SUMMARY
To infect plants, Pseudomonas syringae pv. tomato delivers ~30 type III effector proteins into host cells, many of which interfere with PAMP-triggered immunity (PTI). One effector, AvrPtoB, suppresses PTI using a central domain to bind host BAK1, a kinase that acts with several pattern recognition receptors to activate defense signaling. A second AvrPtoB domain binds and suppresses the PTI-associated kinase Bti9 but is conversely recognized by the protein kinase Pto to activate effector-triggered immunity. We report the crystal structure of the AvrPtoB-BAK1 complex, which revealed structural similarity between these two AvrPtoB domains, suggesting that they arose by intragenic duplication. The BAK1 kinase domain is structurally similar to Pto, and a conserved region within both BAK1 and Pto interacts with AvrPtoB. BAK1 kinase activity is inhibited by AvrPtoB, and mutations at the interaction interface disrupt AvrPtoB virulence activity. These results shed light on a structural mechanism underlying host-pathogen coevolution.
INTRODUCTION
Animals and plants defend themselves against potential pathogens by detecting non-self molecules known as pathogen/microbe-associated molecular patterns (PAMPs/ MAMPs). These conserved microbial components are perceived by pattern recognition receptors (PRRs) leading to activation of an immune response (Ronald and Beutler, 2010). In plants, this layer of innate immunity is referred to as PAMP-triggered immunity (PTI) and results in the production of antimicrobial compounds, reactive oxygen species, fortification of the plant cell wall, and ultimately pathogen arrest (Chisholm et al., 2006).
A well-studied PTI response in plants involves recognition of the bacterial PAMP, flagellin, by its cognate PRR, FLS2 (Gomez-Gomez and Boller, 2000). FLS2 is a leucine-rich repeat-containing receptor-like kinase (LRR-RLK) that binds flagellin at the plant plasma membrane, leading to its association with the regulatory protein BAK1, phosphorylation of the cyto-plasmic kinases BIK1 and PBL1, activation of MAP kinase signaling, and transcriptional regulation of downstream genes (He et al., 2006; Lu et al., 2010; Schulze et al., 2010; Shan et al., 2008; Zhang et al., 2010). BAK1 is a conserved member of the SERK family of LRR-RLKs and was originally identified as a component of brassinosteroid (BR) signaling, specifically as a regulator of the BR receptor, BRI1 (Clouse, 2011). In addition to its roles in BR and flagellin signaling, BAK1 serves as a regulator for EFR, a PRR that recognizes bacterial elongation factor Tu, and is also involved in PTI responses to csp22, HrpZ, peptidoglycan, and lipopolysaccharide, highlighting its broad importance in immunity (Heese et al., 2007; Segonzac and Zipfel, 2011; Shan et al., 2008; Zipfel et al., 2006). Interestingly, the dual roles of BAK1 in BR signaling and PTI are separable, as demonstrated by the identification of a mutant allele in Arabidopsis (Schwessinger et al., 2011).
Elucidation of the function of BAK1 in PTI was mostly accomplished using the Arabidopsis-Pseudomonas syringae pathosystem. While the bacterial pathogen P. syringae pv. tomato strain DC3000 (Pst) has multiple PAMPs that reveal its presence to the plant surveillance system, it also injects ~30 effector proteins through its type three secretion system into the plant cell. Collectively, these effectors suppress PTI to the benefit of the bacteria that reside in the intercellular spaces of the leaf, resulting in their multiplication and the onset of disease (Cunnac et al., 2009). AvrPtoB is a well-studied Pst effector with an N-terminal region (amino acids 1–387) that interacts with the kinase domain of BAK1 and FLS2 and suppresses signaling following flagellin perception (Gohre et al., 2008; Shan et al., 2008). The C-terminal E3 ligase domain of AvrPtoB may facilitate degradation of FLS2 (Gohre et al., 2008). A shorter fragment, AvrPtoB1–307, is unable to suppress BAK1/FLS2 but interacts and interferes with another PTI-associated kinase, Bti9/CERK1 (Figure 1A) (Gimenez-Ibanez et al., 2009; He et al., 2006; Shan et al., 2008; Zeng et al., 2011). AvrPtoB1–387 also enhances Pst virulence in susceptible tomato plants (Xiao et al., 2007b), likely by targeting BAK1. Consistent with its inhibition of upstream components of PTI, AvrPtoB acts early in the infection process and is required for the growth-promoting and symptom-enhancing activities of other effectors (Cunnac et al., 2011). Furthermore, homologs of avrPtoB are present in many sequenced P. syringae strains, in line with its important virulence function (O’Brien et al., 2011).
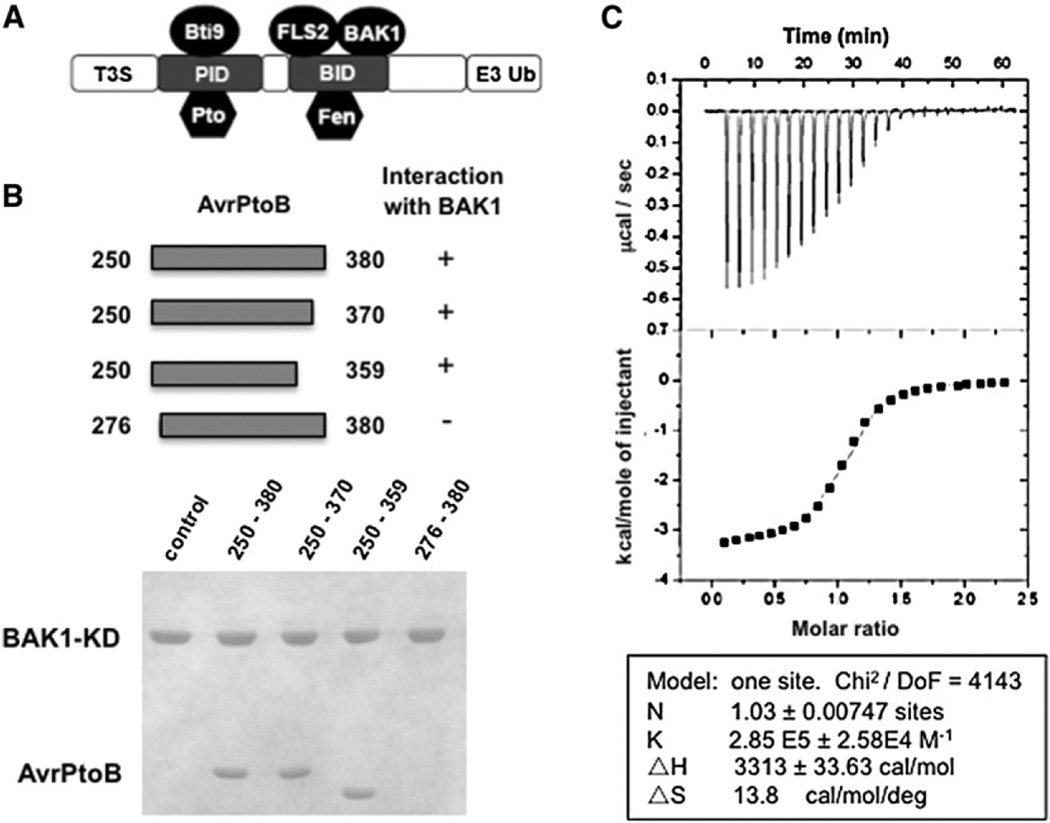
Figure 1. AvrPtoB250–359 Is Sufficient for Interaction with the BAK1 Kinase Domain.
(A) A schematic diagram of the structural organization of AvrPtoB. T3S, type III secretion sequence; PID, Pto-interacting domain; BID, BAK1-interacting domain; E3 Ub, E3 ubiquitin ligase.
(B) The upper panel depicts the AvrPtoB fragments tested for interaction with BAK1-KD (residues 250–591). Each AvrPtoB fragment was individually incubated with BAK1-KD and subjected to gel filtration analysis. Aliquots of the peak fraction corresponding to BAK1-KD were visualized by Coomassie blue staining following SDS-PAGE, as observed in the lower panel.
(C) Quantification of binding affinity between BAK1-KD and BID by isothermal titration calorimetry (ITC). Twenty-four injections of AvrPtoB250–359 solution were added to the BAK1-KD protein solution in the ITC cell. The area of each injection peak corresponds to the total heat released for that injection. The integrated heat is plotted against the molar ratio of AvrPtoB250–359 added to BAK1-KD in the cell. Data fitting revealed a binding affinity of 3.5 µM.
In addition to PTI, plants have a second layer of defense termed effector-triggered immunity (ETI), which involves the direct or indirect perception of effectors, typically resulting in complete halting of the infection (Chisholm et al., 2006). The same region of AvrPtoB that is required for its interaction with BAK1 and PTI suppression is recognized by the tomato resistance (R) protein Fen, activating ETI (Figure 1A) (Abramovitch et al., 2003; Rosebrock et al., 2007). Another tomato R protein, Pto, activates ETI by recognizing the region of AvrPtoB that binds Bti9/CERK1 (Gimenez-Ibanez et al., 2009; Zeng et al., 2011). Like BAK1 and Bti9/CERK1, both Fen and Pto are kinases. However, the R proteins lack transmembrane and extracellular LRR domains and require a nucleotide binding-LRR (NB-LRR) protein, Prf, for function (Abramovitch et al., 2003; Kim et al., 2002). Based on their similarity to the virulence targets of AvrPtoB, it has been proposed that proteins like Pto and Fen are molecular mimics of host virulence targets to activate ETI (van der Hoorn and Kamoun, 2008; Xing et al., 2007). The AvrPtoB E3 ubiquitin ligase domain ubiquitinates and thereby facilitates degradation of Fen (Abramovitch et al., 2006; Janjusevic et al., 2006; Rosebrock et al., 2007). Interestingly, Pto is able to resist this ubiquitination possibly by phosphorylating the C-terminal region of AvrPtoB (Ntoukakis et al., 2009; Rosebrock et al., 2007).
Previously, a structural analysis of AvrPtoB121–205 in complex with the Pto kinase shed light on ETI activation in tomato (Dong et al., 2009). Here we define the domain of AvrPtoB (AvrPtoB250–359) that interacts with BAK1 and report the crystal structure of the AvrPtoB250–359-BAK1 complex. Structural analysis elucidated the mechanism of AvrPtoB-mediated suppression of PTI. Surprisingly, the structure of AvrPtoB250–359 has significant similarity to AvrPtoB121–205, likely due to an ancient intragenic duplication. Structure-based mutagenesis and functional studies confirmed the structural predictions and indicate that AvrPtoB250–359 interferes with BAK1 by inhibiting its kinase activity. These observations are discussed in light of the possible evolutionary history between tomato and Pst.
RESULTS
Biochemical Characterization of the AvrPtoB-BAK1 Interaction
AvrPtoB1–387, but not AvrPtoB1–307, interacts with the BAK1 kinase domain, indicating that the C-terminal portion of AvrPtoB1–387 is important for the association of these proteins (Figure 1A) (Shan et al., 2008). To determine the BAK1-interacting domain (BID) of AvrPtoB and facilitate structural analysis of the AvrPtoB-BAK1 complex, we purified the BAK1 kinase domain (amino acids 250–591, hereafter BAK1-KD) and investigated its interaction with AvrPtoB fragments by gel filtration. A C-terminal region, AvrPtoB250–380, comigrated with BAK1-KD, indicating that they form a stable complex (Figure 1B). Removal of 16 residues from the N terminus of this fragment (AvrPtoB276–380) caused a loss of interaction with BAK1-KD. In contrast, a C-terminal deletion of 21 residues (AvrPtoB250–359) did not affect its interaction with BAK1-KD. This defined a minimal BID as AvrPtoB250–359. Isothermal titration calorimetry (ITC) showed that AvrPtoB250–359 and BAK1-KD interact with a dissociation constant of 3.5 uM, providing further evidence that these proteins form a stable complex (Figure 1C).
To understand the molecular mechanisms underlying the BAK1-KD-AvrPtoB250–359 interaction, we determined the crystal structure of the complex. The correct stoichiometry of the complex was obtained by passing a mixture of the two purified proteins through size exclusion chromatography and harvesting the peak fractions corresponding to the complex for crystallization. The structure of the complex was determined by molecular replacement using the coordinates of Pto as the initial searching model for BAK1 (see Table S1, available with this article online). Four complex molecules exist in the crystallo-graphic asymmetric unit. The structures of the four complex molecules in one asymmetric unit are nearly identical, with a maximum root-mean-square deviation (rmsd) of 0.66 Å between two complex molecules. The structure of the BAK1-KD-AvrPtoB250–359 complex was refined to a resolution of 2.5 Å, with an R factor and an Rfree of 18.5% and 23.8%, respectively.
AvrPtoB250–359 Is Homologous to the Pto Interaction Domain, AvrPtoB121–205
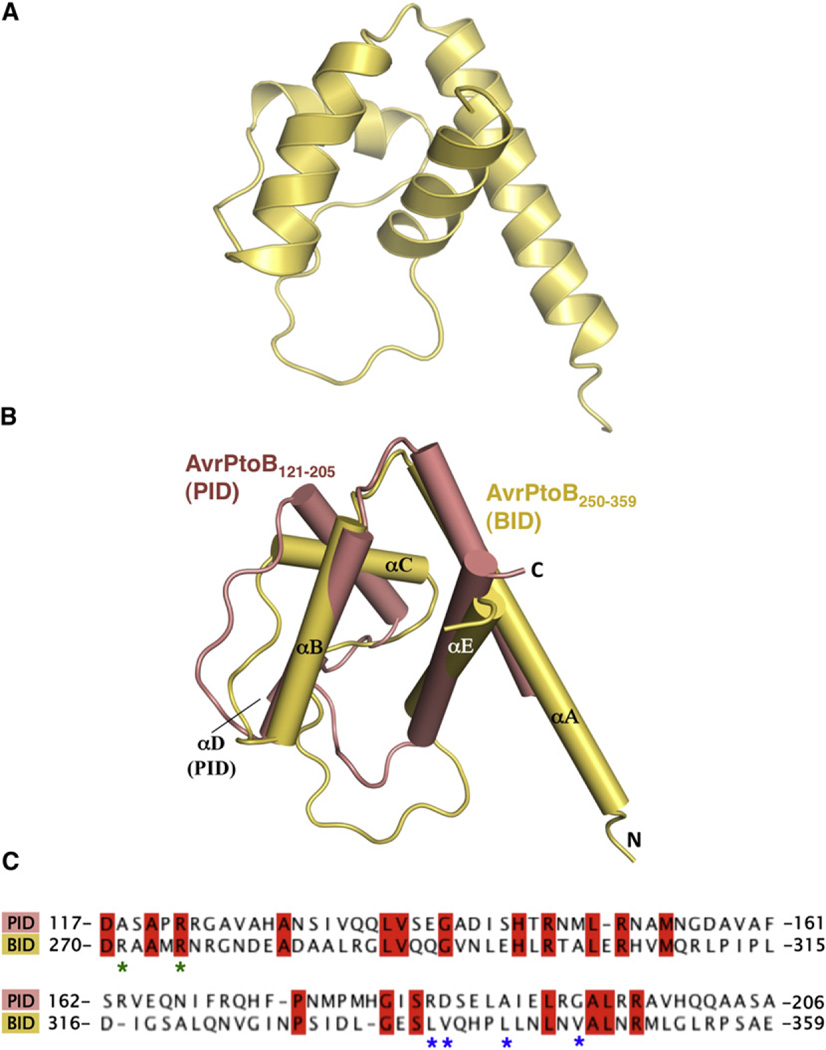
The structure of AvrPtoB250–359 bound to BAK1-KD consists of four α helices forming a globular helix bundle (Figure 2A). The helices were designated αA, αB, αC, and αE, from the N to the C terminus, following the structural nomenclature of AvrPtoB121–205 (Dong et al., 2009).
Figure 2. AvrPtoB250–359 Is a Structural Homolog of AvrPtoB121–205.
(A) BID is composed of a four-helix bundle.
(B) Structural superimposition of AvrPtoB121–205 (salmon) and AvrPtoB250–359 (light yellow). The structural elements are labeled.
(C) Primary sequence alignment of AvrPtoB121–205 and AvrPtoB250–359. Identical amino acids are highlighted in red. Asterisks indicate amino acids involved in the interaction and mutated in subsequent experiments. R271/ R275 (green asterisks) were mutated together, whereas as the others were mutated individually (see also Figure S1).
We used DALI (Holm and Rosenström, 2010) to identify structural homologs of AvrPtoB250–359. Unexpectedly, the structure of the BID was found to be most similar to the Pto-interacting domain (PID) of AvrPtoB (AvrPtoB121–205), with a Z score of 7.0 and an rmsd of 2.2 Å over 66 Cα atoms. In addition to the four helices, the BID has an extended loop between αC and αE, similar to that observed in the PID (Figure 2B). One striking difference, however, is that αA of the BID is much longer than αA in the PID. Despite these similarities, the two AvrPtoB domains interact with their respective host kinases in different orientations (see the Discussion). A structure-based primary sequence alignment indicated that the two domains of AvrPtoB share only 20% amino acid identity and 38% nucleotide identity (Figure 2C and Figure S1). The conserved amino acids are largely hydrophobic and are involved in the formation of the four-helix bundle. These results suggest that the BID and PID of AvrPtoB are evolutionarily related, probably having derived from a common ancestral DNA sequence.
Structural Analysis of the Complexed BAK1 Kinase Domain
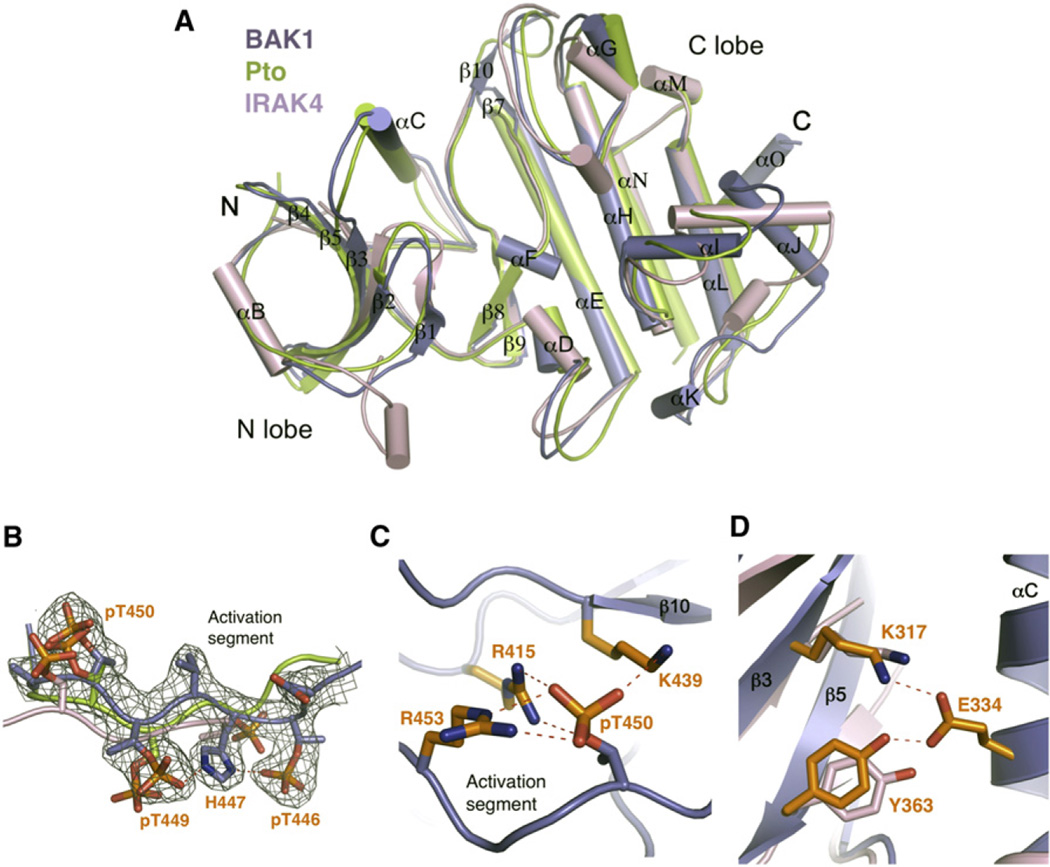
The BAK1-KD has the canonical fold of protein kinases (Figure 3A and Table S1), with a small N-terminal lobe and a larger C-terminal lobe. As defined by DALI, the active form of Pto and IRAK4 were the closest structural homologs of BAK1-KD, with an rmsd of 2.1 Å (over 260 Cα atoms) and 2.2 Å, respectively (Figure 3A). All three are RD kinases, whereas many other immunity-associated kinases including FLS2, EFR, and IRAK1 are non-RD kinases (Ronald and Beutler, 2010). The activation segment of BAK1-KD, flanked by the DFG and APE motifs, is well defined in the structure and adopts a similar conformation to those of Pto and IRAK4, suggesting BAK1-KD in the complex assumes an active conformation. Activation of a serine/threonine kinase is usually regulated by phosphorylation of serines or threonines within the activation segment. Indeed, electron density showed that three residues in this region, BAK1T446, T449, T450, are phosphorylated (Figure 3B). Phosphorylation of these residues has previously been observed both in vitro and in vivo (Wang et al., 2008). The phosphorylated residue T450 (pBAK1T450) appears to be important to maintain BAK1 in the active conformation, as the phosphate forms salt bridges with BAK1R415, R453, K439 (Figure 3C). In contrast, pBAK1T449 points outward to the solvent region and forms a BAK1H447-mediated interaction with pBAK1T446. Structural comparisons revealed that, except for Pto and IRAK4, all top matches with rmsd less than 2.5 Å (over ~250 Cα atoms) are tyrosine kinases. Tyrosine-262 in IRAK4, termed the tyrosine gatekeeper and shown to be critical for selection of various kinase inhibitors, is a unique feature of the IRAK family of kinases (Wang et al., 2006). This residue is conserved in the BAK1-KD structure (Y363; Figure 3D), indicating that BAK1 is a member of the IRAK family of kinases, although the residue is not important for BAK1 kinase activity (Oh et al., 2010).
Figure 3. BAK1-KDBoundbyAvrPtoB250–359 Is in an Active Conformation.
(A) Structural comparison of BAK1-KD, Pto, and IRAK4. The coordinates of active Pto and IRAK4 were used for structural superimposition. The secondary structural elements in BAK1-KD are labeled. “N” and “C” represent the N termini and C termini, respectively. The color codes are as indicated.
(B) The activation segment of BAK1-KD adopts a similar conformation to those of Pto and IRAK4. Shown is superimposition of BAK1-KD, Pto, and IRAK4 activation segments. The electron density (2Fo–Fc) contoured at 1.2 σ around the activation segment of BAK1-KD is shown. The phosphorylated residues are displayed as sticks, and those from BAK1-KD are labeled.
(C) The phosphate group of pT450 in BAK1-KD forms ionic interactions with its neighboring basic residues.
(D) The conserved “tyrosine gate” in BAK1-KD and IRAK4 has a similar conformation. Shown is a close-up view of the structural comparison between BAK1-KD and IRAK4 around BAK1Tyr363. The side chains from BAK1-KD and IRAK4 are shown in orange and pink, respectively. Residues labeled are from BAK1-KD (see also Table S1).
Analysis of Contact Surfaces between BAK1-KD and AvrPtoB250–359
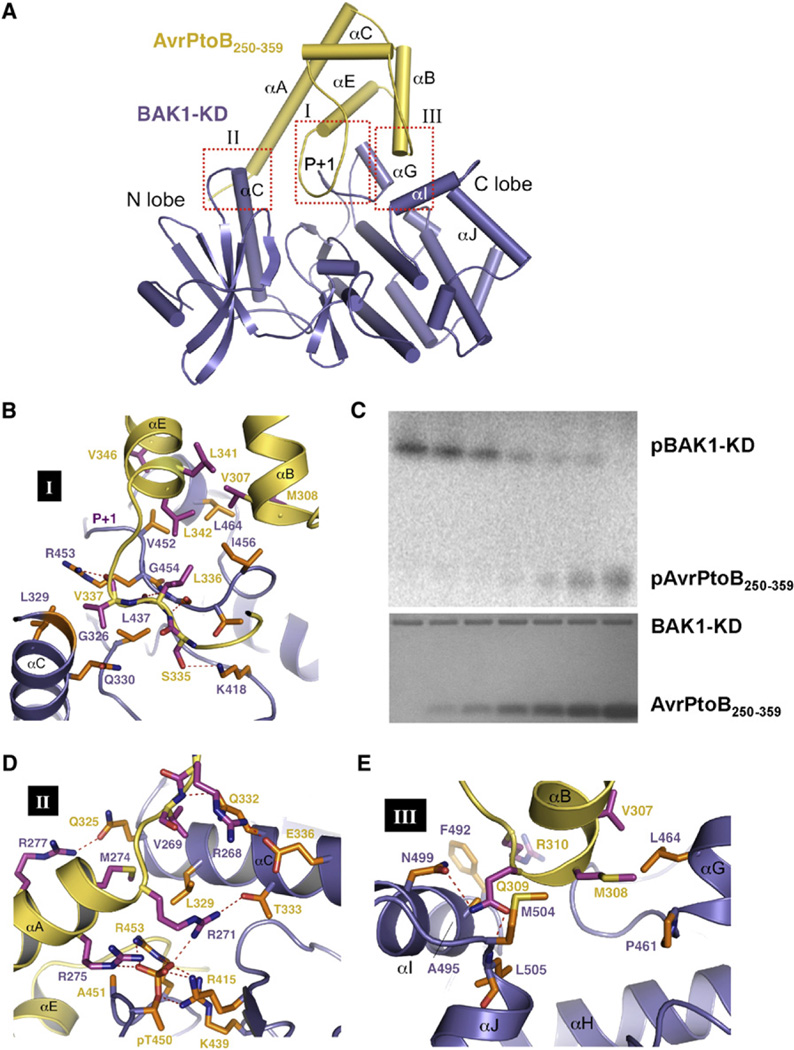
The BAK1-KD-AvrPtoB250–359 complex assumes a compact globular structure (Figures 4A). In crystals, the interaction of these two proteins results in a 1:1 stoichiometric complex, consistent with the ITC data (Figure 1C). Analysis of the interfaces by PISA showed the AvrPtoB250–359-BAK1-KD complex is stable in solution; the buried surface area upon complex formation is 2740 Å2 (Figure S2). Three interfaces are present in the complex (Figure 4A). Dominating the first interface is binding of the loop linking αC and αE (referred to as the P+1-interacting loop) in AvrPtoB250–359 to the catalytic cleft of BAK1-KD located at the junction of the N- and C-terminal lobes through contacts in the P+1 loop. Additional interactions around this interface include packing of one end of αG immediately preceding the P+1 loop against the N-terminal side of αE in AvrPtoB250–359. The N-terminal portion of αA together with the N-terminal extension in AvrPtoB250–359 wedges between αC and the N-terminal side of the activation segment, resulting in formation of the second interface. The third interface involves contacts of aB of AvrPtoB250–359 with αG and αI of BAK1-KD.
Figure 4. Specific Recognition of AvrPtoB by BAK1-KD.
(A) A cartoon representation of the overall structure of the AvrPtoB250–359-BAK1-KD complex. AvrPtoB250–359 and BAK1-KD are colored in yellow and purple, respectively. The three interfaces, I, II, and III, within the complex are highlighted in the red dashed frames. The P+1 loop of BAK1-KD is indicated. Some of the secondary structural elements in the BAK1-KD are labeled. (B), (D), and (E) show the detailed interactions of the interfaces I, II, and III in (A),respectively. The side chains from AvrPtoB250–359 and BAK1-KD are colored in orange and magenta, respectively. Relevant amino acid residues are numbered. Red dashed lines represent polar interactions. (C) AvrPtoB BID inhibits BAK1-KD kinase activity and is phosphorylated by BAK-KD in vitro. To determine the effect of the BID onBAK1 kinase activity, BAK1-KD autophosphorylation assays were performed in the presence of different concentrations of the BID (0, 2.17 µM, 4.34 µM, 8.68 µM, 17.36 µM, 119.5 µM, and 141.2 µM; bottom panel, Coomassie blue-stained gel). All reactions were incubated at 30 degrees for 30 min and terminated by adding an equal volume (50 ml) of 23× SDS buffer. SDS-polyacrylamide gel electrophoresis was used to fractionate proteins, and the phosphorylated BAK1 (pBAK1-KD) and AvrPtoB (pAvrPtoB250–359) were visualized with a phosphorimager (top panel) (see also Figure S2).
Interactions in the complex are established through a combination of polar and hydrophobic interactions. The first interface is closely packed, and the P+1 loop forms backbone hydrogen bonds with the P+1-interacting loop of AvrPtoB250–359, similar to that observed in the AvrPto-Pto and AvrPtoB121–205-Pto complexes (Figure 2A; Dong et al., 2009). However, unlike the latter two complexes, in which interactions involving the P+1 loop are exclusively mediated by hydrogen bonding between backbones, it is the side chain of BAK1R453 in the P+1 loop that hydrogen bonds with the carbonyl oxygen of AvrPtoBV337 and the hydroxyl group of AvrPtoBS335 that hydrogen bonds with BAK1K418 (Figure 4B). In addition to the intricate core network of hydrogen bonds, surrounding van der Waals contacts fortify interactions at this interface including hydrophobic contacts between BAK1I456 and AvrPtoBL342, L336.
The P+1 loop of kinases is located in the region of substrate binding where phosphorylation occurs. Our observation that AvrPtoB250–359 directly contacts the P+1 loop of BAK1-KD suggests AvrPtoB250–359 inhibits BAK1 kinase activity. To investigate this possibility, we tested the effects of AvrPtoB250–359 on BAK1-KD autophosphorylation. The proteins were purified and the kinase activity of BAK1-KD was examined in the presence of AvrPtoB250–359. As shown previously, BAK1-KD exhibited strong autophosphorylation (Figure 4C; Wang et al., 2008). BAK1-KD autophosphorylation was attenuated by the addition of AvrPtoB250–359 and was progressively reduced with increasing amounts of AvrPtoB250–359 (Figure 4C). AvrPtoB variants that are unable to interact with BAK1 were unable to suppress BAK1 kinase activity (Figure S2). Notably, AvrPtoB is phosphorylated by BAK1 in this assay, and it is possible that BAK1 contributes to the previously reported phosphorylation of residue S258 in AvrPtoB (Xiao et al., 2007a).
Insertion of the side chain of AvrPtoBR271 into the pocket formed by αA of AvrPtoB and the N-terminal side of the BAK1 P+1 loop constitutes the center of the second interface (Figure 4D). AvrPtoBR271 makes van der Waals contacts with BAK1L329, R453 and also forms ionic interactions with the phosphate of pBAK1T450 and hydrogen bonds with BAK1T333. In addition to AvrPtoBR271, the phosphate of pBAK1T450 forms a pair of salts bridges with AvrPtoBR275. These observations explain why AvrPtoB276–380 does not interact with BAK1-KD. At the periphery of this contact interface, the amide nitrogen of AvrPtoBV269 forms a hydrogen bond with BAK1Q332, whereas its side chain packs tightly against the Ca atom of BAK1L329.
Compared to the other two interfaces, the third interface is primarily hydrophobic and less packed. Packing of AvrPtoBV307, M308, V346 against α2 of BAK1 and α3 appears to mainly contribute to the interactions around the interface (Figure 4E).
Structure-Informed Mutagenesis of BAK1-KD and AvrPtoB250–359 Disrupts the Complex
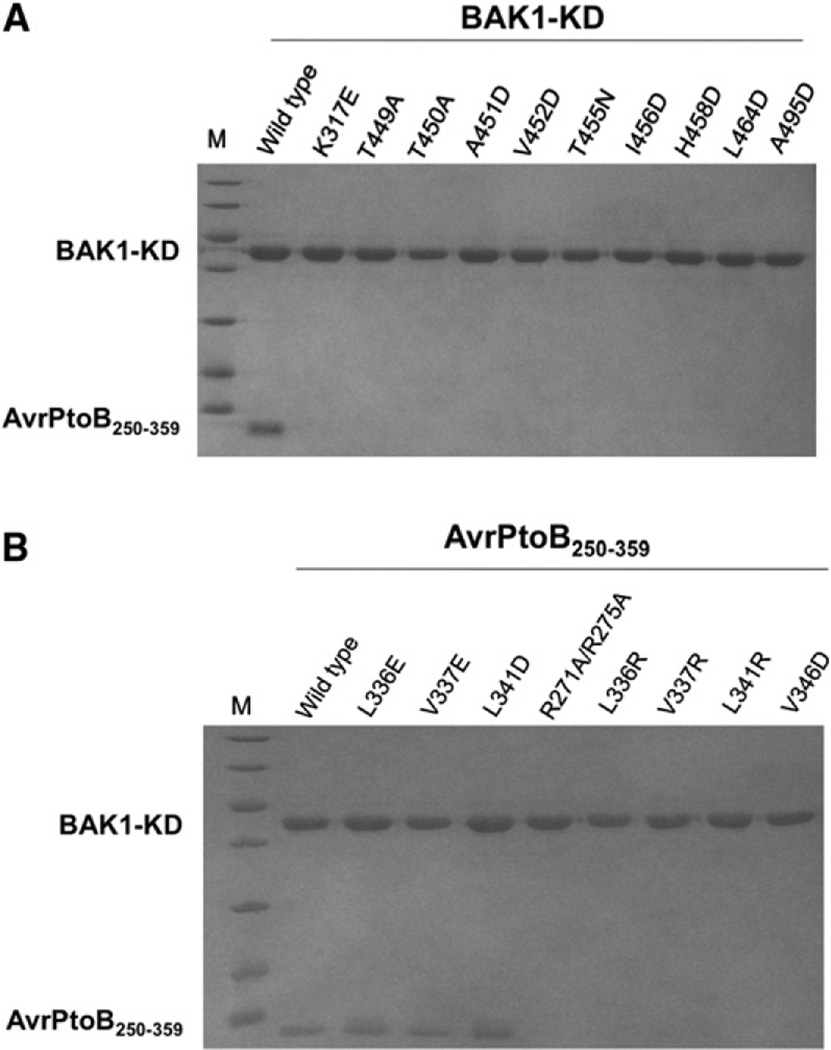
We next engineered amino acid substitutions in BAK1-KD and evaluated their impact on the interaction with AvrPtoB250–359 using gel filtration. Circular dichroism experiments indicated that the substitutions did not affect the global structure of BAK1-KD (Figure S3). As a positive control, the wild-type BAK1-KD protein formed a stable complex with AvrPtoB250–359 (Figure 5A). In contrast, BAK1-KD substitutions that were predicted to disrupt the first interface, BAK1-KDI456D, L464D, resulted in a loss of interaction with AvrPtoB250–359. BAK1-KDA451 and BAK1-KDA495 are positioned in small hydrophobic pockets at the second and third interfaces, respectively. As expected, substitutions of these amino acids with the bulkier, charged residue glutamic acid disrupted the interaction with AvrPtoB250–359.
Figure 5. Mutagenesis Analyses of AvrPtoB250–359-BAK1-KD Complex.
(A) Effects of point mutations in BAK1 on the interaction with AvrPtoB. BAK1-KD variant proteins were individually incubated with AvrPtoB250–359 and subjected to gel filtration. Aliquots of the peak fraction corresponding to BAK1-KD were visualized by Coomassie blue staining following SDS-PAGE.
(B) Effects of point mutations in AvrPtoB on the interaction with BAK1. The assay is similar to (A) except that AvrPtoB mutant proteins were used to test interaction with wild-type BAK1-KD (see also Figure S3).
Our analysis above indicates that BAK1-KD adopts an active conformation in the complex. Although the residues BAK1V452, T455, H458 are not directly involved in binding AvrPtoB250–359, they are important for maintaining the active conformation of the activation segment by making interactions with their neighboring residues. As predicted, gel filtration revealed that substitutions in these amino acids of BAK1-KD disrupted their ability to interact with AvrPtoB250–359, suggesting the active conformation of BAK1 is important for its interaction with AvrPtoB (Figure 5A). To test if phosphorylation of BAK1T450 is required for the interaction with AvrPtoB250–359, we made the substitution BAK1T450A, which is expected to disrupt the ionic interaction between the phosphate group and its surrounding basic residues at the second interface. This variant protein did not interact with AvrPtoB250–359 (Figure 5A). A T450A substitution was previously reported to abolish the ability of BAK1 to function in FLS2 signaling (Wang et al., 2008), reinforcing the importance of this residue.
We tested the kinase activity of additional BAK1 variant proteins and found that most of the proteins that lacked kinase activity were also unable to interact with AvrPtoB (Figure S3). The only exception is T449A, which had comparable kinase activity to wild-type BAK1 but exhibited no interaction with AvrPtoB250–359. The reason for this may be that phosphorylation of T449 leads to its interaction with BAK1 H447 (Figure 3B), which can play a role in maintaining proper conformation of the P+1 loop required for interaction with AvrPtoB250–359 but not BAK1 substrates. Although the precise mechanisms await further investigation, our structural and biochemical data support the idea that kinase activity of BAK1 is important for its ability to bind AvrPtoB.
To further test the contact surfaces inferred from the BAK1-KD-AvrPtoB250–359 structure, we used gel filtration to assess the interaction between AvrPtoB250–359 variants and BAK-KD. Five variants, AvrPtoB250–359R271A/R275A, L336R, V337R, L341R, and V346D, were unable to interact with BAK1-KD (Figure 5B). Circular dichroism experiments of these AvrPtoB variants indicated that two of them (V337R and L341R) deviated from the wild-type protein, suggesting that these substitutions disturbed the global conformation of AvrPtoB250–359 (Figure S3). However, loss of BAK1-AvrPtoB250–359 interaction caused by other substitutions in AvrPtoB250–359 as well as those in BAK1 supports the crystal structure. In particular, mutation of AvrPtoB residues R271/ R275, which interact with the phosphate group of T450, did not affect global AvrPtoB structure but abolished interaction with BAK1.
Structure-Informed Functional Analysis of AvrPtoB Residues Involved in the BAK1 Complex
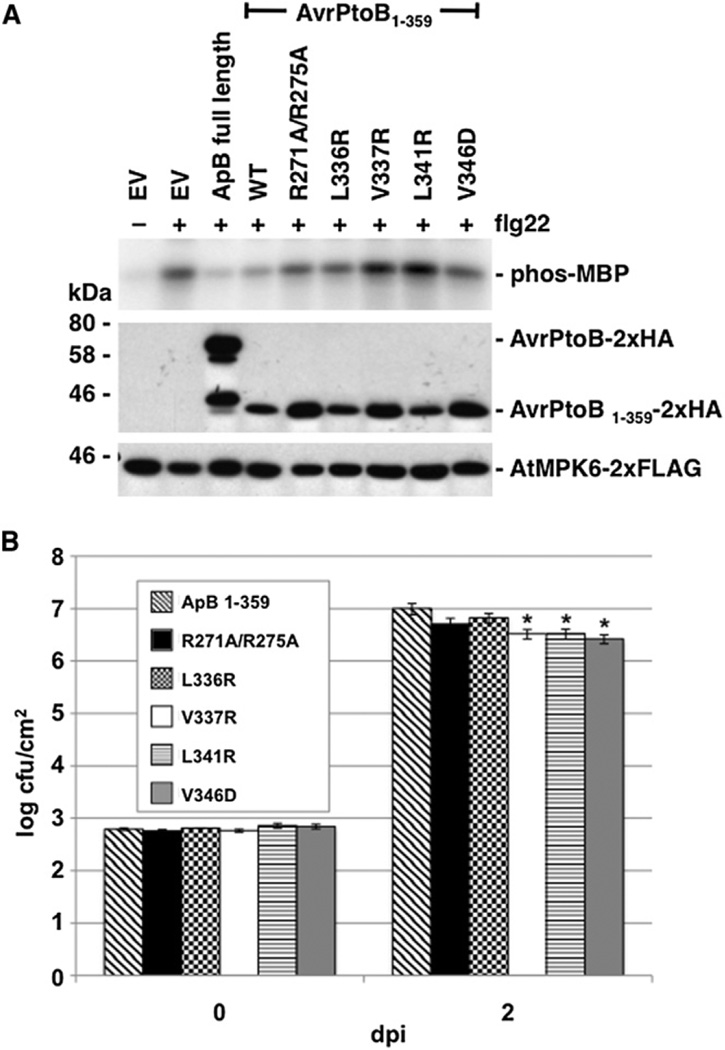
To functionally test the structure, we first identified a minimal fragment of AvrPtoB sufficient for virulence activity. The N-terminal 387 amino acids of AvrPtoB are known to suppress MAPK activation in response to flg22 stimulation in Arabidopsis protoplasts expressing a tagged version of AtMPK6, whereas AvrPtoB1–307 cannot do so (He et al., 2006). This suppression of MAPK activity presumably results from AvrPtoB-BAK1 interaction (Shan et al., 2008). To determine the minimal biologically active fragment of AvrPtoB, we used the MAPK suppression assay and found AvrPtoB1–359 to be sufficient for MAPK suppression and essentially equal to AvrPtoB in its activity (Figure 6A). AvrPtoB250–359 accumulated poorly in protoplasts and did not suppress AtMPK6 activation (data not shown).
Figure 6. Mutant Forms of AvrPtoB1–359 Unable to Interact with BAK1-KD In Vitro Are Impaired in Virulence.
(A) AvrPtoB (ApB) variants unable to interact with BAK1-KD in vitro have reduced ability to suppress MPK6 activation in Arabidopsis Col-0 protoplasts. Protoplasts expressing AtMPK6 and AvrPtoB were treated with 1 µM flg22 for 10 min. AtMPK6 activity was assessed in an immunocomplex kinase assay with MBP as the substrate (upper panel shows autoradiograph). AtMPK6 and AvrPtoB protein abundance were detected by immunoblot analysis (middle and lower panels). The smaller protein in the full-length AvrPtoB lane is probably a degradation product of the effector.
(B) AvrPtoB variants unable to interact with BAK1-KD in vitro have reduced virulence in tomato. Pst DC3000 ΔavrPtoΔavrPtoB strains expressing AvrPtoB1–359 wild-type or its variants were vacuum infiltrated into RG-prf-3 leaves at 3 × 104 cfu/ml. Bacterial growth per leaf area was determined at 0 and 2 days postinoculation (dpi). The figure is derived from data from one representative experiment using four biological replicates per strain. *Significantly different from wild-type at p < 0.05. The statistical analysis was performed on data derived from seven experiments, analyzed as the fold increase between days 0 and 2, using a one-way ANOVA with Tukey’s HSD as the correction; bars represent ± standard error (see also Figure S4 and Table S2).
We then assessed the contribution of amino acids identified in the structural analysis as important for the interaction with BAK1-KD to the biological activity of AvrPtoB1–359. Five variants of AvrPtoB1–359 were tested for their ability to suppress AtMPK6 activation in the MAPK assay. All five variants, AvrPtoB1–359R271A/R275A, L336R, V337R, L341R, and V346D, were compromised for biological function to various degrees when compared with wild-type (Figure 6A), although, as noted above, the global structure of two of these variant proteins was disrupted when expressed as AvrPtoB250–359 proteins, and they are therefore less informative.
We next determined if our results from the molecular assay were reflective of bacterial virulence in plants. Because AvrPtoB1–359 virulence activity has not been characterized for Pst DC3000 infection assays in Arabidopsis, we instead tested this activity in the Pst-tomato pathosystem (Xiao et al., 2007b). Shortened forms of AvrPtoB, lacking the E3 ubiquitin ligase domain, are sufficient for enhancing virulence of Pst DC3000 in susceptible tomato plants (Xiao et al., 2007b). The kinase domains of the two proteins in tomato with closest similarity to Arabidopsis BAK1 (SlSERK3A [GenBank accession number HQ438098] and SlSERK3B [HQ438099]) are 95% identical to that of AtBAK1, and all of the residues tested in Figure 5A are identical in these two proteins. This conservation supports the use of this pathosystem for AvrPtoB virulence assays.
We tested strains of DC3000ΔavrPtoΔavrPtoB expressing each of the five AvrPtoB1–359 variants under the control of a hrp-inducible promoter for bacterial growth in susceptible Rio Grande (RG)-prf-3 tomato plants in comparison with wildtype AvrPtoB1–359. DC3000ΔavrPtoΔavrPtoB strains expressing three of the variants, AvrPtoB1–359V337R, L341R, and V346D, were compromised in growth in the lower leaves at 2 days post-inoculation (Figure 6B). The difference was small but statistically significant as analyzed over seven experiments. Additionally, disease symptoms caused by the strains expressing AvrPtoB1–359V337R, L341R, and V346D were frequently but not always attenuated on the lower leaves of the plants (Figure S4).
Each of the AvrPtoB1–359 variants was detectable after induction in hrp-inducing conditions (Figure S4). However, consistent with the circular dichroism data, the AvrPtoB1–359(L341R) variant accumulated poorly, which may account for its reduced virulence activity. The AvrPtoB1–359(V337R) protein was detected by Pto-expressing plants, suggesting this longer form may be less affected than the spectroscopy results with the AvrPtoB250–359 suggest. To further test protein stability and also verify delivery from Pst, we infected RG-PtoR tomato plants that express Pto conferring immunity to bacteria expressing AvrPtoB1–307 with the same strains (Xiao et al., 2007b). As expected, all the strains activated immunity, with the exception of the AvrPtoB1–359L341R variant (Table S2A).
The domain of AvrPtoB required for interaction with BAK1-KD overlaps with the Fen-interacting domain (Rosebrock et al., 2007; Shan et al., 2008), and we hypothesized that some of the AvrPtoB substitutions might also affect the interaction with Fen. This assay would effectively test the hypothesis that Fen acts as a molecular mimic of BAK1. The same Pst strains expressing the AvrPtoB variants were inoculated into RG-pto11 tomato plants; these plants express Fen, but have a point mutation in Pto rendering it ineffective. Strains expressing AvrPtoB1–359R271A/R275A, L341R, or V346D did not activate Fenmediated immunity and caused disease, indicating a loss of Fen interaction (Table S2A). Notably, this subset of variants did not completely correlate with those that lost virulence activity in susceptible plants. Therefore, if Fen evolved as a molecular mimic of BAK1, it is not a perfect one, as it has structural differences in its contact surfaces with AvrPtoB.
DISCUSSION
Acting as an accessory protein to multiple PRRs, BAK1 plays a central role in PTI signaling. The targeting of BAK1 by AvrPtoB, then, is a particularly effective way to suppress PTI and enhance bacterial virulence. The molecular mechanisms by which AvrPtoB acts on BAK1 to inhibit downstream signaling, however, are largely unknown. In order to further characterize this mechanism, we used structural and functional analyses to examine the BAK1-AvrPtoB interaction in relation to the virulence activity of AvrPtoB.
Two N-terminal domains of AvrPtoB interact with multiple host kinases (Figure 1A) (Gimenez-Ibanez et al., 2009; Rosebrock et al., 2007; Shan et al., 2008; Xiao et al., 2007b). In Arabidopsis, AvrPtoB1–307, which encompasses the most N-terminal kinasebinding domain (amino acids 121–205), is sufficient to interact with the Bti9/CERK1 kinase, but not the BAK1 kinase (Gimenez-Ibanez et al., 2009; Shan et al., 2008; Zeng et al., 2011). In tomato, the Pto kinase recognizes AvrPtoB121–205 to activate immunity. The Pto family member Fen, which shares ~80% identity with Pto, does not recognize AvrPtoB121–205 (Dong et al., 2009). BAK1, Fen, and likely FLS2 require a different kinase-binding domain (amino acids 250–359) for interaction with AvrPtoB, although the exact domain for the interaction with Fen and FLS2 is unknown (Gohre et al., 2008; Rosebrock et al., 2007; Shan et al., 2008). These differential interactions demonstrate the kinase-binding specificity of AvrPtoB.
Like AvrPtoB121–205, AvrPto, a Pst effector that lacks homology to AvrPtoB, interacts with Pto but not with Fen (Tang et al., 1996; Xing et al., 2007). Our previous work revealed that Pto utilizes two distinct surfaces adjacent to its P+1 loop that are not conserved in Fen to distinguish between AvrPto and AvrPtoB121–205 (Dong et al., 2009). This not only offers an explanation for the inability of Fen to recognize AvrPto and AvrPtoB121–205, but also suggests that the specificity determinants within the kinase are located in the nonconserved regions flanking the P+1 loop.
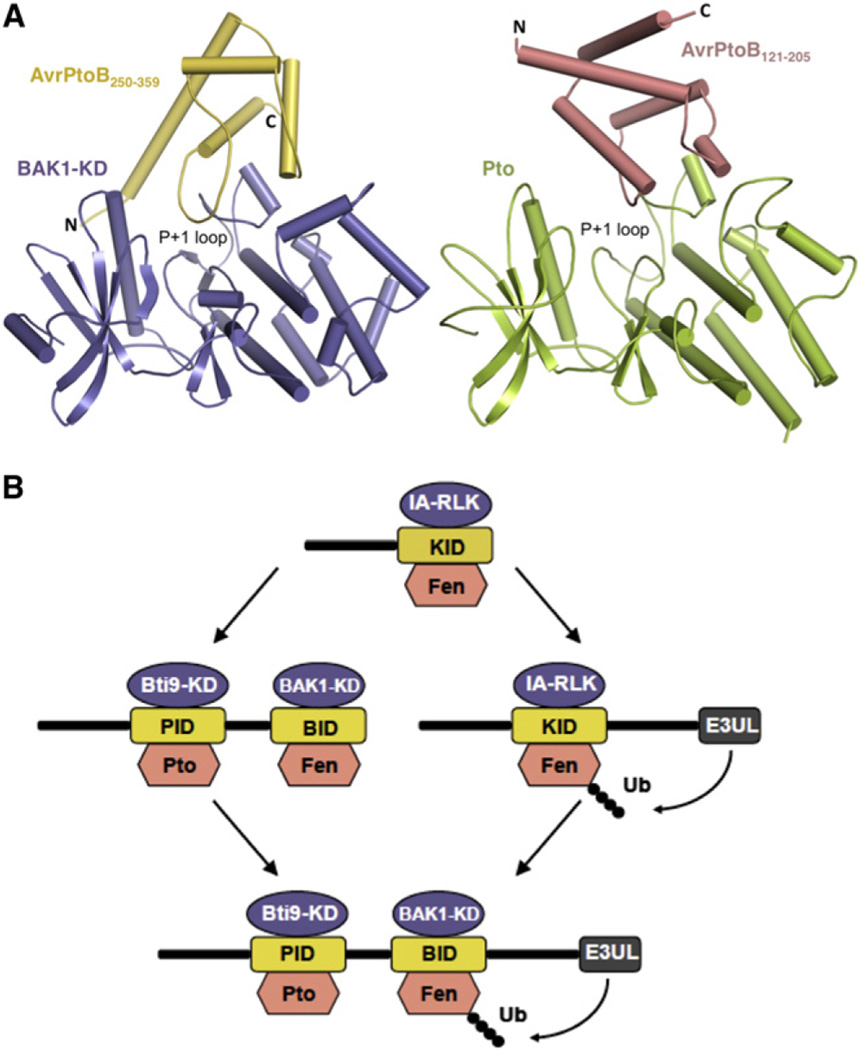
Despite the specificity with which AvrPtoB121–205 and AvrPtoB250–359 interact with different host kinases, our structural comparison revealed striking similarities between these two domains. Interestingly, AvrPtoB121–205 and AvrPtoB250–359 bind Pto and BAK1-KD, respectively, in different orientations (Figure 7). In order for AvrPtoB121–205 to interact with BAK1-KD, it would have to maintain its interactions with the P+1 loop and adopt a similar orientation to that in complex with Pto. Such positioning of AvrPtoB121–205, however, would lead to a loss of the contact surface governed by PtoF213 because the equivalent amino acid, BAK1L464, is not conserved. Furthermore, substitution of PtoV51 with BAK1G295 would add additional instability to the putative formation of AvrPtoB121–205-BAK1 complex. These subtle structural differences likely play an important role in the inability of AvrPtoB121–205 to interact with BAK1.
Figure 7. Structural Comparison and Evolutionary Model of the AvrPtoB121–205-Pto and AvrPtoB250–359-BAK1 Complexes.
(A) The AvrPtoB250–359-BAK1 complex is shown on the left and the AvrPtoB121–205-Pto complex on the right. The BID and PID in their respective complex have different orientations despite their similar overall structure.
(B) An evolutionary model for AvrPtoB virulence activity and host recognition. Two possible paths are illustrated. Kinases shown in blue normally activate PTI in response to PAMPS but are suppressed upon AvrPtoB binding. Kinases shown in salmon activate ETI after binding to specific domains of AvrPtoB. The AvrPtoB E3 ligase ubiquitinates Fen to overcome ETI (Rosebrock et al., 2007). IA-RLK, immunity-associated receptor-like kinase; KID, kinase-interacting domain; E3UL, E3 ubiquitin ligase; Ubq, ubiquitin. See the Discussion for more detail.
Fen and BAK1 both bind the same AvrPtoB domain (Rosebrock et al., 2007; Shan et al., 2008). Here we delimit the BID to AvrPtoB250–359. We hypothesize that this smaller region also interacts with Fen, but this remains to be tested experimentally. By aligning the P+1 loop of Fen and BAK1, Fen would be expected to maintain two of the three contact surfaces with AvrPtoB250–359. The amino acids that are involved in the interaction with AvrPtoB250–359 at the first interface, BAK1K418, R453, I456, are conserved in Fen. Compared to AvrPtoB121–205, AvrPtoB250–359 possesses an N-terminal extension that is directly involved in binding BAK1-KD at the second interface, mainly through polar interactions with the phosphate of BAK1pT450. The equivalent amino acid, T197, is conserved in Fen, although its phosphorylation status is unknown. Additional residues around the second interface involved in interaction with AvrPtoB250–359, including BAK1R453 and BAK1T333, are also conserved in Fen. Thus the N-terminal extension of AvrPtoB250–359 is expected to bind the second interface of Fen, which may compensate for the predicted compromised interaction around the third interface caused by variations of residues from α2 and α3 of Fen.
We found that AvrPtoB250–359 functions as an inhibitor of the BAK1 kinase in vitro, most likely by competitive interference at the substrate-binding site. Whether this inhibition occurs when AvrPtoB is delivered into the plant cell by Pst is unknown. However, several bacterial effector proteins are known to inhibit the activity of host kinases (Li et al., 2007; Xiang et al., 2008; Xing et al., 2007; Zhang et al., 2010). In most cases, the effectors require enzymatic activity for inhibition, permitting each effector molecule to act on multiple kinase molecules after delivery into the host. In the case of AvrPtoB, its only known enzymatic domain is an E3 ubiquitin ligase, but this activity is dispensable for the inhibition of BAK1 kinase activity and also for virulence in the natural host-pathogen system (Shan et al., 2008; Xiao et al., 2007a; Zeng et al., 2011). Thus a one-to-one correlation of AvrPtoB molecules to specific host kinases would be needed to inactive them. This is plausible if we consider immune responses to invading bacteria to be extremely localized. AvrPtoB would be delivered at an early time point into the plant cell and bind to and paralyze each activated immune kinase encountered in the vicinity of the point of delivery. Other effectors would then be delivered to suppress additional host components of PTI, eventually leading to disease. AvrPto has also been shown to inhibit kinase activity despite its lack of characterized enzymatic activity (Xiang et al., 2008; Xing et al., 2007). Our understanding of kinase inhibition by AvrPto and AvrPtoB may become clearer as the temporal delivery and spatial distribution of these proteins within the host cell becomes known.
Structural analyses directed our attention to six amino acids in AvrPtoB that when mutated would be likely to disrupt the interaction with BAK1 and abolish the virulence activity of AvrPtoB1–359. Introduction of substitutions at these residues disrupted the in vitro interaction between AvrPtoB250–359 and BAK1-KD. These substitutions also largely reduced the ability of AvrPtoB1–359 to suppress MPK6 activation. However, when delivered from Pst, only three of the AvrPtoB1–359 variants led to a statistically significant decrease in bacterial growth compared with the wild-type protein. One of the three, AvrPtoB1–359L341R, was poorly expressed and led to global destabilization of AvrPtoB250–359, providing an explanation for its reduced virulence activity.
The contributions of individual effectors to bacterial growth of Pst in planta are typically small, likely due to redundancy within the effector repertoire (Cunnac et al., 2009). Moreover, AvrPtoB is known to interact with multiple host kinases, and it is possible it targets additional proteins (Gimenez-Ibanez et al., 2009; Gohre et al., 2008; Kim et al., 2002; Rosebrock et al., 2007; Shan et al., 2008). Potentially, the AvrPtoB mutations we have tested disrupt the interaction with a single host target, BAK1, while retaining the ability to act on others. This may explain the minor decreases in virulence we observed in our tomato virulence assays. Alternatively, additional contacts between amino acids 1–250 of AvrPtoB and BAK1-KD may stabilize the interaction and reduce the effects of AvrPtoB mutations on this phenotype.
We hypothesized that in tomato Fen might have evolved as a molecular mimic (a decoy) of BAK1 leading to ETI (van der Hoorn and Kamoun, 2008; Xing et al., 2007). One way to test this is to determine whether the AvrPtoB amino acids that play a role in BAK1 interaction and virulence activity are also necessary for recognition by Fen. Of the two well-expressed AvrPtoB variants, one did not interact with BAK1 and was compromised in all virulence phenotypes; it also was not recognized by Fen. It is possible that Fen is a decoy, but it does not appear to be a perfect mimic of BAK1. The existence of an “imperfect decoy” may be beneficial to the plant by allowing the decoy to avoid unintended interactions with downstream components of the PTI signaling machinery. Collectively, our analyses suggest that AvrPtoB121–205 and AvrPtoB250–359 have evolved to target the structurally conserved portion of kinases to inhibit their activity, and also certain divergent structural elements to achieve specificity for distinct kinases.
Given their lack of obvious sequence similarity, the finding that AvrPtoB121–205 and AvrPtoB250–359 are structurally similar was surprising. However, upon further analysis we found the two domains share ~20% amino acid identity. The proximity of the two kinase-binding domains in AvrPtoB and presence of sequence similarity suggest that these domains have arisen long ago by tandem duplication of a progenitor kinase-interacting domain (KID). Tandem duplication and divergence is a well-known evolutionary force, and it is common for sequences to diverge beyond the point of similarity recognition by computational methods even though they may share the same evolutionary origin (Chothia and Gough, 2009; Gough, 2005).
Our current model posits that AvrPtoB initially had a type III secretion signal linked to a single KID (Figure 7B). The KID was sufficient to suppress the activity of an immunity-associated receptor-like kinase (IA-RLK). A progenitor of Fen evolved to recognize this domain. There are then two evolutionary paths that present-day AvrPtoB may have taken. The first involves tandem duplication of the KID followed by divergence of the duplicated domain to interact with other IA-RLKs. In order to recognize the new virulence activity of the duplicated KID, the Fen progenitor also sustained a duplication event leading to the divergence of Fen and Pto (these genes are members of a clustered gene family in tomato). Based on the widespread occurrence of Fen-like resistance in wild relatives of tomato, we have proposed previously that Fen evolved prior to Pto (Rosebrock et al., 2007). A subsequent evolutionary event leading to the present form of AvrPtoB was the acquisition of an E3 ubiquitin ligase domain. The second path may have involved the linkage of the E3 ubiquitin ligase domain to the single KID, followed by the tandem duplication of the KID and divergence to interact with different IA-RLKs and Pto. Regardless of the evolutionary sequence, AvrPtoB has acquired unusually effective virulence domains.
EXPERIMENTAL PROCEDURES
Crystallization, Data Collection, Structure Determination, and Refinement
Details of protein expression and purification, gel filtration, and the ITC experiments are described in the Supplemental Experimental Procedures. Crystallization conditions for BAK1-KD in complex with AvrPtoB250–359 were initially determined from the sparse matrix screen (Hampton Research). Screening was performed using hanging drop vapor diffusion by combining 2 µl of protein solution with an equal volume of well buffer. Crystals of the complex were eventually grown in the buffer containing 0.6 M NH4Ac, 17.5% PEG3350 (v/v) at room temperature. Typically, the crystals grew to their maximum size (0.1 × 0.2 × 0.2 mm3) within 5 days. The crystals were transferred to the mother liquor containing an additional 25% (v/v) glycerol and flash cooled in liquid nitrogen. The diffraction data were collected at the Shanghai Synchrotron Radiation Facility (SSRF) at beamline BL17U1 using a CCD detector. The data were processed using HKL2000 (Otwinowski and Minor, 1997). Further details of the structural analysis are described in the Supplemental Experimental Procedures. The crystallographic data are summarized in Table S1.
Kinase Assay
The assay was performed as described (Xing et al., 2007). The reaction buffer contained 50 mM HEPES (pH 7.4), 10 mM MgCl2, 1 mM DTT, 10 uM ATP, and 1 ul [γ−32P]ATP. For autophosphorylation reactions, 0.5 µg of BAK1-KD was added to 30 µl of reaction buffer. BAK1 was not dephosphorylated, as it was known from previous work (Wang et al., 2008) that BAK1 purified from E. coli is not phosphorylated on all of its potential in planta phosphorylation sites. The reaction mixture was incubated at 30°C for 0.5 hr and terminated by adding an equal volume of 2× SDS buffer. To determine the effect of AvrPtoB250–359 on BAK1-KD activity, BAK1-KD autophosphorylation was assayed in the presence of various concentrations of AvrPtoB250–359: 0, 2.17 µM, 4.34 µM, 8.68 µM, 17.36 µM, 119.5 µM, and 141.2 µM. Protein samples were separated by SDS polyacrylamide electrophoresis, and phosphorylated proteins were visualized with a phosphorimager.
MAPK Suppression Assay
Protoplasts were prepared from 3- to 4-week-old Arabidopsis thaliana Col-0 plants grown in long day conditions with a light intensity of ~70 µE/m2/s using the Tape sandwich method followed by transformation with AtMPK6-2xFLAG and the AvrPtoB variants using the TEAMP method (Wu et al., 2009; Yoo et al., 2007). MAPK suppression assays were carried out according to the methods of He et al. (2006). Primers and plasmids used for the AvrPtoB constructs are provided in Table S2. Further details are provided in the Supplemental Experimental Procedures.
AvrPtoB Virulence Assays in Tomato
AvrPtoB1–359 lacking a stop codon was cloned into the SmaI site of pJM51 for use in the Gateway recombination system and sequence confirmed. Site-directed mutagenesis was performed on the resulting construct to produce the following variants of AvrPtoB1–359: R271A/R275A, L336R, V337R, L341R, and V346D. Entry vectors containing wild-type AvrPtoB1–359 and its variants were recombined into pCPP5372, a Gateway destination vector containing the AvrPto hrp promoter and a C-terminal HA tag (Oh et al., 2007) via the LR recombination reaction (Invitrogen). Virulence assays were carried out similar to the methods of Xiao et al. (2007b). Further details are provided in the Supplemental Experimental Procedures.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Outstanding Young Scholar Science Foundation of the National Natural Science Foundation of China (20101331722) and State Key Program of National Natural Science of China (31130063) to J.C. and by the National Science Foundation (IOS-1025642) and the National Institutes of Health (R01-GM078021) to G.B.M.
Footnotes
ACCESSION NUMBERS
The coordinates and structure factors of the crystal structures presented in this paper have been deposited in the Protein Data Bank with the accession code 3TL8.
SUPPLEMENTAL INFORMATION
Supplemental Information includes four figures, two tables, Supplemental Experimental Procedures, and Supplemental References and can be found with this article online at doi:10.1016/j.chom.2011.10.013.
REFERENCES
- Abramovitch RB, Kim Y-J, Chen S, Dickman MB, Martin GB. Pseudomonas type III effector AvrPtoB induces plant disease susceptibility by inhibition of host programmed cell death. EMBO J. 2003;22:60–69. doi: 10.1093/emboj/cdg006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramovitch RB, Janjusevic R, Stebbins CE, Martin GB. Type III effector AvrPtoB requires intrinsic E3 ubiquitin ligase activity to suppress plant cell death and immunity. Proc. Natl. Acad. Sci. USA. 2006;103:2851–2856. doi: 10.1073/pnas.0507892103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm ST, Coaker G, Day B, Staskawicz BJ. Hostmicrobe interactions: shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Chothia C, Gough J. Genomic and structural aspects of protein evolution. Biochem. J. 2009;419:15–28. doi: 10.1042/BJ20090122. [DOI] [PubMed] [Google Scholar]
- Clouse SD. Brassinosteroid signal transduction: from receptor kinase activation to transcriptional networks regulating plant development. Plant Cell. 2011;23:1219–1230. doi: 10.1105/tpc.111.084475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnac S, Lindeberg M, Collmer A. Pseudomonas syringae type III secretion system effectors: repertoires in search of functions. Curr. Opin. Microbiol. 2009;12:53–60. doi: 10.1016/j.mib.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Cunnac S, Chakravarthy S, Kvitko BH, Russell AB, Martin GB, Collmer A. Genetic disassembly and combinatorial reassembly identify a minimal functional repertoire of type III effectors in Pseudomonas syringae. Proc. Natl. Acad. Sci. USA. 2011;108:2975–2980. doi: 10.1073/pnas.1013031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Xiao F, Fan F, Gu L, Cang H, Martin GB, Chai J. Crystal structure of the complex between Pseudomonas effector AvrPtoB and the tomato Pto kinase reveals both a shared and a unique interface compared with AvrPto-Pto. Plant Cell. 2009;21:1846–1859. doi: 10.1105/tpc.109.066878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Ibanez S, Hann DR, Ntoukakis V, Petutschnig E, Lipka V, Rathjen JP. AvrPtoB targets the LysM receptor kinase CERK1 to promote bacterial virulence on plants. Curr. Biol. 2009;19:423–429. doi: 10.1016/j.cub.2009.01.054. [DOI] [PubMed] [Google Scholar]
- Gohre V, Spallek T, Haweker H, Mersmann S, Mentzel T, Boller T, de Torres M, Mansfield JW, Robatzek S. Plantpattern-recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Curr. Biol. 2008;18:1824–1832. doi: 10.1016/j.cub.2008.10.063. [DOI] [PubMed] [Google Scholar]
- Gomez-Gomez L, Boller T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis . Mol. Cell. 2000;5:1003–1011. doi: 10.1016/s1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- Gough J. Convergent evolution of domain architectures (is rare) Bioinformatics. 2005;21:1464–1471. doi: 10.1093/bioinformatics/bti204. [DOI] [PubMed] [Google Scholar]
- He P, Shan L, Lin NC, Martin GB, Kemmerling B, Nurnberger T, Sheen J. Specific bacterial suppressors of MAMP signaling upstream of MAPKKK in Arabidopsis innate immunity. Cell. 2006;125:563–575. doi: 10.1016/j.cell.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Heese A, Hann DR, Gimenez-Ibanez S, Jones AM, He K, Li J, Schroeder JI, Peck SC, Rathjen JP. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc. Natl. Acad. Sci. USA. 2007;104:12217–12222. doi: 10.1073/pnas.0705306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm L, Rosenström P. Dali server: conservation mapping in 3D. Nucleic Acids Res. 2010;38:W545–W549. doi: 10.1093/nar/gkq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjusevic R, Abramovitch RB, Martin GB, Stebbins CE. A bacterial inhibitor of host programmed cell death defenses is an E3 ubiquitin ligase. Science. 2006;311:222–226. doi: 10.1126/science.1120131. [DOI] [PubMed] [Google Scholar]
- Kim Y-J, Lin N-C, Martin GB. Two distinct Pseudomonas effector proteins interact with the Pto kinase and activate plant immunity. Cell. 2002;109:589–598. doi: 10.1016/s0092-8674(02)00743-2. [DOI] [PubMed] [Google Scholar]
- Li H, Xu H, Zhou Y, Zhang J, Long C, Li S, Chen S, Zhou JM, Shao F. The phosphothreonine lyase activity of a bacterial type III effector family. Science. 2007;315:1000–1003. doi: 10.1126/science.1138960. [DOI] [PubMed] [Google Scholar]
- Lu D, Wu S, Gao X, Zhang Y, Shan L, He P. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc. Natl. Acad. Sci. USA. 2010;107:496–501. doi: 10.1073/pnas.0909705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntoukakis V, Mucyn TS, Gimenez-Ibanez S, Chapman HC, Gutierrez JR, Balmuth AL, Jones AM, Rathjen JP. Host inhibition of a bacterial virulence effector triggers immunity to infection. Science. 2009;324:784–787. doi: 10.1126/science.1169430. [DOI] [PubMed] [Google Scholar]
- O’Brien HE, Desveaux D, Guttman DS. Next-generation genomics of Pseudomonas syringae. Curr. Opin. Microbiol. 2011;14:24–30. doi: 10.1016/j.mib.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Oh HS, Kvitko BH, Morello JE, Collmer A. Pseudomonas syringae lytic transglycosylases coregulated with the type III secretion system contribute to the translocation of effector proteins into plant cells. J. Bacteriol. 2007;189:8277–8289. doi: 10.1128/JB.00998-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MH, Wang X, Wu X, Zhao Y, Clouse SD, Huber SC. Autophosphorylation of Tyr-610 in the receptor kinase BAK1 plays a role in brassinosteroid signaling and basal defense gene expression. Proc. Natl. Acad. Sci. USA. 2010;107:17827–17832. doi: 10.1073/pnas.0915064107. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Ronald PC, Beutler B. Plant and animal sensors of conserved microbial signatures. Science. 2010;330:1061–1064. doi: 10.1126/science.1189468. [DOI] [PubMed] [Google Scholar]
- Rosebrock TR, Zeng L, Brady JJ, Abramovitch RB, Xiao F, Martin GB. A bacterial E3 ubiquitin ligase targets a host protein kinase to disrupt plant immunity. Nature. 2007;448:370–374. doi: 10.1038/nature05966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze B, Mentzel T, Jehle A, Mueller K, Beeler S, Boller T, Felix G, Chinchilla D. Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J. Biol. Chem. 2010;285:9444–9451. doi: 10.1074/jbc.M109.096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwessinger B, Roux M, Kadota Y, Ntoukakis V, Sklenar J, Jones A, Zipfel C. Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet. 2011;7:e1002046. doi: 10.1371/journal.pgen.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonzac C, Zipfel C. Activation of plant pattern-recognition receptors by bacteria. Curr. Opin. Microbiol. 2011;14:54–61. doi: 10.1016/j.mib.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Shan L, He P, Li J, Heese A, Peck SC, Nurnberger T, Martin GB, Sheen J. Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe. 2008;4:17–27. doi: 10.1016/j.chom.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Frederick RD, Zhou J, Halterman DA, Jia Y, Martin GB. Initiation of plant disease resistance by physical interaction of AvrPto and Pto kinase. Science. 1996;274:2060–2063. doi: 10.1126/science.274.5295.2060. [DOI] [PubMed] [Google Scholar]
- van der Hoorn RA, Kamoun S. From Guard to Decoy: a new model for perception of plant pathogen effectors. Plant Cell. 2008;20:2009–2017. doi: 10.1105/tpc.108.060194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu J, Sudom A, Ayres M, Li S, Wesche H, Powers JP, Walker NP. Crystal structures of IRAK-4 kinase in complex with inhibitors: a serine/threonine kinase with tyrosine as a gatekeeper. Structure. 2006;14:1835–1844. doi: 10.1016/j.str.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Wang X, Kota U, He K, Blackburn K, Li J, Goshe MB, Huber SC, Clouse SD. Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev. Cell. 2008;15:220–235. doi: 10.1016/j.devcel.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Wu FH, Shen SC, Lee LY, Lee SH, Chan MT, Lin CS. Tape-Arabidopsis Sandwich—a simpler Arabidopsis protoplast isolation method. Plant Methods. 2009;5:16. doi: 10.1186/1746-4811-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang T, Zong N, Zou Y, Wu Y, Zhang J, Xing W, Li Y, Tang X, Zhu L, Chai J, et al. Pseudomonas syringae effector AvrPto blocks innate immunity by targeting receptor kinases. Curr. Biol. 2008;18:74–80. doi: 10.1016/j.cub.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Xiao F, Giavalisco P, Martin GB. Pseudomonas syringae type III effector AvrPtoB is phosphorylated in plant cells on serine 258, promoting its virulence activity. J. Biol. Chem. 2007a;282:30737–30744. doi: 10.1074/jbc.M705565200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, He P, Abramovitch RB, Dawson JE, Nicholson LK, Sheen J, Martin GB. The N-terminal region of Pseudomonas type III effector AvrPtoB elicits Pto-dependent immunity and has two distinct virulence determinants. Plant J. 2007b;52:595–614. doi: 10.1111/j.1365-313X.2007.03259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing W, Zou Y, Liu Q, Liu J, Luo X, Huang Q, Chen S, Zhu L, Bi R, Hao Q, et al. The structural basis for activation of plant immunity by bacterial effector protein AvrPto. Nature. 2007;449:243–247. doi: 10.1038/nature06109. [DOI] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2007;2:1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]
- Zeng L, Velasquez AC, Munkvold KR, Zhang J, Martin GB. A tomato LysM receptor-like kinase promotes immunity and its kinase activity is inhibited by AvrPtoB. Plant J. 2011 doi: 10.1111/j.1365-313X.2011.04773.x. Published online August 31, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li W, Xiang T, Liu Z, Laluk K, Ding X, Zou Y, Gao M, Zhang X, Chen S, et al. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe. 2010;7:290–301. doi: 10.1016/j.chom.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–760. doi: 10.1016/j.cell.2006.03.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.