Abstract
Background
Self-monitoring of blood glucose (SMBG) is the most accessible way to assess glycemic patterns, and interpretation of these patterns can provide reasons for poor glycemic control and suggest management strategies. Furthermore, diabetes management based on blood glucose (BG) patterns is associated with improved patient outcomes. The aim of this review is therefore to evaluate the impact of pattern management in clinical practice.
Methods
We included a review of available literature, a discussion of obstacles to implementation of SMBG and pattern management, and suggestions on how clinicians and patients might work together to optimize this management feature.
Results
The literature review revealed eight publications specifically describing structured approaches to SMBG and pattern management. Specific information on how SMBG might be structured to detect BG patterns, however, remains limited. Barriers to pattern management include not just practical reasons, but emotional and psychological reasons as well.
Conclusions
Patterns are not always easy to detect or interpret, but on-meter and web-based tools can support both patients and clinicians. Ultimately, successful pattern management requires education and mutual commitment from the clinician and patient—ongoing collaboration is needed to obtain, review, and interpret SMBG values and to make changes based on the patterns.
Keywords: glycemic variability, pattern management, self-monitoring of blood glucose, tools
Introduction
Glycemic control, i.e., correction of hyperglycemia without hypoglycemia, is a major objective of diabetes management. Intensive glycemic control has been associated with reduced rates of microvascular complications in both type 1 and type 2 diabetes mellitus (T1DM, T2DM).1,2 However, these benefits come at the cost of a higher risk of severe hypoglycemic episodes.3–5 Fear of hypoglycemia is a recognized obstacle to improving glycemic control,6–8 driving patients and clinicians to be cautious in their treatment. The ability to predict hypoglycemic episodes opens up the opportunity to prevent them8 and could alleviate this fear.
Intraday glycemic variability, i.e., the occurrence of hypoglycemic or hyperglycemic episodes, makes insulin dose adjustments necessary. However, achieving intensive glycemic control is complicated by the interday glycemic variability observed in some patients, which obscures glycemic patterns and makes insulin dose adjustment difficult.9–11 The only way to detect glycemic variability and blood glucose (BG) patterns is by frequent measurement and documentation of BG or continuous glucose monitoring (CGM). While CGM provides a more complete view of BG values, this technology is not yet widely available. In this review, we focus on self-monitoring of blood glucose (SMBG), as this is the most widely used way to assess patterns of BG and determine changes in therapy.12 A BG pattern (high or low pattern) may be defined as a series of BG readings taken at the same time each day that fall outside the individual’s target range. Analysis of BG patterns can guide on a daily basis the treatment needed to stabilize BG and improve hemoglobin A1c (HbA1c) levels. Unlike HbA1c, which provides a long-term perspective, BG patterns can be used to analyze day-to-day and within-day glycemic fluctuations.13,14 A number of studies have based interventions on data from 3 consecutive days, with readings taken at the same time of day, although the specific timing and complexity of patterns varied.15–17 By looking for patterns in SMBG data, one can create order from disorder.
Programs for individuals with T1DM18–21 and T2DM15 that include a structured approach to SMBG and education on how to act on these data have demonstrated that it is possible to improve glycemic control without the increased rate of severe hypoglycemia seen in the Diabetes Control and Complications Trial. In real life, however, patients often do not regularly measure BG or adjust diet or therapy in response to out-of-range BG values, even after appropriate education,20,22 due to a variety of barriers, which can be due to practical, emotional, or psychological reasons.23–25
This review evaluates the impact of pattern management based on SMBG in clinical practice. We present a review of the literature relating to structured approaches to SMBG and pattern management, discuss obstacles to implementation of SMBG and pattern management, and offer suggestions for how physicians and patients can work together to make the most of this key feature of diabetes management.
Review of Literature: Method
A structured approach to SMBG and pattern management is crucial in diabetes management. However, there is no consensus in the literature as to how SMBG should be structured to provide the necessary data to detect BG patterns. A search on PubMed was executed using the search terms “pattern(s) management diabetes SMBG,” “structured testing diabetes SMBG,” and “pattern(s) analysis diabetes SMBG.” Limiting the search to publications written in the English language over June 2002–June 2012 (excluding published conference abstracts), we found 23 publications. From these, we selected those publications that described results of clinical trials assessing structured SMBG, either prospectively or retrospectively. The resulting eight publications are summarized in Table 1 and later. The remaining papers were reviews or opinion articles.
Table 1.
Summary of Clinical Studies Assessing Pattern Management
| First author | Number of study participants, study duration | Comparators | Main outcomes |
| Polonsky16 |
|
Cluster randomized to structured testing using a paper tool and seven-point testing + education or active control receiving routine care (ACG) |
|
| Polonsky26 |
|
Cluster randomized to structured testing (STG) using a paper tool and seven-point testing + education or active control receiving routine care (ACG) |
|
| Polonsky27 | 36 primary care professionals and 25 internists |
|
|
| Lalic28 |
|
Structured glucose monitoring and lifestyle adjustments: seven-point SMBG profile on three consecutive days prior to baseline and months 1, 2, and 3; routine BG test (>1 test per day); use of Accu-Chek 360o |
|
| Rodbard29 | 288 clinicians: 40% family physicians, 38% internists, and 22% nurse practitioners | Review of 30 cases with either: structured SMBG data, structured SMBG with decision support tool, structured SMBG with DVD, or structured SMBG with decision support tool + DVD |
|
| Cox30 |
|
Retrospective evaluation of SMBG data to look for prediction of severe hypoglycemia events |
|
| Kempf31 |
|
SMBG + lifestyle intervention: seven-point SMBG profile at baseline and at weeks 4, 8, and 12; healthy diet and physical activity; make changes to lifestyle based on SMBG results |
|
| Hansen32 | 1076 individuals with T1DM |
|
|
Much of the information that is currently available about how SMBG might be structured to optimize outcomes comes from the Structured Testing Program (STeP) study. The STeP study was a multicenter trial involving 483 individuals with poorly controlled, insulin-naive T2DM from 34 primary care practices in the United States. The practices were cluster randomized to an active control group (ACG) with enhanced usual care or a structured testing group (STG) with enhanced usual care and at least quarterly use of structured SMBG results. STG patients used a paper tool that graphed seven-point glucose profiles over 3 consecutive days while physicians received a treatment algorithm based on SMBG patterns. The primary endpoint was HbA1c level measured at 12 months. Findings from the study16,26 revealed that appropriate use of structured SMBG data led to earlier, more frequent, and more effective treatment modification recommendations and improved glycemic control in these patients. Compared with ACG patients, significantly more STG patients received treatment modification recommendations, experienced significantly greater reductions in HbA1c, and received more timely/aggressive treatment changes. Polonsky and coauthors27 explored whether primary care physicians could utilize data collection tools (DCTs), consisting of SMBG data presented in five different formats, to identify glycemic abnormalities accurately in structured, episodic SMBG data and whether use of these data would influence their therapeutic decisions. The five formats were as follows: DCT A, 3-day, seven-point glucose profile; DCT B, 3-day fasting and three post-prandial readings; DCT C, 7-day fasting with postprandial supper; log sheet A, standard log book with two facing pages containing a daily seven-point testing profile (premeal, postmeal, and bedtime) for each day of the week, with daily values aligned vertically; and log sheet B, standard log book on one page containing a daily seven-point testing profile (premeal, postmeal, and bedtime) for each day of the week, with daily values aligned horizontally, with additional space for the patient to calculate and record differences between preprandial and postprandial values. Next, data were presented in different formats to primary care physicians who were asked to evaluate the cases based on HbA1c data alone and then combined with SMBG data, looking for specific glucose patterns, and to determine and select specific therapeutic changes. Most (78%) identified the same primary BG feature identified by diabetes specialists, and 94% agreed with the diabetes care specialists regarding the need for therapy modification. The study showed that primary care physicians were able to use SMBG data appropriately. Correct identification was higher, with more specific formats. Lalic and coauthors28 explored whether a modified version of the STeP intervention could be used in a real-world clinical setting. Individuals with T1DM and T2DM in 11 countries were asked to generate a BG profile once a month for three consecutive months using a paper-based BG analysis tool (Accu-Chek® 360° View® BG analysis system, Roche Diagnostics, Mannheim, Germany).28 Measurements were performed before and 2h after main meals and before bedtime on three consecutive days. This intervention resulted in improvements in diabetes management, with significant improvements in HbA1c, BG and lipid parameters, and blood pressure; acceptance of the structured SMBG tool among patients and physicians was high. In another analysis from the STeP study,29 the addition of an automated decision support tool and/or an educational DVD to structured SMBG data improved the ability of clinicians to identify significant glycemic patterns correctly and to make appropriate therapeutic decisions to address those patterns. This study also highlights the importance of educational approaches in optimizing the use of structured SMBG data.
Information from three other studies has added to the STeP study data or have highlighted the lack of routine SMBG among diabetes patients. In the study by Cox and coauthors,30 routine SMBG readings were retrieved from memory meters and combined with information about episodes of severe hypoglycemia; this information was used to estimate the relative risk for such events. The relative risk of severe hypoglycemia was found to increase significantly in the 24 h before the episodes. A sliding algorithm predicted 58–60% of episodes of severe hypoglycemia when three SMBG readings were available, which increased to 63–75% if five SMBG readings were available, demonstrating the utility of pattern management in predicting severe hypoglycemia. Kempf and coauthors31 evaluated a 12-week lifestyle intervention in the Retrospective Study Self-Monitoring of Blood Glucose and Outcome in Patients with Type-2-Diabetes study. Non-insulin-treated patients with diabetes generated an SMBG profile (seven-point BG diurnal profile) every 4 weeks, in addition to weight, waist circumference, and physical activity (steps/day). Patients who completed the program showed significant reduction in weight, body mass index, waist circumference, BG, blood pressure, low-density lipoprotein cholesterol, and HbA1c, accompanied by increased physical and mental health and reduced depression measurements. The study by Hansen and coauthors32 assessed the frequency of and motives for SMBG using data from a cross-sectional survey of individuals with T1DM and demonstrated just how few SMBG data might be available for pattern management. Patient compliance with SMBG was limited; almost two-thirds of patients did not perform daily SMBG, and one-third did not perform routine tests. Lower HbA1c was associated with more frequent testing.
While these studies have provided valuable information on the value of a structured approach to SMBG and pattern management in diabetes management, specific information on how SMBG might be structured to detect BG patterns, however, remains limited; it is likely that information on pattern management protocols is buried within educational curricula.
While there is no clinical trial data suggesting the appropriate amount of data that should be collected, the American Association of Clinical Endocrinologists recommend that intensive episodic SMBG could be done in patients who (a) have been having recurrent hypoglycemia; (b) are undergoing changes in medication, i.e., steroids; and (c) have worsening HbA1c values.
Addressing Barriers to Pattern Management
Even after receiving training, patients may not perform recommended therapeutic tasks such as measuring BG or dose-adjusting insulin in response to BG patterns for a variety of emotional/psychological reasons23–25 (Figure 1). These reasons may be complex and include lack of motivation (they do not wish to do it), lack of appropriate beliefs (they believe that they cannot do it, that it is unnecessary, that the hassle of adherence outweighs the potential advantage of the reduced risk of long-term complications), or fear of negative consequences. Fear of negative consequences, for example, may lead patients to enter incorrect data in their log books; studies have shown that a large proportion of patients with T1DM (65%) recorded entries in a way that obscured hyperglycemia or hypoglycemia, and one-third of patients with T2DM keep inaccurate diaries.33
Figure 1.
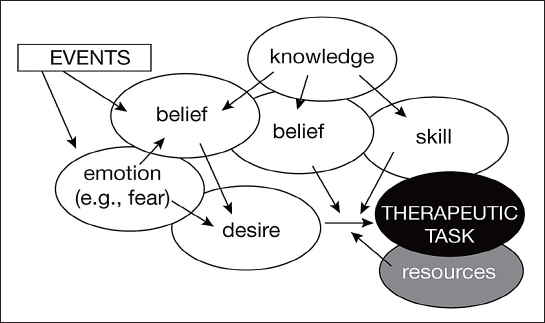
A mental-states-based model of adherence. Desire, associated with appropriate beliefs, is the driving force, while knowledge and skills have an indirect role. Emotions can lead to a revision of beliefs and desires. An example is the fear triggered by a severe hypoglycemic episode. Resources have a permissive role. Adapted with permission from Diabetes and Metabolism.24
Awareness of decision-making theories may help us understand the psychology underlying the “lack of appropriate beliefs” obstacle to dose adjustment and pattern management.34 First, immediacy as well as concreteness of reward may influence decision making.35 A key principle of diabetes management is that patients are indeed asked to engage in difficult diet and lifestyle changes and to adhere to therapy that may have short-term unpleasant side effects. In return, they may gain a reduced risk of serious—but theoretical and seemingly distant—consequences. The immediate, certain, positive outcome of avoiding the inconvenience of BG measurement, pattern assessment, and dose adjustment is assigned more weight than the long-term, uncertain, possible positive outcomes of reduced diabetes complications. In short, “people often prefer smaller rewards sooner to larger rewards later.”36 This discrepancy may represent a barrier to the efficiency of patient education. Kahneman and Tversky’s prospect theory points out that aversion to losses is greater than attraction to gains37, and this phenomenon may also affect patient behaviors concerning insulin dose adjustment.25
Secondly, according to Kahneman and Tversky,38 when having to make a decision under uncertainty, people rely on heuristics—rapid thinking processes that are usually efficient but can lead to bias. For instance, the availability heuristic is used when individuals are asked to predict the likelihood of an event: they base their answer on how easily an example of such an event happening previously comes to mind. In the case of SMBG, it will tend to overestimate the risk for hypoglycemia because patients remember hypoglycemic events more readily than normal BG values; this is due to the greater power of bad events over good ones in learning processes.39 People use heuristics when they have a difficult question to answer, and one can understand how, in the case of diabetes management, glycemic variability represents an incentive to use them.
We suggest that a structured approach to SMBG and automated pattern recognition may address obstacles to effective diabetes management (Table 2). First, SMBG provides immediate, positive, and concrete feedback. Also, SMBG and the accompanying education about interpreting the data may potentially enable the patient to see the impact of adjustment to diet, activity, or insulin dose on BG in the short term and to feel rewarded for his or her action; studies are needed to test this hypothesis. Second, when patients are confronted with glycemic variability (both intraday and interday), an obstacle to pattern management may be the time and tedium involved with BG measurement and analysis, followed by calculation of appropriate dose adjustment. This leads patients to use Kahneman and Tversky’s38 heuristics with their source of bias, including the overestimation of the risk of hypoglycemia. Automating as many of these functions as possible may help support patients and increase use of pattern analysis in diabetes management. This is discussed in the next section of this article.
Table 2.
Creating Order From Disorder: How SMBG and Automated Pattern Recognition Can Address Barriers to Self-Adjustment of Insulin Doses
| Obstacle | Solution |
|
|
|
|
|
|
|
|
Tools for Pattern Detection and Management
Incorporating pattern management into practice requires detection of patterns. A first step in this is establishing target and baseline values for preprandial and postprandial BG. Patients must document carbohydrate intake, medication use (including rotation of the insulin injection site),40 physical activity, and psychological factors (such as stress) that may affect BG levels.
Pattern management further involves uncovering how a pattern originates, followed by conducting a retrospective review to connect meals, medication, or other causes to an excursion. Patterns may identify issues with the basic structure of the regime, i.e., inadequate overnight insulin or inadequate prandial insulin or may identify specific behaviors such as inadequate or overzealous correction of high or low readings or hypoglycemia caused due to exercise or alcohol. Looking at “modal day” data can provide some information toward structural issues, and looking at outlier data, especially episodes of hypoglycemia or very high glucose, can provide information on some behavioral aspects. Knowing about common patterns can help identify them through what can be quite a daunting maze of numbers. Information on exercise, alcohol, and food intake are essential in making sense of the data.
Tools to Aid Pattern Recognition
Paper Log Book
The traditional paper log book offers the advantages of simplicity and a minimal learning curve. Filling in the log book may also help the patient to appreciate the real frequency of high, normal, or low BG, though this has not been proven empirically. It has the disadvantage of being labor intensive for both the patient and health care provider (HCP). An exploratory study determined that 78% of primary care physicians were able to identify the primary glucose abnormality in sample cases using a validated paper log (Accu-Chek 360° View BG analysis system).27 Augmenting this paper log book with an automated decision support tool improved the ability of primary care physicians to identify the primary glycemic abnormalities correctly compared with the paper tool alone. The automated decision support tool algorithm analyzes SMBG data recorded in the paper log book, generates a report identifying the primary glycemic abnormality, and recommends therapeutic options.41
Meters with Add-On Devices or Applications
There are a number of meters and smartphone applications available that can use data such as insulin-to-carbohydrate ratio, BG targets, and insulin sensitivity to calculate appropriate short-acting insulin such as FreeStyle InsuLinx®, Abbot Diabetes Care, Alameda, CA; Roche Expert, Roche; and iBGStar® (Sanofi-Aventis, Frankfurt, Germany). These devices to provide a “calculator” function but do not actively monitor patterns or offer advice on changing those settings in response to patterns.
On-Meter Software
The OneTouch® Verio®Pro and VerioIQ BG meters (LifeScan Inc., Milpitas, CA) have pattern detection on the actual meter via the high and low pattern tool. The high and low pattern tool can be set to alert users automatically in real time to the possible development of high or low BG patterns, with high and low limits customized for each patient.42 These are currently the only systems on the market that have this on-meter pattern alert.
Off-Meter Software
Most manufacturers of home BG meters offer SMBG analysis software solutions. The off-meter OneTouch Diabetes Management Software and the OneTouch ZoomPro software (both from LifeScan Inc.) offer periodical assessment of seven-point testing. OneTouch Diabetes Management Software highlights before- and after-meal patterns, while OneTouch ZoomPro includes an optional pattern recognition feature. One limitation is that the software examines absolute values, whereas relative changes are of more importance for pattern recognition.
Online Tools
Several online tools enable patients to upload data from BG meters for viewing and analysis by HCPs. Clinicians may then obtain a variety of reports. Examples of these platforms include CareLink Pro (http://www.medtronic.com/for-healthcare-professionals/products-therapies/diabetes/diabetes-management-software/careLink-pro-diabetes-therapy-management-software/index.htm), Diasend (http://diasend.com/site/index.php?lang=en), and DIABASS (http://www.mediaspects.com/index.hp?lang=en&key=diabass5). These tools aim to facilitate communication between HCPs and patients. The CareLink Pro software does offer some pattern recognition facilities, identifying issues that may predispose to high or low readings, However, this requires at least five days of CGM over the past week.43
Technological support offers the advantages of improved accuracy and convenience over paper logs, the capacity to display data in a variety of forms that may be impactful for patients and HCPs, and the ability to store and share findings electronically. Standardizing pattern analysis algorithms in software would ensure consistent application of the desired protocols. Addressing issues of electronic information privacy and virus contamination may facilitate the use of this technology.
We would like to include a caveat here: by knowing that the data are stored in the meter’s memory, there is the possibility that the patient does not fill their log book, does not use the technology, or does not adjust the insulin doses and gets emotionally disconnected from the data and the appropriate actions. An important task of HCPs is to prevent this potential disconnect. Indeed, it is important to point out that when we speak about the “memory” of a glucometer, it is only a metaphor: human memory not only stores data, but in addition is governed by emotions and thus has a teaching effect aimed at optimizing future actions.39 This emotional effect may be missing in the passive filling of the meter’s “memory,” disconnecting emotions from memory.
Optimizing the Patient–Health Care Provider Relationship
Self-monitoring of BG and pattern management require significant commitment from both the patient and the HCP. Guidelines stress the importance (in both patients and HCPs) of sufficient understanding, training, skills, and willingness to undertake SMBG, as well as desire to use this information to adjust therapy to agreed treatment goals.44,45 One of the key principles underlying successful pattern management is that HCPs must ensure that patients are appropriately trained in SMBG use and interpretation (Table 3). Another key principle is that HCPs must review SMBG results consistently, use the BG data to guide changes in therapy, and communicate to patients how BG data influence their care so that pattern management has motivational value.
Table 3.
| Patient requirements | HCP requirements |
|
|
|
|
|
|
|
|
|
|
It is important to optimize the patient–HCP relationship. Some patients report frustration that HCPs show no interest in their BG records or BG fluctuations, making therapeutic adjustments based on HbA1c values only.47 A similar complaint may be around the difficulty in obtaining data from devices from different companies, the clinician’s computer often resembling an octopus with numerous cords for each manufacturer. This also leads to problems in identifying data from different software programs. This problem can be mitigated to some degree through some commercial software solutions such as Diasend, which can import data into a common platform, making it easier for the clinician to access relevant information. Commending patients for taking the time to collect BG data, along with assisting them in creating a simple action plan with instructions for responding to high or low BG readings can improve BG patterns, may encourage patients to persist with SMBG, and may improve the patient–HCP relationship. Health care professionals should also ensure that they react to patient SMBG data in a nonjudgmental manner to avoid discouraging individuals who obtain insufficient readings or respond inappropriately to a BG finding.45
Conclusions
Interpretation of BG data patterns can shed light on the reasons for poor glycemic control and suggest possible management strategies. Diabetes management based on BG patterns with appropriate education has been associated with reduced HbA1c levels, lower incidence of hypoglycemia, and prediction of severe hypoglycemia, but more clinical trials are warranted to confirm the clinical relevance of these associations.
Self-monitoring of BG is the most accessible way to assess glycemic patterns in real time. Patterns are not always easy to detect or interpret, but on-meter and web-based tools can support both patients and clinicians. Successful pattern management requires education and mutual commitment from the HCP and patient. The two must establish an ongoing collaboration to obtain, review, and interpret SMBG values and to make changes based on the patterns in those values.
Glossary
- (ACG)
active control group
- (BG)
blood glucose
- (CGM)
continuous glucose monitoring
- (DCT)
data collection tools
- (HbA1c)
hemoglobin Ale
- (HCP)
health care provider
- (SMBG)
self-monitoring of blood glucose
- (STeP)
Structured Testing Program
- (STG)
structured testing group
- (T1DM)
type 1 diabetes mellitus
- (T2DM)
type 2 diabetes mellitus
Funding
The authors received editorial and writing support from Excerpta Medica, sponsored by LifeScan Inc., in the preparation of the initial draft. This work was supported by an unrestricted grant from LifeScan Inc. The views expressed in this publication are those of the authors; the sponsors did not have access to the manuscript prior to publication.
Disclosures
Pratik Choudhary has served as an advisory board member for and has received speaker fees from LifeScan Inc., Animas, Roche, and Medtronic. Stefano Genovese has participated in advisory board meetings for LifeScan Inc., Abbott Laboratories, Boehringer-Ingelheim GmbH, Eli Lilly and Co., and Takeda Pharmaceutical Company Limited and has received speaker fees from GlaxoSmithKline plc, Novartis International AG, LifeScan Inc., Eli Lilly and Co., and Abbott Laboratories. Gérard Reach has received speaker fees from Novo-Nordisk AS, Eli Lilly and Co., Novartis International AG, Sanofi-Aventis, Merck and Co., GlaxoSmithKline plc, Ipsen, Abbott Laboratories, Bristol-Myers Squibb, Pfizer Inc., Roche, LifeScan Inc., and Bayer and research grant support from LifeScan Inc. and has participated in advisory board meetings for LifeScan Inc., Bayer, Eli Lilly and Co., and Novo-Nordisk AS.
References
- 1.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complications Trial Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1997;46(2):271–286. [PubMed] [Google Scholar]
- 4.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010;33(6):1389–1394. doi: 10.2337/dc09-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57(12):3169–3176. doi: 10.2337/db08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCrimmon RJ, Frier BM. Hypoglycaemia, the most feared complication of insulin therapy. Diabete Metab. 1994;20(6):503–512. [PubMed] [Google Scholar]
- 8.Thompson CJ, Cummings JF, Chalmers J, Gould C, Newton RW. How have patients reacted to the implications of the DCCT? Diabetes Care. 1996;19(8):876–879. doi: 10.2337/diacare.19.8.876. [DOI] [PubMed] [Google Scholar]
- 9.Pickup JC, Kidd J, Burmiston S, Yemane N. Determinants of glycaemic control in type 1 diabetes during intensified therapy with multiple daily insulin injections or continuous subcutaneous insulin infusion: importance of blood glucose variability. Diabetes Metab Res Rev. 2006;22(3):232–237. doi: 10.1002/dmrr.614. [DOI] [PubMed] [Google Scholar]
- 10.Alemzadeh R, Loppnow C, Parton E, Kirby M. Glucose sensor evaluation of glycemic instability in pediatric type 1 diabetes mellitus. Diabetes Technol Ther. 2003;5(2):167–173. doi: 10.1089/152091503321827821. [DOI] [PubMed] [Google Scholar]
- 11.Service FJ, O’Brien PC. The relation of glycaemia to the risk of development and progression of retinopathy in the Diabetic Control and Complications Trial. Diabetologia. 2001;44(10):1215–1220. doi: 10.1007/s001250100635. [DOI] [PubMed] [Google Scholar]
- 12.Bergenstal RM, Gavin JR 3rd. Global Consensus Conference on Glucose Monitoring Panel. The role of self-monitoring of blood glucose in the care of people with diabetes: report of a global consensus conference. Am J Med. 2005;118(Suppl 9A):1S–6S. doi: 10.1016/j.amjmed.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 13.Dailey G. Assessing glycemic control with self-monitoring of blood glucose and hemoglobin A(1c) measurements. Mayo Clin Proc. 2007;82(2):229–236. doi: 10.4065/82.2.229. [DOI] [PubMed] [Google Scholar]
- 14.St John A, Davis WA, Price CP, Davis TM. The value of self-monitoring of blood glucose: a review of recent evidence. J Diabetes Complications. 2010;24(2):129–141. doi: 10.1016/j.jdiacomp.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R. ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28(6):1282–1288. doi: 10.2337/diacare.28.6.1282. [DOI] [PubMed] [Google Scholar]
- 16.Polonsky WH, Fisher L, Schikman CH, Hinnen DA, Parkin CG, Jelsovsky Z, Petersen B, Schweitzer M, Wagner RS. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34(2):262–267. doi: 10.2337/dc10-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson J, Bergenstal R. Fine-tuning control: pattern management versus supplementation. View 1: pattern management: an essential component of effective insulin management. Diabetes Spectr. 2001;14:75–78. [Google Scholar]
- 18.DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ. 2002;325(7367):746. doi: 10.1136/bmj.325.7367.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sämann A, Mühlhauser I, Bender R, Hunger-Dathe W, Kloos C, Müller UA. Flexible intensive insulin therapy in adults with type 1 diabetes and high risk for severe hypoglycemia and diabetic ketoacidosis. Diabetes Care. 2006;29(10):2196–2199. doi: 10.2337/dc06-0751. [DOI] [PubMed] [Google Scholar]
- 20.Sämann A, Mühlhauser I, Bender R, Kloos Ch, Müller UA. Glycaemic control and severe hypoglycaemia following training in flexible, intensive insulin therapy to enable dietary freedom in people with type 1 diabetes: a prospective implementation study. Diabetologia. 2005;48(10):1965–1970. doi: 10.1007/s00125-005-1905-1. [DOI] [PubMed] [Google Scholar]
- 21.Pieber TR, Brunner GA, Schnedl WJ, Schattenberg S, Kaufmann P, Krejs GJ. Evaluation of a structured outpatient group education program for intensive insulin therapy. Diabetes Care. 1995;18(5):625–630. doi: 10.2337/diacare.18.5.625. [DOI] [PubMed] [Google Scholar]
- 22.Choleau C, Albisser AM, Bar-Hen A, Bihan H, Campinos C, Gherbi Z, Jomaa R, Aich M, Cohen R, Reach G. A novel method for assessing insulin dose adjustments by patients with diabetes. J Diabetes Sci Technol. 2007;1(1):3–7. doi: 10.1177/193229680700100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reach G. Can technology improve adherence to long-term therapies? J Diabetes Sci Technol. 2009;3(3):492–499. doi: 10.1177/193229680900300313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reach G. Patient non-adherence and healthcare-provider inertia are clinical myopia. Diabetes Metab. 2008;34(4 Pt 1):382–385. doi: 10.1016/j.diabet.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Reach G. A psychophysical account of patient non-adherence to medical prescriptions. The case of insulin dose adjustment. Diabetes Metab. 2013;39(1):50–55. doi: 10.1016/j.diabet.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Polonsky WH, Fisher L, Schikman CH, Hinnen DA, Parkin CG, Jelsovsky Z, Axel-Schweitzer M, Petersen B, Wagner RS. A structured self-monitoring of blood glucose approach in type 2 diabetes encourages more frequent, intensive, and effective physician interventions: results from the STeP study. Diabetes Technol Ther. 2011;13(8):797–802. doi: 10.1089/dia.2011.0073. [DOI] [PubMed] [Google Scholar]
- 27.Polonsky WH, Jelsovsky Z, Panzera S, Parkin CG, Wagner RS. Primary care physicians identify and act upon glycemic abnormalities found in structured, episodic blood glucose monitoring data from non-insulin-treated type 2 diabetes. Diabetes Technol Ther. 2009;11(5):283–291. doi: 10.1089/dia.2008.0087. [DOI] [PubMed] [Google Scholar]
- 28.Lalic N, Tankova T, Nourredine M, Parkin C, Schweppe U, Amann-Zalan I. Value and utility of structured self-monitoring of blood glucose in real world clinical practice: findings from a multinational observational study. Diabetes Technol Ther. 2012;14(4):338–343. doi: 10.1089/dia.2011.0186. [DOI] [PubMed] [Google Scholar]
- 29.Rodbard HW, Schnell O, Unger J, Rees C, Amstutz L, Parkin CG, Jelsovsky Z, Wegmann N, Axel-Schweitzer M, Wagner RS. Use of an automated decision support tool optimizes clinicians’ ability to interpret and appropriately respond to structured self-monitoring of blood glucose data. Diabetes Care. 2012;35(4):693–698. doi: 10.2337/dc11-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox DJ, Gonder-Frederick L, Ritterband L, Clarke W, Kovatchev BP. Prediction of severe hypoglycemia. Diabetes Care. 2007;30(6):1370–1373. doi: 10.2337/dc06-1386. [DOI] [PubMed] [Google Scholar]
- 31.Kempf K, Kruse J, Martin S. ROSSO-in-praxi: a self-monitoring of blood glucose-structured 12-week lifestyle intervention significantly improves glucometabolic control of patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2010;12(7):547–553. doi: 10.1089/dia.2010.0008. [DOI] [PubMed] [Google Scholar]
- 32.Hansen MV, Pedersen-Bjergaard U, Heller SR, Wallace TM, Rasmussen AK, Jørgensen HV, Pramming S, Thorsteinsson B. Frequency and motives of blood glucose self-monitoring in type 1 diabetes. Diabetes Res Clin Pract. 2009;85(2):183–188. doi: 10.1016/j.diabres.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 33.Mazze RS, Shamoon H, Pasmantier R, Lucido D, Murphy J, Hartmann K, Kuykendall V, Lopatin W. Reliability of blood glucose monitoring by patients with diabetes mellitus. Am J Med. 1984;77(2):211–217. doi: 10.1016/0002-9343(84)90693-4. [DOI] [PubMed] [Google Scholar]
- 34.Reach G. Two character traits associated with adherence to long term therapies. Diabetes Res Clin Pract. 2012;98(1):19–25. doi: 10.1016/j.diabres.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Ainslie G. Précis of breakdown of will. Behav Brain Sci. 2005;28(5):635–673. doi: 10.1017/S0140525X05000117. [DOI] [PubMed] [Google Scholar]
- 36.Reach G. Obstacles to patient education in chronic diseases: a trans-theoretical analysis. Patient Educ Couns. 2009;77(2):192–196. doi: 10.1016/j.pec.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk. Econometrica. 1979;47(2):263–292. [Google Scholar]
- 38.Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124–1131. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- 39.Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Rev Gen Psychol. 2001;5(4):323–370. [Google Scholar]
- 40.Teft G. Lipohypertrophy: patient awareness and implications for practice. J Diabetes Nurs. 2002;6(1):20–23. [Google Scholar]
- 41.Rodbard HW, Schnell O, Unger J, Rees C, Amstutz L, Parkin CG, Jelsovsky Z, Wegmann N, Axel-Schweitzer M, Wagner RS. Use of an automated decision support tool optimizes clinicians’ ability to interpret and appropriately respond to structured self-monitoring of blood glucose data. Diabetes Care. 2012;35(4):693–698. doi: 10.2337/dc11-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LifeScan. OneTouch® Verio™Pro. Owner’s booklet. http://www.lifescan.co.uk/sites/default/files/pdf/booklets/06687701AOTVROOBGBenzugR8Webnew.pdf Accessed April 2012.
- 43.Welsh JB, Myers SJ, Uhrinak AN, Kaufman FR, Lee SW. User acceptability and perceived benefits of new reports in CareLink Pro 3.0 Therapy Management Software for Diabetes. J Diabetes Sci Technol. 2012;6(2):481–482. doi: 10.1177/193229681200600240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.International Diabetes Federation. Guideline. Self-monitoring of blood glucose in non-insulin treated type 2 diabetes. 2009. http://www.idf.org/webdata/docs/SMBG_EN2.pdf Accessed April 2012.
- 45.Klonoff DC, Bergenstal R, Blonde L, Boren SA, Church TS, Gaffaney J, Jovanovic L, Kendall DM, Kollman C, Kovatchev BP, Leippert C, Owens DR, Polonsky WH, Reach G, Renard E, Riddell MC, Rubin RR, Schnell O, Siminiero LM, Vigersky RA, Wilson DM, Wollitzer AO. Consensus report of the coalition for clinical research-self-monitoring of blood glucose. J Diabetes Sci Technol. 2008;2(6):1030–1053. doi: 10.1177/193229680800200612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parkin CG, Davidson JA. Value of self-monitoring blood glucose pattern analysis in improving diabetes outcomes. J Diabetes Sci Technol. 2009;3(3):500–508. doi: 10.1177/193229680900300314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peel E, Douglas M, Lawton J. Self monitoring of blood glucose in type 2 diabetes: longitudinal qualitative study of patients’ perspectives. BMJ. 2007;335(7618):493. doi: 10.1136/bmj.39302.444572.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]


