Abstract
Background
Partial pressure of oxygen (pO2) in blood samples can affect blood glucose (BG) measurements, particularly in systems that employ the glucose oxidase (GOx) enzyme reaction on test strips. In this study, we assessed the impact of different pO2 values on the performance of five GOx systems and one glucose dehydrogenase (GDH) system. Two of the GOx systems are labeled by the manufacturers to be sensitive to increased blood oxygen content, while the other three GOx systems are not.
Methods
Aliquots of 20 venous samples were adjusted to the following pO2 values: <45, ~70, and ≥150 mmHg. For each system, five consecutive measurements on each sample aliquot were performed using the same test strip lot. Relative differences between the mean BG results at pO2 ~70 mmHg, which is considered to be similar to pO2 in capillary blood samples, and the mean BG result at pO2 <45 and ≥150 mmHg were calculated.
Results
For all tested GOx systems, mean relative differences in the BG measurement results were between 6.1% and 22.6% at pO2 <45 mmHg and between -7.9% and -14.9% at pO2 ≥150 mmHg. For both pO2 levels, relative differences of all tested GOx systems were significant (p < .0001). The GDH system showed mean relative differences of -1.0% and -0.4% at pO2 values <45 and ≥150 mmHg, respectively, which were not significant.
Conclusions
These data suggest that capillary blood pO2 variations lead to clinically relevant BG measurement deviations in GOx systems, even in GOx systems that are not labeled as being oxygen sensitive.
Keywords: blood glucose monitoring systems, glucose dehydrogenase, glucose oxidase, partial pressure of oxygen, self-monitoring of blood glucose
Introduction
Today, many available systems for self-monitoring of blood glucose (SMBG) utilize a glucose oxidase (GOx) enzyme reaction on test strips (hereafter denoted GOx systems). It is widely known that GOx systems are prone to oxygen interference, as oxygen is the physiological electron acceptor of GOx.1–4 In order to minimize oxygen dependency, biosensors of these systems usually contain a nonphysiological electron acceptor (mediator).1,2,5 The characteristics of the mediator, e.g., stability, kinetics, and ability to compete with oxygen, are important aspects with regard to the extent of oxygen dependency.1,2
Other SMBG systems employ a glucose dehydrogenase (GDH) enzyme reaction on test strips (hereafter denoted GDH systems). Since oxygen is not involved in the electrochemical reaction catalyzed by GDH, these systems are oxygen insensitive.6
The influence of the blood sample’s partial pressure of oxygen (pO2) on blood glucose (BG) measurement results has received attention mainly in studies that are concerned with the clinical performance of portable glucose monitoring systems in critically ill patients with high and unpredictably varying blood pO2 values.7,8 In these studies, GOx systems underestimated BG values when the blood samples’ pO2 was >100 mmHg.
Information concerning the influence and clinical impact of decreased pO2 values on GOx systems’ measurement results is limited. Decreased pO2 levels can be expected, e.g., during long-distance flights or in patients with respiratory diseases.9–13 In addition, for many GOx systems, detailed information, i.e., the pO2 range in which the system operates well, is not provided in the manufacturers’ labeling.
In one study, we found that the impact of different pO2 levels on BG measurements considerably varies among GOx systems that were labeled to be sensitive to increased oxygen content of the blood sample.14 In this study, we investigated whether GOx systems, which, according to the manufacturer, are not oxygen sensitive, are affected by the pO2 value of the blood sample. For this purpose we assessed the influence of different pO2 levels on BG measurements with three such GOx systems in comparison with two GOx systems that are labeled as being sensitive to increased blood oxygen content and one GDH system.
Materials and Methods
The study was conducted in June 2013 in compliance with the German Medical Devices Act at the Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm, Ulm, Germany. The study protocol was approved by the responsible ethics committee. Informed consent forms were signed by all participants prior to the study procedures.
Subjects
In this study, 26 subjects were enrolled. Twenty subjects were included in the evaluation [10 female, 10 male; mean age 47 years (range 24 to 65 years); 17 subjects with type 1 diabetes, 2 subjects with type 2 diabetes, and 1 subject without diabetes]. For six subjects with initial pO2 values ≥45 mmHg, sample preparation, measurement procedures, and data analysis were not performed (as described later). The subjects’ anamnesis and medication were reviewed and compared with interfering substances indicated in the manufacturers’ labeling.
Blood Glucose Monitoring Systems
In this study, five GOx systems (systems 1 to 5) and one GDH system (system 6) from six different manufacturers were evaluated. All systems were purchased from pharmacies.
In the test strip package insert of GOx system 1, it is labeled that in patients receiving oxygen therapy, falsely low BG values can be measured. For GOx system 2, it is stated that high oxygen concentrations in the blood sample can lead to lower measurement results. Glucose oxidase systems 1 and 2 were further also referred to as “systems with labeled oxygen sensitivity,” although only the influence of increased pO2 is mentioned in their package inserts. For GOx systems 3, 4, and 5, potential influences of pO2 on measurement results are not indicated in their respective user manual or in the package insert of test strips. Glucose oxidase systems 1 and 2 as well as the GDH system had also been evaluated in a previous study focusing on pO2 influences on BG measurements.14
All systems were stored, used, and maintained in compliance with the manufacturers’ instructions. Control measurements were performed daily prior to the experimental procedure and for each test strip vial.
Sample Preparation and Measurement Procedure
Sample preparation and test procedures were performed by trained personnel in a laboratory setting with controlled room temperature (20–24 °C) and humidity (39–54%).
Although capillary blood is the intended sample for BG measurements with all five tested GOx systems, in this study, venous blood samples were used for the assessment of potential influences of different pO2 values on BG measurement results. The study procedure required a larger blood volume, and in addition, for the adjustments of different pO2 values, blood samples with initial pO2 values <45 mmHg were required (venous blood pO2 values are usually around 40 mmHg).15
Three aliquots of each blood sample were adjusted to the following three pO2 levels: ~70 mmHg, which is considered to be similar to pO2 values in capillary blood samples;16,17 <45 mmHg, also referred to as “low”; and ≥150 mmHg, also referred to as “high.” For this purpose, a venous blood sample from each of the 26 subjects was collected in a lithium heparin tube. Immediately after sample collection, the initial pO2 value was determined by using a blood gas analyzer (OPTI™ CCA-TS Analysator, OPTI Medical System Inc., Roswell, GA). The blood gas analyzer was maintained, handled, and controlled according to the manufacturer’s labeling. Additionally, regular internal and external quality control measurements were performed, as required by the German national guidelines.18
Since the adjustment of samples designated for pO2 values <45 mmHg was not intended, only samples with initial pO2 values <45 mmHg (n = 20) were included and evaluated. For six subjects with initial pO2 values between 46 and 66 mmHg, sample preparation and measurement procedures were not performed.
After determination of the initial pO2, the hematocrit value of the blood sample was determined in duplicate by using heparinized capillaries in order to ensure a hematocrit value within the range specified by the manufacturers. For this purpose, the capillaries were centrifuged and the hematocrit values were determined using an alignment chart. Hematocrit values of the 20 test samples were between 35.0% and 47.5%.
For the preparation of test samples with three different pO2 levels (as described earlier), three aliquots of the venous blood sample were collected in one syringe each (~2.5 ml). Since the initial pO2 values of the 20 venous blood samples (as described earlier) ranged between 21 and 41 mmHg, pO2 adjustment was not performed for the aliquot designated for pO2 values <45 mmHg. The syringe with this test sample was immediately deaerated, sealed airtight, and placed on a rotating mixer for sample incubation until the measurement procedure with the SMBG systems was performed. To achieve blood samples with pO2 values ~70 and ≥150 mmHg, a volume of up to ~3 ml of air was added to the aliquots in the syringe before being sealed airtight and incubated on a rotating mixer. During incubation, the pO2 values of these samples were checked repeatedly, and the syringe was deaerated as soon as the desired pO2 value was reached in order to prevent any further pO2 increase.
Glucose measurements in these blood samples were performed with all SMBG systems after the blood had reached the designated pO2 value. For each of the six systems, five consecutive measurements on a given blood sample were performed using the same test strip lot. In order to maintain pO2 values and BG levels as constant as possible, blood samples were measured as quickly as possible by using five different meters per system. Before and after the measurements with the SMBG systems, samples for measurements with a standard laboratory glucose analyzer were collected and centrifuged. The plasma was then separated, and glucose was measured by using a hexokinase method (cobas c111, Roche Instrument Center, Rotkreuz, Switzerland); glucose measurement results ranged between 82 and 179 mg/dl. During the adjustment of the samples’ pO2 values, small changes in the glucose concentrations were possible, resulting in slight differences between samples with <45, ~70, and ≥150 mmHg generated from one initial sample. The laboratory method was used primarily for compensation of these differences. It was not intended to compare the measurement results of SMBG systems with the laboratory device (see equation).
The pO2 of the blood samples was also determined immediately before and after the measurements, with the SMBG systems showing a maximum pO2 change during the measurement procedure of ~12%. For the 20 venous blood samples, the following mean pO2 values were achieved:
| pO2 level ~70 mmHg: | 71 mmHg, | ranging from 68 to 77 mmHg; |
| pO2 level <45 mmHg: | 30 mmHg, | ranging from 21 to 41 mmHg; |
| pO2 level ≥150 mmHg: | 164 mmHg, | ranging from 152 to 180 mmHg. |
Data Analysis
Data management and evaluation were performed at the study site.
For each of the 20 samples, normalized relative differences between the mean BG value (five consecutive measurements per sample) of a given SMBG system at pO2 values <45 and ≥150 mmHg and the mean BG value of that system at a pO2 value ~70 mmHg were calculated, taking the differences in laboratory analyzer measurement results into account (see equation).
where Sn is the mean BG value for a specific SMBG system at pO2 n (n = “low,” “~70 mmHg ,” or ”high”), Ln is the mean laboratory method result at pO2 n (n = “low,” “~70 mmHg,” or “high”), L70 is the mean laboratory method result at pO2 ~70 mmHg, S70 is the mean BG value for a specific SMBG system at pO2 ~70 mmHg, and dnorm is the normalized relative difference.
By applying this equation, the normalized relative difference at pO2 value ~70 mmHg was set to zero. This was done because all tested GOx systems are intended for use with capillary blood samples, and pO2 values ~70 mmHg are considered to be similar to pO2 values in fresh capillary blood samples.16,17
For each SMBG system and each pO2 level, the normalized relative difference of each of the 20 samples (five measurements per sample) was calculated (Figures 1 and 2). The mean value over all 20 normalized relative differences was also calculated (Table 1).
Figure 1.
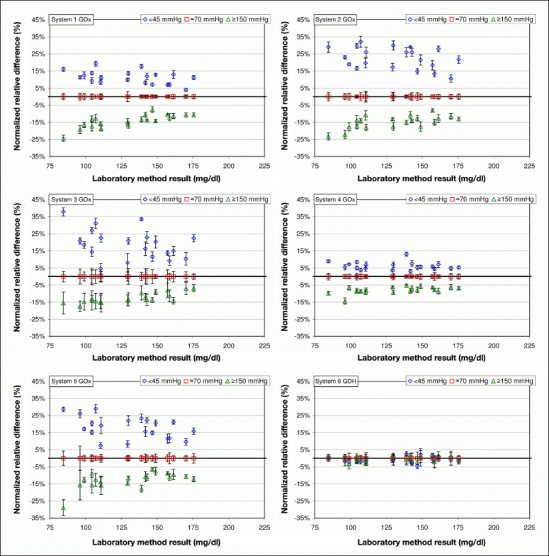
Normalized relative differences at pO2 values <45 and ≥150 mmHg are displayed for the respective BG concentration (20 samples each, 5 measurements per sample). Normalized relative differences at pO2 value ~70 mmHg were set to zero (see Data Analysis).
Figure 2.
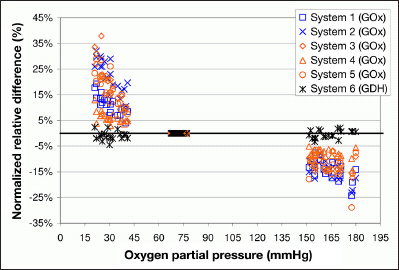
Normalized relative differences of BG measurements are plotted for pO2 values <45 and ≥150 mmHg, respectively (20 samples each, 5 measurements per sample). Normalized relative difference at pO2 value ~70 mmHg were set to zero (see Data Analysis). For systems 1 and 2, sensitivity to increased blood pO2 was labeled in the package inserts. For systems 3, 4, and 5, potential influences of pO2 on measurement results were not indicated in their user manual/package inserts.
Table 1.
Mean Normalized Relative differences of GOx Systems 1–5 and GDH System 6 at pO2 Values <45 and ≥150 mmHg (20 Samples Each, 5 Measurements per Sample)a
| Oxygen sensitivity | GOx systems | GDH system | ||||
| Labeledb | Not labeledc | |||||
| System 1 | System 2 | System 3 | System 4 | System 5 | System 6 | |
| Relative differences pO2 <45 mmHg | ||||||
| Mean | 11.2% | 22.6% | 19.1% | 6.1% | 18.0% | -1.0% |
| Standard deviation | 3.8% | 6.2% | 8.7% | 2.3% | 6.4% | 1.7% |
| Minimum | 4.0% | 10.6% | 4.7% | 3.0% | 7.4% | -0.2% |
| Maximum | 19.2% | 32.1% | 37.9% | 13.0% | 29.0% | -4.5% |
| padjusted | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.1505 |
| Relative differences pO2 ≥150 mmHg | ||||||
| Mean | -14.2% | -14.9% | -12.6% | -7.9% | -12.8% | -0.4% |
| Standard deviation | 3.8% | 3.8% | 3.0% | 2.0% | 4.8% | 1.6% |
| Minimum | -7.7% | -8.0% | -6.8% | -5.1% | -6.5% | 0.6% |
| Maximum | -24.3% | -23.2% | -17.3% | -14.4% | -28.9% | -3.4% |
| padjusted | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 1.0 |
The p values adjusted according to Bonferroni show whether normalized relative mean differences were significantly different from zero.
Sensitivity to increased blood pO2 was labeled in the package inserts.
Potential influences of pO2 on measurement results were not labeled in the user manual/package inserts.
Sample size was calculated based on data of a previous study, a level of significance of α = 0.05/12 and 90% power, corresponding to a Bonferroni adjustment for 12 statistical tests. The estimated sample size was below 20; however, 20 subjects were scheduled in order to obtain more stable estimates for effect sizes. To assess whether normalized relative differences were significantly different from zero, two-tailed one-sample t-tests were used. The resulting p-values were adjusted according to Bonferroni (padjusted = min (12 pnonadjusted, 1)).
Results
With pO2 values <45 mmHg, the mean normalized relative differences among the GOx systems that are indicated to be oxygen sensitive were 11.2% (system 1) and 22.6% (system 2; Table 1). The GOx systems not indicated to be oxygen sensitive (systems 3, 4, and 5) showed mean normalized relative differences of 19.1%, 6.1%, and 18.0%, respectively. With pO2 values ≥150 mmHg, the differences with systems 1 and 2 were -14.2% and -14.9%, respectively, while systems 3, 4, and 5 showed differences of -12.6%, -7.9%, and -12.8%, respectively. For all tested GOx systems, the relative differences at pO2 values <45 and ≥150 mmHg (Figures 1 and 2) were different from zero (padjusted < .0001).
The GDH system showed mean relative differences of -1.0% at pO2 values <45 mmHg and -0.4% at pO2 values ≥150 mmHg. Relative differences at pO2 values <45 and ≥150 mmHg (Figures 1 and 2) were not different from zero (pO2 values <45 mmHg, padjusted = .1505 and pnonadjusted = .0125; ≥150 mmHg, padjusted = 1 and pnonadjusted = . 2483).
Discussion
In the present study, we investigated the influence of different pO2 levels on BG measurements with three GOx systems that are not labeled by the manufacturer as being oxygen sensitive, two GOx systems that are labeled to be sensitive to increased blood oxygen content, and one GDH system. The influence of different pO2 levels on measurements was assessed for each system individually, and thus the study’s results are not suitable for a direct comparison of the measurement performance between the evaluated systems. The study was performed in a controlled laboratory setting, in which other interfering factors were reduced to a minimum.
Our results show that BG measurements with all tested GOx systems are affected by pO2 values <45 and ≥150 mmHg in the blood sample.
These results are in agreement with a previous study14 in which the two GOx systems labeled to be oxygen sensitive and the GDH system had also been evaluated: GOx systems that were labeled to be sensitive to increased pO2 levels showed underestimated results at pO2 values ≥150 mmHg and overestimated results at pO2 values ≤45 mmHg. Measurement results of the oxygen-insensitive GDH system were not affected by the pO2 value of the sample.
In the current study, both GOx systems labeled as being sensitive to oxygen showed differences >±10% when measurement results at “high” and “low” pO2 were compared with measurement results at pO2 ~70 mmHg. Interestingly, similar results were found for two of the three tested GOx systems that are not labeled by the manufacturer to be oxygen sensitive. Only one of these systems showed differences <±10% when measurement results at “high” and “low” pO2 were compared with measurement results at pO2 ~70 mmHg. In general, all tested GOx systems without labeled oxygen sensitivity also showed overestimated measurement results at pO2 values <45 mmHg and underestimated measurement results at pO2 values ≥150 mmHg.
In the standard of the International Organization for Standardization (ISO), EN ISO 15197:2003,19 it is stipulated that any interfering substances, sample conditions, or physiological conditions known to affect the accuracy of results should be included in the instructions for use. In the revision of this standard ISO 15197:2013,20 it is stipulated that interference effects shall be described in the instructions for use if the difference between the test sample and the control sample exceeds 10% (10 mg/dl) for BG values ≥100 mg/dl (<100 mg/dl). Mandatory compliance to this standard is recommended after a transition period of 36 months.20 It is also stipulated in this revised standard that the conditions required to obtain accurate measurement results shall be specified if the system is affected by environmental factors, such as oxygen. Although oxygen is not clearly specified in the list of possible interfering factors in ISO 15197:2013, results of our study show that oxygen interferences should be considered when the performance of GOx systems is assessed.
In this context, it should also be considered that for system accuracy evaluation according to the ISO 15197 standard, blood samples in the highest and lowest glucose concentration categories are allowed to be adjusted. As such preparation procedures might potentially lead to altered pO2 values of blood samples, systematic measurement bias on oxygen-dependent SMBG systems may occur.
The five tested GOx systems are labeled for use with capillary blood only. In this study, venous blood was adjusted to different pO2 levels in order to assess the difference between measurement results from samples with pO2 levels of <45 and ≥150 mmHg and measurement results from samples with pO2 values ~70 mmHg for each system. Venous blood was used for this evaluation since the study procedure required a sample with larger blood volume, and secondly, no adjustments of pO2 values <45 mmHg was required (venous blood pO2 values are usually around 40 mmHg15). In addition, venous blood is also recommended in the ISO 15197 standard, e.g., for interference testing20 and precision evaluation.19,20
In our study, pO2 values ~70 mmHg are considered to be similar to pO2 values in capillary blood samples.16,17 In order to investigate pO2 effects on measurement results, considerably decreased/increased pO2 ranges (<45/≥150 mmHg) were generated, which might be relevant in particular clinical situations. To support the clinical relevance of our finding, further investigations focusing on pO2 variations in capillary blood of people with diabetes and their impact on BG measurement would be helpful.
We only focused on pO2 effects in blood samples with BG concentrations (82–179 mg/dl) and hematocrit values (35.0–47.5%) in a limited range. Therefore, when interpreting the results, it is important to take into account that varying BG or hematocrit levels might also have an impact on the extent of pO2 effects.
Nevertheless, the results show that different blood-oxygen contents can affect BG measurements with GOx systems. Currently, some GOx systems are indicated to be sensitive to increased blood oxygen content as it occurs, for example, in patients receiving oxygen therapy. However, for most of the available GOx systems, detailed information concerning the pO2 limits of the system is not provided, especially with regard to potential interferences in conditions with decreased oxygen content in the blood sample. Since decreased pO2 levels are expected to occur in the daily life conditions of many people with diabetes, the considerable overestimation of BG measurements at “low” pO2 values (≥18% for three of the tested GOx systems) observed here might be of clinical relevance.
Arterial pO2 values of approximately 90 mmHg can be found in healthy young adults at sea level.21 At higher altitudes or during long-distance flights, studies reported decreased arterial pO2 values of approximately 55–65 mmHg in healthy adults.9,10,22 In addition, in patients with respiratory diseases, arterial pO2 values <70 mmHg can be found.11,13 Considering the fact that the frequent respiratory disease chronic obstructive pulmonary disease is associated with increased risk of developing type 2 diabetes,23,24 decreased pO2 values may occur in a considerable number of people with type 2 diabetes.
Regarding physiological blood circulation, arteries carry oxygenated blood from the heart to the small arterioles and into the capillary network in which oxygen, nutrients, and metabolites are exchanged between blood and tissue cells.16 Since there is a physiological pO2 gradient from arterial to capillary blood, in conditions with decreased arterial pO2 values, decreased capillary pO2 values can be expected too. Results of our study show that in such conditions, oxygen-sensitive systems that are intended for capillary pO2 values under normal conditions may overestimate BG measurements with the potential risk of not being able to detect hypoglycemic events adequately and to take appropriate therapeutic actions.
Nevertheless, it is important to be aware that not only the blood samples’ pO2, but also other factors can affect measurement results not only in GOx, but also in GDH systems. Therefore, for all SMBG systems, a detailed description of all interfering factors is important to enable the choice of an adequate system for specific conditions or a specific group of patients.
Conclusion
Results of this study indicate that BG measurements with GOx systems might be affected by the blood sample’s pO2 value. Only one of the three GOx systems that are not labeled to be oxygen sensitive showed measurement deviations <±10% when pO2 levels were altered. In general, increased pO2 values lead to underestimated measurement results, and decreased pO2 values lead to considerably overestimated measurement results. In conditions with decreased pO2 levels, e.g., during long-distance flights, staying at high altitude, or in patients with respiratory disease, measurements with oxygen-sensitive systems might bear the risk that hypoglycemic events are not detected in time. In order to ensure an adequate use of GOx systems in the daily life conditions of people with diabetes, it would be desirable that patients are sufficiently informed about potential oxygen interferences in oxygen-sensitive systems, also taking into account conditions with considerably decreased blood pO2.
Acknowledgments
We thank Lutz Heinemann, Volker Lodwig, Peter Müller, Joachim Hönes, and Andrew Hattemer for their valuable input.
Glossary
- (BG)
blood glucose
- (GDH)
glucose dehydrogenase
- (GOx)
glucose oxidase
- (ISO)
International Organizations for Standardization
- (pO2)
partial pressure of oxygen
- (SMBG)
self-monitoring of blood glucose
Funding
This study was funded by a grant from Roche Diagnostics GmbH, Mannheim, Germany.
Disclosures
All authors are employees of the Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm (IDT), Ulm, Germany. Guido Freckmann is general manager of the IDT, which carries out studies evaluating BG meters and medical devices for diabetes therapy on behalf of various companies. Guido Freckmann/IDT have received speakers’ honoraria or consulting fees from Abbott, Bayer, Menarini Diagnostics, Roche Diagnostics, Sanofi, and Ypsomed.
References
- 1.Yoo EH, Lee SY. Glucose biosensors: an overview of use in clinical practice. Sensors (Basel) 2010;10(5):4558–4576. doi: 10.3390/s100504558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaubey A, Malhotra BD. Mediated biosensors. Biosens Bioelectron. 2002;17(6-7):441–456. doi: 10.1016/s0956-5663(01)00313-x. [DOI] [PubMed] [Google Scholar]
- 3.Hönes J, Müller P, Surridge N. The technology behind glucose meters: test strips. Diabetes Technol Ther. 2008;10(Suppl 1):S10–S26. [Google Scholar]
- 4.Chun TY, Hirose M, Sawa T, Harada M, Hosokawa T, Tanaka Y, Miyazaki M. The effect of the partial pressure of oxygen on blood glucose concentration examined using glucose oxidase with ferricyan ion. Anesth Analg. 1994;79(5):993–997. doi: 10.1213/00000539-199411000-00030. [DOI] [PubMed] [Google Scholar]
- 5.Wang J. Electrochemical glucose biosensors. Chem Rev. 2008;108(2):814–825. doi: 10.1021/cr068123a. [DOI] [PubMed] [Google Scholar]
- 6.Ferri S, Kojima K, Sode K. Review of glucose oxidases and glucose dehydrogenases: a bird’s eye view of glucose sensing enzymes. J Diabetes Sci Technol. 2011;5(5):1068–1076. doi: 10.1177/193229681100500507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang Z, Louie RF, Payes M, Chang KC, Kost GJ. Oxygen effects on glucose measurements with a reference analyzer and three handheld meters. Diabetes Technol Ther. 2000;2(3):349–362. doi: 10.1089/15209150050194215. [DOI] [PubMed] [Google Scholar]
- 8.Tang Z, Louie RF, Lee JH, Lee DM, Miller EE, Kost GJ. Oxygen effects on glucose meter measurements with glucose dehydrogenase- and oxidase-based test strips for point-of-care testing. Crit Care Med. 2001;29(5):1062–1070. doi: 10.1097/00003246-200105000-00038. [DOI] [PubMed] [Google Scholar]
- 9.Coker RK, Partridge MR. What happens to patients with respiratory disease when they fly? Thorax. 2004;59(11):919–920. doi: 10.1136/thx.2004.025940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García Río F, Borderías Clau L, Casanova Macario C, Celli BR, Escarrabill Sanglás J, González Mangado N, Roca Torrent J, Uresandi Romero F. SEPAR. [Air travel and respiratory diseases] Arch Bronconeumol. 2007;43(2):101–125. doi: 10.1016/S1579-2129(07)60031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Connor TM, Barry PJ, Jahangir A, Finn C, Buckley BM, El-Gammal A. Comparison of arterial and venous blood gases and the effects of analysis delay and air contamination on arterial samples in patients with chronic obstructive pulmonary disease and healthy controls. Respiration. 2011;81(1):18–25. doi: 10.1159/000281879. [DOI] [PubMed] [Google Scholar]
- 12.Mays EE. An arterial blood gas diagram for clinical use. Chest. 1973;63(5):793–800. doi: 10.1378/chest.63.5.793. [DOI] [PubMed] [Google Scholar]
- 13.Ak A, Ogun CO, Bayir A, Kayis SA, Koylu R. Prediction of arterial blood gas values from venous blood gas values in patients with acute exacerbation of chronic obstructive pulmonary disease. Tohoku J Exp Med. 2006;210(4):285–290. doi: 10.1620/tjem.210.285. [DOI] [PubMed] [Google Scholar]
- 14.Schmid C, Baumstark A, Pleus S, Haug C, Tesar M, Freckmann G. Impact of partial pressure of oxygen in blood samples on the performance of systems for self-monitoring of blood glucose. Diabetes Technol Ther. doi: 10.1089/dia.2013.0184. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehmke H. Stuttgart: Georg Thieme Verlag; 2012. Atmung. In: Duale Reihe Physiologie; pp. 224–265. [Google Scholar]
- 16.Higgins C. Capillary blood gases: to arterialize or not. MLO Med Lab Obs. 2008;40(11):42, 44–47. [PubMed] [Google Scholar]
- 17.Freckmann G, Schmid C, Baumstark A, Pleus S, Link M, Haug C. Partial pressure of oxygen in capillary blood samples from the fingertip. J Diabetes Sci Technol. 2013;7(6):1648–9. doi: 10.1177/193229681300700627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bundesärztekammer Richtlinie der Bundesärztekammer zur Qualitätssicherung laboratoriumsmedizinischer Untersuchungen. Deutsches Ärzteblatt. 2008;105(7):341–355. [Google Scholar]
- 19.International Organization for Standardization. Geneva: International Organization for Standardization; 2003. ISO 15197:2003: In vitro diagnostic test systems -- requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. [Google Scholar]
- 20.International Organization for Standardization. Geneva: International Organization for Standardization; 2013. ISO 15197:2013: In vitro diagnostic test systems -- requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. [Google Scholar]
- 21.Scheid P. Stuttgart: Georg Thieme Verlag; 2005. Atmung. In: Physiologie; pp. 255–305. [Google Scholar]
- 22.Fischer R. High altitude and pulmonary diseases. Deutsche Zeitschrift Sportmedizin. 2000;51(12):412–417. [Google Scholar]
- 23.Rana JS, Mittleman MA, Sheikh J, Hu FB, Manson JE, Colditz GA, Speizer FE, Barr RG, Camargo CA., Jr Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women. Diabetes Care. 2004;27(10):2478–2484. doi: 10.2337/diacare.27.10.2478. [DOI] [PubMed] [Google Scholar]
- 24.Mirrakhimov AE. Chronic obstructive pulmonary disease and glucose metabolism: a bitter sweet symphony. Cardiovasc Diabetol. 2012;11:132. doi: 10.1186/1475-2840-11-132. [DOI] [PMC free article] [PubMed] [Google Scholar]


