Abstract
Ras proteins are guanine nucleotide-binding proteins that are highly conserved among eukaryotes. They are involved in signal transduction pathways and are tightly regulated by two sets of antagonistic proteins: GTPase-activating proteins (GAPs) inhibit Ras proteins, whereas guanine exchange factors activate them. In this work, we describe Tfs1p, the first physiological inhibitor of a Ras GAP, Ira2p, in Saccharomyces cerevisiae. TFS1 is a multicopy suppressor of the cdc25-1 mutation in yeast and corresponds to the so-called Ic CPY cytoplasmic inhibitor. Moreover, Tfs1p belongs to the phosphatidylethanolamine-binding protein (PEBP) family, one member of which is RKIP, a kinase and serine protease inhibitor and a metastasis inhibitor in prostate cancer. In this work, the results of (i) a two-hybrid screen of a yeast genomic library, (ii) glutathione S-transferase pulldown experiments, (iii) multicopy suppressor tests of cdc25-1 mutants, and (iv) stress resistance tests to evaluate the activation level of Ras demonstrate that Tfs1p interacts with and inhibits Ira2p. We further show that the conserved ligand-binding pocket of Tfs1—the hallmark of the PEBP family—is important for its inhibitory activity.
RAS genes encode small guanine nucleotide-binding proteins that serve as molecular switches in signal transduction pathways and thus control cell growth and differentiation (35). Ras proteins cycle between an active GTP-bound form and an inactive GDP-bound form. The GDP/GTP exchange reaction that converts inactive Ras into its active form is enhanced by guanine exchange factors (GEFs) (4). The GTP-bound form of Ras is converted into its GDP-bound form by a GTPase activity stimulated by GTPase-activating proteins (GAPs) (4).
Ras proteins are highly conserved among eukaryotes. Furthermore, the fact that the functional domains of GEF and GAP are homologous suggests that their activity is regulated by similar mechanisms. Indeed, Saccharomyces cerevisiae possesses two Ras proteins, Ras1 and Ras2, which are activated by two GEFs, Cdc25 and Sdc25 (12, 13), and inhibited by two GAPs, Ira1 and Ira2 (43, 44). All of these proteins display sequence and functional homology with their mammalian counterparts (18, 26, 30, 42, 53).
In yeast, Ras proteins activate the cyclic AMP (cAMP)/protein kinase A (PKA) pathway, which controls metabolism, stress resistance, growth, and meiosis (10, 45, 46). In this pathway, cAMP is synthesized by adenylate cyclase (27, 48). cAMP then activates the cAMP-dependent PKA by binding to its regulatory subunit (47). The numerous downstream targets of PKA include enzymes involved in intermediate metabolism, proteins that are important for cell cycle control, and transcription factors involved in the expression of stress response element (STRE)-controlled genes and ribosomal protein genes (29, 32, 34, 41).
In this work, we describe the first physiological inhibitor of Ras GAP activity: that of the yeast protein, Ira2. The inhibitor protein is named Tfs1p (for “Twenty-Five suppressor”) and was previously isolated by Robinson and Tatchell (33) as a multicopy suppressor of the cdc25-1 mutant. TFS1 is an STRE-regulated gene. It is overexpressed in many stress conditions such as oxidative stress, diauxic shift, and heat shock (19, 8, 7). Tfs1p has also been shown to inhibit CPY (11), and it has recently been shown that the acetylation of its N terminus is required for this activity (28).
Tfs1p belongs to the conserved phosphatidylethanolamine-binding protein (PEBP) family (present in many organisms such as mammals, plants, worms, Drosophila, yeast, and bacteria) (37, 38, 22). The mammalian PEBP has a number of putative functions: (i) lipid binding (37), (ii) inhibition of serine proteases (21), (iii) being the precursor of a neurostimulatory peptide (HCNP) that is important in the development of hippocampus (49), (iv) inhibition of the Raf-1 kinase and the mitogen-activated protein kinase Raf/MEK/ERK signaling pathway (in this study the protein was called RKIP [for “Raf kinase inhibitor protein”]) (55), (v) inhibition of two kinases involved in the NFκB activation pathway (56), (vi) affecting heterotrimeric G protein-dependent signaling by inhibiting GRK-2 (G-protein-coupled receptor kinase 2) (24, 25), and (vii) suppressing metastasis in prostate cancer (16). The PEBP homologs in plants are furthermore involved in the control of a morphogenic switch between shoot growth and flower structures (9).
Here, we describe a new type of function for one of the two members of the PEBP family in yeast. Our work is based on three sets of data demonstrating (i) that Tfs1p and Ira2p interact directly and specifically, (ii) that there is a direct link between the ability of Tfs1p to interact with Ira2p and its ability to be a multicopy suppressor of the cdc25-1 mutation, and (iii) that Tfs1p directly affects the activation level of the Ras/cAMP/PKA pathway. We also demonstrate that conserved residues (involved in the formation of the cavity in members of the PEBP family) are required for Tfs1p function.
MATERIALS AND METHODS
Strains, media, and general methods.
Escherichia coli DH5α [endA1 hsdR17 (rk− mk+) gln V44 thi-1 recA1 gyrA (Nalr) relA1Δ(lac1ZYA-argF) U169 deoR (φ80dlacΔ(lacZ)M15)] was used for all plasmid amplifications and isolations. The E. coli BL21(DE3) [F− ompT hsdSB(rB− mB−) gal dcm (DE3)] strain was used to produce 6His-Tfs1, and E. coli SCS1 (recA1 endA1 gyrA96 thi-1 hsdR17 (rk− mk+) SupE44 relA1) was used to produce GST-Ira2p-704. The yeast strains used in this study are listed in Table 1. The cultivation of yeast and bacterial strains and DNA manipulations and transformations were performed as described previously (19a, 26a). Each gene deletion was confirmed by PCR. A QuickChange XL site-directed mutagenesis kit (Stratagene) was used for site-directed mutagenesis. A DyAzymeII DNA polymerase system (Finnzyme) or an Expand high-fidelity PCR system (Roche) was used for PCR. Yeast cells were grown in cultures on yeast extract-peptone-dextrose (YPD) in the presence or absence of Geneticin (200 μg ml−1) or on synthetic dropout medium lacking the supplements required to maintain plasmids. The carbon source was glucose (2%) except when the Ira2 TBD (for “Tfs1-binding domain”) and the Ira1p homologous domain were overproduced, in which case the medium contained 2% galactose and 1% raffinose.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| BY4742 | MATα his3Δ1 leu2Δ0 lysΔ0 ura3Δ0 | Euroscarf |
| BY4742ΔT | BY4742 tfs1::kan | This study |
| BY4742ΔTY | BY4742 tfs1 ylr179c::kan | This study |
| LRB27 | MATα his4 leu2 ura3 cdc25-1 | L. Robinson |
| LRB27Δira2TBD | LRB27 ira2 (2232-2347)::kanMX4 | This study |
| LRB27Δira2GAP | LRB27 ira2 (1644-2347)::kanMX4 | This study |
| LRB27Δira2CT | LRB27 ira2 (2360-2670)::kanMX4 | This study |
| LRB27Δrim11 | LRB27 rim11::kanMX4 | This study |
| EGY48 | MATα trp1 his3 ura3 leu2::6 LexAop-LEU2 | MoBiTec |
Plasmids and oligonucleotides.
The plasmids and oligonucleotides used are listed in Table 2 and Table 3, respectively. Unless otherwise stated, all PCRs were performed with genomic DNA from the BY4742 strain as the template and the products were subcloned into pCR2.1-TOPO TA-cloning plasmid (Invitrogen).
TABLE 2.
Plasmids used in this study
| Plasmid | Description | Source or reference |
|---|---|---|
| Yep352 | 2μm URA3 | 22a |
| Yep352T | Yep352, TFS1 | See text |
| Yep352TP99L | Yep352, TFS1(P99L) | See text |
| Yep352TH111A | Yep352, TFS1(H111A) | See text |
| Yep352TR162A | Yep352, TFS1(R162A) | See text |
| Yep352Y | Yep352, YLR179c | See text |
| pYX243 | 2μm LEU2 | R&D Systems |
| pYXIra2TBD | pYX243, GAL1-IRA2 TBD-HA | See text |
| pYXIra1 | pYX243, GAL1-IRA1 (1987-2358)-HA | See text |
| pKA | kanMX′ | 14 |
| pKAT | pKA, TFS1′ | See text |
| pKAY | pKA, YLR179c′ | See text |
| pAN | ′kanMX | 14 |
| pANT | pAN, ′TFS1 | See text |
| pEGKG | 2μm LEU2-d, URA3, GST-fusion expression vector | 28a |
| pEGKGIra2TBD | pEGKG, IRA2 TBD | See text |
| pGEXIra2-383 | Ampr, plac, GST-Ira2 (1644-2026) | 30 |
| pGEXIra2-704 | GST-Ira2 (1644-2347) | See text |
| pEG202 | 2μm HIS3, LexA DBD, NLS | MoBiTec |
| pEG202T | pEG202, TFS1 | See text |
| pEG202TP99L | pEG202, TFS1(P99L) | See text |
| pEG202TH111A | pEG202, TFS1(H111A) | See text |
| pEG202TR162A | pEG202, TFS1(R162A) | See text |
| pEG202Y | pEG202, YLR179c | See text |
| pEG202Ira2TBD | pEG202, IRA2 TBD | See text |
| pJG4-5 | 2μm TRP1, transcriptional AD | MoBiTec |
| pJG4-5b | pJG4-5, IRA2 (1983-2432) | See text |
| pJG4-5c | pJG4-5, IRA2 (1992-2444) | See text |
| pJG4-5f | pJG4-5, IRA2 (1920-2347) | See text |
| pJGIra2TBD | pJG4-5, IRA2 TBD | See text |
| pJGIra2TBDΔ | pJG4-5, IRA2 TBD Δ 2232-2347 | See text |
| pJGIra1 | pJG4-5, IRA1 (1987-2358) | See text |
| pJGT | pJG4-5, TFS1 | See text |
| pJGY | pJG4-5, YLR179c | See text |
| pGNG | 2μm URA3, LexAop-GFP | MoBiTec |
| pET14-b | Ampr, pT7-His6 | Novagen |
| pET14T | pET14-b, pT7-His6-TFS1 | See text |
| pCR2.1 TOPO | Ampr, Knr, 3′-T, topoI activated | Invitrogen |
TABLE 3.
Oligonucleotides used in this study
| Name | Sequence (5′ to 3′) |
|---|---|
| HB1 | GATCATATGAACCAAGCAATAGACTTCGCAC |
| HB2 | ACTCGAGGATCCTTATTTCGTTTCCGCATAGAAGAA |
| HB3 | GCAGAGCTCGAGTTGAATCCGAAGTTA |
| HB4 | ACTGCAGACTGACCCGGACGCGCCC |
| HB5 | ACTGAGCTCTACGGCAAGTATCCC |
| HB6 | GTCTGCAGTGCGGCCGCTCGTGGACACCTTGGGC |
| HB7 | GGAATTCGGTGACATTGATGGTGACG |
| HB8 | CGCTCGAGTTAAAATGACGCCTCAGTGGCAGC |
| HB10 | GCTCGAGTTATACGTCGAATAAAGTACC |
| HB11 | CGCTCGAGACTGTTATGCTCAACGACAAAATTC |
| HB12 | CGCTCGAGTTAGTTGCCACGAGAAGGTTGTATG |
| HB13 | TAGCGGCCGCCACCAAGCAATAGACTTCGCA |
| HB14 | GTGAATTCTCTAGTGCCATCGTTGCTAAA |
| HB15 | ACTCGAGGATCCTTACATGTCATAGTTGAAATCA |
| HB16 | CCGATCCGGATGCTCTATCTAAGACAGATC |
| HB17 | TGATCTGTCTTAGATAGAGCATCCGGATCG |
| HB18 | CGCATTCTACCAAAGCGCAGAATTCAGACC |
| HB19 | GGTCTGAATTCTGCGCTTTGGTAGAATGCG |
| HB20 | GCTTATATAACAAGAAAACATAAGCGTGGGGACCGGATCCC |
| HB21 | GGGATCCGGTCCCCACGCTTATGTTTTCTTGTTATATAAGC |
| HB22 | CGTCTAGACGGTGACATTGATGGTGACG |
| HB23 | GCCATGGCTGAACTTTGTAGAACAGATCGC |
| HB24 | CAAGCTTAGCTATTAGGTTATACGAACTG |
| HB25 | GCCATGGGCACTGTTATGCTCAACGACAAAATTC |
| HB26 | CGACGCGTCGTTGCCACGAGAAGGTTGTA |
| HB27 | CTGGTACCGTCTATTGCTTGGTTCAT |
| HB28 | ACCCGGGGAGTTGAATCCGAAGTTA |
| HB29 | CGGATCCGAAACGAAATAGGTATAT |
| HB30 | CTCCCGGGGGCACTAGACATAATATATAT |
| HB31 | GATGTCTACGAAATTAGCCGAATATTTGAAGTACATGTTTCGTCAATAACTGCCAGCGACATGGAGGCCC |
| HB32 | GTTGTAGTATCTTCGGGGACGTACACCTCGGGAGAGCGATGAAAATGGGCATTCACATACGATTGACGC |
| HB33 | CCAATGTTGATGCGAGTTTGCAGTTCACCTTACCGATGGGCTATTCCGGCCAGCGACATGGAGGCCC |
| HB34 | CCATTTTCATCGCTCTCCCGAGGTGTACGTCCCCGAAGATACTACAACACCAGCGACATGGAGGCCC |
| HB35 | CAAGACATATTTCTTGTATCTTGTCTTCCACACGTTTGCTTCGTACGTTGACATACGATTGACGC |
| HB36 | TACATTACAACACGGCAACACTAATCACGCAACGCTAGAACTCGCGCAGGCCAGCGACATGGAGGCCC |
| HB37 | CCTTCCTTCTCCCATTATTCTTGCCTGGGCTCCCTCCGGTGCTATCACATTCACATACGATTGACGC |
TFS1 was amplified by PCR using the primers HB1 and HB2. After digestion with NdeI and XhoI, the gene was inserted into pET14-b that had been opened with the same enzymes. The resulting construct, pET14T, contains the entire coding sequence of Tfs1 inserted in frame downstream of a six-His tag.
The TFS1 and YLR179c open reading frames were amplified by PCR with primer pair HB13 and HB2 and primer pair HB14 and HB15, respectively. After cleavage with EcoRI and XhoI, the PCR fragments were ligated into the EcoRI and XhoI sites of both pEG202 and pJG4-5. This generated pEG202T and pEG202Y, which contain the entire coding sequences of TFS1 and YLR179c, respectively, inserted in frame downstream of a set of two DNA sequences encoding the simian virus 40 nuclear localization signal and LexA. This also generated pJGT and pJGY, which contain the TFS1 and YLR179c sequences, respectively, inserted in frame downstream of a set of three DNA sequences encoding the simian virus 40 nuclear localization signal, the B42 activator, and the hemagglutinin (HA) epitope tag.
A DNA fragment encoding amino acids (aa) 1983 to 2347 of Ira2 was PCR amplified with the primer pair HB7 and HB8. After cleavage with EcoRI and XhoI, the PCR fragments were ligated into the EcoRI and XhoI sites of pEG202 and pJG4-5. The fragments were inserted in frame downstream of LexA in pEG202 and in frame downstream of HA in pJG4-5, generating pEG202Ira2TBD and pJGIra2TBD, respectively.
To construct pJGIra2TBDΔ, the same procedure was followed except that the DNA fragment encoding the deleted Ira2TBD was amplified with the primer pair HB7 and HB10.
DNA encoding the Ira1p fragment homologous to Ira2 TBD was amplified from genomic DNA with the primers HB11 and HB12. As an EcoRI site is present in this fragment, it was cloned into the dephosphorylated XhoI site of pJG4-5 to give pJGIra1.
Yep352T and Yep352Y are multicopy plasmids that contain large DNA fragments (2,075 and 1,888 bp, respectively) comprising the TFS1 and YLR179c genes, respectively, with their own promoter sequences; each fragment was PCR amplified using primer pair HB3 and HB4 and primer pair HB5 and HB6, respectively. The fragments were then cut with SacI and PstI and cloned into Yep352 that had been cut with the same enzymes.
Tfs1p was altered by performing site-directed mutagenesis on the Yep352T plasmid through the use of a Stratagene kit and the primer pairs HB16 and HB17 for the P99L mutation, HB18 and HB19 for the H111A mutation, and HB20 and HB21 for the R162A mutation. The resulting plasmids (Yep352TP99L, Yep352TH111A, and Yep352TR162A) were used as templates to obtain PCR fragments with primers HB13 and HB2; these fragments were then cloned into pEG202 that had been cut with EcoRI and XhoI, yielding pEG202TP99L, pEG202TH111A, and pEG202TR162A, respectively.
pEGKGIra2TBD contains the DNA sequence encoding Ira2TBD inserted in frame downstream of glutathione S-transferase (GST). The primer pair HB22 and HB8 was used to amplify the DNA fragment from genomic DNA. The resulting fragment was inserted into pCR2.1-TOPO. The plasmid obtained was cut with XbaI and HindIII, and the fragment encoding Ira2 TBD could be subsequently cloned into pEGKG that had been cut with the same enzymes.
The IRA2 fragment encoding TBD was inserted in the pGEXIra2-383 plasmid (Parrini et al. [30]) encoding Ira2p-383 (aa 1644 to 2026), thereby allowing the production of Ira2p-704 (aa 1644 and 2347) containing the GAP-like catalytic domain and the TBD fused to the GST in the N terminus. For this purpose, the PCR fragment encoding Ira2TBD was amplified with primers HB7 and HB8. It was cut with the NdeI and XhoI enzymes and inserted into the NdeI and EcoRI sites of pGEXIra2-383 after filling in the opened EcoRI and XhoI sites with the Klenow enzyme.
In pYXIra2TBD, the production of Ira2TBD is under the control of the Gal10 promoter. The PCR fragment encoding Ira2TBD was amplified with primers HB23 and HB24. It was inserted into the NcoI and HindIII sites of pYX243 such that it was in frame upstream of the HA tag.
pYXIra1 was obtained in a similar way. The primer pair HB25 and HB26 was used to obtain a PCR fragment encoding the IRA1 fragment, which was then inserted between the NcoI and MluI sites of pYX243.
pKAT contains a 660-bp DNA fragment corresponding to the region immediately upstream of the TFS1 gene. This fragment was amplified with primers HB27 and HB4. It was cloned into the pCR2.1 TOPO plasmid, which allowed its excision with the KpnI and BamHI enzymes and its subsequent cloning into the same sites of pKA. pANT contains a 770-bp DNA fragment corresponding to a DNA region localized immediately downstream of the TFS1 gene. This fragment was amplified using primers HB28 and HB29. After cutting with XmaI and BamHI was performed, the resulting fragment was cloned into pAN.
pKAY contains an 880-bp DNA fragment corresponding to a region localized immediately upstream of the YLR179C gene. This fragment was amplified with the primers HB30 and HB6. It was cloned into pCR2.1 TOPO, which allowed its excision with XmaI and BamHI enzymes and its subsequent cloning into pKA opened with the same sites.
Strain construction.
TFS1 was deleted from BY4742 by “split-marker” recombination (14) with pKAT and pANT. In the resulting disrupted strain, BY4742ΔT, TFS1 was replaced by the kanMX gene (51). TFS1 and YLR179C are adjacent; therefore, the two genes were disrupted simultaneously in BY4742 by the kanMX gene through the use of the pKAY and the pANT plasmids. The resulting strain was designated BY4742ΔTY. LRB27Δira2TBD was constructed by replacing the IRA2 fragment encoding aa 2232 to 2347 of the TBD with kanMX4 in LRB27. This fragment of IRA2 was replaced by transforming a PCR-generated DNA fragment containing kanMX4 flanked by 50 bases homologous to a region located immediately upstream of the first amino acid of this region and directly downstream of the last amino acid of this region. Primers HB31 and HB32 were used to amplify this fragment. The same procedure was used to construct LRB27Δira2GAP and LRB27Δira2CT. For LRB27Δira2GAP, the fragment of IRA2 encoding the GAP and TBDs was deleted with kanMX4. For this purpose, LRB27 cells were transformed with a PCR-generated DNA fragment containing kanMX4 flanked by 50 bases homologous to a region located immediately upstream of the first amino acid of this region (aa 1644) and directly downstream of the last amino acid of this region (aa 2347). Primers HB33 and HB32 were used to amplify this fragment. For LRB27Δira2CT, the fragment of IRA2 encoding aa 2360 to 2670 and located downstream of that encoding the TBD was deleted with kanMX4, resulting in the loss of the residues 2360 to 3079 of the Ira2 protein. Primers HB34 and HB35 were used to amplify the PCR fragment used for this disruption.
LRB27Δrim11 was constructed along the same line by inserting the kanMX4 marker into the RIM11 gene. HB36 and HB37 were used to amplify the PCR fragment dedicated to this disruption.
GST pulldown.
For the in vivo GST pulldown, GST and GST-Ira2 TBD were produced by growing 50 ml of BY4742 cells transformed with pEGKG and pEGKGIra2TBD, respectively, to an optical density at 600 nm of 1 at 30°C in SDGal/Raf-Ura medium. Cell extracts were obtained by breaking the cells open with glass beads (Sigma) (0.5-mm diameter) in buffer A (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA, 10% glycerol) containing Complete protease inhibitor cocktail (Roche), phenylmethylsulfonyl fluoride (1 mM), and lysozyme (5%). Cleared cell lysates (named “input”) were analyzed by Western blotting, and 300 μl was incubated with glutathione Sepharose beads (50 μl) (Amersham) for 1 h at 4°C. After three washes with buffer A containing 0.08% Triton X-100 and two washes with buffer A without Triton or glycerol, the beads were boiled for 5 min in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. The eluted material (named “Beads” [see Fig. 2]) was separated from the beads and analyzed by Western blotting.
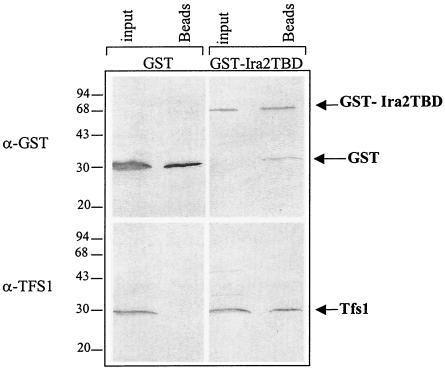
FIG. 2.
Tfs1p specifically interacts with the GST-Ira2 TBD in vivo. Wild-type yeast cells producing GST alone or GST fused at the N terminus of Ira2 TBD (GST-Ira2TBD) were lysed. An aliquot of these cell extracts (input) was directly analyzed by Western blotting with the antibodies indicated on the left side of the figure. Another aliquot was incubated with glutathione beads, which specifically bind to GST. After several washes, the material bound to the beads was eluted with SDS-PAGE sample buffer (Beads) and analyzed by Western blotting. The molecular-mass markers are indicated (in kilodaltons) on the left side of the figure.
For the in vitro GST pulldown, GST-Ira2p-704 was produced from SCS1 E. coli cells after overnight induction at 17°C after addition of IPTG (isopropyl-β-d-thiogalactopyranoside). Cells were lysed in Tris 20 mM (pH 7.5)-NaCl (75 mM)-NP-40 (0.1%). 500 μl of cells lysate were incubated with glutathione beads (25 μl) in this buffer for 1 h at 4°C. After five washes with the same buffer and a last wash in this buffer containing 5% lysozyme, the beads were incubated with 6His-Tfs1 and YNL281W-6His (6 μg) in a total volume of 700 μl of the latter buffer for 1 h at 4°C. After three washes with this buffer and two washes without lysozyme and NP-40, the beads were incubated in the presence of 10 mM glutathione (50 μl) for 5 min at room temperature and the eluted material was analyzed by Western blotting.
Two-hybrid assays.
We used the LexA-based version of the yeast two-hybrid system originally developed by Fields and Song (15). The components used for these assays (pEG202, pJG4-5, and pGNG plasmids and the EGY48 strain) were obtained from MoBiTec GmbH (Göttingen, Germany), and all assays were carried out as suggested by the manufacturer. The bait, pEG202T, was created as described above. The pJG4-5 yeast genomic library was obtained from OriGene Technologies (Rockville, Md.) and introduced into the reporter strain EGY48 that had been transformed with the bait plasmid and the pGNG reporter plasmid, allowing the production of GFPuv, a green fluorescent protein (GFP) variant optimized for maximal fluorescence by UV light excitation. A total of 1.2 × 108 transformants were selected for uracil, histidine, tryptophan, and leucine prototrophy and fluorescence under UV light. We subsequently analyzed 80 of the 217 isolated positive-testing colonies.
For each interaction tested, the bait (LexA-Tfs1 or LexA-Ylr179c) and the prey (B42-HA-Ira2TBD, B42-HA-Ira2TBDΔ2232-2347, or B42-HA-Ira1) were sought by Western blotting using different antibodies. Anti-LexA antibodies were used for detection of baits and anti-HA antibodies were used for detection of preys.
Heat shock and oxidative stress resistance.
Heat shock and H202 resistance assays were performed on BY4742, BY4742ΔT, or BY4742ΔTY cells transformed or not transformed with pYep352 or pYep352T and grown to saturation. Cells were first incubated at 37°C for 1 h. For the H2O2 resistance assays, they were then cooled and spotted in 10-fold serial dilutions onto YPD plates containing 0, 2, or 3 mM H2O2. For the heat shock assays, the cells were incubated for various times at 55°C before being spotted onto YPD plates.
Protein purification and antibody preparation.
6His-Tfs1 was purified from the cell lysate of BL21(DE3) cells transformed with pET14T and induced for 120 min by IPTG (100 μM) essentially as described by Serre et al. (38). A cleared lysate was obtained by sonicating the cells in buffer B (50 mM sodium phosphate buffer [pH 8.0], 10 mM Tris-HCl [pH 8.0], 50 mM NaCl, 5 mM imidazole, 5 mM β-mercaptoethanol) containing protease inhibitors (Sigma), lysozyme (100 μg/ml), DNase (5 μg/ml), and MgCl2 (10 mM) and then centrifuging for 2 h at 40,000 × g. This lysate was incubated with Talon TM cobalt affinity resin (Clontech) for 30 min at room temperature. The resin was washed several times with buffer B for 20 min, and the protein was eluted by two 20-min incubations in buffer B containing 400 mM imidazole. The eluate was dialyzed against 25 mM Tris-HCl (pH 7.5)-30 mM NaCl-7 mM β-mercaptoethanol and concentrated to 1 mg/ml.
Anti-Tfs1 antibodies were raised by injecting rabbits with purified 6His-Tfs1. The rabbits received four injections each containing 150 μg of protein mixed with Freund's adjuvant over a period of 40 days. The antiserum obtained was used at a 1/500 dilution.
YNL281W-6His was a kind gift of N. Coste. 6His-YLR179C purification and anti-YLR179C antibodies will be described elsewhere.
Protein analysis.
Except for the cell extracts used in the GST pulldown experiments, all cell extracts were prepared by a method adapted from Yaffe and Schatz (54). Cells were grown to an optical density at 600 nm of 1 and then centrifuged and resuspended in 500 μl of water containing Complete protease inhibitor cocktail (Roche) and phenylmethylsulfonyl fluoride (1 mM). We then added 150 μl of freshly prepared 2 M NaOH and 8% β-mercaptoethanol to the cells; after incubation for 10 min on ice, trichloroacetic acid (10% final concentration) was added. After a further 10-min incubation on ice, samples were centrifuged for 10 min at 10,000 × g. The pellet was then resuspended in 50 μl of SDS-PAGE sample buffer and boiled for 5 min. Western blot analyses were performed as described previously (50) and were probed with anti-HA rat antibodies (Roche), anti-lexA rabbit antibodies (a kind gift from R. Lloubès), anti-Tfs1 or anti-YLR179C rabbit antibodies, anti-GST monoclonal antibodies (Roche), and anti-six-His tag monoclonal antibodies (Invitrogen). Bound antibodies were detected using anti-rat, anti-mouse, or anti-rabbit secondary antibodies coupled to alkaline phosphatase (Promega). The presence of these secondary antibodies was revealed using BCIP (5-bromo-4-chloro-3-indolylphosphate) and Nitro Blue Tetrazolium as described by the manufacturer (Promega).
RESULTS
Interaction of Tfs1p with Ira2p. (i) Two-hybrid screening to identify proteins that interact with Tfs1p.
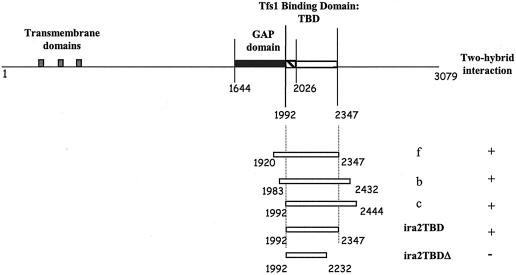
The entire coding sequence of TFS1 was used as a bait (pEG202T) to screen an S. cerevisiae genomic library containing 4 × 106 independent clones (OriGene Technologies, Rockville, Md.). We screened a total of 1.2 × 108 prey clones and identified 217 positive-testing clones that were prototrophic for uracil, histidine, tryptophan, and leucine and fluorescent under UV light. We studied 80 of these 217 clones further. A total of 62 of them encoded overlapping DNA fragments (named f, c, and b) corresponding to the IRA2 gene (Fig. 1), and 18 of them gave false-positive test results and did not give positive test results after a second screening with the bait.
FIG. 1.
Ira2p domains and truncated Ira2 protein constructs. The putative membrane-spanning segments are indicated by gray-shaded boxes. The GAP domain is indicated by a filled box; the TBD is indicated by an open box. The overlap between the two domains is hatched. The first and last residues of Ira2p and the limits of the GAP and TBDs are indicated. f, b, and c designate the three IRA2 DNA fragments isolated from positive-testing prey plasmids. + indicates that an interaction was detected by the two-hybrid system; − indicates that no interaction was detected.
The minimum DNA fragment common to all the positive prey clones (between residues 1992 and 2347 of Ira2p) was cloned into pJG4-5 and appeared to interact with Tfs1p as a bait (Table 4). This domain of Ira2p was called TBD (Fig. 1).
TABLE 4.
Two-hybrid pairings tested for GFP fluorescence and Leu2 prototrophy
| Bait | Result for indicated preya
|
||
|---|---|---|---|
| Ira2 TBD (Leu prototrophy and GFP activity) | Ira2TBDΔ (Leu prototrophy and GFP activity) | Ira1 (Leu prototrophy and GFP activity) | |
| Tfs1 | Yes | No | No |
| Tfs1: P99L | No | NDb | ND |
| Tfs1: H111A | No | ND | ND |
| Tfs1: R162A | No | ND | ND |
| YLR179c | No | ND | No |
Yes stands for Leu prototrophy and GFP activity in the two-hybrid test. No stands for no Leu prototrophy or GFP activity in the two-hybrid test.
ND, not determined.
The transcription of GFP and LEU2 was activated regardless of whether the sequences encoding TFS1 or the Ira2 TBD were in pEG202 or pJG4-5. Furthermore, none of the constructs activated GFP and LEU2 transcription with the empty vector, confirming that the interaction between Tfs1p and the Ira2 TBD was required for the expression of these genes.
(ii) Tfs1p coprecipitates with Ira2 TBD and Ira2 GAP TBD (Ira2p-704) tagged with GST in vivo and in vitro.
Ira2p is a 3,079-residue integral membrane protein. Its N terminus is predicted to contain three transmembrane domains, and the rest of the protein is predicted to be cytoplasmic. The “GAP domain” is contained within a 383-residue fragment (aa 1644 to 2026) (11) (Fig. 1). The Ira2 TBD region identified here overlaps the C-terminal part of the Ira2p GAP domain and extends over 364 residues (Fig. 1).
To confirm the two-hybrid screen data, we carried out two GST pulldown experiments, one in vivo in yeast and the other one in vitro with purified proteins.
To perform the in vivo pulldown experiment, the DNA fragment encoding the Ira2 TBD was cloned into pEGKG to produce a GST-Ira2 TBD fusion protein. Sepharose glutathione beads were then used to affinity purify GST-Ira2 TBD or GST alone as a negative control. Tfs1p was specifically coprecipitated with GST-Ira2 TBD but not with GST alone by glutathione Sepharose beads (Fig. 2). Therefore, it can be concluded that Tfs1p and the Ira2 TBD interact in vivo in yeast cells.
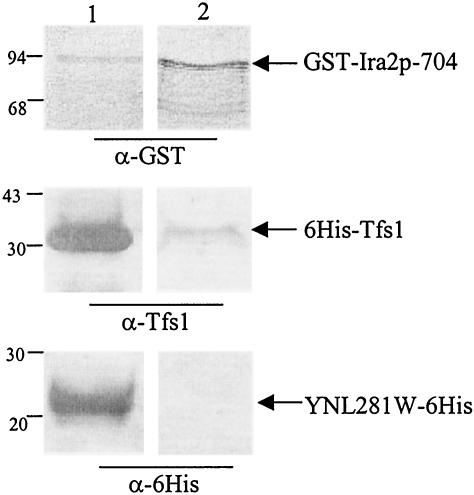
To prove that no other yeast protein was needed for this interaction to take place, a GST pulldown experiment was carried out in vitro with purified recombinant GST-Ira2p-704 and Tfs1 proteins. First, Sepharose glutathione beads were used to affinity purify GST-Ira2p-704 from E. coli cell extracts. Then, these beads were incubated with 6His-Tfs1 or YNL281W-6His (as a negative control) purified from E. coli extracts. Figure 3 shows that 6His-Tfs1 but not YNL281W-6His specifically coprecipitates with GST-Ira2p-704. Indeed, 6His-Tfs1 didn't bind to glutathione beads in the absence of GST-Ira2p-704 (data not shown).
FIG. 3.
6His-Tfs1 and GST-Ira2p704 interact in vitro. Purified 6His-Tfs1 or YNL281W-6His (an aliquot of which was analyzed [lane 1] with anti-Tfs1 and anti-six-His antibodies, respectively) was incubated with glutathione beads previously used for affinity purification of GST-Ira2p-704 produced from E. coli (10 μl of extracts was loaded in lane 1 and revealed with anti-GST antibodies). After several washes, the material bound to the beads was eluted with glutathione and analyzed by Western blot analysis with antibodies directed against GST, Tfs1, or six-His (as indicated) (lane 2). The molecular mass markers are indicated (in kilodaltons) on the left side of the figure.
The suppression of cdc25-1 by TFS1 requires the Ira2 TBD.
cdc25-1 cells (LRB27) are unable to grow at the restrictive temperature of 37°C on glucose or at the semipermissive temperature of 30°C on nonfermentable carbon sources. The overproduction of Tfs1p in that strain allows it to grow at 37°C on glucose but not at 30°C on glycerol (33). The fact that the overexpression of TFS1 suppressed the Cdc25-1 allelic mutation of CDC25 more efficiently than the other allelic mutations suggested that Cdc25p and Tfs1p might interact directly. Our finding that Tfs1p interacts with Ira2p rather than with Cdc25p suggests that this interaction is responsible for the suppression effect. To address this issue, we constructed an Ira2p mutant lacking the TBD and looked for the maintenance or loss of the TFS1 suppression of cdc25-1.
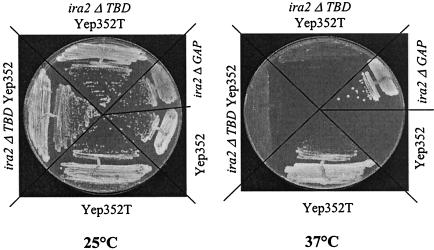
The deletion of the 115 C-terminal residues of Ira2 TBD resulted in an Ira2 fragment (Ira2TBDΔ2232-2347) that was unable to interact with Tfs1p in the two-hybrid system (Table 4) and in the GST pulldown assay (data not shown). The chromosomal IRA2 gene was mutated in a cdc25-1 strain such that the DNA region encoding residues 2232 to 2347 of Ira2p was replaced by the Kana marker (LRB27Δira2TBD). This marker insertion in the gene also resulted in the loss of the residues 2348 to 3079 of the Ira2 protein. As a control, another cdc25-1 strain was constructed in which the Kana marker was inserted downstream of the DNA region encoding TBD, resulting in the loss of residues 2360 to 3079 of the Ira2 protein (LRB27Δira2CT). These strains were unable to grow on glucose at 37°C (Fig. 4 and data not shown). Thus, these IRA2 deletions do not suppress the cdc25-1 mutation; therefore, the GAP activity of the resulting Ira2 protein is likely not affected. In contrast, the deletion of the Ira2 GAP domain (LRB27Δira2GAP) restored growth at 37°C (Fig. 4).
FIG. 4.
The TFS1 multicopy suppressor phenotype of cdc25-1 mutants is dependent on the presence of an intact Ira2 TBD. LRB27 cells expressing a wild-type Ira2p (LRB27), Ira2p lacking its 1,435 C-terminal residues (LRB27Δira2GAP), or Ira2 lacking its 847 C-terminal residues (LRB27Δira2TBD) was transformed or not transformed with the following plasmids: Yep352 and Yep352T. Each was then streaked out for single colonies and grown at 25°C or at the restrictive temperature of 37°C on YPD medium.
We tested the effect of Tfs1p overproduction in the LRB27Δira2TBD and LRB27Δira2CT strains grown at 37°C on glucose. The IRA2 deletion affecting the TBD region but not that affecting the region in the C terminus of TBD prevented Tfs1p from exerting its suppressor effect on the cdc25-1 mutation (Fig. 4 and data not shown). This unambiguously demonstrates that the TFS1 dose-dependent suppressor effect requires the interaction between Tfs1p and Ira2 TBD and strongly suggests that Tfs1p inhibits Ira2 GAP activity.
To confirm this result we developed a competition assay to prevent the suppressive effect of TFS1 upon cdc25-1 thermosensitivity.
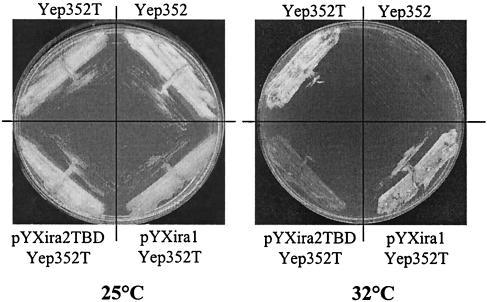
The Ira2 TBD was overproduced in cdc25-1 cells (LRB27). The DNA fragment encoding the Ira2 TBD was cloned into pYX243 behind the Gal10-inducible promoter and in frame with the HA epitope in the C terminus (pYXIra2TBD). The overproduction of Ira2 TBD in the presence of galactose was checked by Western blot analysis using anti-HA antibodies (data not shown). We tested the growth of LRB27 cells harboring or not harboring Yep352T at different temperatures on galactose, and it appeared that 32°C was the best temperature at which to observe the multicopy suppressor phenotype of TFS1 on galactose. When LRB27 Yep352T cells were transformed with pYXIra2TBD, they could no longer grow at 32°C on YPGal (Fig. 5). This phenotype is specific of Ira2 TBD production, as the overproduction of the Ira1p domain homologous to the Ira2 TBD did not impair cell growth at 32°C (Fig. 5). These results show that Ira2 TBD overproduction directly affects TFS1 suppressor function. Therefore, the Ira2 TBD fragments appear to compete with the natural Ira2 protein for Tfs1p, thereby strongly suggesting that the TFS1 dose-dependent suppressor effect involves the inhibition of Ira2 activity.
FIG. 5.
The overproduction of Ira2 TBD specifically inhibits the TFS1 multicopy suppressor phenotype of cdc25-1 mutants. LRB27 cells were transformed with the following plasmids: Yep352, Yep352T, pYXIra2TBD, and pYXIra1. They were then streaked out for single colonies and grown at 25°C or at the restrictive temperature of 32°C on YPGal medium to allow Ira2 TBD or Ira1p fragment overproduction.
Tfs1p does not interact with Ira1p or interfere with its function.
The functional similarity between Ira1p and Ira2p suggested that they were both regulated by Tfs1p. The overall sequences of the two Ira proteins are 45% identical, and their TBD regions are 48.5% identical.
The two-hybrid technique revealed no interaction between Tfs1p and the Ira1p domain that is homologous to the Ira2 TBD (Table 4). As described above, furthermore, TFS1 could only exert its suppressor effect on cdc25-1 mutants via Ira2 TBD and the presence of a wild-type IRA1 gene in these strains did not allow the Tfs1p dose-dependent suppressor function to take place. Finally, the Ira1p domain homologous to the Ira2 TBD was cloned into the pYX243 plasmid (pYXira1) in frame with the HA epitope in the C terminus. We checked (by Western blot analysis using anti-HA antibodies) that the Ira1 fragment was indeed overproduced in the presence of galactose (data not shown). The overproduction of Ira1 fragment in cdc25-1 cells did not interfere with the multicopy suppressor phenotype of TFS1 (Fig. 5). Therefore, Tfs1p inhibits only Ira2p function; this is one of many functional differences between the two Ira proteins (46).
YLR179C encodes a Tfs1p homolog that does not display the same function.
YLR179C, which is adjacent to TFS1 (YLR178C) on chromosome XII, encodes a protein that is 40% identical to Tfs1p. In contrast to the results seen with TFS1, YLR179C has not been reported to be overexpressed in different stress conditions. Indeed, the promoter region of TFS1 bears two STREs whereas the YLR179C promoter is devoid of them.
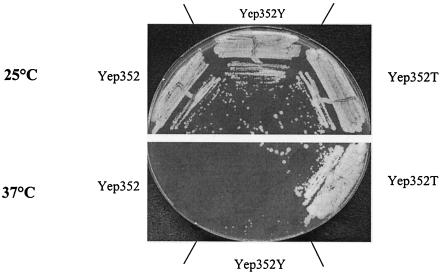
To test whether the product of YLR179C shares some of the functional properties of Tfs1p, we used the two-hybrid system to assess its interaction with the Ira2 TBD and checked whether the overexpression of YLR179C could mimic the dose-dependent suppressor phenotype of TFS1 in cdc25-1 strains. Whatever the vector chosen for cloning the corresponding DNA fragments (pEG202 or pJG4-5), the Ira2 TBD and the product of YLR179C did not display any interaction in the two-hybrid system (Table 4). Similarly, cdc25-1 cells overproducing Tfs1p (LRB27 Yep352T), but not cdc25-1 cells overexpressing YLR179C (LRB27 Yep352Y) or transformed with the corresponding empty vector (LRB27 Yep352), could grow on glucose at 37°C (Fig. 6). Indeed, the overproduction of YLR179C gene product was verified by Western blot analysis using anti-YLR179C antibodies (data not shown). Therefore, although they are homologous, the TFS1 and YLR179C gene products interact with different proteins and fulfill different functions (at least with regard to the suppression of cdc25-1 mutants).
FIG. 6.
YLR179c does not exert a multicopy suppressor function on cdc25-1 mutants. LRB27 cells were transformed with the following plasmids: Yep352, Yep352T, and Yep352Y. They were then streaked out for single colonies at 25°C or at the restrictive temperature of 37°C on YPD medium.
The deletion of TFS1 enhances the stress resistance of the cells and the overexpression of TFS1 causes the opposite phenotype.
As the overexpression of TFS1 appeared to suppress cdc25-deficient phenotypes by inhibiting Ira2 GAP activity, we predicted that cells overproducing Tfs1p would contain more Ras-activated proteins than cells lacking TFS1. Robinson and Tatchell (33) showed that the overexpression of TFS1 had a small effect on the cAMP intracellular concentration, as cells that overexpressed TFS1 accumulated less glycogen than did wild-type cells and appeared to express the β-galactosidase gene more from a cAMP-regulated promoter.
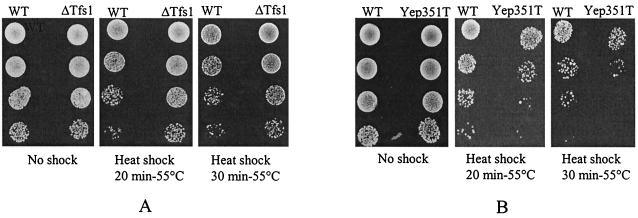
We investigated the resistance of cells to heat shock and hydrogen peroxide stress. The tests were performed in conditions that naturally allowed the high-level expression of TFS1 in wild-type cells. According to Gasch et al. (17), when cells that had been grown at 25°C were shifted to 37°C for 1 h STRE-responsive genes are strongly induced. Therefore, the heat shock and H202 resistance tests were performed after preincubating the cells at 37°C for 1 h. Cells overproducing Tfs1p were more sensitive (between 5- and 10-fold) to heat shock than wild-type cells, whereas TFS1 deletants were more resistant (between 5- and 10-fold) than wild-type cells (Fig. 7). As expected, the tfs1 ylr179c double mutant was not more resistant to the stresses than the tfs1 single mutant (data not shown). The same results were obtained in the H2O2 stress tests (data not shown).
FIG. 7.
Physiological effects of TFS1 disruption or overexpression. (A) BY4742 cells disrupted or not disrupted in TFS1 (ΔT and wild type [WT], respectively) were grown to saturation for 2 days, incubated at 37°C for 1 h, and then incubated for various times at 55°C. They were then cooled, and 10-fold serial dilutions were plated on YPD to compare survival. (B) The same experiment was performed with BY4742 cells overproducing or not overproducing Tfs1p (Yep352T or Yep352, respectively).
Although the effects were moderate, these results suggest that the overexpression of TFS1 is associated with a higher level of Ras activity and that the deletion of TFS1 is associated with the opposite phenotype. Furthermore, the phenotypes of TFS1 disruptants demonstrate that the inactivation of Ira2p by Tfs1p is physiological and is not a side effect of the artificial overproduction of one of the two proteins.
Functional importance of conserved residues in Tfs1p.
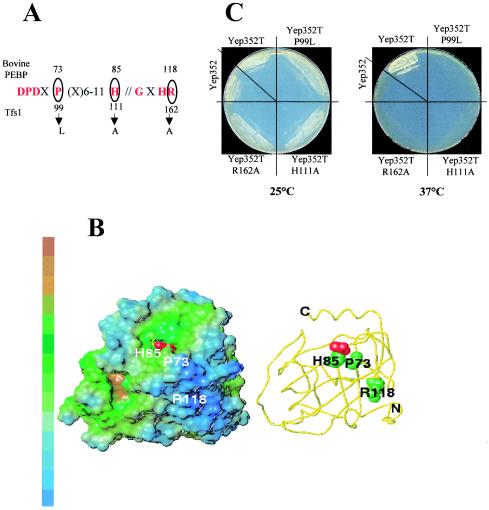
Tfs1p and the product of YLR179C belong to a large family of proteins called PEBP. The three-dimensional structures of several members of this family have been resolved and appear to be remarkably similar (2, 3, 38-40). The conserved regions always delimit a unique and small binding pocket at the surface of the proteins and are thought to participate in the formation of the ligand-binding site, which can accommodate different anions. To determine whether the putative ligand-binding site of Tfs1p is important for the interaction with the Ira2 TBD, we created different point mutations in the conserved residues of the PEBP family signature of Tfs1p (P99L, H111A, and R162A) (Fig. 8A). His111 corresponds to the His85 of the bovine PEBP, which directly interacts with phosphorylethanolamine and acetate (39). R162 corresponds to R119 of human PEBP, which establishes contacts with the N terminus of the protein and with three residues in the ligand-binding site (2). The Pro99L mutation mimics the natural mutation of the tomato PEBP (Self-Pruning gene product), resulting in drastic inflorescence anomalies (31). For each mutant, the interaction with the Ira2 TBD was tested by the two-hybrid system (Table 4). Each mutant was also overproduced in cdc25-1 strains. After the stability of each mutant protein had been checked by Western blot analysis with anti-Tfs1p antibodies (data not shown), we tested growth at 37°C on glucose (Fig. 8C). None of the Tfs1p mutants interacted with the Ira2 TBD (Table 4), and none of them were able to restore the growth of cdc25-1 mutants at 37°C like wild-type Tfs1p. These results demonstrate that different conserved residues of Tfs1p (thought to participate in the formation of the ligand-binding site of the protein) are involved in the interaction with Ira2 TBD.
FIG. 8.
Functional importance of conserved residues in Tfs1p. (A) Mutations in the conserved region of Tfs1p. Residues in the conserved regions are indicated in one-letter code. “X” stands for any residue. The circled residues were mutated. The numbers at the top indicate the positions of the corresponding residues in bovine PEBP. The numbers at the bottom indicate the positions of the mutated residues of Tfs1p, and the arrows indicate the mutations. (B) Positions of the mutated residues in the 3D structure of bovine PEBP, showing their locations relative to the putative active site of the bovine PEBP. The residues are numbered as for PEBP. The acetate ligand of the X-ray structure (45) is displayed in a red spacefill representation. Left panel: lipophilic potentials on the surface. The MOLCAD surface (SYBYL software; TRIPOS, St. Louis, Mo.) is colored from blue to brown (indicating increasing hydrophobicity). Right panel (with the same orientation): the PEBP backbone is displayed as a yellow tube; the heavy atoms of the mutated residues P73, H85, and R118 are represented in a spacefill representation. (C) Multicopy suppressor phenotype of Tfs1p mutants. LRB27 cells were transformed with the following plasmids: Yep352, Yep352T, Yep352TP99L, Yep352TH111A, and Yep352TR162A. They were then streaked out for single colonies at 25°C or at the restrictive temperature of 37°C on YPD medium.
DISCUSSION
This work shows that Tfs1p, previously characterized as a multicopy suppressor of cdc25-1 mutation (Twenty-Five suppressor) (33) and as a CPY inhibitor (26), specifically interacts with Ira2p and inhibits its activity in vivo. The suppressor effect of TFS1 was reported to be independent of its inhibitory activity on CPY (26) and more efficient on cdc25-1 strains than on other mutant alleles of CDC25, thereby suggesting an allele specificity for the efficiency of the suppressor phenotype (22). Our results (showing that Tfs1p directly interacts with Ira2p and that such an interaction is absolutely required for the suppressor effect of TFS1) allowed us to interpret this phenotype differently and to demonstrate by genetic means a new physiological role for Tfs1p. The defect in Ras protein activation of the cdc25 mutants (more or less pronounced depending on the cdc25 alleles) is compensated (more or less partially), because Tfs1p inhibits a Ras inhibitor, Ira2p. ira1 mutations have been described as being more efficient at suppressing the growth defect in cdc25 mutants than ira2 mutations (6). This is consistent with the fact that Tfs1p overproduction can partially suppress cdc25 alleles but is unable to bypass the essential requirement for CDC25 function.
Although Ira1p and Ira2p both inactivate Ras proteins, they display functional differences (43). First, some preference seems to exist between Ras and Ira proteins. Indeed, mutations in ras1 or ras2 do not equally well suppress phenotypes associated to mutations in ira1 and ira2. Second, the normal production or even the overproduction of Ira1p or Ira2p does not completely suppress the effects of disrupting the corresponding IRA gene. Third, ira1 is more efficient at suppressing the growth defect in cdc25 mutants than is ira2 mutation, although ira2 phenotypes are more severe than ira1 phenotypes. Our results showing that Tfs1p inhibits Ira2p but not Ira1p represent a new functional difference between these two GAPs. Together with the amino acid differences observed in the N-terminal regions of the two proteins, Tfs1p-specific inhibition of Ira2p might play a role in the phenotypic differences observed between the two ira mutants.
NF1, the closest human homolog to Ira1 and Ira2, is responsible for the human autosomal dominant disease neurofibromatosis type I. Specific lipids modulate NF1 GAP activity in vitro (5, 20). Furthermore, NF1 associates with microtubules and the interaction with tubulin (thought to involve an 80-residue segment upstream of the GAP domain) inhibits GAP activity in vitro (6). Conversely, Parrini et al. (30) have shown that the GAP activity of Ira2p is also inhibited by tubulin in vitro and that the 95 N-terminal residues flanking its catalytic domain might be involved in tubulin binding. In this work, we describe a new physiological inhibitor of Ira2p in S. cerevisiae. This also involves the 40 C-terminal residues of the Ira2p GAP domain, which are important for the interaction with Tfs1p (H. Chautard, personal communication). This Tfs1p-binding site extends to a 324-residue C-terminal flanking region. By analogy with tubulin, one of the human homologs of Tfs1p might inhibit the GAP activity of NF1 by interacting with a region located at the C terminus of its catalytic domain. The existence of an extended area of similarity (23.5% identity and 45.5% similarity) of around 800 residues immediately downstream of the GAP domain of NF1 and Ira suggests that this region has a conserved function (52). However, we cannot exclude the possibility that this type of inhibition is restricted to yeast. Indeed, the yeast Ira proteins display specific features such as a strict specificity towards the yeast Ras proteins (42-44, 52, 30), whereas mammalian GAPs act on both mammalian and yeast Ras proteins (18, 52).
Biochemical and structural analyses have shown that the most important function of GAPs is to supply the active site of Ras with an arginine (the finger) localized at the tip of a loop (the finger loop) joining two α-helices, thus stabilizing the transition state of the GTPase reaction and playing a crucial role in catalysis (52, 1, 36). Te Biesebeke et al. (44a) subsequently showed that this 19-residue finger loop (centered on Arg1742 in Ira2p) is also a determinant for the specificity toward Ras proteins from yeast or mammals. The inhibitory action of Tfs1p on Ira2p might be explained by sterical hindrance resulting from the formation of a complex close to the finger loop. Another possibility is that interaction with Tfs1p induces a conformational change in the GAP domain, thereby impairing its ability to bind to Ras and/or inhibiting its stimulating role in catalysis. Another possibility is that Tfs1p affects Ira2p localization or stability. We are currently testing these different hypotheses.
Tfs1p belongs to the large PEBP family, members of which are structurally characterized by a small cavity at their surface that is able to interact with different anions. Two stretches of conserved residues are involved in the formation of this cavity. We mutated three of these residues in Tfs1p. His111, which is directly involved in the interaction between the bovine PEBP and its ligands (phosphorylethanolamine and acetate) (39) was mutated into Ala. Pro99 was mutated into Leu to mimic the natural mutation of the tomato PEBP (Self-Pruning gene product) that results in drastic inflorescence anomalies (31). Arg162, which forms contacts with the N terminus and three residues of the cavity in the bovine PEBP, was mutated into Ala. Our results demonstrate that these mutations completely eliminate the interaction of Tfs1p with Ira2p and its subsequent inhibition. Modeling experiments suggested that each of these mutations has a direct or indirect effect on the accessibility or the binding properties of the cavity (D. Sy, personal communication). Indeed, H111A might remove a binding site for negatively charged ligands; P99L (localized on a turn at the border of the cavity) might affect the accessibility of the cavity to ligands; finally, R162A might have an indirect effect on the ligand-binding site via its contacts with residues located in the cavity.
These results strongly suggest that the conserved cavity of Tfs1p is important for its interaction with Ira2p. Given the possible contacts between R162 and the N terminus of the protein, however, we cannot exclude the possibility that the N terminus of Tfs1p participates in the interaction with Ira2p. This idea is strengthened by the fact that Ira2p does not interact with YLR179c, a Tfs1p homolog, even though its cavity seems to be quite similar (D. Sy, personal communication) despite the fact that its N-terminal extremity is quite different. Phosphorylated residues have already been identified as being possible ligands for members of the PEBP family (46, 47). Different putative PKA-dependent phosphorylation sites have been proposed for Ira2p; however, none of them are located in the Ira2 TBD fragment (43). Recently, the TAP (tandem affinity protein)-Tag technique showed that Ira2p interacts with the serine-threonine kinase Rim11p (23). However, RIM11 disruption in the cdc25-1 context does not prevent Tfs1 from exerting its suppressor effect (H. Chautard, personal communication), thereby demonstrating that Tfs1p could act independently of a putative Rim11p phosphorylation.
It is noteworthy that following the disruption or overexpression of TFS1, the phenotypes associated with carbohydrate storage or stress resistance are weak. This might be due to the fact that Tfs1p regulates only one of the two GAPs. Another nonexclusive possibility is that Tfs1p exerts its role only in certain physiological conditions (which differ from those used in the phenotypic analysis). These conditions might be related to stress conditions, because TFS1 is a STRE-regulated gene that is overproduced in many stress conditions. In conclusion, TFS1 constitutes a link between the general stress response pathway and the cAMP/PKA pathway. By helping to activate the cAMP/PKA pathway by downregulating Ira2p, Tfs1p may participate in a feedback loop of the general stress response pathway.
Acknowledgments
This work was supported by a “Jeune Equipe” grant of the Life Science Department of the CNRS and “la Ligue Départementale contre le Cancer, Comité du Loiret.” H. Chautard was the recipient of a CNRS/Region Centre fellowship.
We thank F. Godin for technical assistance, A. Rigal for his expertise in obtaining anti-Tfs1 and anti-YLR179C antibodies, R. Bouchard for the construction of pEG202T, G. Massart for the construction of Tfs1p mutants, L. Robinson for the LRB27 strain and helpful comments, and E. Boy-Marcotte and H. Garreau for helpful discussions and useful plasmids. We are also very grateful to D. Sy for providing the three-dimensional-structure modelization of the Tfs1p cavity and her help in analyzing the most probable effects of the mutations. We also thank V. Géli and M. N. Simon for their advice and for the numerous plasmids they gave us.
REFERENCES
- 1.Ahmadian, M. R., P. Stege, K. Scheffzek, and A. Wittinghofer. 1997. Confirmation of the arginine-finger hypothesis for the GAP-stimulated GTP-hydrolysis reaction of Ras. Nat. Struct. Biol. 4:686-689. [DOI] [PubMed] [Google Scholar]
- 2.Banfield, M. J., J. J. Barker, A. C. Perry, and R. L. Brady. 1998. Function from structure? The crystal structure of human phosphatidylethanolamine-binding protein suggests a role in membrane signal transduction. Structure 6:1245-1254. [DOI] [PubMed] [Google Scholar]
- 3.Banfield, M. J., and R. L. Brady. 2000. The structure of Antirrhinum centroradialis protein (CEN) suggests a role as a kinase regulator. J. Mol. Biol. 297:1159-1170. [DOI] [PubMed] [Google Scholar]
- 4.Boguski, M. S., and F. McCormick. 1993. Proteins regulating Ras and its relatives. Nature 366:643-654. [DOI] [PubMed] [Google Scholar]
- 5.Bollag, G., and F. McCormick. 1991. Differential regulation of rasGAP and neurofibromatosis gene product activities. Nature 351:576-579. [DOI] [PubMed] [Google Scholar]
- 6.Bollag, G., F. McCormick, and R. Clark. 1993. Characterization of full-length neurofibromin: tubulin inhibits Ras GAP activity. EMBO J. 12:1923-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boy-Marcotte, E., G. Lagniel, M. Perrot, F. Bussereau, A. Boudsocq, M. Jacquet, and J. Labarre. 1999. The heat shock response in yeast: differential regulations and contributions of the Msn2p/Msn4p and Hsf1p regulons. Mol. Microbiol. 33:274-283. [DOI] [PubMed] [Google Scholar]
- 8.Boy-Marcotte, E., M. Perrot, F. Bussereau, H. Boucherie, and M. Jacquet. 1998. Msn2p and Msn4p control a large number of genes induced at the diauxic transition which are repressed by cyclic AMP in Saccharomyces cerevisiae. J. Bacteriol. 180:1044-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley, D., R. Carpenter, L. Copsey, C. Vincent, S. Rothstein, and E. Coen. 1996. Control of inflorescence architecture in Antirrhinum. Nature 379:791-797. [DOI] [PubMed] [Google Scholar]
- 10.Broach, J. R. 1991. Ras-regulated signaling processes in Saccharomyces cerevisiae. Curr. Opin. Genet. Dev. 1:370-377. [DOI] [PubMed] [Google Scholar]
- 11.Bruun, A. W., I. Svendsen, S. O. Sorensen, M. C. Kielland-Brandt, and J. R. Winther. 1998. A high-affinity inhibitor of yeast carboxypeptidase Y is encoded by TFS1 and shows homology to a family of lipid binding proteins. Biochemistry 37:3351-3357. [DOI] [PubMed] [Google Scholar]
- 12.Crechet, J. B., P. Poullet, J. Camonis, M. Jacquet, and A. Parmeggiani. 1990. Different kinetic properties of the two mutants, RAS2Ile152 and RAS2Val19, that suppress the CDC25 requirement in RAS/adenylate cyclase pathway in Saccharomyces cerevisiae. J. Biol. Chem. 265:1563-1568. [PubMed] [Google Scholar]
- 13.Crechet, J. B., P. Poullet, M. Y. Mistou, A. Parmeggiani, J. Camonis, E. Boy-Marcotte, F. Damak, and M. Jacquet. 1990. Enhancement of the GDP-GTP exchange of RAS proteins by the carboxyl-terminal domain of SCD25. Science 248:866-868. [DOI] [PubMed] [Google Scholar]
- 14.Fairhead, C., B. Llorente, F. Denis, M. Soler, and B. Dujon. 1996. New vectors for combinatorial deletions in yeast chromosomes and for gap-repair cloning using 'split-marker' recombination. Yeast 12:1439-1457. [DOI] [PubMed] [Google Scholar]
- 15.Fields, S., and O. Song. 1989. A novel genetic system to detect protein-protein interactions. Nature 340:245-246. [DOI] [PubMed] [Google Scholar]
- 16.Fu, Z., P. C. Smith, L. Zhang, M. A. Rubin, R. L. Dunn, Z. Yao, and E. T. Keller. 2003. Effects of raf kinase inhibitor protein expression on suppression of prostate cancer metastasis. J. Natl. Cancer Inst. 95:878-889. [DOI] [PubMed] [Google Scholar]
- 17.Gasch, A. P., P. T. Spellman, C. M. Kao, O. Carmel-Harel, M. B. Eisen, G. Storz, D. Botstein, and P. O. Brown. 2000. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11:4241-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibbs, J. B., and M. S. Marshall. 1989. The ras oncogene—an important regulatory element in lower eucaryotic organisms. Microbiol. Rev. 53:171-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godon, C., G. Lagniel, J. Lee, J. M. Buhler, S. Kieffer, M. Perrot, H. Boucherie, M. B. Toledano, and J. Labarre. 1998. The H2O2 stimulon in Saccharomyces cerevisiae. J. Biol. Chem. 273:22480-22489. [DOI] [PubMed] [Google Scholar]
- 19a.Guthrie, C., and G. R. Fink. 1991. Guide to yeast genetics and molecular biology. Academic Press, New York, N.Y.
- 20.Han, J. W., F. McCormick, and I. G. Macara. 1991. Regulation of Ras-GAP and the neurofibromatosis-1 gene product by eicosanoids. Science 252:576-579. [DOI] [PubMed] [Google Scholar]
- 21.Hengst, U., H. Albrecht, D. Hess, and D. Monard. 2001. The phosphatidylethanolamine-binding protein is the prototype of a novel family of serine protease inhibitors. J. Biol. Chem. 276:535-540. [DOI] [PubMed] [Google Scholar]
- 22.Hickox, D. M., G. Gibbs, J. R. Morrison, K. Sebire, K. Edgar, H. H. Keah, K. Alter, K. L. Loveland, M. T. Hearn, D. M. de Kretser, and M. K. O'Bryan. 2002. Identification of a novel testis-specific member of the phosphatidylethanolamine binding protein family, pebp-2. Biol. Reprod. 67:917-927. [DOI] [PubMed] [Google Scholar]
- 22a.Hill, J. E., A. M. Myers, T. J. Koerner, and A. Tzagoloff. 1986. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast 2:163-167. [DOI] [PubMed] [Google Scholar]
- 23.Ho, Y., A. Gruhler, A. Heilbut, G. D. Bader, L. Moore, S. L. Adams, A. Millar, P. Taylor, K. Bennett, K. Boutilier, L. Yang, C. Wolting, I. Donaldson, S. Schandorff, J. Shewnarane, M. Vo, J. Taggart, M. Goudreault, B. Muskat, C. Alfarano, D. Dewar, Z. Lin, K. Michalickova, A. R. Willems, H. Sassi, P. A. Nielsen, K. J. Rasmussen, J. R. Andersen, L. E. Johansen, L. H. Hansen, H. Jespersen, A. Podtelejnikov, E. Nielsen, J. Crawford, V. Poulsen, B. D. Sorensen, J. Matthiesen, R. C. Hendrickson, F. Gleeson, T. Pawson, M. F. Moran, D. Durocher, M. Mann, C. W. Hogue, D. Figeys, and M. Tyers. 2002. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415:180-183. [DOI] [PubMed] [Google Scholar]
- 24.Kroslak, T., T. Koch, E. Kahl, and V. Hollt. 2001. Human phosphatidylethanolamine-binding protein facilitates heterotrimeric G protein-dependent signaling. J. Biol. Chem. 276:39772-39778. [DOI] [PubMed] [Google Scholar]
- 25.Lorenz, K., M. J. Lohse, and U. Quitterer. 2003. Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2. Nature 426:574-579. [DOI] [PubMed] [Google Scholar]
- 26.Maekawa, M., S. Li, A. Iwamatsu, T. Morishita, K. Yokota, Y. Imai, S. Kohsaka, S. Nakamura, and S. Hattori. 1994. A novel mammalian Ras GTPase-activating protein which has phospholipid-binding and Btk homology regions. Mol. Cell. Biol. 14:6879-6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Matsumoto, K., I. Uno, and T. Ishikawa. 1984. Regulation of repressible acid phosphatase by cyclic AMP in Saccharomyces cerevisiae. Genetics 108:53-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mima, J., T. Kondo, and R. Hayashi. 2002. N-terminal acetyl group is essential for the inhibitory function of carboxypeptidase Y inhibitor (I(C)). FEBS Lett. 532:207-210. [DOI] [PubMed] [Google Scholar]
- 28a.Mitchell, D. A., T. K. Marshall, and R. J. Deschenes. 1993. Vectors for the inducible overexpression of glutathione S-transferase fusion proteins in yeast. Yeast 9:715-723. [DOI] [PubMed] [Google Scholar]
- 29.Neuman-Silberberg, F. S., S. Bhattacharya, and J. R. Broach. 1995. Nutrient availability and the RAS/cyclic AMP pathway both induce expression of ribosomal protein genes in Saccharomyces cerevisiae but by different mechanisms. Mol. Cell. Biol. 15:3187-3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parrini, M. C., E. Jacquet, A. Bernardi, M. Jacquet, and A. Parmeggiani. 1995. Properties and regulation of the catalytic domain of Ira2p, a Saccharomyces cerevisiae GTPase-activating protein of Ras2p. Biochemistry 34:13776-13783. [DOI] [PubMed] [Google Scholar]
- 31.Pnueli, L., L. Carmel-Goren, D. Hareven, T. Gutfinger, J. Alvarez, M. Ganal, D. Zamir, and E. Lifschitz. 1998. The SELF-PRUNING gene of tomato regulates vegetative to reproductive switching of sympodial meristems and is the ortholog of CEN and TFL1. Development 125:1979-1989. [DOI] [PubMed] [Google Scholar]
- 32.Reinders, A., N. Burckert, T. Boller, A. Wiemken, and C. De Virgilio. 1998. Saccharomyces cerevisiae cAMP-dependent protein kinase controls entry into stationary phase through the Rim15p protein kinase. Genes Dev. 12:2943-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson, L. C., and K. Tatchell. 1991. TFS1: a suppressor of cdc25 mutations in Saccharomyces cerevisiae. Mol. Gen. Genet. 230:241-250. [DOI] [PubMed] [Google Scholar]
- 34.Sagee, S., A. Sherman, G. Shenhar, K. Robzyk, N. Ben-Doy, G. Simchen, and Y. Kassir. 1998. Multiple and distinct activation and repression sequences mediate the regulated transcription of IME1, a transcriptional activator of meiosis-specific genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 18:1985-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satoh, T., M. Nakafuku, and Y. Kaziro. 1992. Function of Ras as a molecular switch in signal transduction. J. Biol. Chem. 267:24149-24152. [PubMed] [Google Scholar]
- 36.Scheffzek, K., M. R. Ahmadian, L. Wiesmuller, W. Kabsch, P. Stege, F. Schmitz, and A. Wittinghofer. 1998. Structural analysis of the GAP-related domain from neurofibromin and its implications. EMBO J. 17:4313-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoentgen, F., and P. Jolles. 1995. From structure to function: possible biological roles of a new widespread protein family binding hydrophobic ligands and displaying a nucleotide binding site. FEBS Lett. 369:22-26. [DOI] [PubMed] [Google Scholar]
- 38.Serre, L., K. Pereira de Jesus, C. Zelwer, N. Bureaud, F. Schoentgen, and H. Benedetti. 2001. Crystal structures of YBHB and YBCL from Escherichia coli, two bacterial homologues to a Raf kinase inhibitor protein. J. Mol. Biol. 310:617-634. [DOI] [PubMed] [Google Scholar]
- 39.Serre, L., B. Vallee, N. Bureaud, F. Schoentgen, and C. Zelwer. 1998. Crystal structure of the phosphatidylethanolamine-binding protein from bovine brain: a novel structural class of phospholipid-binding proteins. Structure 6:1255-1265. [DOI] [PubMed] [Google Scholar]
- 40.Simister, P. C., M. J. Banfield, and R. L. Brady. 2002. The crystal structure of PEBP-2, a homologue of the PEBP/RKIP family. Acta Crystallogr. Sect. D Biol. Crystallogr. 58:1077-1080. [DOI] [PubMed] [Google Scholar]
- 41.Smith, A., M. P. Ward, and S. Garrett. 1998. Yeast PKA represses Msn2p/Msn4p-dependent gene expression to regulate growth, stress response and glycogen accumulation. EMBO J. 17:3556-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka, K., B. K. Lin, D. R. Wood, and F. Tamanoi. 1991. IRA2, an upstream negative regulator of RAS in yeast, is a RAS GTPase-activating protein. Proc. Natl. Acad. Sci. USA 88:468-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanaka, K., M. Nakafuku, T. Satoh, M. S. Marshall, J. B. Gibbs, K. Matsumoto, Y. Kaziro, and A. Toh-e. 1990. S. cerevisiae genes IRA1 and IRA2 encode proteins that may be functionally equivalent to mammalian ras GTPase activating protein. Cell 60:803-807. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka, K., M. Nakafuku, F. Tamanoi, Y. Kaziro, K. Matsumoto, and A. Toh-e. 1990. IRA2, a second gene of Saccharomyces cerevisiae that encodes a protein with a domain homologous to mammalian ras GTPase-activating protein. Mol. Cell. Biol. 10:4303-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44a.Te Biesebeke, R., I. M. Krab, and A. Parmeggiani. 2001. The arginine finger loop of yeast and human GAP is a determinant for the specificity toward Ras GTPase. Biochemistry 40:7474-7479. [DOI] [PubMed] [Google Scholar]
- 45.Thevelein, J. M. 1994. Signal transduction in yeast. Yeast 10:1753-1790. [DOI] [PubMed] [Google Scholar]
- 46.Thevelein, J. M., and J. H. de Winde. 1999. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 33:904-918. [DOI] [PubMed] [Google Scholar]
- 47.Toda, T., S. Cameron, P. Sass, M. Zoller, and M. Wigler. 1987. Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell 50:277-287. [DOI] [PubMed] [Google Scholar]
- 48.Toda, T., I. Uno, T. Ishikawa, S. Powers, T. Kataoka, D. Broek, S. Cameron, J. Broach, K. Matsumoto, and M. Wigler. 1985. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell 40:27-36. [DOI] [PubMed] [Google Scholar]
- 49.Tohdoh, N., S. Tojo, H. Agui, and K. Ojika. 1995. Sequence homology of rat and human HCNP precursor proteins, bovine phosphatidylethanolamine-binding protein and rat 23-kDa protein associated with the opioid-binding protein. Brain Res. Mol. Brain Res. 30:381-384. [DOI] [PubMed] [Google Scholar]
- 50.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA. 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wach, A., A. Brachat, R. Pohlmann, and P. Philippsen. 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10:1793-1808. [DOI] [PubMed] [Google Scholar]
- 52.Xu, G. F., B. Lin, K. Tanaka, D. Dunn, D. Wood, R. Gesteland, R. White, R. Weiss, and F. Tamanoi. 1990. The catalytic domain of the neurofibromatosis type 1 gene product stimulates ras GTPase and complements ira mutants of S. cerevisiae. Cell 63:835-841. [DOI] [PubMed] [Google Scholar]
- 53.Xu, G. F., P. O'Connell, D. Viskochil, R. Cawthon, M. Robertson, M. Culver, D. Dunn, J. Stevens, R. Gesteland, R. White, et al. 1990. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell 62:599-608. [DOI] [PubMed] [Google Scholar]
- 54.Yaffe, M. P., and G. Schatz. 1984. Two nuclear mutations that block mitochondrial protein import in yeast. Proc. Natl. Acad. Sci. USA 81:4819-4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yeung, K., T. Seitz, S. Li, P. Janosch, B. McFerran, C. Kaiser, F. Fee, K. D. Katsanakis, D. W. Rose, H. Mischak, J. M. Sedivy, and W. Kolch. 1999. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature 401:173-177. [DOI] [PubMed] [Google Scholar]
- 56.Yeung, K. C., D. W. Rose, A. S. Dhillon, D. Yaros, M. Gustafsson, D. Chatterjee, B. McFerran, J. Wyche, W. Kolch, and J. M. Sedivy. 2001. Raf kinase inhibitor protein interacts with NF-κB-inducing kinase and TAK1 and inhibits NF-κB activation. Mol. Cell. Biol. 21:7207-7217. [DOI] [PMC free article] [PubMed] [Google Scholar]