Abstract
Background
Hyperglycemia and hypoglycemia in hospitalized patients have been associated with increased morbidity and mortality. Improvements in glucose monitoring technology may be helpful in the clinical management of critically ill patients with abnormal glucose levels. A first-generation intravenous blood glucose monitoring (IVBG) system was developed to facilitate glycemic control therapy in hospitalized patients. A nonrandomized, single-arm, multicenter study was performed to evaluate the safety and accuracy of the IVBG system in insulin-treated subjects with diabetes mellitus.
Methods
The IVBG system is a bedside monitor that automatically measures venous blood glucose (BG) concentration. In this study, BG was measured every 7.5 min by the IVBG system. Reference samples [venous blood samples measured on the Yellow Springs Instruments (YSI) glucose analyzer] were drawn every 15 min during inpatient studies on days 1, 2, and 3. Fifty insulin-treated healthy volunteers with diabetes were studied, and a maximum of 72 reference samples were collected. Effectiveness was primarily evaluated by assessing the proportion of IVBG BG measurements within the 15 mg/dl or 20% criterion [15 mg/dl (for YSI <75 mg/dl) or 20% (for YSI ≥75 mg/dl)] compared with YSI. Adverse events and adverse device effects were evaluated.
Results
A total of 95% of all IVBG values were within the 15 mg/dl or 20% criterion. The IVBG system BG measurement showed significant linear relationship with the laboratory YSI standard. Catheter insertion site irritation was mild and infrequent. No serious adverse events were reported. A total of 33% of the sensors were replaced during the 3-day use due to problematic IV lines or sensor/system errors.
Conclusions
This clinical performance evaluation demonstrates that the IVBG system provides accurate and safe continuous BG measurements in healthy insulin-treated patients with diabetes.
Keywords: accuracy, glucose sensor, intravenous, near-continuous glucose monitoring, safety, YSI
Introduction
Studies have demonstrated increased morbidity and mortality in hospitalized patients with hyperglycemia, regardless of an established diagnosis of diabetes.1 Several prospective clinical trials were published,2–4 the most important in 2001 by Van den Berghe and coauthors,4 which catapulted efforts to improve glycemic control in critically ill patients into the mainstream. Increased support for tight glycemic control was reinforced by data published in 2006 in the medical intensive care unit (ICU) setting.5 In the subsequent years, most hospitals contemplated and many initiated programs with the goal of aggressively lowering glucose levels in hospitalized patients.
The primary barriers to implementing an intensive glycemic control program are obtaining timely accurate glucose measurements, cost, and nursing workload.6,7 While the clinical laboratory can measure glucose precisely and at low cost, the turnaround time is too long to implement a glucose control algorithm using this methodology. Standard point-of-care glucose testing using capillary measurements provides more timely data, but this can be time-consuming,6 imprecise, and subject to interfering substances and effects of other conditions such as anemia.8 consequently, most efforts to regulate glucose more stringently have been confined to the ICUs.
Targeting aggressive glycemic goals brings the potential risks of such an intervention (e.g., hypoglycemia, excessive cost) to the forefront. Technology that has the potential to minimize these risks is therefore of great importance. The NICE-SUGAR study9 was the primary study that highlighted the potential pitfalls of aggressive glucose control efforts with its findings of both increased severe hypoglycemia and mortality. However, the technology used in this study may have led to an overestimation of BG that could have contributed to the increase in severe hypoglycemia.8
Appropriate glycemic goals may differ among certain groups of hospitalized patients. Regardless of the absolute goals that are adopted, technology that can assist in maintaining glucose within a goal range is likely to be of great utility in clinical management.
An accurate, automatic, and frequent sampling method to measure BG concentration would be helpful to assess more optimally the glycemic status of patients with hyperglycemia and hypoglycemia. This approach has the potential to implement glucose regulation protocols more effectively.
Edwards Lifesciences LLC (Irvine, CA) and Dexcom Inc. (San Diego, CA) developed a first-generation intravenous blood glucose monitoring (IVBG) system (GlucoClear System, EV900, Edwards Lifesciences) to facilitate glycemic control therapy in hospitalized patients. This IVBG system uses an intravenous (IV) sensor for measuring blood glucose (BG) concentration and is intended for use in hospitalized patients, including critically ill patients.
This study was performed in otherwise healthy volunteers with diabetes to provide information that cannot be readily obtained through additional nonclinical assessments or assessments in the intended use population. This information includes evaluating accuracy of the IVBG system across the entire reportable range of the IVBG system (i.e., 40– 400 mg/dl) and across various rates of change during the entire 72 h use life of the sensor.
System Description
Intravenous Blood Glucose Monitoring System
This first-generation IVBG system consists of a glucose sensor placed within a 20 G peripheral IV catheter and a bedside monitor that has fluidic control and displays the glucose information (Figure 1). A single-use IV tubing set (tubing set) connects the sensor to the fluidics control system and a reference solution. The system was designed to maximize accuracy under extreme and variable conditions of the ICU. The system is designed for automatic sampling and displays BG measurements every 7.5 min for up to 72 h.
Figure 1.
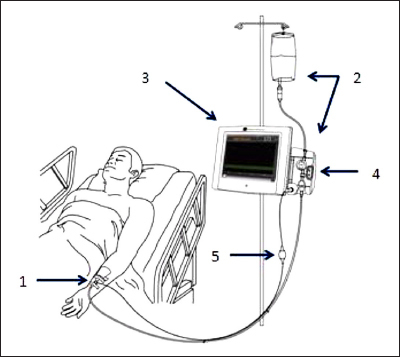
The components of the IVBG system. (1) IVBG sensor and patient IV catheter, (2) reference solution and IVBG tubing set, (3) EV900 monitor, (4) fow control valve unit, and (5) EV900 patient cable.
Intravenous Blood Glucose Monitoring Sensor
The sensor is a small glucose-oxidase-based electrochemical electrode array containing two sensing electrodes that generates an electric current proportional to the concentration of BG (Figure 2). The first sensing electrode consists of a membrane that contains glucose oxidase and has been designed for improved stability and interferent blocking. The second sensing electrode consists of the same membrane but does not contain the glucose-oxidase enzyme. This second electrode is used to measure and subtract background electrochemical signal caused by endogenous electroactive species, interferences such as acetaminophen, and other sources of noise. The resulting subtracted signal is selective to glucose, resulting in an accurate raw sensor signal. The IVBG system provides additional inputs for diagnostic monitoring of the fluidic sampling and indicates if interferents are present, providing additional safeguards on accuracy. The sensor array is sterile, single use, integrated within a Luer-Lock fluid connector, and electrically connected to the monitoring cable as demonstrated in Figure 2.
Figure 2.
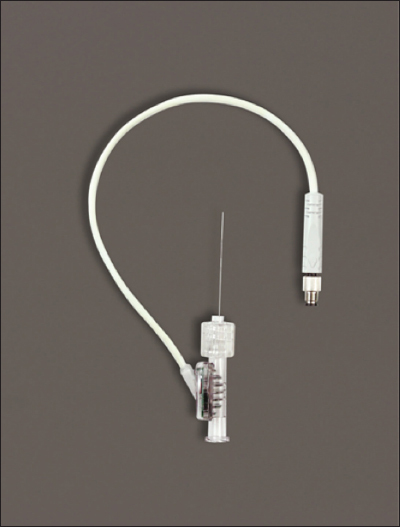
The IVBG sensor. The sensor wires are connected to the sensor hub, which is connected to a signal cable for electrical connection to the patient cable.
Fluidic Control and Reference Solution
The IVBG system is controlled by a flow control valve that operates by aspirating approximately 40 ml of blood into the IV catheter. The sensor contained in the catheter measures the aspirated blood sample, and the blood is returned to the patient, resulting in no blood loss. A reference solution (200 mg/dl glucose, 2 IU/ml heparin in normal saline) is then flushed through the IV catheter to calibrate the sensor and clear the IV catheter of residual blood. The sensor is calibrated before every sample measurement to ensure accuracy and to monitor the performance of the sensor and fluidics. Abrupt changes in sensitivity are flagged and can trigger fail-safes if they are outside expectations. The reference solution contains heparin for rinsing and minimizing the risk for thrombus formation. Within the 7.5 min cycle, the sensor is exposed to blood for only 2.5 min, allowing the sensor to spend most of its use life in a well-controlled and stable media. Less than 20 IU of heparin and 20 mg of dextrose are delivered to the subject per hour. This continuous cycle amounts to less than 10 ml of reference solution delivered to the patient per hour and causes no loss of blood from the patient.
Monitor
The monitor has a touch screen display through which the operator initiates IVBG system functions such as starting and stopping glucose measurement. The monitor displays glucose values, trends, and alarms. For this clinical evaluation, the BG data (values, trends, and alarms) were masked to the patient and physician.
Patient Cable
The patient cable is electrically connected to the sensor and contains a potentiostat for amperometric determination of glucose concentration. The potentiostat continuously measures the sensor glucose signal (concentration). The patient cable, flow control valve, and monitor are reusable and nonsterile components of the IVBG system.
Upon startup of the IVBG system, an automatic sensor initialization process is performed for approximately 1 h. After this sensor initialization is completed, the IVBG system requests a calibration that requires the user to draw a venous blood sample from the subject and measure the BG of the sample with a laboratory analyzer. The measured BG is then entered into the monitor using the touch screen. The IVBG system requires this blood calibration approximately every 24 h.
Methods
This clinical study was a nonrandomized, single-armed, blinded study to evaluate the safety and accuracy of the IVBG system. The study was performed at three investigational sites, all of which were located in the United States (AMCR Institute Inc., Escondido, CA; DGD Research Inc., San Antonio, TX; and Profil Institute for Clinical Research, Chula Vista, CA). The study was conducted in accordance with the Declaration of Helsinki and according to Good Clinical Practices. Prior to study enrollment, each subject or legally authorized representative documented his or her consent by signing an informed consent form. For the purposes of this clinical study, the BG measured by the IVBG system was masked (i.e., real-time glucose values, trend graphs, alerts, and alarms were not provided to the end user).
Patient Population
Fifty adult males and females with insulin-treated diabetes mellitus who were otherwise healthy were enrolled in June and July 2009 (10 subjects enrolled at AMCR Institute Inc., 14 subjects at DGD Research Inc., and 26 subjects at Profil Institute for Clinical Research, Inc.). Patients did not have skin conditions or existing (or planned) medical instrumentation and/or dressings that precluded wearing the sensor or dressings, a contraindication to placement of a dedicated peripheral IV line, or a history of heparin-induced thrombocytopenia and had not participated in another investigational study within the previous 30 days. The majority of subjects were male (72%) and Caucasian (88%; Table 1). Subjects had a mean ± standard deviation (SD) age of 41 ± 13.1 years (range 19–67 years) and a mean ± SD body mass index of 29.7 ± 8.0 kg/m2. Thirty-three subjects (66%) had type 1 diabetes, and 17 subjects (34%) had type 2 diabetes. Fourteen subjects (28%) used continuous subcutaneous insulin infusion for insulin therapy, and 36 subjects (72%) were on multiple daily injection therapy.
Table 1.
Baseline Characteristics of Study Subjects
| Characteristic | Percentage or mean (SD) |
| Number of subjects | 50 |
| Age (years) | 41 (13.1) |
| Female sex (%) | 28 |
| Height (cm) | 175.2 (9.0) |
| Weight (kg) | 90.6 (21.9) |
| Body mass index (kg/m2) | 29.7 (8.0) |
| Diabetes Type (%) | |
| Type 1 | 66 |
| Type 2 | 34 |
| Duration of diabetes (years) | 15.8 (8.3) |
| Insulin Therapy Type (%) | |
| Continuous subcutaneous insulin infusion | 28 |
| Multiple daily injections | 72 |
| Hemoglobin A1c (%) | 8.4 (1.8) |
| Hematocrit (%) | 43.0 (4.4) |
Comparative Glucose Measurements
During the 72 h in-clinic session, comparative samples were obtained from the Yellow Springs Instruments (YSI) glucose analyzer (YSI-2300 Stat Plus, Yellow Springs, OH). For YSI assessment, a maximum of four venous blood draws were taken per hour from a separate IV catheter inserted in the contralateral upper extremity (relative to placement of the IVBG sensor). Carbohydrate intake and insulin dosing were adjusted to allow for collection of YSI samples across the entire reportable range of the IVBG system (i.e., 40–400 mg/dl). Frequent comparative BG measurements (up to four times per hour) were collected immediately after the IVBG system was calibrated, during the final 4–8 h of sensor insertion, and following glucose excursions premeals/postmeals during the in-clinic session. Glucose values from the subjects’ personal BG analyzers were used to guide diabetes management decisions throughout the study.
End Points
The primary objective of this study was to show that the accuracy of the IVBG system met the accuracy criterion described in ISO 15197:2003, i.e., that at least 95% of IVBG–YSI matched pairs differed by ≤15 mg/dl (for YSI values <75 mg/dl) or by ≤20% (for YSI values ≥75 mg/dl), which will be referred to as the “15/20% criterion.”
The secondary accuracy metrics that were evaluated were (all refer to IVBG–YSI matched pairs)
IVBG system bias, as calculated with regression methods;
relative difference and absolute relative difference between the IVBG and YSI readings; and
traditional Clarke error grid (CEG) analysis.
Safety
Safety was assessed by adverse device effects (ADEs), serious adverse device events, and unanticipated ADEs occurring after device insertion. Sensor insertion site and adhesive area were examined for erythema, edema, and bruising.
Statistical Analyses
The primary efficacy population consisted of all subjects for whom at least one IVBG–YSI matched pair was collected. All subjects who had undergone IVBG sensor insertion were included in the safety analysis.
For the purpose of matching a YSI BG measurement with an IVBG BG measurement (IVBG–YSI matched pair), the pairing window was ±3 min and 45 s around the YSI time stamp. These IVBG–YSI matched pairs were considered the primary efficacy data set to be evaluated. The agreement and bias between IVBG BG measurements compared with YSI BG measurements on the IVBG–YSI matched pairs were evaluated in linear ordinary least squares (OLS) regressions.
Summary statistics for continuous variables included the mean, SD, median, and range. Categorical variables were presented as counts and percentages. Differences between the IVBG BG measurements and YSI BG measurements were calculated and analyzed using regression analysis and comparative methods defined in ISO 15197. Any formal hypothesis test was carried out at the 5% level of significance using a two-sided alternative hypothesis, and all confidence intervals provided were calculated with 95% confidence level, unless otherwise noted.
Results
Intravenous Blood Glucose System Accuracy
All 50 enrolled subjects contributed at least one IVBG–YSI matched pair and were included in the primary efficacy analysis. A total of 2815 IVBG–YSI matched pairs were included in the primary efficacy analysis data. A total of 75 sensors were inserted for a mean (±SD) of 65.7 (±13.8) h per subject. Twenty-five sensors were removed and replaced. The sensor replacements were due to suspected sensor or system errors (12 sensors) or IV occlusion/dislodgement (13 sensors). Forty-four sensors lasted until completion of the study, day 3 (49–72 h; 44/75 = 58.7%).
The prespecified primary efficacy end point (meeting the 15/20% criterion) was satisfied, indicating that the IVBG system is accurate. As shown in Table 2 , 95% of the individual IVBG values met the 15/20% criterion. The exact binomial 95% confidence interval for this proportion was 94%, 96%. The concentration for the subjects’ BG ranged from 40 to 400 mg/dl (46.7% of matched pairs were within 81 to 200 mg/dl). The rate of change for the subjects’ BG ranged from <-3 to >3 mg/dl/min (87.5% of matched pairs were within rate of change -1 to 1 mg/dl/min). The reliability of the IVBG system, in terms of the percentage of attempted BG measurements that resulted in a glucose measurement reported, was 79.0%.
Table 2.
Accuracy within Sensor Day: IVBG System Compared with YSI
| Sensor day | Matched pairs (n) | Matched pairs within 15/20% criterion (%) | 95% Confidence interval |
| All 3 days | 2815 | 94.9 | 94.0, 95.6 |
| Day 1 | 1071 | 92.8 | 91.1, 94.3 |
| Day 2 | 958 | 95.9 | 94.5, 97.1 |
| Day 3 | 783 | 96.3 | 94.7, 97.5 |
Regression Analysis
Regression analysis of IVBG versus YSI indicated a linear relationship between the IVBG system and the YSI. The OLS regression line with associated parameter estimate, the line of identity (y = x), and the acceptance boundary according to the 15/20% criterion line were superimposed on the regression plot in Figure 3. The regression statistics are summarized in Table 3.
Figure 3.
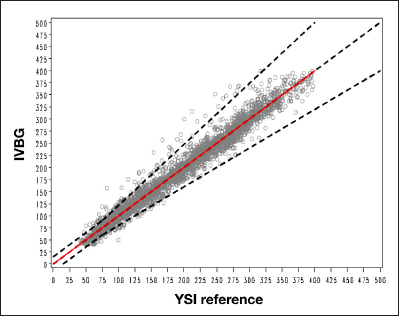
Ordinary least squares. The black dotted line indicates the 15/20% acceptance criteria. The red line represents the regression line. OLS root mean square error = 15.626814493; OLS slope = 0.9997129318; Intercept = 0.2067628554.
Table 3.
Ordinary Least Squares Regression Analysis Parameter Estimates between IVBG and YSI
| Matched pairs | Intercept | 95% Confidence interval intercept | Slope | 95% Confidence interval slope |
| 2815 | 0.21 | (-1.20, 1.61) | 1.00 | (0.99, 1.01) |
Intravenous Blood Glucose System versus YSI Relative Difference and Absolute Relative Difference
The IVBG system had a mean absolute relative difference of 6.6% or a mean absolute difference of 10.7 mg/dl in reference to laboratory standard YSI across the 40 to 400 mg/dl measurement range (Table 4).
Table 4.
Absolute Difference and Absolute Relative Diference within Glucose Concentration: IVBG System Compared with YSI
| YSI (mg/dl) | Matched pairs (n) | Absolute difference (mg/dl) | Absolute relative difference (%) | ||
| Median | Mean | Median | Mean | ||
| 40–400 | 2815 | 7.0 | 10.7 | 4.2 | 6.6 |
| 40–80 | 287 | 5.1 | 7.3 | 7.6 | 11.0 |
| 81–120 | 487 | 6.0 | 8.5 | 5.9 | 8.5 |
| 121–200 | 829 | 6.5 | 10.0 | 4.1 | 6.4 |
| 201–300 | 881 | 9.0 | 12.5 | 3.5 | 5.0 |
| 301–400 | 331 | 11.0 | 13.7 | 3.3 | 4.1 |
Traditional Clarke Error Grid Results
A total of 99.5% of the IVBG–YSI matched pairs were in zone A and B (Figure 4 and Table 5).
Figure 4.
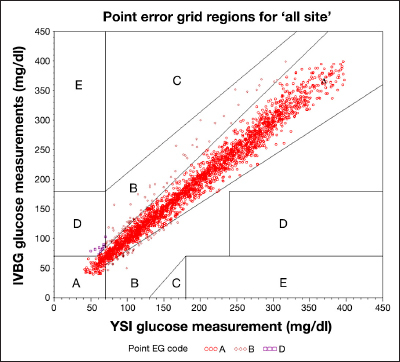
Traditional CEG. EG, error grid. IVBG system glucose measurements between 40 and 400 mg/dl, inclusive, are included.
Table 5.
Clarke Error Grid Results: IVBG System Compared with YSI
| CEG zone | Matched pairs within zone n (%) |
| All zones | 2815 (100%) |
| A | 2677 (95.1%) |
| B | 124 (4.4%) |
| C | 0 (0.0%) |
| D | 14 (0.5%) |
| E | 0 (0.0%) |
Safety
No serious adverse events were reported. All adverse events reported during the study were resolved by study completion. No moderate or severe irritation was recorded at the IV catheter insertion area for any device removals. Ten subjects experienced device-related erythema, and one of these subjects also experienced edema, which are consistent with routine clinical use of a peripheral IV catheter. Nausea, emesis, diarrhea, and headache were also reported during the study. However, these are more likely related to the large swings of glucose and time spent in hypoglycemia and hyperglycemia induced as part of the study design and not caused by the IVBG system.
Discussion
The IVBG system has the potential to make intensive, insulin-based therapy safer and more acceptable in patients. Frequent glucose monitoring and glycemic control may improve outcomes in hospitalized patients with and without diabetes.1–3 Trends from a continuous glucose monitor may assist in facilitating glycemic control thus preventing hypoglycemic or hyperglycemic conditions in patients.
The IVBG system satisfied the 15/20% evaluation criterion of ISO 15197, demonstrating that the device is accurate across the entire reportable range of the IVBG system (40 to 400 mg/dl). The IVBG system glucose measurements showed significant linear relationship with the laboratory YSI standard values. The IVBG system measurements were accurate over the 72 h period of the device use and were also consistent across a broad range of glucose rates of change. The CEG analysis provided a measure of the overall clinical utility of the IVBG system glucose readings. The IVBG system provided accurate readings in clinical utility CEG analysis. Since carbohydrate intake and insulin dosing were adjusted to allow for collection of YSI samples across the entire reportable range of the IVBG system (40–400 mg/dl), this study ensured that the IVBG system was able to display accuracy across the entire range and at rapid rates of change in glucose values. The sensor replacements were due to difficult blood sample acquisition through the peripheral IV catheter or suspected sensor/system errors. A total of 17% of the sensors failed because of issues with peripheral IV catheters. Standardizing the securement method of the IV line is anticipated to improve this failure rate. The additional failures were due to the mechanical and fluidic design of the sensor and are recognized as an area for optimization. To address the high blood sampling failure rate, the sensor and fluidics system have been redesigned on the second-generation IVBG system. This second-generation of the IVBG system has received Communauté Européenne mark for sale in Europe.
The IVBG system was well tolerated by the subjects. No serious ADEs were reported. The nonserious ADEs that were observed consisted of expected events associated with the use of any small, peripheral venous catheter.
A limitation in this study was the potential presence of YSI preanalytical errors (i.e., blood sample diluted from saline flushes or hemolysis). These YSI errors need to be prevented or detected and removed from the accuracy analyses, as they negatively impact the calculated accuracy of the statistical analysis.
The subject population for this study consisted of individuals with stable treatment of diabetes mellitus who were otherwise healthy. Other than standard insulin treatment, few medications were taken in the 7 days prior to the study. The IVBG system is intended for use in critically ill hospitalized patients, where the subject population is more likely to have many concomitant medications. The potential interference of medications could not be assessed during this study. This study did not include subjects who were acutely ill, so its findings must be confirmed to ensure that the characteristics frequently seen in critically ill patients (e.g., anemia) do not affect the accuracy of glucose results.
However, the IVBG system was subsequently tested in adult patients hospitalized for elective and nonemergent surgery and/or medical/surgical ICU care under a separate study protocol.10 The IVBG system demonstrated a similar accuracy in 100 ICU patients, with 93.3% of IVBG values meeting the 15/20% accuracy criterion. The slight reduction in accuracy can be attributed to the variable blood sources measured on the YSI.
Conclusions
The safety and accuracy characteristics of the IVBG system were demonstrated in 50 diabetes subjects. The data suggest that this first-generation of the IVBG system provides accurate BG values, which may assist clinicians in glycemic control of patients by providing frequent BG measurements with trend data. Based on the experience in this study, less overall staff effort in monitoring glucose levels is expected. Further trials in hospitalized, ill ICU patients are required to validate this finding.
Acknowledgments
The authors thank the volunteers who participated in this multicenter clinical trial, the research nurses who participated in data collection, and the investigators who assisted with patient recruitment, data collection, and/or analysis.
Glossary
- (ADE)
adverse device effect
- (BG)
blood glucose
- (CEG)
Clarke error grid
- (ICU)
intensive care unit
- (IV)
intravenous
- (IVBG)
intravenous blood glucose monitoring
- (OLS)
ordinary least squares
- (SD)
standard deviation
- (YSI)
Yellow Springs Instruments
Funding
This study was funded by Dexcom.
Disclosures
Angela M. Gulino and Michael Higgins are full-time employees of Edwards Lifesciences. Peter C. Simpson, Jacob Leach, and Apurv Kamath are employees of Dexcom.
References
- 1.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–982. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 2.Malmberg K. Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group. BMJ. 1997;314(7093):1512–1515. doi: 10.1136/bmj.314.7093.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, Floten HS, Starr A. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125(5):1007–1021. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 4.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 5.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 6.Aragon D. Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control. Am J Crit Care. 2006;15(4):370–377. [PubMed] [Google Scholar]
- 7.Malesker MA, Foral PA, McPhillips AC, Christensen KJ, Chang JA, Hilleman DE. An efficiency evaluation of protocols for tight glycemic control in intensive care units. Am J Crit Care. 2007;16(6):589–598. [PubMed] [Google Scholar]
- 8.Cembrowski GS, Tran DV, Slater-Maclean L, Chin D, Gibney RT, Jacka M. Could susceptibility to low hematocrit interference have compromised the results of the NICE-SUGAR trial? Clin Chem. 2010;56(7):1193–1195. doi: 10.1373/clinchem.2010.146217. [DOI] [PubMed] [Google Scholar]
- 9.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 10.Bochicchio G, Joseph J, Magee M, Gulino A, Higgins M.L, Peyser T, Simpson P, Leach J, Kamath A. Multicenter evaluation of a first generation automated blood glucose monitor in the OR/ICU. Crit Care Med. 2011;39(12):55. [Google Scholar]


