The artificial pancreas (AP) offers an ideal solution to many patients with diabetes treated by insulin because it covers the triple aim of diabetes therapy: (i) close-to-normal mean glucose control in order to prevent long-term diabetes complications, (ii) maintenance of glucose levels in a safe range in order to avoid acute brain-debilitating glucose excursions, and (iii) minimal burden in disease management thanks to a discrete wearable system, including an insulin infuser, a glucose monitor, and a diabetes controller.
Building the Components of the Artificial Pancreas
In the 1970s, the insulin infuser was the first component of the AP developed, resulting in current miniaturized, safe, and reliable insulin pumps, including catheter-free models, devices equipped by bolus calculators that assist patients in decisions for insulin doses, sensor-augmented pumps, and fully implantable systems allowing intraperitoneal insulin delivery. When used as “open-loop” systems, i.e., requiring patient action to command insulin infusion, insulin pumps have been demonstrated to be more effective in glucose control than multiple daily insulin injections and safer in terms of prevention of severe hypoglycemia.1 Insulin pumps are nowadays commonly adopted as a convenient wearable tool for diabetes therapy by patients with type 1 diabetes in the developed parts of the world.
Glucose monitoring was limited to frequent self-monitoring of blood glucose through finger sticks until continuous glucose monitoring (CGM) systems became available in the clinical field in 1999. Based on enzymatic measurement of glucose levels in the subcutaneous interstitial fluid, CGM devices provide increasingly accurate estimations of blood glucose levels thanks to continuous improvements in the biocompatibility of the inserted “sensing needle,” in signal filtering and in calibration procedures. Some CGM systems reach a mean absolute relative difference between sensor glucose and blood glucose gradually closer to 10%, over the whole physiological glucose range. When connected to or combined with an insulin pump, CGM systems allow further improvement in hemoglobin A1c levels and, thanks to warning alarms of glucose deviations out of a set range, can reduce occurrence of hypoglycemia.2
However, this “sensor-augmented open-loop” concept only benefits patients who tightly manage sensor information and therefore tends to increase the patient’s burden for glucose control.
Since 2006, developments have focused on the controller component of the AP toward creation of a safe and effective closed-loop insulin delivery system.3 This task represents a true challenge, because adjustment of insulin delivery has to take into account a 30–60 min delay of insulin action when infused in the subcutaneous tissue and a 1–5 min delay in glucose assessment by the CGM device when measured in the interstitial fluid. Studies are underway using intraperitoneal and inhaled insulin delivery in an attempt to mimic both the onset and offset of endogenous insulin action.4–6 Because of the reduced occurrence of interfering events during nighttime sleep, especially in a controlled hospital setting, most algorithms have succeeded in keeping glucose in a safe range during this period. As a matter of fact, the controller only needs to intervene in case of gradual deviations of glucose toward hypoglycemia or hyperglycemia while basal insulin infusion is set according to usual patient needs. Mealtime periods and exercise bouts are much more challenging for the controllers because of quick variations in glucose levels that need to be anticipated, prevented, or corrected. In this frame, model-predictive controllers have shown their unique ability to reduce hyperglycemic deviations with no later hypoglycemia, thanks to the development of insulin infusion “brakes” in addition to the main control algorithm so that prevention of hypoglycemia comes first. Controller models could combine a reduction of mean blood glucose level and an increased time spent in a safe glucose range versus open-loop insulin infusion in a hospital setting.7
Moving to Outpatient Artificial Pancreas: A Step-by-Step Approach
Expanding the use of these AP systems in daily life opens a “new frontier.” Indeed, moving to ambulatory conditions creates instability at many levels: variations of insulin needs due to any stressful physical or psychological event, possible physical stress at the sensor and at the infusion sites, physical constraints to communication between devices, and variable attention paid by the patient to the functioning of the system.
To accommodate such a changing environment, the first move toward outpatient use of AP needs an intermediary step: “home-like” environment. Early experiences reported with an AP in outpatients have followed this mode: hotel, diabetes camp, resort villa.8–10 Closing the loop step-by-step is another component of this new adventure. Initially, closed-loop can be considered at nighttime only or during excursions to town with technicians or nurses as “body guards,” while daytime activities can be covered by a more relaxed hyper/hypo mitigation controller. The goal of these feasibility studies has been to identify the weak links that may corrupt the system’s function and to describe the procedures needed to solve the occurring issues, such as defaulting to patient self-management of the insulin pump as the fallback mode. Moving to “control-to-range” at all times will represent the next step.
Patient selection for AP use in outpatient conditions becomes a hot topic, and prolonged experiments are now designed. Although the AP is expected to bring ultimately the most to patients who hardly manage diabetes due to limited compliance to glucose monitoring and poor decision ability in adjusting insulin delivery, initial candidates for an outpatient AP will likely be patients deeply involved in diabetes care. Indeed, the first prototypes for an outpatient AP will request sufficient skills in device use and a thorough attention paid by the enrolled patients in order to detect early system failures, to refer to the monitoring team as soon as needed, and to comply to fallback or correction procedures when applicable. The inclusion criteria for the first sustained outpatient use of AP will comprise several months of insulin pump therapy, some experience with CGM use, and a good ability in being trained to manage devices and eventual system failures. Once these initial trials are accomplished, first indications for an outpatient AP will likely include patients who fail to reach stable glucose control either because of overzealous dose adjustments that increase the occurrence of hypoglycemia or, on the contrary, because of pusillanimous behavior toward tuning insulin delivery, resulting in sustained hyperglycemia. Thanks to the decisions taken by the controller, glucose control should be significantly improved in these patient profiles. Poorly compliant patients with reluctance for training and low involvement in diabetes management will not be good candidates for outpatient AP as long as contributions will be expected from the patient, e.g., for meal announcement or for correction procedures in case of system warning of expected out-of-range glucose deviation beyond the ability of the controller to prevent it by itself.
A New Diabetes Ecosystem
In terms of technology, a parallel objective is to make the AP system compatible with personal, social, and professional life, i.e., prevent “astronaut suit” mode. The various criteria to fulfill can be summarized as a list of expected features for outpatient AP: discrete, wearable, wireless, user-friendly, monitored, and, ultimately, integrated while keeping safety and efficacy of glucose control as the leading objectives. So far, the first approach has been to reduce the size of the control platform from the laptop used in the hospital setting to a smartphone or tablet-like device.8,10 This discrete and wearable format is combined with an easy-to-manage interface that provides online the key information on glucose control, system functioning, and potential need for intervention. The reliability and stability of the connections between this platform, the insulin pump, and the CGM system have emerged as crucial elements to be consolidated. The move toward wireless connections appears as the key objective, including a sufficient range for communication between the devices. Given that the power sources of these devices need to be stable, there is a need to develop energy-sparing communication modes to prevent recurrent battery replacements, which are incompatible with everyday life. Early trials of portable outpatient AP have been aided by remote monitoring systems that allow distant monitoring of the AP system as an added safety measure.10
Ultimately, the AP will move to a vertically integrated system that includes local functions running on devices near the subject and global functions executed in the cloud. Figure 1 presents a schematic of such an AP system and outlines its key functions:
At the base of the AP ecosystem is a medical-grade operating environment, which will ensure the proper use of the AP as an outpatient medical device. System security, the security of communications, priority power management, and other basic functions will be embedded at this level.
The core of the local AP functions is based on a local database service that handles communication with peripheral devices (e.g., sensors, patch pumps, or others) and ensures data availability and integrity for the operation of various levels of closed-loop control.
The closed-loop control functions reside in a modular system3 that can execute various functions, depending on the desired configuration. The first key module is safety, which is responsible for monitoring the patient, preventing hypoglycemia via insulin attenuation or glucagon injection, and other critical functions.
At higher control levels, control-to-range or control-to-target modules will ensure optimal insulin or dual-hormone delivery to keep the patient within a predetermined range or bring blood glucose levels to a desired target.
The local functions of the AP ecosystem are communicated to the patient via patient-oriented user interface, which needs to be developed in human-factor studies and with the help of experts in interface design. One example included in Figure 1 is the user interface of the DiAs (diabetes assistant platform for artificial pancreas) system used in early feasibility outpatient studies.8,10
Figure 1.
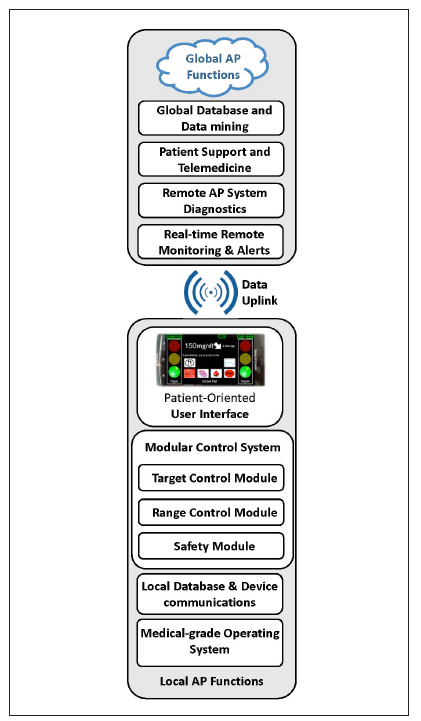
The AP ecosystem of the near future.
It is likely that local functions 1–3 will reside and will be executed by a sensor-augmented insulin pump. Functions 4 and 5 could reside on the pump as well or could be distributed between the pump and a companion device, such as a smartphone—an approach taken by the early feasibility studies.8,10
The global function of the AP ecosystem will reside in the cloud and could range from straightforward real-time remote monitoring already employed in some studies10 to remote AP diagnostics, telemedicine, and data mining, all of which are likely to be built in the future. The advantages of a well-designed global cloud component are evident and range from the ability to alert relatives or emergency responders about arising critical situations to rapid database searches and pattern recognition, assisting patients and health care providers with their decision making.
In summary, the integration of critical AP ecosystem functions into one device and the further distribution of less critical functions to companion devices or to the cloud, as well as the availability of very-fast-acting insulin solutions and glucose sensors with high accuracy, will ultimately provide patients with access to reliable optimal diabetes control.
Acknowledgments
We thank our partners of the “Bringing Artificial Pancreas at Home” E.U. project consortium as well as our collaborators who are contributing to the development of the AP in our research teams: Jerome Place and Anne Farret at Montpellier University Hospital and University of Montpellier, France; Francis J. Doyle III, Eyal Dassau, and Wendy Bevier at the University of California and Sansum Diabetes Research Institute; Stacey Anderson, Sue Brown, Marc Breton, Steve Patek, and Patrick Keith-Hynes at the University of Virginia; Lalo Magni at the University of Pavia, Pavia, Italy; and Simone Del Favero, Roberto Visentin, Daniela Bruttomesso, and Angelo Avogaro at the University of Padova and Padova University Hospital, Padova, Italy.
Glossary
- (AP)
artificial pancreas
- (CGM)
continuous glucose monitoring
Funding
This work was supported by the European Community Framework Program 7 (FP7-ICT-2009–4 Grant #247138), the National Institutes of Health (DP3 DK094331, R01 DK085628, R01 DK085623), and the JDRF (17–2010–765, 17–2011–515, 22–2011–637, 22–2011–649)
Disclosures
Eric Renard is a consultant/advisor for A. Menarini Diagnostics, Abbott, Cellnovo, Dexcom, Eli-Lilly, Johnson & Johnson (Animas, LifeScan), Medtronic, Novo-Nordisk, Roche Diagnostics, and Sanofi-Aventis and has received research grants/material support from Abbott, Dexcom, Insulet, and Roche Diagnostics. Claudio Cobelli holds patents related to AP technology, is a consultant/advisor for Dexcom, has received research grants from Dexcom, and has received study material support from Dexcom, Insulet, and Roche. Howard C. Zisser holds patents related to AP technology; is a consultant/advisor for Animas, CellNovo, Insulet, MannKind, and Roche; and has received research grants/product support from Animas, Abbott, Dexcom, Eli Lilly, GluMetrics, Insulet, LifeScan, Medtronic, Novo Nordisk, Roche, and Sanofi. Boris P. Kovatchev holds patents related to AP technology, is a consultant/advisor for Animas and Sanofi-Aventis, has received research grants from Sanofi-Aventis, and has received study material support from Abbott, Dexcom, Insulet, LifeScan, and Tandem.
References
- 1.Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in Type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med. 2008;25(7):765–774. doi: 10.1111/j.1464-5491.2008.02486.x. [DOI] [PubMed] [Google Scholar]
- 2.Langendam M, Luijf YM, Hooft L, Devries JH, Mudde AH, Scholten RJ. Continuous glucose monitoring systems for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2012;1:CD008101. doi: 10.1002/14651858.CD008101.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cobelli C, Renard E, Kovatchev B. Artificial pancreas: past, present, future. Diabetes. 2011;60(11):2672–2682. doi: 10.2337/db11-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ClinicalTrials.gov. Feasibility study using Zone-MPC controller, HMS and Technosphere® Insulin Inhalation System from MannnKind Corp. http://www.clinicaltrials.gov/ct2/show/NCT01874392.
- 5.Lee JJ, Zisser H, Dassau E, Farret A, Place J, Pelletier MJ, Harvey RA, Doyle FJ, III, Renard E. Clinical results of artificial pancreas using intraperitoneal insulin delivery. Diabetes. 2013;62:A4–5. Suppl 1. [Google Scholar]
- 6.Zisser H. Clinical hurdles and possible solutions in the implementation of closed-loop control in type 1 diabetes mellitus. J Diabetes Sci Tech nol. 2011;5(5):1283–1286. doi: 10.1177/193229681100500537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breton M, Farret A, Bruttomesso D, Anderson S, Magni L, Patek S, Dalla Man C, Place J, Demartini S, Del Favero S, Tofanin C, Hughes-Karvetski C, Dassau E, Zisser H, Doyle FJ, III, De Nicolao G, Avogaro A, Cobelli C, Renard E, Kovatchev B. International Artifcial Pancreas Study Group. Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes. 2012;61(9):2230–2237. doi: 10.2337/db11-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobelli C, Renard E, Kovatchev BP, Keith-Hynes P, Ben Brahim N, Place J, Del Favero S, Breton MD, Farret A, Bruttomesso D, Dassau E, Zisser H, Doyle FJ, III, Patek S, Avogaro A. Pilot studies of wearable artificial pancreas in type 1 diabetes. Diabetes Care. 2012;35(9):e65–e67. doi: 10.2337/dc12-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillip M, Battelino T, Atlas E, Kordonouri O, Bratina N, Miller S, Biester T, Stefanija MA, Muller I, Nimri R, Danne T. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368(9):824–833. doi: 10.1056/NEJMoa1206881. [DOI] [PubMed] [Google Scholar]
- 10.Kovatchev BP, Renard E, Cobelli C, Zisser HC, Keith-Hynes P, Anderson SM, Brown SA, Chernavvsky DR, Breton MD, Farret A, Pelletier MJ, Place J, Bruttomesso D, Del Favero S, Visentin R, Filippi A, Scotton R, Avogaro A, Doyle FJ., III Feasibility of outpatient fully integrated closed-loop control: first studies of wearable artificial pancreas. Diabetes Care. 2013;36(7):1851–1858. doi: 10.2337/dc12-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]


