mammography. Austral Phys Eng Sci Med. 1996;19(4):207–216. [PubMed] [Google Scholar]
- 21.Law J. The development of mammography. Phys Med Biol. 2006;51(13):R155–R167. doi: 10.1088/0031-9155/51/13/R10. [DOI] [PubMed] [Google Scholar]
- 22.Dance DR, Skinner CL, Alm Carlsson G. Breast dosimetry. Appl Radiat Isot. 1999;50(1):185–203. doi: 10.1016/s0969-8043(98)00047-5. [DOI] [PubMed] [Google Scholar]
- 23.Hammerstein GR, Miller DW, White DR, Masterson ME, Woodard HQ, Laughlin JS. Absorbed radiation dose in mammography. Radiology. 1979;130(2):485–491. doi: 10.1148/130.2.485. [DOI] [PubMed] [Google Scholar]
- 24.Woodward HQ, White DR. The composition of body tissues. Br J Radiol. 1986;59:1209–1219. doi: 10.1259/0007-1285-59-708-1209. [DOI] [PubMed] [Google Scholar]
- 25.Klein R, Aichinger H, Dierker J, Jansen JT, Joite-Barfuss S, Sabel M, et al. Determination of average glandular dose with modern mammography units for two large groups of patients. Phys Med Biol. 1997;42(4):651–671. doi: 10.1088/0031-9155/42/4/004. [DOI] [PubMed] [Google Scholar]
- 26.Geise RA, Palchevsky A. Composition of mammographic phantom materials. Radiology. 1996;198(2):347–250. doi: 10.1148/radiology.198.2.8596830. [DOI] [PubMed] [Google Scholar]
- 27.Kruger RL, Schueler BA. A survey of clinical factors and patient dose in mammography. Med Phys. 2001;28(7):1449–1454. doi: 10.1118/1.1382606. [DOI] [PubMed] [Google Scholar]
- 28.Yaffe MJ, Boone JM, Packard N, Onzo-Proulx O, Huang SY, Peressotti CL, et al. The myth of the 50-50 breast. Med Phys. 2009;36(12):5437–5443. doi: 10.1118/1.3250863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abramson RG, Mavi A, Cermik T, Basu S, Wehrli NE, Houseni M, et al. Age-related structural and functional changes in the breast: multimodality correlation with digital mammography, computed tomography, magnetic resonance imaging, and positron emission tomography. Semin Nucl Med. 2007;37(3):146–153. doi: 10.1053/j.semnuclmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Bulling SM, Nicoll JJ. Level And Distribution of the Radiation Dose to the Population from a Mammography Screening Programme in New Zealand. Radiat Prot Dosim. 1995;57:455–458. [Google Scholar]
- 31.Young KC, Ramsdale ML, Rust A, Cooke J. Effect of automatic kV selection on dose and contrast for a mammographic X-ray system. Br J Radiol. 1997;70:1036–1042. doi: 10.1259/bjr.70.838.9404208. [DOI] [PubMed] [Google Scholar]
- 32.Thilander-Klang AC, Ackerholm PH, Berlin IC, Bjurstam NG, Mattsson SL, Mansson LG, et al. Influence of anode-filter combinations on image quality and radiation dose in 965 women undergoing mammography. Radiology. 1997;203(2):348–354. doi: 10.1148/radiology.203.2.9114087. [DOI] [PubMed] [Google Scholar]
- 33.Young KC, Burch A. Radiation doses received in the UK Breast Screening Programme in 1997 and 1998. Br J Radiol. 2000;73(867):278–287. doi: 10.1259/bjr.73.867.10817044. [DOI] [PubMed] [Google Scholar]
- 34.Burch A, Goodman DA. A pilot survey of radiation doses received in the United Kingdom Breast Screening Programme. Br J Radiol. 1998;71(845):517–527. doi: 10.1259/bjr.71.845.9691897. [DOI] [PubMed] [Google Scholar]
- 35.Funke M. Magnification survey and spot view mammography with a new microfocus X-ray unit: detail resolution and radiation exposure. Eur Radiol. 1998;8(3):386–390. doi: 10.1007/s003300050399. [DOI] [PubMed] [Google Scholar]
- 36.Leitz W, Jönsson H. Patient dose levels from X-ray examinations in Sweden (Synthesis of results from public medical service 1999) Stockholm: Swedish Radiation Protection Authority; 2001. SSI Report 2001: 01. [Google Scholar]
- 37.Moran P, Chevalier M, Vaño E. Comparative study of dose values and image quality in mammography in the area of Madrid. Br J Radiol. 1994;67:556–563. doi: 10.1259/0007-1285-67-798-556. [DOI] [PubMed] [Google Scholar]
- 38.Moran P, Chevalier M, Pombar M, Lobato R, Vaño E. Breast Doses from Patients and from a Standard Phantom: Analysis of Differences. Radiat Prot Dosim. 2000;90(1–2):117–121. [Google Scholar]
- 39.Warren RM, Duffy S. A comparison of the effectiveness of 28 kV (grid) versus 25 kV (no grid) mammographic techniques for breast screening. Br J Radiol. 1997;70(838):1022–1027. doi: 10.1259/bjr.70.838.9404206. [DOI] [PubMed] [Google Scholar]
- 40.Young KC, Ramsdale ML, Bignell F. Review of dosimetric methods for mammography in the UK breast screening programme. Radiat Prot Dosim. 1998;80(1–3):183–186. [Google Scholar]
- 41.Perry N, Broeders M, de Wolf C, Törnberg S, Holland R, von Karsa L, editors. European Commission European guidelines for quality assurance in breast cancer screening and diagnosis. 4th ed. Luxembourg: Office for Official Publications of the European Communities; 2006. [DOI] [PubMed] [Google Scholar]
- 42.Baker LH. Breast Cancer Detection Demonstration Project: five-year summary report. CA Cancer J Clin. 1982;32(4):194–225. doi: 10.3322/canjclin.32.4.194. [DOI] [PubMed] [Google Scholar]
- 43.Salomon A. Beitrage zur Phatologic und Klinic der Mammakarzinoms. Arch Klin Chir. 1913;101:573–668. [Google Scholar]
- 44.Wolfe JN. Developments in mammography. Am J Obstet Gynecol. 1976;124(3):312–323. doi: 10.1016/0002-9378(76)90164-2. [DOI] [PubMed] [Google Scholar]
- 45.Leborgne R. Diagnostic of tumors in the breast by simple roentgenography. Calcification in carcinomas. Am J Roentgenol Radium Ther. 1951;65(1):1–11. [PubMed] [Google Scholar]
- 46.Egan RL. Mammography. Am J Surg. 1963;106:421–429. doi: 10.1016/0002-9610(63)90125-9. [DOI] [PubMed] [Google Scholar]
- 47.Gershon-Cohen J, Berger SM, Delpino L. Mammography: Some remarks on techniques. Radiol Clin North Am. 1965;3(3):389–401. [PubMed] [Google Scholar]
- 48.Gilbertson JD, Randall MG, Fingerhut AG. Evaluation of roentgen exposure in mammography. II. Four views. Radiology. 1970;97(3):641–648. doi: 10.1148/97.3.641. [DOI] [PubMed] [Google Scholar]
- 49.Palmer RC, Egan RL, Barrett BS. Preliminary evaluation of absorbed dose in Mammography. Six views. Radiology. 1970;95:395–397. doi: 10.1148/95.2.395. [DOI] [PubMed] [Google Scholar]
- 50.Ostrum BJ, Becker W, Isard HJ. Low-dose mammography. Radiology. 1973;109:323–326. doi: 10.1148/109.2.323. [DOI] [PubMed] [Google Scholar]
- 51.Rothenberg LN, Kirch RLA, Snyder RE. Patient exposures from film and xeroradiographic mammographic techniques. Radiology. 1975;117:701–703. doi: 10.1148/117.3.701. [DOI] [PubMed] [Google Scholar]
- 52.Snyder RE, Masterson ME, Kirch RLA, Rothenberg LN, Hammerstein GR, Laughlin JS. Image quality control and radiation exposure in mammography. In: Logan Wende., editor. Breast Carcinoma, the radiologist's expanded view. New York: John Wiley & Sons, Inc; 1977. pp. 167–176. [Google Scholar]
- 53.Conway BJ, McCrohan J-L, Rueter FG, Suleiman OH. Mammography in the eighties. Radiology. 1990;177:335–339. doi: 10.1148/radiology.177.2.2217765. [DOI] [PubMed] [Google Scholar]
- 54.Rueter FG, Conway BJ, McCrohan J-L, Slayton OH, Suleiman OH. Assessement of skin entrance kerma in the United States: the nationwide evaluation of X-ray trends (NEXT) Radiat Prot Dosim. 1992;43(1–4):71–73. [Google Scholar]
- 55.Conway BJ, Suleiman OH, Rueter FG, Anstonsen RG, Slayton RJ. National survey of mammographic facilities in 1985, 1988 and 1992. Radiology. 1994;191(2):323–330. doi: 10.1148/radiology.191.2.8153301. [DOI] [PubMed] [Google Scholar]
- 56.Suleiman OH, Spelic DC, McCrohan JL, Symonds GR, Houn F. Mammography in the 1990s: the United States and Canada. Radiology. 1999;210(2):345–351. doi: 10.1148/radiology.210.2.r99fe45345. [DOI] [PubMed] [Google Scholar]
- 57.Suleiman OH, Stern SH, Spelic DC. Patient dosimetry activities in the United States: the nationwide evaluation of X-ray trends (NEXT) and tissue dose handbooks. Appl Radiat Isot. 1999;50:247–259. doi: 10.1016/s0969-8043(98)00073-6. [DOI] [PubMed] [Google Scholar]
- 58.Spelic D, Kaczmarek R, Hilohi M, Belella S. United States radiological health activities: inspection results of mammography facilities. Biomed Imaging Interv J. 2007;3(2):e35. doi: 10.2349/biij.3.2.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.National Council on Radiation Protection Measurements (NCRP) Mammography - a user's guide. Bethesda (MD): Library of congress; 1987. NCRP report 85; p. 178. [Google Scholar]
- 60.National Council on Radiation Protection Measurements (NCRP) A guide to mammography and other breast imaging procedures. Bethesda (MD): Library of congress; 2004. NCRP report 149; p. 379. [Google Scholar]
- 61.Moran P, Chevalier M, Ten JI, Fernandez Soto JM, Vaño E. A survey of patient dose and clinical factors in a full-field digital mammography system. Radiat Prot Dosim. 2005;114(1–3):375–379. doi: 10.1093/rpd/nch514. [DOI] [PubMed] [Google Scholar]
- 62.Bor D, Tukel S, Olgar T, Aydin E. Variations in breast doses for an automatic mammography unit. Diagn Interv Radiol. 2008;14(3):122–126. [PubMed] [Google Scholar]
- 63.Oliveira LC, Dias TK, Lopes RT, Kodlulovich S. Evaluation of the entrance surface air kerma in mammographic examinations in Rio de Janeiro, Brazil. Radiat Prot Dosim. 2009;133(3):136–143. doi: 10.1093/rpd/ncp031. [DOI] [PubMed] [Google Scholar]
- 64.Beaumont MA, Zhang W, Balding DJ. Approximate Bayesian computation in population genetics. Genetics. 2002;162(4):2025–2035. doi: 10.1093/genetics/162.4.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.European Commission. European protocol for dosimetry in mammography. Luxembourg: Office for Official Publications of the European Communities; 1998. Report EUR 16263; p. 76. [Google Scholar]
- 66.Stanton L, Villafana T, Day JL, Lightfoot DA. Dosage evaluation in mammography. Radiology. 1984;150:577–584. doi: 10.1148/radiology.150.2.6691119. [DOI] [PubMed] [Google Scholar]
- 67.Wu X, Barnes GT, Tucker DM. Spectral dependence of glandular tissue dose in screen-film mammography. Radiology. 1991;179:143–148. doi: 10.1148/radiology.179.1.2006265. [DOI] [PubMed] [Google Scholar]
- 68.Zoetelief J, Jansen JTM. Calculation of air kerma to average glandular tissue dose conversion factors for mammography. Radiat Prot Dosim. 1995;57(1–4):397–400. [Google Scholar]
- 69.Institute of Physics and Engineering in Medicine (IPEM) Catalogue of Diagnostic X-Ray Spectra and Other Data. York, UK: Institute of Physics and Engineering; 1997. [Google Scholar]
- 70.Boone JM, Fewell TR, Jennings RJ. Molybdenum, rhodium, and tungsten anode spectral models using interpolating polynomials with application to mammography. Med Phys. 1997;24(12):1863–1874. doi: 10.1118/1.598100. [DOI] [PubMed] [Google Scholar]
- 71.Gros CM. [Methodology] J Radiol Electrol Med Nucl. 1967;48(11):638–655. [PubMed] [Google Scholar]
- 72.Whall MA, Roberts PJ. Radiation dose in relation to compressed breast thickness for screening mammography. Rad Prot Dosim. 1992;43(1/4):253–255. [Google Scholar]
- 73.Jamal N. A study of mean glandular dose during diagnostic mammography in Malaysia and some of the factors affecting it. Br J Radiol. 2003;76(904):238–245. doi: 10.1259/bjr/66428508. [DOI] [PubMed] [Google Scholar]
- 74.Tsapaki V, Tsalafoutas IAI, Poga V, Louizi A, Kottou S, Koulentianos E. Investigation of breast dose in five screening mammography centers in Greece. J Radiol Prot. 2008;28:337–346. doi: 10.1088/0952-4746/28/3/004. [DOI] [PubMed] [Google Scholar]
- 75.Stanton L, Lightfoot DA. The selection of optimum mammography technic. Radiology. 1964;83:442–454. doi: 10.1148/83.3.442. [DOI] [PubMed] [Google Scholar]
- 76.United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) Sources and Effects of Ionizing Radiation. New York: United Nations; 1977. [Google Scholar]
- 77.Carney PA, Miglioretti DL, Yankaskas BC, Kerlikowske K, Rosenberg R, Rutter CM, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138(3):168–175. doi: 10.7326/0003-4819-138-3-200302040-00008. [DOI] [PubMed] [Google Scholar]
- 78.Stomper PC, D'Souza DJ, DiNitto PA, Arredondo MA. Analysis of parenchymal density on mammograms in 1353 women 25–79 years old. Am J Roentgenol. 1996;167(5):1261–1265. doi: 10.2214/ajr.167.5.8911192. [DOI] [PubMed] [Google Scholar]
- 79.Minagi H, Tennant JC, Youker JE. Coning and breast compression. An aid in mammographic diagnosis. Radiology. 1968;91(2):379–381. doi: 10.1148/91.2.379. [DOI] [PubMed] [Google Scholar]
- 80.Young KC. Radiation doses in the UK trial of breast screening in women aged 40–48 years. Br J Radiol. 2002;75(892):362–370. doi: 10.1259/bjr.75.892.750362. [DOI] [PubMed] [Google Scholar]
- 81.McParland BJ. Image quality and dose in film-screen magnification mammography. Br J Radiol. 2000;73(874):1068–1077. doi: 10.1259/bjr.73.874.11271899. [DOI] [PubMed] [Google Scholar]
- 82.Dance DR, Thilander AK, Sandborg M, Skinner CL, Castellano IA, Carlsson GA. Influence of anode/filter material and tube potential on contrast, signal-to-noise ratio and average absorbed dose in mammography: a Monte Carlo study. Br J Radiol. 2000;73(874):1056–1067. doi: 10.1259/bjr.73.874.11271898. [DOI] [PubMed] [Google Scholar]
- 83.Hermann KP, Obenauer S, Funke M, Grabbe E. Magnification mammography: a comparison of full-field digital mammography and screen-film mammography for the detection of simulated small masses and microcalcifications. Eur Radiol. 2002;12(9):2188–2191. doi: 10.1007/s00330-002-1356-8. [DOI] [PubMed] [Google Scholar]
- 84.Hendrick RE, Pisano ED, Averbukh A, Moran C, Berns EA, Yaffe MJ, et al. Comparison of acquisition parameters and breast dose in digital mammography and screen-film mammography in the American College of Radiology Imaging Network digital mammographic imaging screening trial. Am J Roentgenol. 2010;194(2):362–369. doi: 10.2214/AJR.08.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bailar JC., III Mammography: a contrary view. Ann Intern Med. 1976;84(1):77–84. doi: 10.7326/0003-4819-84-1-77. [DOI] [PubMed] [Google Scholar]
- 86.Muntz EP, Wikinson E, George FW. Mammography at reduced doses: Present performance and future possibilities. Am J Roentgenol. 1980;134:741–747. doi: 10.2214/ajr.134.4.741. [DOI] [PubMed] [Google Scholar]
- 87.Hricak H, Brenner DJ, Adelstein SJ, Frush DP, Hall EJ, Howell RW, et al. Managing radiation use in medical imaging: a multifaceted challenge. Radiology. 2011;258(3):889–905. doi: 10.1148/radiol.10101157. [DOI] [PubMed] [Google Scholar]
- 88.Gershon-Cohen J. Breast Roentgenology. Historical review. Am J Roentgenol Radium Ther Nuc Med. 1961;86:879–883. [PubMed] [Google Scholar]
- 89.Wolfe JN, McCaughey RS, Lewicki AM, Kalayjian BS. Radiographic examination of the breast. J Mich State Med Soc. 1963;62:374–379. [PubMed] [Google Scholar]
- 90.Egan RL. Experience with mammography in a tumor institution. Evaluation of 1,000 studies. Radiology. 1960;75:894–900. doi: 10.1148/75.6.894. [DOI] [PubMed] [Google Scholar]
- 91.Gershon-Cohen J, Boreadis Borden AG. Detection of Unsuspected Breast Cancer By Mammography. Ann NY Acad Sci. 1964;114(2):782–793. [PubMed] [Google Scholar]
- 92.Gershon-Cohen J, Hermel MB. Modalities in breast cancer detection: Xeroradiography, mammography, thermography and mammometry. Cancer. 1969;24(6):1226–1230. doi: 10.1002/1097-0142(196912)24:6<1226::aid-cncr2820240627>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 93.Moss NH, Gershon-Cohen J. Breast cancer management improved by mammography. Acta Unio Int Contra Cancrum. 1964;20:1849–1850. [PubMed] [Google Scholar]
- 94.Wolfe JN. Xerography of the breast. Radiology. 1968;91:231–240. doi: 10.1148/91.2.231. [DOI] [PubMed] [Google Scholar]
- 95.Hranitzky EB, Shalek RJ. Skin doses from glass and beryllium window x-ray tubes in mammography. Radiology. 1967;88(4):668–671. doi: 10.1148/88.4.668. [DOI] [PubMed] [Google Scholar]
- 96.Egan RL. Mammography. Springfield (IL); 1964. [Google Scholar]
- 97.Stanton L, Lightfoot DA, Boyle JJJ, Cullinan JE. Physical aspects of breast radiography. Radiology. 1963;81(1):1–16. doi: 10.1148/81.1.1. [DOI] [PubMed] [Google Scholar]
- 98.Wolfe JN. Mammography: Report on its use in women with breasts abnormal and normal on physical examination. Radiology. 1964;83:244–254. doi: 10.1148/83.2.244. [DOI] [PubMed] [Google Scholar]
- 99.Price JL, Butler PD. The reduction of radiation and exposure time in mammography. Br J Radiol. 1970;43:251–255. doi: 10.1259/0007-1285-43-508-251. [DOI] [PubMed] [Google Scholar]
- 100.Jensen JE, Butler PF. Breast exposure: nationwide trends; a mammographic quality assurance program-results to date. Radiol Technol. 1978;50(3):251–257. [PubMed] [Google Scholar]
- 101.Sickles EA, Doi K, Genant HK. Magnification film mammography: image quality and clinical studies. Radiology. 1977;125(1):69–76. doi: 10.1148/125.1.69. [DOI] [PubMed] [Google Scholar]
- 102.Wootton P. Mammographic exposures at the breast cancer detection demonstration project screening centers. Am J Roentgenol. 1976;127:531–533. doi: 10.2214/ajr.127.3.531. [DOI] [PubMed] [Google Scholar]
- 103.Jans RG. A federal/State quality assurance program in mammography. In: Logan Wende., editor. Breast Carcinoma, the radiologist's expanded view. New York: John Wiley & Sons, Inc; 1977. pp. 121–127. [Google Scholar]
- 104.Miller DW. Center for Radiologic physics: mammography review procedures and results. In: Logan Wende., editor. Breast Carcinoma, the Radiologist’s expanded view. New York: John Wiley & Sons, Inc; 1977. pp. 129–133. [Google Scholar]
- 105.Iskwi AP, Ritchie JH, Gefter IM, Moore RS, Rainbow AJ. Use of a breast phantom to improve image quality and patient exposure in Ontario mammography facilities. Rad Prot Dosim. 1993;49(1/3):203–206. [Google Scholar]
- 106.Zoetelief J, Broerse JJ, Thijssen MAO. A Dutch protocol for quality control in mammography screening: dosimetric aspects. Rad Prot Dosim. 1992;43(1/4):261–264. [Google Scholar]
- 107.Food and Drug Administration (FDA) Nationwide Evaluation of X-ray Trends-1992 Mammography X-ray data. Silver Spring MD: US Department of Health and Human Services; 1995. [Google Scholar]
- 108.Helvie MA, Chan H-P, Adler DD, Boyd PG. Breast thickness in routine mammograms: effect on image quality and radiation dose. Am J Radiol. 1994;163:1371–1374. doi: 10.2214/ajr.163.6.7992731. [DOI] [PubMed] [Google Scholar]
- 109.Schubauer-Berigan MK. Mammography dose in relation to body mass index, race, and menopausal status. Rad Prot Dosim. 2002;98(4):425–432. doi: 10.1093/oxfordjournals.rpd.a006733. [DOI] [PubMed] [Google Scholar]
- 110.Gonzalez L, Vaño E, Oliete S, Manrique J, Hernaez JM, Lahuerta J, et al. Report of an image quality and dose audit according to Directive 97/43/Euratom at Spanish private radiodiagnostics facilities. Br J Radiol. 1999;72:186–192. doi: 10.1259/bjr.72.854.10365071. [DOI] [PubMed] [Google Scholar]
- 111.Servomaa A, Rannikko S, Parviainen T, Holmberg P, Kuus E, Muursepp T, et al. Quality control and patient dose from x ray examinations in some hospitals in Estonia. Radiat Prot Dosim. 1995;57(1–4):297–300. [Google Scholar]
- 112.Olerud HM, Olsen JH, Widmark A, Fosmark H. A Norwegian survey of image quality, doses and film processing in mammography, with reference to two technical phantoms. Radiat Prot Dosim. 1996;67(3):199–210. [Google Scholar]
- 113.Faulkner K, Law J, Robson KJ. Assessment of mean glandular dose in mammography. Br J Radiol. 1995;68:877–881. doi: 10.1259/0007-1285-68-812-877. [DOI] [PubMed] [Google Scholar]
- 114.Young KC, Ramsdale ML. Image quality and dose measurement phantoms in the UK breast screening programme. Radiat Prot Dosim. 1993;49(1/3):175–177. [Google Scholar]
- 115.Aroua A, Vader J-P, Valley J-F. A Survey on Exposure by Diagnostic and Interventional Radiology in Switzerland in 1998. Lausanne, Switzerland: Institut Universitaire de Radiophysique Appliquée; 2000. [Google Scholar]
- 116.Food and Drug Administration (FDA) Nationwide Evaluation of X-ray Trends-25 years. US Department of Health and Human Services; 2003. [Google Scholar]
- 117.Warren-Forward HM, Duggan L. Towards in vivo TLD dosimetry in mammography. Br J Radiol. 2004;77(917):426–432. doi: 10.1259/bjr/91138314. [DOI] [PubMed] [Google Scholar]
- 118.Gennaro G, Baldelli P, Taibi S, Di Maggio C, Gambaccini M. Patient dose in full-field digital mammography: an Italian survey. Eur Radiol. 2004;14(4):645–652. doi: 10.1007/s00330-003-2010-9. [DOI] [PubMed] [Google Scholar]
- 119.Haus AG. Historical technical developments in mammography. Technol Cancer Res Treat. 2002;1(2):119–126. doi: 10.1177/153303460200100204. [DOI] [PubMed] [Google Scholar]
- 120.Karssemeijer N, Bluekens AM, Beijerinck D, Deurenberg JJ, Beekman M, Visser R, et al. Breast cancer screening results 5 years after introduction of digital mammography in a population-based screening program. Radiology. 2009;253(2):353–358. doi: 10.1148/radiol.2532090225. [DOI] [PubMed] [Google Scholar]
- 121.Sala M, Comas M, Macia F, Martinez J, Casamitjana M, Castells X. Implementation of digital mammography in a population-based breast cancer screening program: effect of screening round on recall rate and cancer detection. Radiology. 2009;252(1):31–39. doi: 10.1148/radiol.2521080696. [DOI] [PubMed] [Google Scholar]
- 122.National Council on Radiation Protection Measurements (NCRP) Mammography. Bethesda MD: Library of congress; 1980. NCRP report 66; p. 185. [Google Scholar]
- 123.Eklund S, Thilander A, Leitz W, Mattsson S. The impact of anatomic variations on absorbed radiation doses in mammography. Radiat Prot Dosim. 1993;49(1–3):167–170. [Google Scholar]
- 124.Jacobson DR. Mammography Radiation Dose and Image Quality. Radiat Prot Dosim. 1998;80(1–3):295–297. [Google Scholar]