Abstract
Background
Hematocrit (HCT) is known to be a confounding factor that interferes with many blood glucose (BG) measurement technologies, resulting in wrong readings. Dynamic electrochemistry has been identified as one possible way to correct for these potential deviations. The purpose of this laboratory investigation was to assess the HCT stability of four BG meters known to employ dynamic electrochemistry (BGStar and iBGStar, Sanofi; Wavesense Jazz, AgaMatrix; Wellion Linus, MedTrust) in comparison with three other devices (GlucoDock, Medisana; OneTouch Verio Pro, LifeScan; FreeStyle Freedom InsuLinx, Abbott-Medisense).
Methods
Venous heparinized blood was immediately aliquoted after draw and manipulated to contain three different BG concentrations (60–90, 130–160, and 280–320 mg/dl) and five different HCT levels (25%, 35%, 45%, 55%, and 60%). After careful oxygenation to normal blood oxygen pressure, each of the resulting 15 different samples was measured six times with three devices and three strip lots of each meter. The YSI Stat 2300 served as laboratory reference method. Stability to HCT influence was assumed when less than 10% difference occurred between the highest and lowest mean glucose deviations in relation to HCT concentrations [hematocrit interference factor (HIF)].
Results
Five of the investigated self-test meters showed a stable performance with the different HCT levels tested in this investigation: BGStar (HIF 4.6%), iBGStar (6.6%), Wavesense Jazz (4.1%), Wellion Linus (8.5%), and OneTouch Verio Pro (6.2%). The two other meters were influenced by HCT (FreeStyle InsuLinx 17.8%; GlucoDock 46.5%).
Conclusions
In this study, meters employing dynamic electrochemistry, as used in the BGStar and iBGStar devices, were shown to correct for potential HCT influence on the meter results. Dynamic electrochemistry appears to be an effective way to handle this interfering condition.
Keywords: blood glucose, dynamic electrochemistry, hematocrit, interference, patient self-monitoring
Introduction
Fequent performance of self-blood glucose measurements is an integral part of the treatment of patients with diabetes mellitus and is recommended by specialist associations worldwide.1,2 As therapeutic decisions, such as insulin dose determinations, are made based on the meter readings, meter accuracy is mandatory to achieve good treatment results. Frequently, however, significant errors and inaccuracies occur that are poorly understood by patients and health care providers.3 The multifactorial reasons for these inaccuracies include, but are not limited to, strip factors (e.g., manufacturing variances, storage conditions, chemical strip composition), physical and environmental factors (e.g., temperature, humidity, altitude), chemical factors [e.g., substance interference, hematocrit (HCT) interference, nutritional interference], and patient factors (e.g., correct device operation, correct hand washing and blood sampling, device and strip storage at home).4
A well-known interfering factor is the ratio of blood cell volume versus plasma water in the sample, which is biochemically expressed by the HCT value. Hematocrit has long been known to affect the accuracy of glucose meters and was considered in treatment guidelines early on.5,6 Many blood glucose (BG) monitoring systems measure the glucose concentration in a measurement chamber on the strip and use the defined chamber size to calculate the finally displayed BG concentration.7 However, only the plasma glucose participates in the measurement reaction on the electrode, and a higher than normal plasma volume (i.e., low HCT) may lead to overestimation of the glucose concentration and vice versa. Classically, BG monitoring systems based on this static electrochemistry technology are calibrated for HCT concentrations of 40–45%. The instructions for use suggest limiting the use of the devices to clinical situations in which HCT levels are within a specific range (typically 30–55%).
However, prevalence of HCT variation is usually underestimated by physicians and diabetes nurse educators and is subject to seasonal variation.8 A recent investigation of the HCT distribution in an urban community has demonstrated HCT values in a range of 30–50% in a healthy reference population, 20–60% in community patients, 10–70% in hospital patients, and 15–40% in intensive care patients.9 In older patients also suffering from various diseases, these variations can be even more pronounced and may have an impact on the patient’s prognosis.9 Deviation from normal HCT levels can be induced by lifestyle interventions (e.g., smoking, prolonged exercise), by environmental conditions (e.g., stay in mountains, seasonal variation), demographic conditions (e.g., age), and disease- and drug-related conditions (e.g., hematological disorders, hypermenorrhea, pregnancy, or renal disease).10–12 In consequence, BG measurements at higher or lower HCT concentration may occur frequently and may have a more substantial impact on treatment quality than expected. We have been able to demonstrate that several devices for patient self-testing (e.g., BGStar and iBGStar) are not affected by HCT interference, which leads to very accurate results in comparison with the reference methods in a laboratory environment13,14 and also in an appropriately performed clinical accuracy evaluation.15We associated this phenomenon with the use of dynamic electrochemistry in these devices, but in another previous accuracy investigation, a device employing the same technology (Wellion Linus) showed only moderate performance and barely met the former International Organization for Standardization accuracy standards, which have meanwhile been replaced by even stricter accuracy acceptance ranges.16
The primary goal of this study was to assess the impact of HCT variation on the performance of new BG meters that employ dynamic electrochemistry technology (BGStar, iBGStar, Wellion Linus, and Wavesense Jazz) in comparison with other BG meters that use alternative electrochemical technologies (OneTouch Verio Pro, GlucoDock, and FreeStyle Freedom InsuLinx).
Materials and Methods
Study Devices
We purchased the study devices and strips from regular pharmacy supplies.
The following glucose meters were included in this study: BGStar and iBGStar (Sanofi), Wavesense Jazz (AgaMatrix), and Wellion Linus [MedTrust; all using glucose oxidase (GOx) with dynamic electrochemistry]; OneTouch Verio Pro LifeScan; glucose dehydrogenase (GDH) with an undisclosed electrochemistry]; FreeStyle Freedom InsuLinx (Abbott Diabetes Care; GDH with coulometry); and GlucoDock (Medisana; GOx with static electrochemistry). The YSI (Yellow Springs Instrument) 2300 STAT PLUS Glucose Analyzer (Life Sciences; GOx method) was utilized as the plasma comparison method. All devices and supplies were stored and operated in accordance with manufacturer’s instructions.
Collection of Blood Samples and Laboratory Settings
Blood samples were collected in compliance with local ethical and legal requirements. Venous, heparinized whole blood was drawn on the day of the experiment and immediately manipulated to contain three different BG concentrations and five different HCT levels (15 different samples in total). Samples were aliquoted and stored at 4 °C until measurement. Before the start of the experiment, glucose concentration, HCT values, and the degree of oxygen saturation were confirmed by means of the ABL80 FLEX CO-OX blood gas analyzer (Drott, Wiener Neudorf, Austria) and carefully adjusted, if necessary. The degree of oxygenation had to remain within physiological capillary values (range 80–100%). Prior to starting the measurements, all glucose meters were checked for proper function with quality control solutions. Three meters of each device and three strip lots were used in this protocol, and each of the 15 samples was tested six times with each meter/lot combination (i.e., 54 measurements/sample/device and a total of 810 measurements/meter type). All tests were carried out simultaneously for each meter by a group of seven trained staff members in a laboratory setting with controlled room temperature (23 ± 5 °C) and humidity (60% ± 5%) to avoid a differential influence of sample aging on the comparative results.
Sample Processing
The freshly drawn blood was spiked to three target glucose concentrations using a 10% concentrated glucose solution (Serag-Wiessner KG, Germany) to the following target ranges: 60–90, 130–160, and 280–320 mg/dl. The blood was gently mixed in a 15 ml test tube and aliquoted. Subsequently, parts of the samples were carefully centrifuged to separate cells from plasma, and both fractions were used to adjust other aliquots to a desired target level (approximately 20–30%, 35–40%, 45–50%, 50–55%, and 55–65%). Both HCT and oxygen pressure were verified in each manipulated sample by means of the ABL analyzer. If the oxygenation saturation was below the meter specifications (i.e., out of physiological range of 80–100%), individual samples were very carefully oxygenized by gentle manual rocking at room temperature. Instead, the sample was discarded and a new sample was prepared. Following repeated HCT and oxygen saturation measurements and confirmation of readjusted values, an aliquot of the individual sample was centrifuged at 300 × g for 5 min at 4 °C in order to separate plasma from red blood cells. The obtained plasma was measured with the YSI comparison device.
Statistical Analyses
Data were collected and tabulated for each meter. Statistical analyses included calculation of the mean values and standard deviations for each meter type/sample combination. The mean of the differences from the comparison method was used for calculating the mean absolute relative difference (MARD) for each meter at the three glucose concentrations. This accuracy analysis as well as the determination of the coefficient of variation (precision) was only performed with the samples that showed a HCT value of 45%. The mean glucose value determined at HCT of 45% was normalized to be 100% in order to determine the potential bias (percentage deviation) occurring at the other HCT levels. The means of the deviations between the different HCT samples were used for calculating a hematocrit interference factor (HIF; largest observed bias above 100% + largest observed bias below 100%) for each meter with the mean relative results obtained from the three glucose concentrations. An HIF <15% for the individual glucose level and a mean HIF over the entire glucose ranges <10% were predefined as indicative for no clinically relevant influence of HCT on the BG readings, as defined previously.14 Comparisons between mean values were calculated by means of the two-sided Student’s t-test. A p value <.05 was considered statistically significant.
Results
Three strip lots were commercially available for all devices except fo the GlucoDock device, for which only one strip lot could be obtained in sufficient quantities for complete testing. However, the three purchased GlucoDock devices came packaged with a limited quantity of 150 strips from a separate lot and were used for an abbreviated testing protocol (two instead of six readings per sample and device). All measurements with all meters for one sample were done within 20 min after release of the sample for the experiment. The final glucose values achieved following laboratory sample manipulations were within 63–68, 141–145, and 272–289 mg/dl at HCT values of 25%, 35%, 45%, 55%, and 60%.
The difference of the results from the value determined at 45% with the three BG ranges and over all glucose ranges is shown in Figure 1 for the four devices employing dynamic electrochemistry. Figure 2 shows the same presentation of the other three tested devices. It can be seen that the four devices in Figure 1 and OneTouch Verio Pro in Figure 2 were not affected in a clinically relevant magnitude by HCT interference. These five unaffected devices and technologies showed comparable performance in this experiment, with individual minor deviations seen with some samples. Wellion Linus appears to be slightly more variable in the results than the other four devices. One of the comparator devices (GlucoDock) showed pronounced deviations from the reference HCT level, with the largest bias in each direction seen for different glucose concentrations (+47.2% at an HCT of 25% at low concentration and -23.3% at 60% HCT at a high BG concentration). Also, FreeStyle Freedom InsuLinx was shown to be affected especially at low HCT concentrations.
Figure 1.
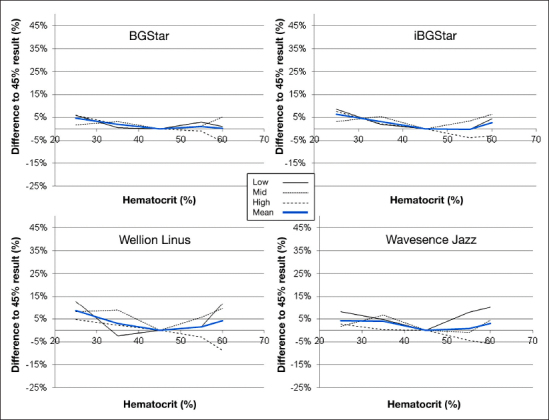
Results of the HCT interference experiment with the devices employing dynamic electrochemistry (BGStar, iBGStar, Wellion Linus, and Wavesense Jazz). The graph shows the impact of different HCT levels on the readings at the three different glucose concentrations (63–68, 141–145, 272–289 mg/dl). The bold line represents the mean value of all glucose levels
Figure 2.
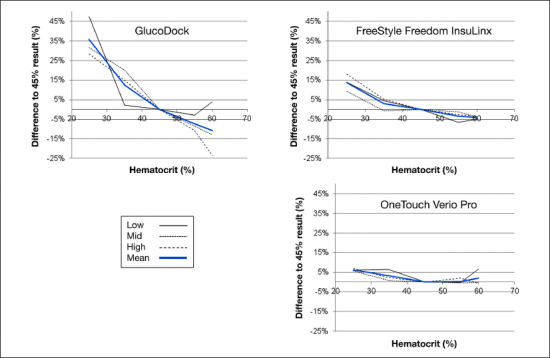
Results of the HCT interference experiment with GlucoDock, FreeStyle Freedom InsuLinx, and OneTouch Verio Pro. The graph shows the impact of different HCT levels on the readings at three different glucose concentrations (63–68, 141–145, 272–289 mg/dl). The bold line represents the mean value of all glucose levels. GlucoDock results were obtained with one strip lot only.
Calculation of the HIF at the different BG concentrations as described previously13,14 resulted in the numbers displayed in Figure 3. An overall HIF below 10% was seen with BGStar (4.6%), iBGStar (6.6%), WaveSense Jazz (4.1%), Wellion Linus (8.5%), and OneTouch Verio Pro (6.2%). Values above 10% were shown by FreeStyle Freedom InsuLinx (17.8%) and GlucoDock (46.5%). All devices employing dynamic electrochemistry were shown to be unaffected by HCT interference.
Figure 3.
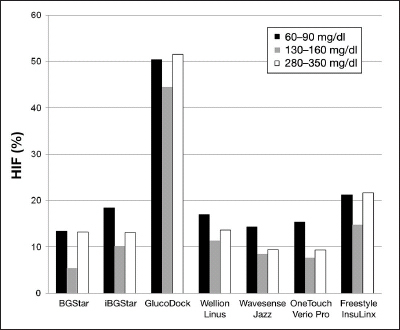
esults of the HIF calculation for all devices for the three different BG concentrations. The degree of HCT interference is not dependent on the BG level. *Only one strip lot was available for GlucoDock.
The accuracy analysis was only performed with the results obtained at 45% HCT for all devices. The mean relative deviation of the BG monitoring system at the different BG levels is shown in Table 1. The devices were also comparable with respect to the analysis of the precision when calculated over all three tested strip lots, which was also only performed with samples showing a 45% HCT concentration. With 3 x 6 determinations, mean precision for BGStar was 3.1% (range 1.7% to 5.4%), iBGStar 3.6% (2.1% to 5.1%), Wellion Linus 3.6% (1.8% to 5.0%), Wavesense Jazz 4.5% (2.7% to 7.8%), OneTouch Verio Pro 2.7% (2.0% to 3.5%), and FreeStyle Freedom InsuLinx 4.0% (2.6% to 5.7%). The precision results for GlucoDock (2.8%, 2.6% to 3.1%) were only obtained from one strip lot and can therefore not be compared with the other device precision values.
Table 1.
Accuracy Analysis: Mean Absolute Relative Deviations from the Reference Method as Calculated for the Different Blood Glucose Levels
| Device | Blood glucose range | ||
| 63–68 mg/dl | 141–145 mg/dl | 272–289 mg/dl | |
| BGStar | 12.2% | 6.4% | 6.0% |
| iBGStar | 13.9% | 6.6% | 5.8% |
| GlucoDocka | 12.0% | 6.0% | 4.2% |
| Wellion Linus | 6.0% | 5.0% | 3.9% |
| WaveSense Jazz | 13.8% | 5.9% | 7.1% |
| OneTouch Verio Pro | 2.9% | 7.3% | 8.9% |
| FreeStyle Freedom InsuLinx | 5.0% | 4.9% | 12.5% |
GlucoDock results were obtained with one strip lot only.
Discussion
Hematocrit interference has long been described as a source for inaccuracies of BG meter readings in daily routine.5,6 The results of previous studies with different glucose meters have indicated that lower-than-normal HCT values (<30% to <35%) result in overestimates of laboratory glucose levels when glucose strip methods are used, whereas HCT values higher than normal (>45%) result in underestimates of laboratory values.17–22The reason is an internal calibration of the analysis process in devices using static electrochemistry, which is based on a standardized 45% HCT value of the sample. Various hypotheses have been proposed to explain the impact of abnormal HCT levels on glucose testing, including but not limited to, altered viscosity of the blood, prevention of plasma from reaching the reaction surface of the test strip, change in diffusion kinetics, and/or increased packed red cell volume and displacement of plasma volume, leading to insufficient plasma volume for accurate testing.23
While many health care professionals believe that HCT is usually quite normal in their community patients, it is generally accepted that HCT may be deviating from the reference values in patients with critical diseases or under very specific conditions, such as pregnancy, extensive exercise, or kidney disease. However, one study has now shown that HCT variance, even in patients visiting family doctors, has been underestimated by far. By analyzing data from 15,108 community patients, Lyon and Lyon9 are reporting HCT ranges from 20% to 60%. They also detected ranges from 10% to 70% when measuring this parameter in 45,260 hospital patients. Both groups obviously include the majority of patients with diabetes mellitus currently treated by public health care. These findings underline the importance of considering HCT as a confounding factor for patients’ self-measurement of blood glucose, which may impact the quality of the subsequent treatment decisions. The instructions for use for handheld BG meters suggest limiting use of the devices to clinical situations in which HCT levels are within a specific range of values (typically 30% to 50%). When considering the findings from Lyon and Lyon,9 use of these devices in daily routine must also occur frequently outside these HCT levels.
One technological solution to reduce HCT interference is the application of a physical and mathematical algorithm for result correction as employed by dynamic electrochemistry. In this mathematical model, it is proposed that each oxidation process leading to an electrode signal can be represented by a unique vector based on a phase angle and a unique vector length and that the concentration of each analyte leading to an electron transfer can be determined by monitoring the change in the admittance magnitude in the direction of the characteristic angle for that particular species when applying different baseline measurement conditions (e.g., frequency, voltage). The total Faradaic admittance for all electroactive species present is given by a linear combination of the independent vectors from the different species. By applying different measurement conditions, the model allows for calculation of the individual contribution of an interfering substance based on the knowledge of the analyte-specific phase angle of the oxidation signal. Based on existing calibration curves and the knowledge about phase angle and basis vectors, glucose in samples containing several electroactive species can be measured by correcting the measured total admittance from several underlying measurement conditions for the influence of a variety of known interfering substances and conditions.24,25 In this laboratory study, these corrections led to unbiased readings independent from HCT variation for BGStar and iBGStar and also for two other devices using the same technology (Wellion Linus and Wavesense Jazz). Similar results for BGStar were obtained already in two previous investigations with a similar experimental design13,14 and in one study with naturally occurring BG variations.23 The method by which the technology of the fifth device with the same stability (OneTouch Verio Pro) reduces the influence of HCT on the meter results has not been disclosed by the manufacturer yet.
Our laboratory study has several limitations, which need to be considered before drawing conclusions with clinical relevance. First, this investigation was performed in an artificial laboratory setting with manipulated venous samples. It is designed to provide information regarding the interfering effect of HCT on the technology of the investigated meters. All tested devices are designed to operate optimally with capillary blood obtained from the fingertip. Therefore, the data provided regarding accuracy can only be interpreted with caution. Second, the OneTouch Verio Pro and the FreeStyle Freedom InsuLinx are based on a GDH method, while we used the GOx-based YSI analyzer as the comparison method. This may have contributed to the differences observed between the other devices with respect to MARD. A negative bias of 3% to 8% has been described between YSI 2300 STAT and the hexokinase-based Olympus AU640 reference method.26,27 As such, different reference methods might introduce a deviation of the values, which is not caused by the device itself. Finally, the device with the largest HCT difference could only be tested with a limited number of strips because of delivery problems. While we believe that this situation does not change the meaning of our findings, it has to be considered when interpreting our data.
In conclusion, BG meters employing dynamic electrochemistry were confirmed to be stable against HCT interference. According to recent studies, HCT levels outside the normal reference ranges are more prevalent than expected. It may be worthwhile to consider these findings when selecting BG meters for patients with diabetes mellitus.
Acknowledgments
Julia Heise of the medical writing group of IKFE-CRO provided editorial/writing support in the preparation of this manuscript, funded by Sanofi. Any authorship of scientists working at IKFE is to be seen independently from this service and is handled in compliance with current ethical industry standards. In particular, no author gets any fee for his/her authorship.
Glossary
- (BG)
blood glucose
- (GDH)
glucose dehydrogenase
- (GOx)
glucose oxidase
- (HCT)
hematocrit
- (HIF)
hematocrit interference factor
- (MARD)
mean absolute relative difference
Funding
This study was funded by Sanofi.
Disclosures
Andreas Pfützner., Petra B. Musholt., and Thomas Forst have received research grants, consultancy fees, and travel expenses from Sanofi. At the time of this work, Petra B. Musholt was an employee of IKFE and later of Profil Mainz, respectively. She is now an employee of Sanofi. Franke Flacke and Jochen Sieber are employees of Sanofi.
References
- 1.Rydén L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, Cosentino F, Jönsson B, Laakso M, Malmberg K, Priori S, Ostergren J, Tuomilehto J, Thrainsdottir I, Vanhorebeek I, Stramba-Badiale M, Lindgren P, Qiao Q, Priori SG, Blanc JJ, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo J, Zamorano JL, Deckers JW, Bertrand M, Charbonnel B, Erdmann E, Ferrannini E, Flyvbjerg A, Gohlke H, Juanatey JR, Graham I, Monteiro PF, Parhofer K, Pyörälä K, Raz I, Schernthaner G, Volpe M, Wood D. Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC); European Association for the Study of Diabetes (EASD). Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD) Eur Heart J. 2007;28(1):88–136. doi: 10.1093/eurheartj/ehl260. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Standards of medical care in diabetes--2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch IB, Bode BW, Childs BP, Close KL, Fisher WA, Gavin JR, Ginsberg BH, Raine CH, Verderese CA. Self-monitoring of blood glucose (SMBG) in insulin- and non-insulin-using adults with diabetes: consensus recommendations for improving SMBG accuracy, utilization, and research. Diabetes Technol Ther. 2008;10(6):419–439. doi: 10.1089/dia.2008.0104. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg BH. Factors affecting blood glucose monitoring: sources of errors in measurement. J Diabetes Sci Technol. 2009;3(4):903–913. doi: 10.1177/193229680900300438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Consensus statement on self-monitoring of blood glucose. Diabetes Care. 1987;10(1):95–99. [PubMed] [Google Scholar]
- 6.American Diabetes Association: clinical practice recommendations 1996. Diabetes Care. 1996;19(Suppl 1):S1–S118. [PubMed] [Google Scholar]
- 7.Fogh-Andersen N, D’Orazio P, Kuwa K, Külpmann WR, Mager G, Larsson L. Recommendation on reporting results for blood glucose (from an IFCC stage I document) IFCC Scientific Division Working Group on Selective Electrodes. eJIFCC. 12(4) http://www.ifcc.org/ifccfles/docs/vol12no4a4.pdf. [PMC free article] [PubMed] [Google Scholar]
- 8.Thirup P. Haematocrit: within-subject and seasonal variation. Sports Med. 2003;33(3):231–243. doi: 10.2165/00007256-200333030-00005. [DOI] [PubMed] [Google Scholar]
- 9.Lyon ME, Lyon AW. Patient acuity exacerbates discrepancy between whole blood and plasma methods through error in molality to molarity conversion: “Mind the gap!”. Clin Biochem. 2011;44(5-6):412–417. doi: 10.1016/j.clinbiochem.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Volkova N, Arab L. Evidence-based systematic literature review of hemoglobin/hematocrit and all-cause mortality in dialysis patients. Am J Kidney Dis. 2006;47(1):24–36. doi: 10.1053/j.ajkd.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Takubo T, Tatsumi N. [Reference values for hematologic laboratory tests and hematologic disorders in the aged] Rinsho Byori. 2000;48(3):207–216. [PubMed] [Google Scholar]
- 12.Macdougall IC, Ritz E. The Normal Haematocrit Trial in dialysis patients with cardiac disease: are we any the less confused about target haemoglobin? Nephrol Dial Transplant. 1998;13(12):3030–3033. doi: 10.1093/ndt/13.12.3030. [DOI] [PubMed] [Google Scholar]
- 13.Musholt PB, Schipper C, Thomé N, Ramljak S, Schmidt M, Forst T, Pfützner A. Dynamic Electrochemistry Corrects for Hematocrit Interference on Blood Glucose Determinations with Patient Self-Measurement Devices. J Diabetes Sci Technol. 2011;5(5):1167–1175. doi: 10.1177/193229681100500520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramljak S, Lock JP, Schipper C, Musholt PB, Forst T, Lyon M, Pfützner A. Hematocrit interference of blood glucose meters for patient self-measurement. J Diabetes Sci Technol. 2013;7(1):179–189. doi: 10.1177/193229681300700123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfützner A, Mitri M, Musholt PB, Sachsenheimer D, Borchert M, Yap A, Forst T. Clinical assessment of the accuracy of blood glucose measurement devices. Curr Med Res Opin. 2012;28(4):525–531. doi: 10.1185/03007995.2012.673479. [DOI] [PubMed] [Google Scholar]
- 16.Freckmann G, Baumstark A, Jendrike N, Zschornack E, Kocher S, Tshiananga J, Heister F. Haug C: System accuracy evaluation of 27 blood glucose monitoring systems according to DIN EN ISO 15197. Diabetes Technol Ther. 2010;12(3):221–231. doi: 10.1089/dia.2009.0128. [DOI] [PubMed] [Google Scholar]
- 17.Pavlicek V, Garzoni D, Urech P, Brändle M. Inaccurate self-monitoring of blood glucose readings in patients on chronic ambulatory peritoneal dialysis with icodextrin. Exp Clin Endocrinol Diabetes. 2006;114(3):124–126. doi: 10.1055/s-2006-924011. [DOI] [PubMed] [Google Scholar]
- 18.Püntmann I, Wosniok W, Haeckel R. Comparison of several point-of-care testing (POCT) glucometers with an established laboratory procedure for the diagnosis of type 2 diabetes using the discordance rate. A new statistical approach. Clin Chem Lab Med. 2003;41(6):809–820. doi: 10.1515/CCLM.2003.123. [DOI] [PubMed] [Google Scholar]
- 19.Tang Z, Lee JH, Louie RF, Kost GJ. Effects of different hematocrit levels on glucose measurements with handheld meters for point-of-care testing. Arch Pathol Lab Med. 2000;124(8):1135–1140. doi: 10.5858/2000-124-1135-EODHLO. [DOI] [PubMed] [Google Scholar]
- 20.Phillipou G, Seaborn CJ, Hooper J, Phillips PJ. Capillary blood glucose measurements in hospital inpatients using portable glucose meters. Aust N Z J Med. 1993;23(6):667–671. doi: 10.1111/j.1445-5994.1993.tb04724.x. [DOI] [PubMed] [Google Scholar]
- 21.Smith EA, Kilpatrick ES. Intra-operative blood glucose measurements. The effect of haematocrit on glucose test strips. Anaesthesia. 1994;49(2):129–132. doi: 10.1111/j.1365-2044.1994.tb03369.x. [DOI] [PubMed] [Google Scholar]
- 22.Louie RF, Tang Z, Sutton DV, Lee JH, Kost GJ. Point-of-care glucose testing: effects of critical care variables, influence of reference instruments, and a modular glucose meter design. Arch Pathol Lab Med. 2000;124(2):257–266. doi: 10.5858/2000-124-0257-POCGT. [DOI] [PubMed] [Google Scholar]
- 23.Pfützner A, Schipper C, Ramljak S, Flacke F, Sieber J, Forst T, Musholt PB. Determination of hematocrit interference in blood samples derived from patients with different blood glucose concentrations. J Diabetes Sci Technol. 2013;7(1):170–178. doi: 10.1177/193229681300700122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iyengar S, Hall EA. Phasor transform to extract glucose and ascorbic acid data in an amperometric sensor. Analyst. 2000;125(11):1987–1992. doi: 10.1039/b005967f. [DOI] [PubMed] [Google Scholar]
- 25.Iyengar S, Wiley M, Nadeau D. Performance of the WaveSense-enabled glucose monitoring system across multiple lots. Diabetes Stofw Herz. 2007;16(1):15–20. [Google Scholar]
- 26.Twomey PJ. Plasma glucose measurement with the Yellow Springs Glucose 2300 STAT and the Olympus AU640. J Clin Pathol. 2004;57(7):752–754. doi: 10.1136/jcp.2003.013417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genter PM, Ipp E. Accuracy of plasma glucose measurements in the hypoglycemic range. Diabetes Care. 1994;17(6):595–598. doi: 10.2337/diacare.17.6.595. [DOI] [PubMed] [Google Scholar]


