Abstract
Input from continuous glucose monitors (CGMs) is a critical component of artificial pancreas (AP) systems, but CGM performance issues continue to limit progress in AP research. While G4 PLATINUM has been integrated into AP systems around the world and used in many successful AP controller feasibility studies, this system was designed to address the needs of ambulatory CGM users as an adjunctive use system. Dexcom and the University of Padova have developed an advanced CGM, called G4AP, to specifically address the heightened performance requirements for future AP studies. The G4AP employs the same sensor and transmitter as the G4 PLATINUM but contains updated denoising and calibration algorithms for improved accuracy and reliability. These algorithms were applied to raw data from an existing G4 PLATINUM clinical study using a simulated prospective procedure. The results show that mean absolute relative difference (MARD) compared with venous plasma glucose was improved from 13.2% with the G4 PLATINUM to 11.7% with the G4AP. Accuracy improvements were seen over all days of sensor wear and across the plasma glucose range (40–400 mg/dl). The greatest improvements occurred in the low glucose range (40–80 mg/dl), in euglycemia (80–120 mg/dl), and on the first day of sensor use. The percentage of sensors with a MARD <15% increased from 69% to 80%. Metrics proposed by the AP research community for addressing specific AP requirements were also computed. The G4AP consistently exhibited improved sensor performance compared with the G4 PLATINUM. These improvements are expected to enable further advances in AP research.
Keywords: accuracy, artificial pancreas, closed loop, continuous glucose monitors
Introduction
Two reviews have highlighted the dramatic progress made in recent years on the development of an artificial pancreas (AP) for treatment and management of glycemia in insulin-dependent diabetes.1,2 In addition, there have now been several reports in peer-reviewed literature of both inpatient and outpatient feasibility studies done to evaluate different algorithmic approaches to the AP. Model predictive algorithms were first proposed by Parker and coauthors3 for automated control of blood glucose in patients with type 1 diabetes. Elleri and coauthors4 from the University of Cambridge reported achieving improved glycemic control in a randomized controlled trial with adolescents over 36 h using a model predictive control algorithm. Breton and coauthors5,6 from the University of Virginia have reported similar improvements in glycemic control with their model predictive control algorithm in both inpatient and supervised outpatient studies. Dassau and coauthors7 have also reported positive results using a model predictive controller that incorporates parameters specific to a given individual. Mauseth and couthors8 and Phillip and coauthors9 have published separate results demonstrating excellent results in AP clinical studies using a fuzzy logic controller. Russell and coauthors10 have shown improved glucose control relative to typical open-loop control in patients with type 1 diabetes using a bihormonal AP system with insulin infusion directed by a model predictive control algorithm and glucagon infusion directed by a proportional-derivative controller.
Continued progress in the development of AP systems will be enabled by further improvements in continuous glucose monitor (CGM) performance. Kovatchev and coauthors6 summarized the many advances in technology that have been responsible for the progress in AP research, but they also noted a number of limitations of current CGM systems, specifically, transient loss of sensitivity and random noise. Kovatchev and coauthors6 conclude that these factors reduce the accuracy of data provided by a CGM. Similarly, Thabit and Hovorka2 identified the development of modern realtime continuous glucose sensing as a crucial step for AP technology, but they too noted that
Suboptimal accuracy and reliability remain one of the biggest obstacles for closed-loop systems. Commercially available CGMs can achieve a median relative absolute difference between sensor and reference glucose measurements of 15% or less, which should be commensurate with closed-loop glucose control. However, transient and persistent deviations of greater magnitude occur. Transient deviations relate to temporal loss or increase of sensor sensitivity, or mechanical perturbation including temporal sensor dislodgment. Persistent deviations are caused by erroneous calibration, an inappropriate calibration algorithm, or slow drift of sensor sensitivity. When a sensor overreads blood glucose levels, the persistent deviations pose the greatest challenge to safe closed-loop insulin delivery, as insulin overdelivery may occur, thus increasing the risk of hypoglycemia.
In guidance provided to clinician researchers and to industry on the development of AP systems, the U.S. Food and Drug Administration (FDA) noted the importance of CGM accuracy and reliability in the safety and efficacy of early stage AP feasibility studies. The FDA recommended that early stage AP system studies demonstrate the ability to identify and compensate for CGM errors before proceeding to less-supervised outpatient studies.11 In their discussion of outpatient studies, the FDA recommended that feasibility studies address the issue of erroneous CGM measurements:
Currently marketed CGMs experience periods when they generate incorrect data, e.g., indicating that glucose levels are significantly above or below the true blood glucose value, or that glucose levels are rising when they are actually falling (or falling when they are actually rising). Incorrect information of this critical nature, if fed into the (artificial pancreas controller), can be life-threatening to the patient. An additional consideration is that CGMs may stop providing data, e.g., they may fail to provide data for several hours or they may stop functioning altogether. This latter condition might also have serious consequences, especially if the patient were sleeping at the time and failed to respond to an alarm.
Accordingly, development of more accurate and reliable CGM systems has been a major focus of research and development groups in the diabetes technology industry. The Dexcom G4 PLATINUM continuous glucose monitoring system received the Communauté Européenne mark and FDA approval in 2012. Christiansen and coauthors10 have published a comparative analysis of the performance of the new G4 PLATINUM and the previous SEVEN PLUS CGM systems.12 The percentage of CGM readings in the clinically accurate Clarke error grid A zone increased from 74% for the SEVEN PLUS to 80% for the G4 PLATINUM. The mean absolute relative difference (MARD) decreased from 15.9% ± 8.6% for the SEVEN PLUS to 13.2% ± 6.7% for the G4 PLATINUM. The G4 PLATINUM has been integrated into AP systems around the world and used in many clinical studies. However, the performance requirements for the G4 PLATINUM CGM were based on the clinical requirements for safe and effective use as an adjunctive device in patients with insulin-dependent diabetes. Dexcom and the University of Padova have developed an advanced CGM, called G4AP, to specifically address the heightened performance requirements of an artificial pancreas system.
Sparacino and coauthors13 described the major elements of their approach to improved CGM sensor performance, the so-called “smart” CGM sensor, including a denoising module, an enhancement module, and a prediction module.13 Facchinetti and coauthors14 applied the “smart” CGM module approach to the SEVEN PLUS and found significant improvements in postprocessed CGM data. As noted by Facchinetti and coauthors,14 CGM accuracy is determined not only by the sensor design and materials, but also by the algorithms used to convert the raw sensor signal into a calibrated glucose value. The G4AP system contains improved algorithms developed in collaboration with the University of Padova following many of the concepts contained in their “smart CGM” approach.15,16 The G4AP system is intended to further improve CGM performance and reduce the occurrence of sensor errors, thereby accelerating future development of commercially viable AP systems.
Methods
The G4AP CGM employs the same sensor and transmitter as the G4 PLATINUM but contains updated denoising and calibration algorithms designed to improve accuracy, day-to-day reliability, and consistency from sensor to sensor. The development of the G4AP focused on the following CGM system attributes believed to be barriers in the advancement of closed-loop AP systems into at-home monitoring studies:
Consistent performance over the entire duration of sensor use: Data from all CGM devices developed to date have exhibited poorer accuracy on day 1 than on subsequent days. This is attributed partly to rapidly changing sensor–tissue interface immediately after insertion and difficulties in estimating glucose early in sensor wear because of lack of blood glucose calibration data. Improvements in CGM reliability throughout the 7 days of sensor use will help deliver more consistent performance of closed-loop controllers.
Time lag1,17 due to signal processing: Large and variable signal processing-induced time delays are known to adversley impact accuracy of CGM devices. These delays and the attendant reduction in sensor performance can also impact performance of closed-loop algorithms. Although this may be mitigated to an extent by the use of model predictive controllers,3,18 which incorporate delays into the model, uncertainty in time delays may still adversely impacts controller performance. Reducing the magnitude and variability of delays in signal processing will enhance the ability of the controllers to react rapidly to changing glucose state.
Large inaccuracies (e.g., CGM readings more than 20% off):19 Large errors in glucose estimates may result in adverse controller performance. These errors are particularly difficult to recover from if they persist for a long time (e.g. greater than 60 min19). Large errors in magnitude and duration may result in large increases or decreases in insulin delivery, the effects of which may last for several hours.
Inconsistent performance from sensor to sensor (i.e., number of sensors with a MARD greater than 20%): Many recent closed-loop studies have focused on overnight control or short-term duration studies lasting several days at most in highly supervised settings in which individual sensor failures could be quickly mitigated by inserting new sensors. Artificial pancreas research groups worldwide are planning future closed-loop studies that are likely to last several weeks to months and move from an in-clinic to home setting. Consistent, accurate sensor performance is important for future AP studies because it translates directly to reliable AP controller performance across multiple, successive sensor wears in a less-supervised out-patient setting.
New algorithms addressing these issues were tested using sensor data collected from clinical studies with the G4 PLATINUM system.12 This data set consists of 72 subjects and 108 sensors (36 subjects wore 2 sensors). Calibrations were performed per the labeled instructions for use, i.e., calibrate once every 12 h using self-monitoring of blood glucose (SMBG). The study protocol called for three 12 h in-clinic sessions to be performed on every subject, with venous blood draws taken every 15 min and analyzed with YSI 2300 Stat Plus Glucose Analyzer (YSI Inc., Yellow Springs, OH). The in-clinic sessions were performed on days 1, 4, and 7. The new algorithms were applied to raw sensor data and calibration data from the clinical study in MATLAB R2013a (MathWorks Inc., Natick, MA) using a simulated prospective procedure.
The performance of the G4AP algorithm was compared with the original G4 PLATINUM algorithm using standard CGM evaluation metrics such as the percentage of points within 20 mg/dl below 80 mg/dl and within 20% above 80 mg/dl (%20/20), mean and median absolute relative differences (ARDs), and mean absolute differences (MADs). Results were stratified to YSI values between 40 and 400 mg/dl and also between 40 and 80 mg/dl to assess low glucose performance.
Accuracy, sensor-to-sensor consistency, and the presence of large errors were also assessed with metrics proposed by active AP researchers. Damiano and coauthors20 from Boston University assessed the accuracy and reliability in a comparative study of three CGM systems: the Navigator (Abbott Diabetes Care Inc., Alameda, CA), the SEVEN PLUS (Dexcom, San Diego, CA), and the Guardian (Medtronic Inc., Northridge, CA). Their approach involves computing the average MARD of each sensor compared with plasma glucose references and then computing the mean and standard deviation (SD) across sensors as metrics of both accuracy and precision (consistency of sensor performance), respectively. In addition, the mean and SD of all CGM and plasma glucose pairs at every plasma glucose concentration (70 to 320 mg/dl) were determined to estimate reliability across the glucose range. Leelarathna and coauthors19 from the University of Cambridge have also proposed novel metrics for assessing CGM inaccuracies that are particularly detrimental to an AP system. Leelarathna and coauthors19 noted that the clinical consequences of erroneous sensor reading for AP applications depends not only on the magnitude of the error, but on its duration as well. Accordingly, they defined three levels of sensor error, where error is estimated as ARD for plasma glucose ≥108 mg/dl, and absolute difference (AD) for plasma glucose <108 mg/dl. Level 1 (least severe) is AD ≥43 mg/dl or ARD ≥40%, level 2 is AD ≥54 mg/dl or ARD ≥50%, and level 3 (most severe) is AD ≥65 mg/dl or ARD ≥60%. The CGM and plasma glucose readings were interpolated to 1 min samples, and then incidence and duration of each level error was quantified. Events separated by at least 30 min were considered independent. The incidence of each level error was separated into sensor over-reading and sensor under-reading, which increase the risk of hypoglycemia and hyperglycemia, respectively. Incidence was reported by converting the measured incidence of level errors into the expected level over 100 days of sensor use. In addition, the percentage of events at each level that persisted for more than 60 min was determined relative to the total number of events. All accuracy and reliability metrics described in this paragraph were restricted to in-clinic sessions on days 4 and 7 for direct comparison to the results published by Damiano and coauthors20 and Leelarathna and coauthors,19 who both excluded day 1 performance.
Results
Figure 1 shows one sensor’s performance using the G4 PLATINUM algorithm and the G4AP algorithm compared to YSI reference values during in-clinic sessions on day 1 (panel A), day 4 (panel B), and day 7 (panel C). Sensor performance in this subject was poor on day 1 with the original G4 PLATINUM algorithm. However, the G4AP algorithm was able to improve day 1 performance as reflected in the MARD which was reduced from 23.6% to 14.4%. The G4AP also improved accuracy on days 4 and 7: the MARD on day 4 was reduced from 10.4% to 5.7% and on day 7 from 10.3% to 9.6%. Figure 1 is representative of G4 PLATINUM performance in general in that day 1 accuracy is worse than later in the week. While G4AP performance also demonstrates the trend of improved accuracy over time, the reduction of sensor errors on day 1 improves the sensor reliability from day to day.
Figure 1.
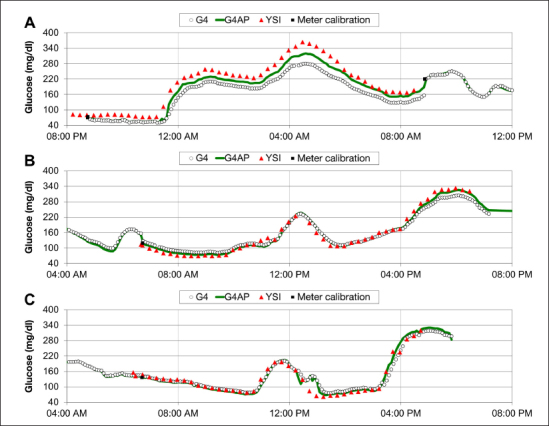
Continuous glucose monitor traces and reference YSI measurements for a representative subject during in-clinic sessions on (A) day 1, (B) day 4, and (C) day 7. G4AP improves accuracy on each day of sensor wear, but the greatest performance improvement occurs on day 1.
Signal-processing-induced time delays were decreased with new G4AP denoising algorithms, which improved accuracy through sensor wear. The reduction in signal processing time delays had minimal impact on the smoothness of the CGM data displayed. An example of the reduced time delays can be seen in Figure 2. Furthermore, the time delay caused by signal processing is more consistent using the G4AP, whereas the G4 PLATINUM can demonstrate larger delays when glucose is changing more rapidly, as evidenced in Figure 2.
Figure 2.
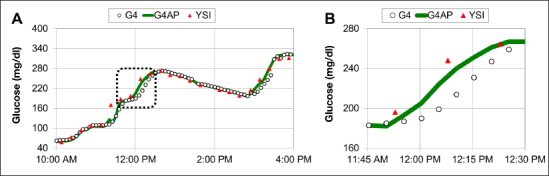
In-clinic session showing reduced time lag between YSI and CGM readings using the G4AP algorithm. The highlighted section of data in the dashed box in panel A is enlarged in panel B, and shows that during a rapid increase in glucose, the G4AP readings precede G4 readings by more than 5 min, resulting in improved accuracy compared with the YSI measurements.
Summary performance of the G4AP algorithm compared with the G4 PLATINUM against YSI reference glucose is shown for a plasma glucose range of 40 to 400 mg/dl (Table 1) and 40 to 80 mg/dl (Table 2) on in-clinic days 1, 4, and 7. The total number of CGM–YSI matched pairs collected (N) is listed by day. In Table 1 , the G4AP shows improved average performance over the glucose range (40–400 mg/dl) in all in-clinic sessions, with the largest improvement on day 1. Referring to Table 2 , low glucose accuracy is improved by the G4AP on days 4 and 7. The G4AP and G4 PLATINUM have comparable low glucose performance on day 1. Overall, the G4AP improved pooled MARD from all in-clinic days and glucose concentrations from 13.2% to 11.7%, and %20/20 from 81.6% to 85.6%.
Table 1.
G4 and G4AP Accuracy versus YSI by In–Clinic Day over the Glucose Range of 40–400 mg/dl
| Algorithm | Day 1 (N = 4524) | Day 4 (N = 4552) | Day 7 (N = 4460) | Days 1–7 (N = 13536) | ||||
| MARD | %20/20 | MARD | %20/20 | MARD | %20/20 | MARD | %20/20 | |
| G4 | 16.8 | 70.8 | 11.0 | 87.8 | 11.8 | 86.3 | 13.2 | 81.6 |
| G4AP | 14.7 | 76.3 | 9.4 | 91.9 | 10.9 | 88.8 | 11.7 | 85.6 |
Table 2.
G4 and G4AP Accuracy versus YSI by In–Clinic Day in the Low Glucose Range of 40–80 mg/dl
| Algorithm | Day 1 (N = 624) | Day 4 (N = 708) | Day 7 (N = 741 ) | Days 1–7 (N = 2073) | ||||
| MAD | %20/20 | MAD | %20/20 | MAD | %20/20 | MAD | %20/20 | |
| G4 | 11.8 | 82.1 | 9.5 | 90.5 | 12.2 | 81.9 | 11.1 | 84.9 |
| G4AP | 12.1 | 82.9 | 7.3 | 96.4 | 9.1 | 88.7 | 9.5 | 89.5 |
Histograms of individual sensor MARD distributions for G4 PLATINUM and G4AP algorithms are shown in Figure 3. Sensor MARD values were computed as the average over all three in-clinic sessions. For the G4 PLATINUM, the average sensor MARD was 13.6%, the median ARD was 12.6%, and the SD was 6.8%. Application of the G4AP algorithm resulted in a reduction of the average sensor MARD to 12.1%, the median ARD to 11.1%, and the SD to 6.0%.
Figure 3.
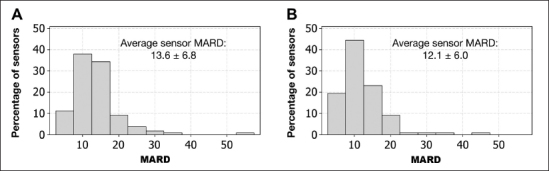
Individual sensor MARD histogram distribution for the G4 PLATINUM algorithm (A) and the G4AP algorithm (B). The G4AP reduced average sensor MARD from 13.6% to 12.1% and SD from 6.8% to 6.0%.
In addition, the number of sensors with MARD less than 15% increased from 68.6% for G4 PLATINUM to 79.6% for G4AP. Similarly, the number of sensors with MARD less than 20% increased from 89.8% to 93.5% for G4AP. Table 3 gives the converse, namely, the percentage of sensors for both G4 PLATINUM and G4AP above MARD threshold values of 15% and 20%.
Table 3.
Percentage of Total Sensors (N = 108) with a Mean Absolute Relative Difference Greater than a Threshold Value
| MARD | G4 | G4AP |
| >15.0 | 31.4% | 20.4% |
| >20.0 | 10.2% | 6.5% |
The box and whisker plots in Figure 4 show the average and SD of the 99 sensor MARD values for the G4AP and G4 PLATINUM, where each sensor MARD is the average of up to 24 h of in-clinic sessions performed on days 4 and 7. There were 9 sensors the total 108 sensors from the G4 PLATINUM study which did not have in-clinic sessions on either day 4 or day 7 for reasons such as subject withdrawal from the study and adhesive or other device-related issues. Figure 4 also shows the average and SD of sensor MARD reported by Damiano and coauthors20 for the Navigator, SEVEN PLUS, and Guardian. The G4AP shows improved average accuracy and precision (SD) compared with the G4 PLATINUM. Furthermore, the G4AP has better average sensor accuracy than the three other CGM devices evaluated by Damiano and coauthors.20 It should be noted that there were experimental differences between the G4 PLATINUM pivotal study and the experiments performed by Damiano and coauthors,20 which make direct comparisons difficult. For example, the study of Damiano and coauthors20 had used a smaller sample size for each device (n = 12). In addition, the in-clinic sessions that covered 48 h over days 2 and 3, used a GlucoScout (International Biomedical, Austin, TX) to measure venous plasma glucose instead of YSI, and were calibrated with plasma glucose measurements rather than capillary SMBG.
Figure 4.
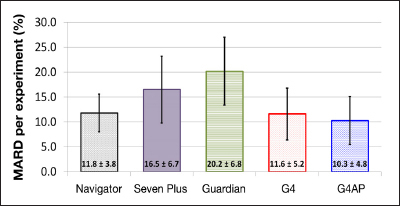
Box and whisker plot of the average and SD of sensor MARD for the G4AP and G4 PLATINUM and the results from the Navigator, SEVEN PLUS, and Guardian CGM systems reported by Damiano and coauthors20 for purposes of comparison. G4AP and G4 PLATINUM data have 99 sensors, reference venous plasma glucose measured with YSI, up to 24 h of in-clinic sessions on days 4 and 7, and calibrations taken from capillary SMBG measurements. Navigator, SEVEN PLUS, and Guardian data have 12 sensors, reference venous plasma glucose measured with GlucoScout, 48 h of in-clinic sessions on days 2 and 3, and calibrations taken from venous blood measurements.
Accuracy and precision (mean and SD) as a function of venous plasma glucose level are shown in Figure 5 for the G4 PLATINUM and G4AP, where paired CGM and plasma glucose measurements were limited to the in-clinic sessions on days 4 and 7. The results by plasma glucose level published by Damiano and coauthors20 for the Navigator, Guardian, and SEVEN PLUS are also shown for comparison. The same experimental differences between the G4 PLATINUM clinical study and the experiments performed by Damiano and coauthors20 that were previously described exist for Figure 5 results as well. While G4 PLATINUM showed similar or improved accuracy and reliability compared with the three other CGM systems evaluated by Damiano and coauthors,20 the G4AP further improved accuracy and reliability, especially in the low glucose range. The average MARD and SD over the glucose range of 70 to 120 mg/dl was improved from 13.7% and 12.0%, respectively, with the G4 PLATINUM to 11.3% and 10.1%, respectively, with the G4AP.
Figure 5.
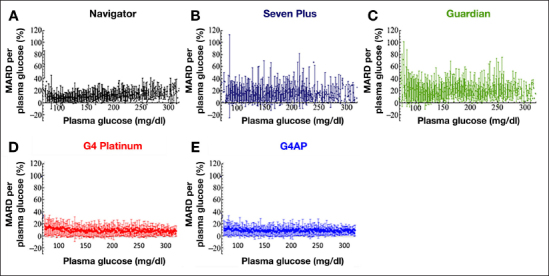
The MARD and SD for the G4 PLATINUM and G4AP algorithms as a function of the plasma glucose. Results for the Navigator, SEVEN PLUS, and Guardian CGM systems published by Damiano and coauthors20 are also shown for purposes of comparison. G4AP and G4 PLATINUM data have n = 99 sensors, reference venous plasma glucose measured with YSI, up to 24 h of in-clinic sessions on days 4 and 7, and calibrations taken from capillary SMBG measurements. Navigator, SEVEN PLUS, and Guardian data have n = 12 sensors, reference venous plasma glucose measured with GlucoScout, 48 h of in-clinic sessions on days 2 and 3, and calibrations taken from venous blood measurements.
The incidence of large errors (levels 1–3) from in-clinic sessions on days 4 and 7 are shown for both the G4 PLATINUM algorithm and the G4AP algorithm in Figure 6. The incidence of large errors for the Navigator and SEVEN PLUS as reported by Leelarathna and coauthors19 is also shown for comparison. Although the incidence of level 1, 2, and 3 errors was reduced with the G4 PLATINUM compared with the SEVEN PLUS, there was a further reduction in level 1, 2, and 3 errors for both over-reading and under-reading using the G4AP algorithm. Direct comparison between the results for the Navigator, SEVEN PLUS, G4 PLATINUM, and G4AP is made difficult by differences in experimental design between the different studies. For example, the in-clinic sessions were of different duration and performed on different days of sensor wear.
Figure 6.
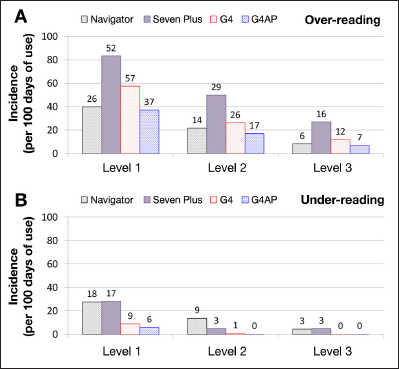
Incidence of sensor over-reading (A) and under-reading (B) by error level for the G4 PLATINUM and G4AP and for the Navigator and SEVEN PLUS as reported by Leelarathna and coauthors.19 The number above each column represents the actual number of episodes during the study period. Incidence was calculated by dividing the study duration (Navigator, 1548 h; SEVEN PLUS, 1440 h; G4 PLATINUM, 2380 h; G4AP, 2380 h) by the number of episodes and normalizing data to 100 days of sensor use.
The occurrence of large errors (levels 1–3) lasting for longer than 1 hour is presented in Figure 7, with occurrence measured as a percentage of all large errors. The occurrence is also shown for the Navigator and Dexcom SEVEN PLUS as reported by Leelarathna and coauthors19 for comparison, although the same experimental differences remain as described earlier. While the G4 PLATINUM reduced the proportion of long duration errors compared with the SEVEN PLUS, G4AP did not reduce it further. However, the G4AP decreased the actual number of long-duration level 1 and 2 errors compared with the G4 PLATINUM. There was one more long duration level 3 error with the G4AP than the G4 PLATINUM. Upon further investigation, it was found that this level 3 error lasted for 62 min using the G4AP and 58 min using the G4 PLATINUM. The single sensor signal responsible for this severe error can be seen in Figure 8, which shows that the poor performance of both G4 algorithms was caused by an inaccurate finger stick calibration (8:00 am).
Figure 7.
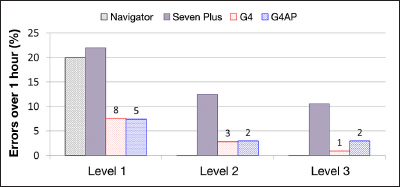
Occurrence of large errors (levels 1–3) longer than 1 h (as a percentage of total events). The number above each column represents the actual number of episodes during the study period. Results for Navigator and SEVEN PLUS are estimated from the published results of Leelarathna and coauthors.19
Figure 8.
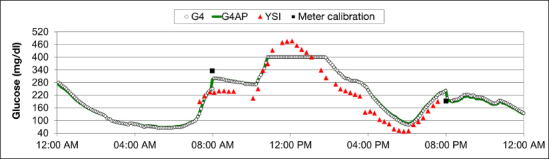
In-clinic session on day 4 with a level 3 error of more than 1 h in duration.
Discussion
The G4AP, through the use of improved denoising and calibration algorithms, was able to significantly improve the accuracy and reliability of the Dexcom G4 PLATINUM CGM system. Continuous glucose monitor performance was more accurate and consistent across the entire duration of use, with the largest improvement on the first day of wear. In addition, the percentage of sensors exhibiting good performance (MARD <15%) was increased, and the SD between sensor MARDs reduced, showing that performance is also more consistent across sensors. The results presented here further show that signal-processing-induced time delays have been reduced and performance at low plasma glucose has been improved. These improvements are expected to enable advancements in AP performance.
The G4AP algorithm was developed to address variability in both CGM system performance and patient physiology. However, while the G4AP reduced the number of large errors (levels 1–3) that can have adverse consequences to AP controller performance, there remained some large errors with duration greater than 1 h. In some instances, these large errors appear to be the result of erroneous finger stick calibrations. Since the routine calibration of both the G4 PLATINUM and G4AP systems is once every 12 h, it can be several hours before large errors are corrected. Future CGM systems with reduced reliance on SMBG calibration may be less susceptible to large errors, especially those of long duration, resulting from erroneous finger stick calibrations.
Finally, it is important to note that the method for integrating real-time data from the G4AP CGM into AP systems is the same as with the G4 PLATINUM system.
Conclusion
Application of elements of the University of Padova’s “smart” CGM technology to the Dexcom G4 PLATINUM CGM system has resulted in an improved version of the system, the G4AP, with greater accuracy and reliability. While the G4 PLATINUM represented a significant improvement in performance relative to the previous-generation SEVEN PLUS device, the G4AP represents a comparable improvement relative to the G4 PLATINUM. Applying G4AP algorithms to previously collected G4 PLATINUM data resulted in an overall MARD reduction from 13.2% to 11.7%. Accuracy was improved over all days, with the largest improvement on the first day of CGM wear. The percentage of individual sensors with MARD <15% increased from 69% with the G4 PLATINUM algorithm to 80% with the G4AP algorithm. The G4AP also exhibited improved sensor performance compared with the G4 PLATINUM using new sensor accuracy metrics proposed within the AP community. The improvements in CGM accuracy and reliability embodied in the G4AP may help to provide more accurate and reliable data input to AP controllers and expedite the development of clinical and commercial implementation of an artificial pancreas.
Glossary
- (%20/20)
percentage of points within 20 mg/dl below 80 mg/dl and within 20% above 80 mg/dl
- (AD)
absolute difference
- (AP)
artificial pancreas
- (ARD)
absolute relative difference
- (CGM)
continuous glucose monitor
- (FDA)
Food and Drug Administration
- (MAD)
mean absolute difference
- (MARD)
mean absolute relative difference
- (SD)
standard deviation
- (SMBG)
self-monitoring of blood glucose
Funding:
This work was funded by Dexcom Inc.
Disclosures:
Naresh C. Bhavaraju, Arturo Garcia, Haripriyan Hampapuram, Apurv Kamath, Thomas Peyser, and Anna Leigh Rack-Gomer are full-time employees of Dexcom. Andrea Facchinetti, Chiara Zecchin, Giovanni Sparacino, and Claudio Cobelli have received research support for this work from Dexcom.
Reference
- 1.Cobelli C, Renard E, Kovatchev B. Artifcial Pancreas: Past, Present, Future. Diabetes. 2011;60(11):2672–2682. doi: 10.2337/db11-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thabit H, Hovorka R. Closed-loop insulin delivery in type 1 diabetes. Endocrinol Metab Clin North Am. 2012;41(1):105–117. doi: 10.1016/j.ecl.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker RS, Doyle FJ, 3rd, Peppas NA. A model-based algorithm for blood glucose control in type I diabetic patients. IEEE Trans Biomed Eng. 1999;46(2):148–157. doi: 10.1109/10.740877. [DOI] [PubMed] [Google Scholar]
- 4.Elleri D, Allen JM, Kumareswaran K, Leelarathna L, Nodale M, Caldwell K, Cheng P, Kollman C, Haidar A, Murphy HR, Wilinska ME, Acerini CL, Dunger DB, Hovorka R. Closed-loop basal insulin delivery over 36 hours in adolescents with type 1 diabetes: randomized clinical trial. Diabetes Care. 2013;36(4):838–844. doi: 10.2337/dc12-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breton M, Farret A, Bruttomesso D, Anderson S, Magni L, Patek S, Dalla Man C, Place J, Demartini S, Del Favero S, Tofanin C, Hughes-Karvetski C, Dassau E, Zisser H, Doyle FJ, 3rd, De Nicolao G, Avogaro A, Cobelli C, Renard E, Kovatchev B. International Artifcial Pancreas Study Group. Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes. 2012;61(9):2230–2237. doi: 10.2337/db11-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovatchev BP, Renard E, Cobelli C, Zisser HC, Keith-Hynes P, Anderson SM, Brown SA, Chernavvsky DR, Breton MD, Farret A, Pelletier MJ, Place J, Bruttomesso D, Del Favero S, Visentin R, Filippi A, Scotton R, Avogaro A, Doyle FJ., 3rd Feasibility of outpatient fully integrated closed-loop control: frst studies of wearable artificial pancreas. Diabetes Care. 2013;36(7):1851–1858. doi: 10.2337/dc12-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dassau E, Zisser H, Harvey RA, Percival MW, Grosman B, Bevier W, Atlas E, Miller S, Nimri R, Jovanovic L, Doyle FJ., 3rd Clinical evaluation of a personalized artificial pancreas. Diabetes Care. 2013;36(4):801–809. doi: 10.2337/dc12-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mauseth R, Hirsch IB, Bollyky J, Kircher R, Matheson D, Sanda S, Greenbaum C. Use of a “fuzzy logic” controller in a closed-loop artificial pancreas. Diabetes Technol Ther. 2013;15(8):628–633. doi: 10.1089/dia.2013.0036. [DOI] [PubMed] [Google Scholar]
- 9.Phillip M, Battelino T, Atlas E, Kordonouri O, Bratina N, Miller S, Biester T, Stefanija MA, Muller I, Nimri R, Danne T. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368(9):824–833. doi: 10.1056/NEJMoa1206881. [DOI] [PubMed] [Google Scholar]
- 10.Russell SJ, El-Khatib FH, Nathan DM, Magyar KL, Jiang J, Damiano ER. Blood glucose control in type 1 diabetes with a bihormonal bionic endocrine pancreas. Diabetes Care. 2012;35(11):2148–2155. doi: 10.2337/dc12-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services; Food and Drug Administration; Center for Devices and Radiological Health. Guidance for industry and Food and Drug Administration staf: the content of investigational device exemption (IDE) and premarket approval (PMA) applications for artificial pancreas device systems. 2012. Nov 9, http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/ GuidanceDocuments/UCM259305.pdf. Accessed July 5, 2013.
- 12.Christiansen M, Bailey T, Watkins E, Liljenquist D, Price D, Nakamura K, Boock R, Peyser T. A new-generation continuous glucose monitoring system: improved accuracy and reliability compared with a previous-generation system. Diabetes Technol Ther. 2013;15(10):881–888. doi: 10.1089/dia.2013.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sparacino G, Zanon M, Facchinetti A, Zecchin C, Maran A, Cobelli C. Italian contributions to the development of continuous glucose monitoring sensors for diabetes management. Sensors (Basel) 2012;12(10):13753–13780. doi: 10.3390/s121013753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Facchinetti A, Sparacino G, Guerra S, Luijf YM, DeVries JH, Mader JK, Ellmerer M, Benesch C, Heinemann L, Bruttomesso D, Avogaro A, Cobelli C. AP@home Consortium. Real-time improvement of continuous glucose monitoring accuracy: the smart sensor concept. Diabetes Care. 2013;36(4):793–800. doi: 10.2337/dc12-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Facchinetti A, Sparacino G, Cobelli C. Online denoising method to handle intraindividual variability of signal-to-noise ratio in continuous glucose monitoring. IEEE Trans Biomed Eng. 2011;58(9):2664–2671. doi: 10.1109/TBME.2011.2161083. [DOI] [PubMed] [Google Scholar]
- 16.Facchinetti A, Sparacino G, Cobelli C. Enhanced accuracy of continuous glucose monitoring by online extended Kalman fltering. Diabetes Technol Ther. 2010;12(5):353–363. doi: 10.1089/dia.2009.0158. [DOI] [PubMed] [Google Scholar]
- 17.Keenan DB, Grosman B, Clark HW, Roy A, Weinzimer SA, Shah RV, Mastrototaro JJ. Continuous glucose monitoring considerations for the development of a closed-loop artificial pancreas system. J Diabetes Sci Technol. 2011;5(6):1327–1336. doi: 10.1177/193229681100500603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patek SD, Magni L, Dassau E, Karvetski C, Tofanin C, De Nicolao G, Del Favero S, Breton M, Man CD, Renard E, Zisser H, Doyle FJ, 3rd, Cobelli C, Kovatchev BP. International Artifcial Pancreas (iAP) Study Group. Modular closed-loop control of diabetes. IEEE Trans Biomed Eng. 2012;59(11):2986–2999. doi: 10.1109/TBME.2012.2192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leelarathna L, Nodale M, Allen JM, Elleri D, Kumareswaran K, Haidar A, Caldwell K, Wilinska ME, Acerini CL, Evans ML, Murphy HR, Dunger DB, Hovorka R. Evaluating the accuracy and large inaccuracy of two continuous glucose monitoring systems. Diabetes Technol Ther. 2013;15(2):143–149. doi: 10.1089/dia.2012.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damiano ER, El-Khatib FH, Zheng H, Nathan DM, Russell SJ. A comparative effectiveness analysis of three continuous glucose monitors. Diabetes Care. 2013;36(2):251–259. doi: 10.2337/dc12-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]


