Abstract
Background
Accuracy of blood glucose readings is (among other things) dependent on the test strip being completely filled with sufficient sample volume. The devices are supposed to display an error message in case of incomplete filling. This laboratory study was performed to test the performance of 31 commercially available devices in case of incomplete strip filling.
Methods
Samples with two different glucose levels (60–90 and 300–350 mg/dl) were used to generate three different sample volumes: 0.20 µl (too low volume for any device), 0.32 µl (borderline volume), and 1.20 µl (low but supposedly sufficient volume for all devices). After a point-of-care capillary reference measurement (StatStrip, NovaBiomedical), the meter strip was filled (6x) with the respective volume, and the response of the meters (two devices) was documented (72 determinations/meter type). Correct response was defined as either an error message indicating incomplete filling or a correct reading (±20% compared with reference reading).
Results
Only five meters showed 100% correct responses [BGStar and iBGStar (both Sanofi), ACCU-CHEK Compact+ and ACCU-CHEK Mobile (both Roche Diagnostics), OneTouch Verio (LifeScan)]. The majority of the meters (17) had up to 10% incorrect reactions [predominantly incorrect readings with sufficient volume; Precision Xceed and Xtra, FreeStyle Lite, and Freedom Lite (all Abbott); GlucoCard+ and GlucoMen GM (both Menarini); Contour, Contour USB, and Breeze2 (all Bayer); OneTouch Ultra Easy, Ultra 2, and Ultra Smart (all LifeScan); Wellion Dialog and Premium (both MedTrust); FineTouch (Terumo); ACCU-CHEK Aviva (Roche); and GlucoTalk (Axis-Shield)]. Ten percent to 20% incorrect reactions were seen with OneTouch Vita (LifeScan), ACCU-CHEK Aviva Nano (Roche), OmniTest+ (BBraun), and AlphaChek+ (Berger Med). More than 20% incorrect reactions were obtained with Pura (Ypsomed), GlucoCard Meter and GlucoMen LX (both Menarini), Elite (Bayer), and MediTouch (Medisana).
Conclusions
In summary, partial and incomplete blood filling of glucose meter strips is often associated with inaccurate reading. These findings underline the importance of appropriate patient education on this aspect of blood glucose self-monitoring.
Keywords: accuracy, blood glucose meter; low-volume alarm; sample volume; strip filling
Introduction
Frequent measurements of blood glucose are common during daily routine in patients with diabetes mellitus, especially in those who are treating themselves with multiple daily insulin injection therapy.1,2 Treatment decisions, such as calculation of insulin doses, are based on the readings of the blood glucose meters (BGMs), thus the devices need to be reliable, accurate, and easy to operate. However, BGM accuracy can be affected by many confounding factors, including, but not limited to, the underlying measurement technology, environmental factors, patient proficiency factors, and interfering substances.3 Advances in blood glucose reading technologies by several manufacturers have improved the robustness of the readings against interfering substances (e.g., hematocrit) in some meters.4
One technical solution to correct for a possible biochemical interference includes parallel measurement, e.g., of hematocrit with a subsequent correction algorithm.5,6 Another more general solution is the application of a mathematical algorithm, which is derived from dynamic electrochemistry as employed by the BGStar and iBGStar (Sanofi, Frankfurt, Germany). Dynamic electrochemistry uses multiple measurements at different conditions (e.g., by varying frequencies and voltage) to readjust the input stimulation signal in response to how the electron transfer kinetics at the electrode and the related chemistry is progressing. This dynamic adjustment results in a richer output signal that the meter’s algorithms can analyze to develop correction factors to minimize the distortion caused by interfering factors.7,8 We have been able to demonstrate that this technology corrects for hematocrit interference4 and results in very accurate and stable performance data for the BGStar and iBGStar when tested with patients in a clinical setting.9 The technology, however, could potentially also correct for environmental conditions and other sources of error, including low blood sample volume.7,8
A critical factor in BGM performance is the “patient factor.” The user also ultimately influences the blood glucose results by either complying or not complying with the instructions for performing the measurement. Even the best technology will result in wrong readings, e.g., if the strips are not stored properly or if the patient forgets to clean the finger before drawing the sample with a finger prick.3 An additional potential source for problems is provided by the blood-drawing mechanism itself. If the patient punctures the finger insufficiently, the resulting amount of blood sample may be insufficient to completely fill the analysis chamber on the strip and to initiate correct device operation.
In many meters, the strip filling situation is assessed during the procedure, and the meter is supposed to display an error message indicating insufficient sample volume and the need to either add more volume or to repeat the entire process. The requisite amount of blood to provide a correct reading has constantly been decreased and has reached volumes below 1 µl in many devices. According to our knowledge, the impact of insufficient strip filling on device performance has never been evaluated in a comparative study, and no information about a thoroughly performed study on this topic can be found in the international literature.
We therefore investigated the device performance of 31 commercially available blood glucose monitoring systems when operated with three different sample volumes: 0.20 µl (below any required volume), 0.32 µl (borderline volume, sufficient for some technologies), and 1.20 µl (low but supposedly sufficient volume for all technologies).
Materials and Methods
This laboratory investigation was performed in accordance with local ethical and regulatory requirements. The patients donating blood for this laboratory study gave written informed consent prior to the blood draw. The goal was to have one sample with a low blood glucose level (between 60 and 90 mg/dl) and one sample with a high blood glucose concentration (300–550 mg/dl), as confirmed by a reference method (Yellow Springs Instrument 2300 STAT PLUS glucose analyzer). Prior to testing, the hematocrit and the oxygen pressure in the venous heparinized whole blood samples were verified by means of the ABL 80 Flex CO/OX point-of-care device (Drott, Wiener Neudorf, Austria). If the oxygen saturation or the hematocrit value of an individual sample was outside of the physiological range (80–100% and 35–50%, respectively), the sample was discarded and the sample preparation was repeated. After release of a sample for testing, the samples were aliquoted, and up to 11 devices were tested in parallel by a similar number of trained investigators to eliminate any potential bias induced by test sequence. The type and nature of responses of the meters to the respective volumes (e.g., alarms and displayed blood glucose result) were recorded.
Sample Volume Preparation
Three sample volumes were generated for this investigation: 0.20 µl (below any required filling volume), 0.32 µl (borderline volume, too low or just acceptable for many devices), and 1.20 µl (low but supposedly sufficient volume for all systems). As samples volumes below 1 µl are very difficult to pipette, we produced the three different volumes as follows. In a previous pilot study, we had confirmed that our internal capillary reference method (StatStrip, NovaBiomedical) requires exactly 1.8 µl for strip filling. In this experiment, we pipetted 2, 2.12, and 3 µl by means of high-precision polymerase chain reaction pipettes on a laboratory film surface, which was positioned in a pharmaceutical high-precision balance (Mettler Toledo, precision 1 × 10–8 g). Performance of a capillary StatStrip measurement removed 1.8 µl from the film, and the respective volume was weighed out for the experiment. In the pilot setting, we showed that all of the remaining volume was sucked into a test strip from the hydrophobic surface of the laboratory film by means of the weight balance reading result (mean remaining weight 0.03 ± 0.02 µg). Each of the six sample categories (three volumes, two blood glucose concentrations) was tested six times with two devices and one strip lot per meter, resulting in a total of 72 measurements per meter type.
The following meters were included in this study: ACCU-CHEK Aviva (sample volume as stated in the instructions for use, 0.6 µl), ACCU-CHEK Aviva Nano (0.6 µl), ACCU-CHEK Compact Plus (1.5µl), and ACCU-CHEK Mobile (0.3 µl; Roche Diagnostics, Mannheim Germany); AlphaCheck+ (0.5 µl; Berger Med, Stadt, Germany); BGStar (0.5 µl) and iBGStar (0.5 µl; Sanofi); Breeze 2 (1.0 µl), Contour (0.6 µl), Contour USB (0.6 µl), and Elite (2.0 µl; Bayer, Wuppertal, Germany); FreeStyle Freedom (0.3 µl), FreeStyle Freedom Lite (0.3 µl), Precision Xceed (1.0 µl), and Precision Xtra (0.6 µl; Abbott Medisense, Wiesbaden, Germany); GlucoMen GM (0.5 µl), GlucoMen LX (0.3 µl), GlucoCard G Meter (0.6 µl), and GlucoCard G+ (0.6 µl; Menarini, Neuss, Germany); OneTouch Ultra 2 (1.0 µl), OneTouch Ultra Easy (1.0 µl), OneTouch Ultra Smart (1.0 µl), OneTouch Ultra Vita (1.0 µl), and OneTouch Verio (0.4 µl; LifeScan, Neckargemünd, Germany); Wellion Calla Dialog (0.6 µl) and Wellion Calla Premium (0.6 µl; MedTrust, Dresden, Germany); FineTouch (1.2 µl; Terumo, Eschborn, Germany); Pura (1.0 µl; Ypsomed, Liederbach, Germany); OmniTest Plus (1.0 µl; BBraun, Melsungen, Germany); MediTouch (0.5.µl; Medisana, Neuss, Germany); and GlucoTalk (0.5 µl; Axis-Shield, Stadt, Germany).
Statistical Analysis
The analysis was performed in a descriptive manner. A correct response to insufficient sample volume was recorded when the device gave a low-volume alarm or displayed an indication of insufficient blood volume. If the device performed a reading without alarm, the displayed value had to be within a ±20% range of the reference value determined immediately before the testing to count as a correct meter performance. The ±20% range was defined by the ISO 13485:2003 guideline, because no sample reference value below 75 mg/dl was measured. No alarm or an incorrect reading outside of the ±20% range without low-volume indication was recorded as device failure. The nature of the response of a given meter was collected and tabulated and the number and percentage of correct responses per volume and blood glucose concentration was calculated. The number of total wrong responses in the different sample categories (defined by the three volumes and the two glucose levels) was determined as an overall performance measure for benchmarking the glucose monitoring systems. Any wrong response in a sample category disqualified the category from being entirely correct. Comparisons between the different devices were performed by means of contingency tables. A p value <0.05 was considered statistically significant.
Results
Processing of blood samples was performed as described earlier. Following careful manipulation of the hematocrit concentrations with confirmation of the hematocrit and oxygen pressure acceptance criteria, all measurements were performed by an appropriate number of laboratory staff members in parallel for each meter within 20 min per sample volume. It turned out that the glucose level in the sample had no significant influence on the results of this investigation.
The meter responses for the individual volumes for the two blood glucose ranges are displayed in Table 1. It can be seen that almost all devices responded correctly during all 24 measurements to the very low volume of 0.2 µl. Only four devices (13%) initiated readings at this very low volume resulting in wrong results [ACCU-CHEK Aviva (one case), FreeStyle Freedom (one case), GlucoCard Meter (five cases), and MediTouch (seven cases)]. Several devices (11/31 devices; 35%) failed occasionally or regularly at the borderline sample volume of 0.32 µl [Contour (1), FreeStyle Freedom Lite (1), OmniTest (1), OneTouch Vita (1), Precision Xtra (2), GlucoMen GM (3), MediTouch (3), GlucoCard + (4), Ypsomed Pura (7), GlucoMen LX (10), and GlucoCard Meter (17)].
Table 1.
Number of Device Failures (No Alarm and Wrong Reading Outside ±20%) as Documented for the Different Blood Volumes with the Different Blood Glucose Monitoring Systemsa
| Device | Sample volume | |||
| 0.20 µl | 0.32 µl | 1.2 µl | All | |
| ACCU-CHEK Aviva | 1/24 (4%) | 0/24 (0%) | 0/24 (0%) | 1/72 (1%) |
| ACCU-CHEK Aviva Nano | 0/24 (0%) | 0/24 (0%) | 8/24 (33%) | 8/72 (11%) |
| ACCU-CHEK Compact+ | 0/24 (0%) | 0/24 (0%) | 0/24 (0%) | 0/72 (0%) |
| ACCU-CHEK Mobile | 0/24 (0%) | 0/24 (0%) | 0/24 (0%) | 0/72 (0%) |
| AlphaCheck+ | 0/24 (0%) | 0/24 (0%) | 8/24 (33%) | 8/72 (11%) |
| BGStar | 0/24 (0%) | 0/24 (0%) | 0/24 (0%) | 0/72 (0%) |
| iBGStar | 0/24 (0%) | 0/24 (0%) | 0/24 (0%) | 0/72 (0%) |
| Breeze 2 | 0/24 (0%) | 0/24 (0%) | 2/24 (8%) | 2/72 (3%) |
| Contour | 0/24 (0%) | 1/24 (4%) | 0/24 (0%) | 1/72 (1%) |
| Contour USB | 0/24 (0%) | 0/24 (0%) | 2/24 (8%) | 2/72 (3%) |
| Elite | 0/24 (0%) | 0/24 (0%) | 17/24 (71%) | 17/72 (24%) |
| FineTouch | 0/24 (0%) | 0/24 (0%) | 5/24 (21%) | 5/72 (7%) |
| FreeStyle Freedom | 1/24 (4%) | 0/24 (0%) | 0/24 (0%) | 1/72 (1%) |
| FreeStyle Freedom Lite | 0/24 (4%) | 1/24 (4%) | 0/24 (0%) | 1/72 (1%) |
| GlucoCard+ | 0/24 (0%) | 4/24 (17%) | 0/24 (0%) | 4/72 (6%) |
| GlucoCard Meter | 5/24 (21%) | 17/24 (71%) | 18/24 (75%) | 40/72 (56%) |
| GlucoMen GM | 0/24 (0%) | 3/24 (13%) | 0/24 (0%) | 3/72 (4%) |
| GlucoMen LX | 0/24 (0%) | 10/24 (42%) | 9/24 (38%) | 19/72 (26%) |
| GlucoTalk | 0/24 (0%) | 0/24 (0%) | 1/24 (4%) | 1/72 (1%) |
| MediTouch | 7/24 (29%) | 3/24 (13%) | 20/24 (83%) | 30/72 (42%) |
| OmniTest | 0/24 (0%) | 1/24 (4%) | 11/24 (46%) | 12/72 (17%) |
| OneTouch Ultra Easy | 0/24 (0%) | 0/24 (0%) | 6/24 (25%) | 6/72 (8%) |
| OneTouch Ultra 2 | 0/24 (0%) | 0/24 (0%) | 3/24 (13%) | 3/72 (4%) |
| OneTouch Ultra Smart | 0/24 (0%) | 0/24 (0%) | 1/24 (4%) | 1/72 (4%) |
| OneTouch Verio | 0/24 (0%) | 0/24 (0%) | 0/24 (0%) | 0/72 (0%) |
| OneTouch Vita | 0/24 (0%) | 1/24 (4%) | 10/24 (42%) | 11/72 (15%) |
| Precision Xceed | 0/24 (0%) | 0/24 (0%) | 17/24 (0%) | 17/72 (24%) |
| Precision Xtra | 0/24 (0%) | 2/24 (8%) | 3/24 (13%) | 5/72 (7%) |
| Wellion Calla Dialog | 0/24 (0%) | 0/24 (0%) | 1/24 (4%) | 1/72 (1%) |
| Wellion Calla Premium | 0/24 (0%) | 0/24 (0%) | 3/24 (13%) | 3/72 (4%) |
| Ypsomed Pura | 0/24 (0%) | 7/24 (29%) | 20/24 (83%) | 27/72 (38%) |
Shadowed fields indicate the volumes with reported device failures.
Notably, the majority of device failures was recorded by devices displaying incorrect readings when tested with a supposedly sufficient but still limited sample volume of 1.2 µl [19/31 devices (61%); OneTouch Ultra Smart (1), Wellion Calla Dialog (1), Breeze 2 (2), Contour USB (2), OneTouch Ultra 2 (3), Precision Xtra (3), Wellion Calla Premium (3), FineTouch (5), OneTouch Ultra Easy (6), ACCU-CHEK Aviva (8), AlphaChek+ (8), GlucoMen LX (9), FreeStyle Freedom (10), OmniTest (11), FreeStyle Freedom Lite (17), Precision Xceed (17), GlucoCard Meter (18), MediTouch (20), and Ypsomed Pura (20)]. Only two devices presented with wrong responses with all three tested volumes (GlucoCard Meter and MediTouch). In almost all cases, the displayed values during a wrong measurement were too low in comparison with the YSI reference.
Five devices did not show any failure in our experimental protocol (ACCU-CHEK Compact+, ACCU-CHEK Mobile, BGStar, iBGStar, and OneTouch Verio). The nature of their response (error or correct reading) at the different sample volumes is presented in Table 2. It appears that the devices that could best handle the low-volume situation and still deliver accurate results were BGStar and OneTouch Verio, followed by iBGStar, ACCU-CHEK Mobile, and ACCU-CHEK Compact+.
Table 2.
Type of Device Response (Initiation of a Correct Reading or Low-Volume Alarm) in Those Devices with 100% Correct Response to the Artificially Provided Low Sample Volumes
| Device | Sample volume | |||
| 0.20 µl | 0.32 µl | 1.2 µl | All | |
| Number of readings | 24 | 24 | 24 | 72 |
| BGStar | ||||
| Low-volume alarm | 8.0% | 12.5% | 0% | 6.8% |
| Correct reading | 92.0% | 87.5% | 100% | 93.2% |
| iBGStar | ||||
| Low-volume alarm | 79.0% | 62.5% | 0% | 47.2% |
| Correct reading | 21.0% | 37.5% | 100% | 52.8% |
| ACCU-CHEK Compact+ | ||||
| Low-volume alarm | 100% | 100% | 66.5% | 88.8% |
| Correct reading | 0% | 0% | 33.5% | 11.2% |
| ACCU-CHEK Mobile | ||||
| Low-volume alarm | 41.5% | 29.0% | 16.5% | 29.0% |
| Correct reading | 48.5% | 71.0% | 83.5% | 71.0% |
| OneTouch Verio | ||||
| Low-volume alarm | 21.0% | 4.0% | 0% | 8.3% |
| Correct reading | 79.0% | 96.0% | 100% | 91.7% |
For benchmark comparison, we performed an analysis on device failure in the six different categories (3 volumes × 2 blood glucose ranges). If any wrong response occurred in a given category, this category was classified as failed. The result of this analysis is shown in Figure 1. It can be seen that the majority of the devices failed in one category (usually wrong readings with low but supposedly sufficient volume). Devices with failures in four or more categories showed a significantly lower performance than the five devices with overall correct responses. The devices with failures in five or more categories performed significantly worse than devices with one or less failing category.
Figure 1.
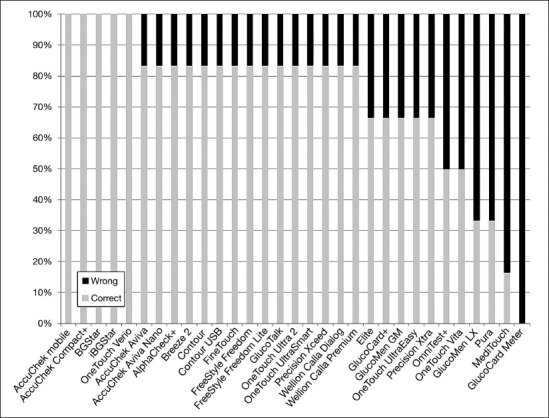
Analysis of responses in the sample categories free of any device failure by device (definition of the six categories: three volumes with two blood glucose ranges). Any single or repeatedly wrong response lead to disqualification of the category (“wrong” versus “correct”) in this benchmark comparison.
Discussion
Regular blood glucose monitoring is an integral part of standard diabetes care, especially in patients on insulin treatment.1,2,10 However, frequently significant errors and inaccuracies occur that are poorly understood by patients and health care providers.11 The reasons for these inaccuracies are manifold and include, but are not limited to, strip factors (e.g., manufacturing variances, storage conditions, and chemical composition), physical and environmental factors (e.g., temperature, humidity, and altitude), chemical factors (e.g., substance interference, hematocrit interference, and nutritional interference), and patient factors (e.g., correct device operation, correct hand washing, and device and strip storage at home).3 One of the device features that is given some attention by the manufacturers is the volume of the blood sample required to perform a correct reading. While the majority of the devices for patient self-blood glucose monitoring required 5–10 µl for an adequate measurement performance at the beginning of this century, the volumes have been significantly lowered to reach volumes of 1 µl or below in many of the currently available strip technologies. These low-volume requirements enable the use of alternative sites for the blood sampling process, which introduced another potential source for inaccuracies and increased the training requirements for patients supposed to perform blood glucose self-testing.12–14 However, by understanding the source of the error and educating the patients on methods of prevention and correction, health care providers can help their patients use the systems more effectively and accurately.
In this laboratory study, we investigated incomplete strip filling as one potential source of device malperformance. All glucose strip sensor technologies have built-in low-volume detection systems as a preventive measure. They are supposed to inform the user by displaying a low-volume alert on the screen in case of incomplete filling of the measurement chamber. From a clinical point of view, a worst case scenario resulting from incomplete strip filling would be the display of an inaccurately high glucose value without indication that something went wrong during the measurement procedure. This could, e.g., lead to wrong and too high insulin dose calculation and potentially result in a hypoglycemic episode that can harm the patient’s wellbeing. According to our knowledge, no thorough investigation of this condition has been published yet in the international literature, so we developed an in-house testing protocol in our laboratory. We exposed the tested systems to three very small sample volumes, which should in the majority of cases result in low-volume alarms, and tested two different glucose concentrations. It turned out that the glucose concentration in the sample had no influence on the study results.
Our lowest volume was 0.2 µl, which is clearly an insufficient volume to completely fill any of the tested blood glucose strips. The vast majority of the devices reacted to this volume with low-volume alerts, and only very few devices (except for two systems with a higher failure rate) had an occasional malfunction. The next volume tested (0.32 µl) represented a borderline volume for our investigation, as some devices give 0.3 µl as the minimally required volume for the strip filling (e.g., FreeStyle Freedom), while others indicate a necessary amount of 0.5 to 1 µl. With this volume, a substantially larger amount of devices showed wrong results (defined as readings outside of a 20% range from the reference device without low-volume indication). The largest number of wrong readings, however, occurred with the 1.2 µl volume, which was supposed to present a sufficient volume for all strips, but one (ACCU-CHEK Compact+) in this investigation. Normally, when puncturing the finger tip correctly with a lancing device, patients can produce much higher blood sample volumes (up to 200 µl according to our own observations) and exposing the strips to an excess sample volume does not represent a problem with respect to system performance. However, low sample volumes may occur as a result of ambient conditions, reduced capillary blood flow, or insufficient depth of the lancing device penetration into the skin.
In contrast to static electrochemistry, dynamic electrochemistry does not determine glucose concentrations based on a fixed measurement chamber volume but based on the electron turnover at the electrodes under different measurement conditions. It is therefore not dependent on a certain volume, which may be the reason for the good results obtained with the BGStar and iBGStar in this investigation. Together with the OneTouch Verio (for which any result correction mechanism has not been made public by the manufacturer yet), these devices reacted with a high number of correct readings even at the very low volume as compared with the other meters, which displayed predominantly error messages. Finally, only five devices showed 100% correct performance under the tested conditions. It therefore appears to be important to educate the patients, in general, on the necessity to test only with sufficient blood volume, because not all patients are using the devices that performed flawlessly or with only minor problems in this investigation.
There are several limitations in this study that need to be addressed prior to drawing conclusions about device performance in a clinical routine setting. First, this was a laboratory investigation, therefore the devices and strips were not operated exactly in accordance with the instructions for use for blood taken directly from the fingertip of a patient. Potential matrix effects between capillary blood and venous blood can heavily influence BGM accuracy but should not affect the low-volume alert function. On the other hand, we have used a previously reported proper laboratory methods for using venous whole blood with an oxygenation procedure, which can result in quite accurate results.15 Second, we used the StatStrip as the capillary comparison method. While we and others have shown that this point-of-care device is very accurate in comparison with reference laboratory methods,6,15,16 it is based on a glucose oxidase method, while several of the tested systems (e.g., the Roche Diagnostics and the Bayer devices) are based on glucose dehydrogenase as the key sensor enzyme. It has been shown that differences of 5–8% can easily occur between different enzymatic methods such as glucose oxidase and hexokinase,17,18 which may account for some of the deviations observed in our study.
Even considering these limitations, however, it becomes clear by our results that an insufficient sample volume represents a potential source of inaccuracy and error in patient self-blood glucose monitoring. This finding underlines the need to address this issue appropriately when performing patient training on blood glucose monitoring in the course of diabetes treatment initiation. It is encouraging that several meters were not affected by this situation, which may be considered advantageous when selecting the appropriate meter for individual patients for daily routine testing.
Acknowledgments
The authors express their gratitude to all collaborators from IKFE laboratory, IKFE research laboratory, and IKFE clinic who participated in the study.
Glossary
- (BGM)
blood glucose meter
Funding
This study was funded by Sanofi.
Disclosures
Andreas Pfützner, Thomas Forst, and Petra B. Musholt have received research grants, travel support, and consultancy fees from Sanofi. Jochen Sieber and Frank Flacke are employees of Sanofi. The authors received editorial/writing support in the preparation of this manuscript provided by Julia Heise of Medical Writing group of IKFE-CRO, funded by Sanofi. Any authorship of scientists working at IKFE is to be seen independently from this service and is handled in compliance with current ethical industry standards. In particular, no author received any fee for his/her authorship.
References
- 1.Rydén L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, Cosentino F, Jönsson B, Laakso M, Malmberg K, Priori S, Ostergren J, Tuomilehto J, Thrainsdottir I, Vanhorebeek I, Stramba-Badiale M, Lindgren P, Qiao Q, Priori SG, Blanc JJ, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo J, Zamorano JL, Deckers JW, Bertrand M, Charbonnel B, Erdmann E, Ferrannini E, Flyvbjerg A, Gohlke H, Juanatey JR, Graham I, Monteiro PF, Parhofer K, Pyörälä K, Raz I, Schernthaner G, Volpe M, Wood D. Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC); European Association for the Study of Diabetes (EASD). Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28(1):88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginsberg BH. Factors affecting blood glucose monitoring: sources of errors in measurement. J Diabetes Sci Technol. 2009;3(4):903–913. doi: 10.1177/193229680900300438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musholt PB, Schipper C, Thomé N, Ramljak S, Schmidt M, Forst T, Pfützner A. Dynamic Electrochemistry Corrects for Hematocrit Interference on Blood Glucose Determinations with Patient Self-Measurement Devices. J Diabetes Sci Technol. 2011;5(5):1167–1175. doi: 10.1177/193229681100500520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramljak S, Lock JP, Schipper C, Musholt PB, Forst T, Lyon M, Pfützner A. Hematocrit interference of blood glucose meters for patient self-measurement. J Diabetes Sci Technol. 2013;7(1):179–189. doi: 10.1177/193229681300700123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfützner A, Harzer O, Musholt PB, Scherer S, Löbig M, Forst T. Performance of blood glucose measurement systems influenced by interfering substances. Diabetes Stoffw Herz. 2009;18:387–392. [Google Scholar]
- 7.Iyengar S, Hall EA. Phasor transform to extract glucose and ascorbic acid data in an amperometric sensor. Analyst. 2000;125(11):1987–1992. doi: 10.1039/b005967f. [DOI] [PubMed] [Google Scholar]
- 8.Iyengar S, Wiley M, Nadeau D. Performance of the WaveSense-enabled glucose monitoring system across multiple lots. Diabetes Stoffw Herz. 2007;16(1):15–20. [Google Scholar]
- 9.Pfützner A, Mitri M, Musholt PB, Sachsenheimer D, Borchert M, Yap A, Forst T. Clinical assessment of the accuracy of blood glucose measurement devices. Curr Med Res Opin. 2012;28(4):525–531. doi: 10.1185/03007995.2012.673479. [DOI] [PubMed] [Google Scholar]
- 10.Bergenstal RM, Gavin JR., 3rd Global Consensus Conference on Glucose Monitoring Panel. The role of self-monitoring of blood glucose in the care of people with diabetes: report of a global consensus conference. Am J Med. 2005;118(Suppl 9A):1S–6S. doi: 10.1016/j.amjmed.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch IB, Bode BW, Childs BP, Close KL, Fisher WA, Gavin JR, Ginsberg BH, Raine CH, Verderese CA. Self-monitoring of blood glucose (SMBG) in insulin- and non-insulin-using adults with diabetes: consensus recommendations for improving SMBG accuracy, utilization, and research. Diabetes Technol Ther. 2008;10(6):419–439. doi: 10.1089/dia.2008.0104. [DOI] [PubMed] [Google Scholar]
- 12.Lucidarme N, Alberti C, Zaccaria I, Claude E, Tubiana-Rufi N. Alternate-site testing is reliable in children and adolescents with type 1 diabetes, except at the forearm for hypoglycemia detection. Diabetes Care. 2005;28(3):710–711. doi: 10.2337/diacare.28.3.710. [DOI] [PubMed] [Google Scholar]
- 13.Pfützner A, Hermanns N, Schröder S, Wegenstein M, Larbig M, Mondok A, Forst T, Haak T. Cross-sectional investigation on the accuracy of alternate site glucose testing using the Soft-Sense glucose meter. Swiss Med Wkly. 2002;132(25-26):351–357. doi: 10.4414/smw.2002.09991. [DOI] [PubMed] [Google Scholar]
- 14.Lock JP, Szuts EZ, Malomo KJ, Anagnostopoulos A, Rao S. Accuracy of alternate site testing--comparing arm and finger blood glucose results in glucose dynamic states. Diabetes Technol Ther. 2002;4(1):87–89. doi: 10.1089/15209150252924157. [DOI] [PubMed] [Google Scholar]
- 15.Rabiee A, Magruder JT, Grant C, Salas-Carrillo R, Gillette A, DuBois J, Shannon RP, Andersen DK, Elahi D. Accuracy and reliability of the Nova StatStrip® glucose meter for real-time blood glucose determinations during glucose clamp studies. J Diabetes Sci Technol. 2010;4(5):1195–1201. doi: 10.1177/193229681000400519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makaya T, Memmott A, Bustani P. Point-of-care glucose monitoring on the neonatal unit. J Paediatr Child Health. 2012;48(4):342–346. doi: 10.1111/j.1440-1754.2011.02253.x. [DOI] [PubMed] [Google Scholar]
- 17.Twomey PJ. Plasma glucose measurement with the Yellow Springs Glucose 2300 STAT and the Olympus AU640. J Clin Pathol. 2004;57(7):752–754. doi: 10.1136/jcp.2003.013417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genter PM, Ipp E. Accuracy of plasma glucose measurements in the hypoglycemic range. Diabetes Care. 1994;17(6):595–598. doi: 10.2337/diacare.17.6.595. [DOI] [PubMed] [Google Scholar]


