Abstract
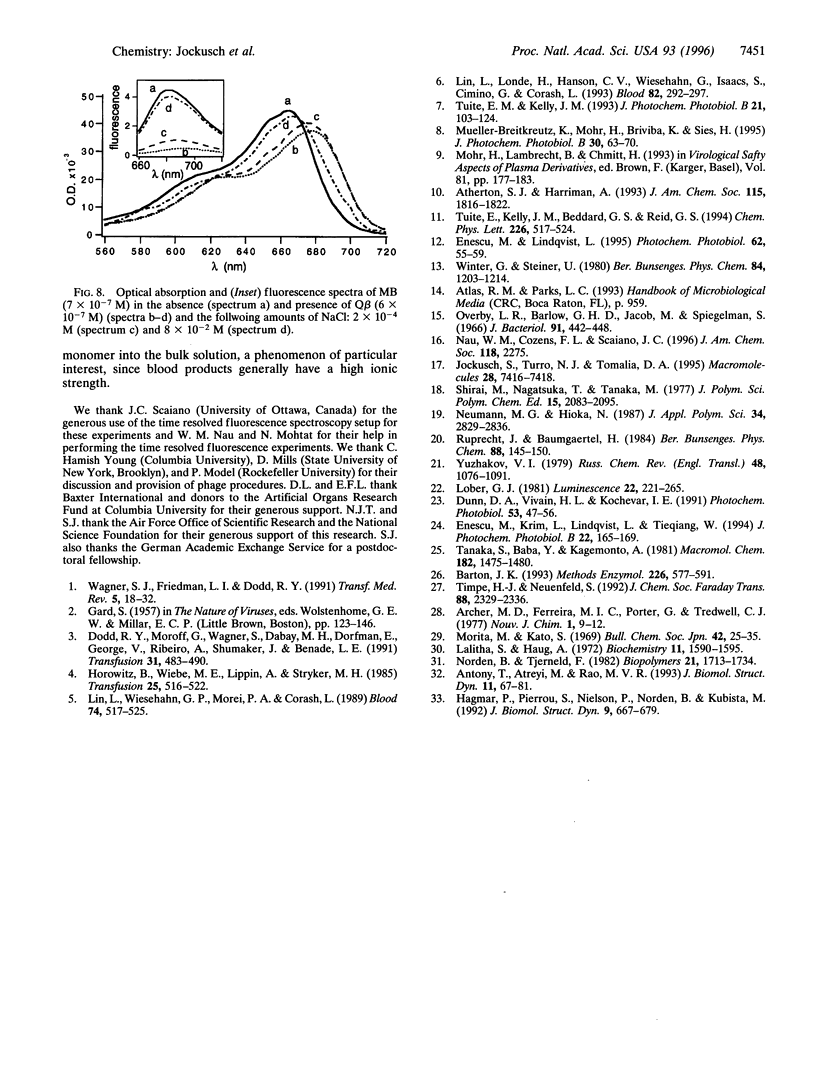
The adsorption of cationic organic dyes (methylene blue, thionine, and thiopyronine) on Qbeta bacteriophage was studied by UV-visible and fluorescence spectroscopy. The dyes have shown a strong affinity to the virus and some have been used as sensitizers for photo-induced inactivation of virus. In the methylene blue concentration range of 0.1-5 microM and at high ratios of dye to virus (greater than 1000 dye molecules per virion), the dyes bind as aggregates on the virus. Aggregation lowers the efficiency of photoinactivation because of self-quenching of the dye. At lower ratios of dye to virus (lower than 500 dye molecules per virion), the dye binds to the virus as a monomer. Fluorescence polarization and time-resolved studies of the fluorescence support the conclusions based on fluorescence quenching. Increasing the ionic strength (adding NaCl) dissociates bound dye aggregates on the virus and releases monomeric dye into the bulk solution.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antony T., Atreyi M., Rao M. V. Spectroscopic studies on the binding of methylene blue to poly(riboadenylic acid). J Biomol Struct Dyn. 1993 Aug;11(1):67–81. doi: 10.1080/07391102.1993.10508710. [DOI] [PubMed] [Google Scholar]
- Dodd R. Y., Moroff G., Wagner S., Dabay M. H., Dorfman E., George V., Ribeiro A., Shumaker J., Benade L. E. Inactivation of viruses in platelet suspensions that retain their in vitro characteristics: comparison of psoralen-ultraviolet A and merocyanine 540-visible light methods. Transfusion. 1991 Jul-Aug;31(6):483–490. doi: 10.1046/j.1537-2995.1991.31691306242.x. [DOI] [PubMed] [Google Scholar]
- Dunn D. A., Lin V. H., Kochevar I. E. The role of ground state complexation in the electron transfer quenching of methylene blue fluorescence by purine nucleotides. Photochem Photobiol. 1991 Jan;53(1):47–56. doi: 10.1111/j.1751-1097.1991.tb08466.x. [DOI] [PubMed] [Google Scholar]
- Hagmar P., Pierrou S., Nielsen P., Nordén B., Kubista M. Ionic strength dependence of the binding of methylene blue to chromatin and calf thymus DNA. J Biomol Struct Dyn. 1992 Feb;9(4):667–679. doi: 10.1080/07391102.1992.10507947. [DOI] [PubMed] [Google Scholar]
- Horowitz B., Wiebe M. E., Lippin A., Stryker M. H. Inactivation of viruses in labile blood derivatives. I. Disruption of lipid-enveloped viruses by tri(n-butyl)phosphate detergent combinations. Transfusion. 1985 Nov-Dec;25(6):516–522. doi: 10.1046/j.1537-2995.1985.25686071422.x. [DOI] [PubMed] [Google Scholar]
- Lalitha S., Haug A. Complex formation and energy transfer from photoexcited thiopyronine to deoxyribonucleic acid. Biochemistry. 1972 Apr 25;11(9):1590–1595. doi: 10.1021/bi00759a007. [DOI] [PubMed] [Google Scholar]
- Lin L., Londe H., Hanson C. V., Wiesehahn G., Isaacs S., Cimino G., Corash L. Photochemical inactivation of cell-associated human immunodeficiency virus in platelet concentrates. Blood. 1993 Jul 1;82(1):292–297. [PubMed] [Google Scholar]
- Lin L., Wiesehahn G. P., Morel P. A., Corash L. Use of 8-methoxypsoralen and long-wavelength ultraviolet radiation for decontamination of platelet concentrates. Blood. 1989 Jul;74(1):517–525. [PubMed] [Google Scholar]
- Müller-Breitkreutz K., Mohr H., Briviba K., Sies H. Inactivation of viruses by chemically and photochemically generated singlet molecular oxygen. J Photochem Photobiol B. 1995 Sep;30(1):63–70. doi: 10.1016/1011-1344(95)07150-z. [DOI] [PubMed] [Google Scholar]
- Nordén B., Tjerneld F. Structure of methylene blue-DNA complexes studied by linear and circular dichroism spectroscopy. Biopolymers. 1982 Sep;21(9):1713–1734. doi: 10.1002/bip.360210904. [DOI] [PubMed] [Google Scholar]
- Overby L. R., Barlow G. H., Doi R. H., Jacob M., Spiegelman S. Comparison of two serologically distinct ribonucleic acid bacteriophages. I. Properties of the viral particles. J Bacteriol. 1966 Jan;91(1):442–448. doi: 10.1128/jb.91.1.442-448.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuite E. M., Kelly J. M. Photochemical interactions of methylene blue and analogues with DNA and other biological substrates. J Photochem Photobiol B. 1993 Dec;21(2-3):103–124. doi: 10.1016/1011-1344(93)80173-7. [DOI] [PubMed] [Google Scholar]
- Wagner S. J., Friedman L. I., Dodd R. Y. Approaches to the reduction of viral infectivity in cellular blood components and single donor plasma. Transfus Med Rev. 1991 Jan;5(1):18–32. doi: 10.1016/s0887-7963(91)70190-9. [DOI] [PubMed] [Google Scholar]


