Abstract
Background
Several clinical trials have been performed to assess safety and efficacy of closed-loop control. Some included physical activity (PA), with the goal of challenging the control algorithms with a rapid change in insulin sensitivity while reducing the risk of hypoglycemia. However, the question remains as to the necessity to inform the control algorithm on the imminent PA. The aim here is to assess in silico (i) if it is necessary to announce upcoming PA and (ii) if this is the case, what is the safest strategy of basal insulin reduction in the context of the closed-loop control.
Methods
We modified the University of Virginia/Padova type 1 diabetes simulator to incorporate the effect of PA based on a study in healthy subjects that demonstrated an almost doubling of insulin sensitivity during PA versus rest. Two in silico experiments, including a PA session, have been simulated on the virtual adult population: one in the absence of and one with different degrees of reductions and durations of basal insulin infusion rates.
Results
Most in silico subjects experienced hypoglycemia in the absence of basal insulin adjustment. We show that, in the absence of patient-specific information, a safe and effective strategy is to reduce basal insulin by 50% starting 90 min before exercise and by 30% during exercise.
Conclusions
Our results suggest that control algorithms could benefit by knowing an upcoming PA Ideally, the control algorithm should be informed about the patient-specific basal insulin reduction pattern. An alternative strategy that has been proposed here has been deemed safe and effective in in silico experiments.
Keywords: physical activity, simulation, type 1 diabetes
Introduction
Due to the inability of the pancreas to produce insulin, patients with type 1 diabetes mellitus (T1DM) use exogenously administered insulin to maintain blood glucose (BG) concentration in the near-normal range (70ߝ180 mg/dl). Severe hypoglycemia is very dangerous, possibly leading to coma and ultimately to death, while sustained hyperglycemia may cause long-term microvascular and macrovascular complications. However, BG control is extremely challenging due to the delay between insulin administration and action, physiological perturbations [meals and physical activity (PA)], and intrasubject variability.
Thanks to the availability of continuous glucose monitoring (CGM) systems and continuous subcutaneous insulin infusion pumps, researchers have focused on the development of a wearable artificial pancreas (AP), a system able to modulate subcutaneous insulin infusion rates automatically based on CGM readings, thus helping in optimally controlling BG1 Artificial pancreas prototypes have been tested successfully in inpatient trials, demonstrating reduced occurrence of hypoglycemia with respect to standard therapy, increased time spent in the target range, and reduction of mean glucose.2–7 Some of these trials6,7 included a session of PA in order to test the ability of the control algorithm to deal with the rapid changes in insulin sensitivity due to exercise and prevent the risk of immediate and delayed hypoglycemia associated with daily activity. In standard therapy, subjects usually, but not always, reduce their basal insulin infusion by a given percentage, starting up to 2 h before exercise session, but each patient follows his/her own routine and physician suggestions.
It remains a question if one needs to inform the control algorithm regarding impending PA and, if this is the case, the degree and duration of changes to insulin infusion rates in relation to PA. In particular, model predictive control algorithms (e.g., Soru and coauthors8 and Patek and coauthors9) may benefit greatly from the availability of this information because they can use it to predict future glucose level and adjust insulin infusion rates accordingly. The issue is particularly important because maintaining unchanged basal infusion rates in the presence of PA often leads to hypoglycemia.
The aim of the present study was to assess in silico (i) if it is necessary to announce upcoming PA and (ii) if this is the case, the safest strategy of basal insulin reduction especially when patient-specific information is lacking. We used the University of Virginia (UVA)/Padova T1DM simulator10,11 and the effect of PA was included in the simulator by using the results of a recent study in healthy subjects12 that reported an almost doubling of insulin sensitivity during PA with respect to rest. Two in silico experiments, including an exercise session, have been simulated on a 100-virtual-adult population with T1DM: one maintaining subject-specific basal insulin infusion profile and the other with different degrees of reductions and durations of basal insulin infusion rates. The optimization strategy aims to explore, in a simulation context, the best combination of basal insulin infusion rate before and during PA that would prevent hypoglycemia during exercise.
Materials and Methods
The University of Virginia/Padova Type 1 Diabetes Simulator
The UVA/Padova T1DM simulator10,11 is a tool accepted by the Food and Drug Administration as a substitute for preclinical trials of certain insulin treatments, including closed-loop control algorithms (Figure 1). It describes the glucose dynamics during a meal by putting in relation plasma glucose, insulin, and glucagon concentrations with glucose fluxes (endogenous glucose production, glucose rate of appearance, glucose utilization, renal excretion), insulin fluxes (rate of insulin appearance from subcutaneous tissue, insulin degradation), and glucagon fluxes (glucagon secretion, glucagon degradation). However, the effect of PA was not yet been included in the simulator.
Figure 1.
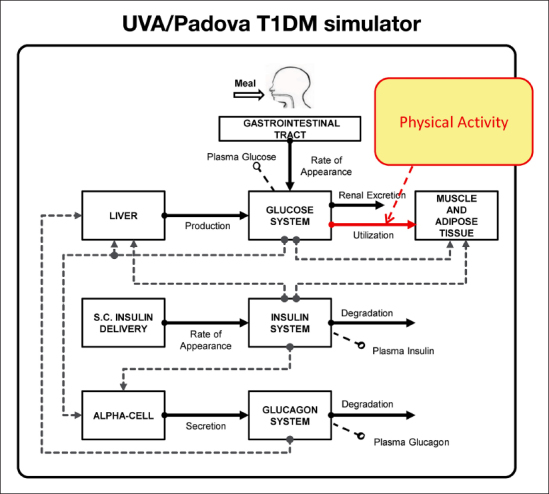
The new version of T1DM simulator11 with the effect of PA evidenced. S.C., subcutaneous.
The primary effect of PA is to enhance glucose utilization by the tissues13 (Figure 1, red arrow). The model of glucose utilization currently implemented in the simulator assumes that glucose kinetics are described by two compartments, that insulin-independent glucose utilization by the brain and the erythrocytes takes place in the first compartment (plasma and rapidly equilibrating tissues) and is constant, while insulin-dependent glucose utilization (Uid) takes place in the remote compartment (slowly equilibrating tissues) and depends nonlinearly from glucose in the tissues:
eqn_1
where Gt(t) is glucose mass in the peripheral compartment; Vm0, Vmx, Km0, and p2U are model parameters; and, in particular, Vmx represents insulin sensitivity, i.e., the ability of insulin to stimulate glucose utilization. Insulin action X(t) on glucose utilization is described by
eqn_2
where p2U is the rate constant of insulin action on the peripheral glucose utilization and I(t) is plasma insulin concentration (suffix b denotes basal state). Moreover, to describe insulin action in the hypoglycemic range, X(t) is modulated by the glucose risk function:14
eqn_3
with Gb the basal glucose, Gth the hypoglycemic threshold (set at 60 mg/dl), and
eqn_4
with r1 and r2 model parameters.
The complete set of model equations can be found from Dalla Man and coauthors.11
Modeling the Effect of Physical Activity
It is an accepted notion that PA increases insulin sensitivity (SI), i.e., the ability of insulin to stimulate glucose utilization, likely due to enhanced glucose uptake by the muscles. The effect size of PA on SI has been estimated in 12 healthy subjects who underwent a moderate-grade PA on a treadmill (~50% of VO2max) for 60 min during a 75 min period, 2 h after having ingested a mixed meal containing 75 g of carbohydrate.12 SI was estimated first using data of the first 120 min after the meal (SIrest), i.e., in the absence of PA and then using data of the whole experiment (lasting 360 min; SIoverall), i.e., in the presence of PA. It was found that SIoverall was almost twice SIrest consistently in all subjects. SIoverall is basically the average of SI in the entire duration of the experiment. To single out the actual value of SI during exercise (SIex), one can assume that SI is equal to SIrest for t ≤ 120, equal to SIex for 120 < t ≤ 195 and decreases linearly from SIex to SIrest in 195 < t ≤ 360 (Figure 2, upper panel, pink line). With this assumption, the average SI during the whole experiment (SIoverall) can be calculated by integrating the SI profile of Figure 2 from tmeal and tend and dividing it by the length of the integration interval (tend–tmeal):
Figure 2.

(Upper panel) Simulation scenario mimicking the experimental protocol reported by Schiavon and coauthors12 with the exercise session (pink line). (Lower panel) Standard premeal insulin bolus given at mealtime and basal insulin infusion rate adjustments, with respect to the patient’s profile, during (red arrow) and/or before (blue and green arrows) the exercise session. CHO, carbohydrate.
eqn_5
thus
eqn_6
Moreover, assuming that SIex is α·SIrest and, as reported in Schiavon and coauthors,12 SIoverall is almost twice SIrest consistently in all subjects, we can explicitly calculate α from
eqn_7
where tmeal and tend are time of the meal and time of the end of experiment, while tex,start and tex,end are time of the start and end of the exercise session, respectively, obtaining α = 3.29.
This effect of exercise on glucose dynamics was incorporated into the UVA/Padova T1DM simulator by modifying accordingly the parameter Vmx, while all other parameters are maintained fixed to standard UVA/Padova T1DM population parameters.
It is well-known that PA also stimulates noninsulin mediated glucose transport, but as discussed by Schiavon and coauthors,12 the contribution of this insulin-independent effect of PA has not yet been quantitatively assessed, and thus it is not possible at this time to simulate the relative contributions of insulin-dependent and insulin-independent effects on glucose utilization.
In Silico Experiments
The simulation scenario mimics the experimental protocol as reported previously.12 It consists of a meal (containing 75g of carbohydrates) administered 3 h after the start of the experiment to 100 in silico adults with T1DM. The exercise session was simulated to start 2 h after the meal and to last 75 min (Figure 2, upper panel) as previously described.12 The simulation of the exercise session was obtained by modifying parameter Vmx as shown in Figure 2 (upper panel, pink line). The virtual subjects received a standard premeal insulin bolus based on each individual’s insulin-to-carbohydrate ratio, while basal insulin infusion rate was first maintained fixed to patient basal profile (experiment 1) and then decreased in various degrees and durations in relation to usual basal insulin infusion rates (experiment 2).
Experiment 1
The virtual subjects received their own basal insulin profile, a subject-specific constant insulin infusion rate able to maintain glucose at steady state in the absence of external disturbances such as meals and PA. This provided the worstcase scenario to which to compare the simulation results of experiment 2.
Experiment 2
The virtual subjects received their own basal insulin profile lowered by step reductions of 10% during and/or before the exercise session to prevent hypoglycemia (Figure 2, lower panel), while outside these ranges, they received constant rates of basal insulin infusion. In particular, before the exercise session, we simulated step reductions of basal insulin rates ranging from 10% to 60%, with a 10% step, starting 90, 60, and 30 min before the beginning of the exercise (Figure 2, lower panel, blue and green arrows). During the exercise, reductions ranging from 10% to 60% with a 10% step have been tested, lasting for the entire exercise duration (Figure 2, lower panel, red arrow). Outside these time intervals, basal insulin infusion rate was maintained constant and equal to the patient’s basal insulin profile (Figure 2, lower panel, black line).
Data Analysis
Results are presented as mean ± standard deviation. Safety (reduction of hypoglycemia) and efficacy (attenuation of hyperglycemia) of different simulated adjustments to basal insulin infusion rates have been assessed by using the control variability grid analysis (CVGA)15 and by computing the time spent in the target range of 90–140 mg/dl. For this calculation, we used simulated glucose profiles from the start of exercise session to 7 h later, i.e., 3 h after that Vmx has returned to its rest value (Figure 2, upper panel).
Results
Experiment 1
Simulated plasma glucose concentrations in the 100 T1DM virtual subjects are shown in Figure 3 (upper-left panel). As expected, most of the subjects experienced hypoglycemia (defined as glucose concentration below 70 mg/dl), with several of them having severe hypoglycemic episodes (defined as glucose concentration below 50 mg/dl). This is confirmed by the CVGA (Figure 3, lower-left panel), which shows 15% of the subjects in the lower C zone, 73% in the lower D zone, no subject in the A zone, and only 12% of the subjects in the B zone. The average time in target was 28.8% ± 16.1%.
Figure 3.
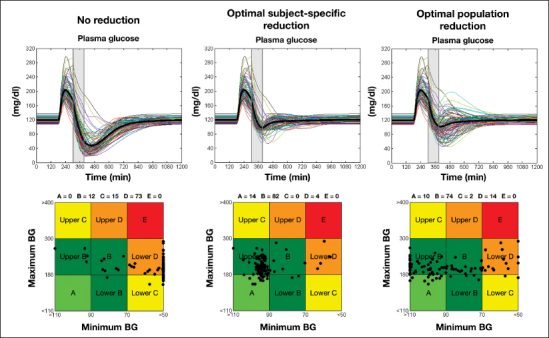
Simulated plasma glucose concentrations (upper panel) and CVGA (lower panel) in the 100 T1DM virtual subjects: no basal insulin infusion rate adjustment (left panel), optimal combination of subject-specific adjustments (middle panel), and optimal combination of population (on average) adjustments (right panel). The shaded box represents the exercise period.
Experiment 2
The distribution of the optimal combination of basal reductions in the 100 in silico subjects are shown in Figure 4. Most of the subjects needed to reduce their basal insulin of 60% (middle panel) from 90 min before the start of exercise (Figure 4, left panel) and maintain this reduction until the end of the PA (Figure 4, right panel). However, there is large intersubject variability, and the optimal reduction strategy is different from the one described earlier in a significant proportion of subjects. Therefore, ideally, one should apply a personalized strategy to each subject. In this case, in fact, our simulations show that the average time in target increases to 90.2% ± 11.4% and that 96% of the subjects do not experience hypoglycemia (Figure 3, upper-middle panel) while the remaining 4% only show mild hypoglycemia episodes (plasma glucose between 50 and 70 mg/dl). This is also confirmed by the CVGA (Figure 3, lower-middle panel), which shows 14% of the subjects in zone A 82% in zone B and only 4% in lower zone D.
Figure 4.
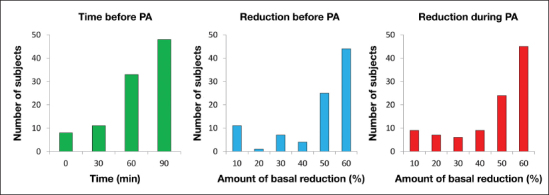
Distribution of the optimal subject-specific combination of basal reductions in the 100 T1DM virtual subjects: time before the start of PA (left), reduction before PA (middle), and reduction during PA (right panel).
Unfortunately, a patient-specific optimal basal reduction strategy is not always available. The simulation results can provide some guidelines in case this patient-specific information is missing. We selected the basal reduction pattern that maximizes efficacy and safety in all the 100 in silico subjects, using as a metric the time spent in the target range of 90–140 mg/dl. We found that a 30% reduction of basal insulin rates during PA preceded by 50% reduction starting 90 min before the start of PA is the combination that most effectively guarantees safety and efficacy in most of the 100 in silico population. As a matter of fact, if this adjustment is applied to all virtual subjects, average time in target is 72.7% ± 23.5%. The glucose-simulated profiles are shown in Figure 3 (upper-right panel), with 84% of the subjects avoiding hypoglycemia. This was confirmed by the CVGA (Figure 3, lower-right panel): 10% of the subjects lie in the A zone, 74% in the B zone, 14% in the lower D zone, and 2% in the lower C zone.
Discussion
Inpatient studies demonstrated that a wearable AP may reduce occurrence of hypoglycemia, increase time spent in the target range, and reduce mean glucose concentrations with respect to standard therapy. However, control algorithms have to deal with many challenges in outpatient conditions such as PA In standard open-loop therapy, subjects usually, but not always, reduce their basal insulin infusion rates in order to prevent the risk of hypoglycemia due to the rapid change in insulin sensitivity caused by exercise. In closed-loop therapy, the need to inform control algorithms regarding impending PA in order to appropriately reduce insulin infusion rates and prevent hypoglycemia both during and after exercise remains debatable.
Physical activity is known to improve insulin action; however, a model of PA effect on glucose–insulin dynamics and the relative contribution of insulin- and non-insulin-mediated effect on muscle glucose uptake has not yet been developed. Here we exploited the results published recently,12 where the quantification of the effect size of PA on insulin sensitivity has been estimated in healthy controls. This effect has been incorporated into the UVA/Padova T1DM simulator, and two experiments, including an exercise session, have been simulated: one maintaining subject-specific basal insulin infusion profile and another with varying degrees of reductions and durations of basal insulin infusion rates. As expected, most of the virtual subjects (88%) experienced hypoglycemia when no announcement of upcoming PA was provided. On the other hand, if the information on modifications to basal insulin infusion rates during PA is provided to the algorithm, then the safest and most effective reduction of basal insulin rates (16% of hypoglycemic events) is a 30% reduction during PA preceded by 50% reduction starting 90 min before the start of PA These results also suggest that it would be useful to provide information about impending PA to the control algorithm in order to intervene promptly to reduce the risk of hypoglycemia and provide guidelines when patient-specific information before/during PA is not available.
A limitation of this study is the assumption of a nonphysiological step increase in insulin sensitivity at the start of PA However, a model of PA on glucose–insulin dynamics has not yet been developed. Simulation can be refined once a model is formulated based on the results reported by Schiavon and coauthors.12
Conclusions
The aim of this study was to use simulation to suggest a best strategy that could be adopted during AP clinical trials that involves a session of moderate PA Our results demonstrate that (i) PA markedly affects glucose control, leading to severe hypoglycemia if not properly addressed; (ii) any control algorithm would benefit by knowing in advance that PA is imminent, because the large delay between subcutaneous insulin infusion and its effect on plasma glucose concentration preclude the possibility of simply stopping subcutaneous basal insulin infusion when PA is detected; (iii) if available, the control algorithm should be informed about patient-specific basal insulin reduction pattern; and (iv) if such information is not available, a “generic” basal reduction strategy is proposed that has been proved to be rather safe and effective.
Glossary
- (AP)
artificial pancreas
- (BG)
blood glucose
- (CGM)
continuous glucose monitoring
- (CVGA)
control variability grid analysis
- (PA)
physical activity
- (T1DM)
type 1 diabetes mellitus
- (UVA)
University of Virginia
Funding
The work was supported by National Institutes of Health Grants DK R01 085561 and DK DP3 094331, grant number UL1 TR000135 from the National Center for Advancing Translational Science of the National Institutes of Health, and the Italian Ministero dell’Istruzione, dell’Università e della Ricerca (Progetto FIRB 2009).
References
- 1.Cobelli C, Renard E, Kovatchev B. Artifcial Pancreas: Past, Present, Future. Diabetes. 2011;60(11):2672–2682. doi: 10.2337/db11-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55(12):3344–3350. doi: 10.2337/db06-0419. [DOI] [PubMed] [Google Scholar]
- 3.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artifcial pancreas. Diabetes Care. 2008;31(5):934–939. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
- 4.Hovorka R, Allen JM, Elleri D, Chassin LJ, Harris J, Xing D, Kollman C, Hovorka T, Larsen AM, Nodale M, De Palma A, Wilinska ME, Acerini CL, Dunger DB. Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet. 2010;375(9716):743–751. doi: 10.1016/S0140-6736(09)61998-X. [DOI] [PubMed] [Google Scholar]
- 5.El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artifcial pancreas for type 1 diabetes. Sci Transl Med. 2010;2(27):27ra27. doi: 10.1126/scitranslmed.3000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breton M, Farret A, Bruttomesso D, Anderson S, Magni L, Patek S, Dalla Man C, Place J, Demartini S, Del Favero S, Tofanin C, Hughes-Karvetski C, Dassau E, Zisser H, Doyle III FJ, De Nicolao G, Avogaro A, Cobelli C, Renard E, Kovatchev B. International Artifcial Pancreas Study Group. Fully integrated artifcial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normo-glycemia. Diabetes. 2012;61(9):2230–2237. doi: 10.2337/db11-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luijf YM, DeVries JH, Zwinderman K, Leelarathna L, Nodale M, Caldwell K, Kumareswaran K, Elleri D, Allen J, Wilinska M, Evans M, Hovorka H, Doll W, Ellmerer M, Mader JK, Renard E, Place J, Farret A, Cobelli C, Del Favero S, Dalla Man C, Avogaro A, Bruttomesso D, Filippi A, Scotton R, Magni L, Giordano L, Di Palma F, Soru P, Tofanin C, De Nicolao G, Arnolds S, Benesch C, Heinemann L. Diabetes Care. 2013. AP@home Consortium. Day and night closed loop control in adults with type 1 diabetes mellitus: a comparison of two closed loop algorithms driving continuous subcutaneous insulin infusion versus patient self management. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soru P, De Nicolao G, Tofanin C, Dalla Man C, Cobelli C, Magni L. AP@home Consortium. MPC based artifcial pancreas: strategies for individualization and meal compensation. Ann Rev Control. 2012;36(1):118–128. [Google Scholar]
- 9.Patek SD, Magni L, Dassau E, Karvetski C, Tofanin C, De Nicolao G, Del Favero S, Breton M, Dalla Man C, Renard E, Zisser H, Doyle III FJ, Cobelli C, Kovatchev BP. International Artifcial Pancreas (iAP) Study Group. Modular closed-loop control of diabetes. IEEE Trans Biomed Eng. 2012;59(11):2986–2999. doi: 10.1109/TBME.2012.2192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovatchev BP, Breton M, Dalla Man C, Cobelli C. In silico preclinical trials: a proof of concept in closed-loop control of type 1 diabetes. J Diabetes Sci Technol. 2009;3(1):44–55. doi: 10.1177/193229680900300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalla Man C, Micheletto F, Dayu L, Breton M, Kovatchev BP, Cobelli C. J Diabetes Sci Technol. The UVA/Padova type 1 diabetes simulator: new features. 2013. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiavon M, Hinshaw L, Mallad A, Dalla Man C, Sparacino G, Johnson M, Carter R, Basu R, Kudva Y, Cobelli C, Basu A. Postprandial glucose fuxes and insulin sensitivity during exercise: a study in healthy individuals. Am J Physiol Endocrinol Metab. 2013;305(4):E557–E566. doi: 10.1152/ajpendo.00182.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter SL, Rennie C, Tarnopolsky MA. Substrate utilization during endurance exercise in men and women after endurance training. Am J Physiol Endocrinol Metab. 2001;280(6):E898–E907. doi: 10.1152/ajpendo.2001.280.6.E898. [DOI] [PubMed] [Google Scholar]
- 14.Kovatchev BP, Straume M, Cox DJ, Farhy LS. Risk analysis of blood glucose data: a quantitative approach to optimizing the control of insulin dependent diabetes. J Theor Med. 2000;3(1):1–10. [Google Scholar]
- 15.Magni L, Raimondo DM, Dalla Man C, Breton M, Patek S, Nicolao GD, Cobelli C, Kovatchev BP. Evaluating the efcacy of closed-loop glucose regulation via control-variability grid analysis. J Diabetes Sci Technol. 2008;2(4):630–635. doi: 10.1177/193229680800200414. [DOI] [PMC free article] [PubMed] [Google Scholar]


