Abstract
Individually, sleep disturbances and type 2 diabetes pose pervasive challenges to health. In addition, the negative symptomology associated with each condition is exacerbated further when presenting concomitantly. This relationship formulates a destructive loop wherein those with diabetes experience decreased sleep quality, which, in turn, worsens a wide range of health threats experienced by those with diabetes, including obesity and glucose intolerance. Because major lifestyle changes and daily care are needed to effectively manage both diabetes and sleep disturbances, an efficient and timely modality of treatment is essential. Advanced technology incorporating telemedicine and telehealth has the potential to enhance treatment by delivering accepted standard of care, medical monitoring, and education quickly and seamlessly—even in rural locations. This type of intervention has the added potential benefit of fostering patient empowerment.
Keywords: diabetes, insulin, obesity, obstructive sleep apnea, sleep disorders, telemedicine
Introduction
Type 2 diabetes is a prolific public health problem resulting in increased mortality, morbidity, and diminished quality of life, affecting an estimated 25.8 million people, and is the sixth leading cause of death in Americans.1–3 Approximately 1.9 million new diabetes cases present each year, and 79 million prediabetes cases are currently being followed. Moreover, it is estimated that these numbers will double by the year 2050.1,3,4 This increase in diabetes diagnoses has been associated with cardiovascular events that may lead to further morbidity and mortality.5,6 Although diabetes management is improving, complications are still common, and diabetes remains the leading cause of visual loss, amputation, and end stage of renal disease in the United States.2,5,6
Similar to diabetes, sleep disturbances are related to myriad health problems, affecting approximately 70 million Americans.1 The extent to which sleep disorders (SDs) affect overall health and functionality has not been clearly ascertained because significant inadequate diagnoses and underdiagnoses of SDs; an estimated 85% of SDs go undiagnosed or untreated.3,7 Investigations into the mechanisms involved in SDs are constrained by difficulty in acquiring valid data from intensive tests such as polysomnography (PSG) and multiple sleep latency tests rather than relying on simple self-report data. The impact of SDs on general health is further impacted by a multitude of intervening variables associated with disease symptomatology.3
The concurrence of SDs and diabetes necessitates aggressive therapy to treat and control both conditions.3 Telemedicine, or the use of computer equipment and communication to provide health care, may serve as an effective treatment option. Through utilizing communication technology, telemedicine can both administer treatment and apply education seamlessly over thousands of miles, avoiding certain barriers that in-person treatment often experiences, such as distance, language, and cultural customs.8,9 It has the ability to quickly collect, transmit, and incorporate data, making it a swift and viable means of communication between patients and their providers. Recently, telemedicine has begun to emerge as an effective alternative modality of care in chronic diseases that require constant care, such as diabetes, heart failure, and chronic obstructive pulmonary disease.10 The increased interest in employing telemedicine techniques in medicine has created a newfound interest in developing telehealth to treat multiple disorders simultaneously, such as diabetes and SDs.
Comorbidity of Diabetes and Sleep Disorders
The comorbidity of diabetes and SDs is complex, as each condition exacerbates the other. This results in a pernicious loop wherein diabetes decreases sleep quality while sleep disturbances increase the risk of developing diabetes.11 Those who experience diabetes most commonly report sleep-disordered breathing, severe hypoxemia, recurrent arousals, poor sleep efficiency, abnormal sleep architecture, and excessive daytime sleepiness.2,5,12,13 Individuals adjusting to diabetes often experience prolonged sleep latency and stay asleep longer because of the emotional and physical distress of the disease, while people with poorly controlled diabetes report poor sleep quality, decreased sleep duration, and higher levels of sleepiness due to shortened sleep.1,14,15 Thus, difficulty initiating and maintaining sleep is commonly reported in people living with diabetes.1
The incidence of diabetes has experienced a significant proliferation in the American population since the 1990s.2 This increased prevalence of SDs and type 2 diabetes diagnoses appears to be in direct correlation to the epidemic of obesity and increasing body mass index (BMI).16 Factors such as insulin resistance, glucose intolerance, abnormal sleep architecture, and sleep apnea also indicate recognizable causality.9 These factors are primarily influenced by family history, aging, weight gain, and lack of exercise.5,12,17 Obesity, particularly abdominal obesity, is recognized as a risk factor for insulin resistance, hypertension, metabolic syndrome, and obstructive sleep apnea (OSA).17 As obesity increases the risk of SDs, hypertension, and insulin resistance, the risk of type 2 diabetes also increases.17
Emerging evidence indicates that SDs may contribute to the onset or exacerbation of glucose intolerance in people with diabetes.1,16,18 Chronic sleep loss may increase obesity and exacerbate diabetes through its effects on appetite and satiety. By altering glucose regulation, increased cortisol concentrations elevate appetite-stimulating hormones that can lead to over consumption.17–19 In people with OSA hypopnea syndrome, leptin levels (associated with satiety) are elevated by approximately 50% compared with controls, indicating leptin resistance.20 Decreased leptin sensitivity in sleep deprivation may contribute to high BMI.1 Ghrelin levels, which increase appetite and decrease metabolic rate, are also elevated in sleep deprivation.1,17–19
This may provide plausible explanations for the interrelatedness of obesity and SDs while supporting a strong association between sleep and diabetes.1,17–19
Currently, the U.S. population as a whole spends less time asleep compared with the past decade; overall sleep duration has decreased by 1.5–2 hours.2,9,16,17 Today, roughly one-third of adults report sleeping less than 6 h each night.10,21 Numerous studies have found that abnormal lengths of sleep (less than 6 h or greater than 9 h) are related to destabilizing health outcomes.2 Sleep duration and sleep quality appear to be significant risk factors for diabetes due to hormonal alterations.17 In fact, research has demonstrated that healthy subjects who experience partial sleep deprivation during a single night had induced insulin resistance in multiple metabolic pathways.1,22 Furthermore, short-term sleep deprivation in people without diabetes results in impaired glucose tolerance, indicating that sleep is essential for metabolic homeostasis.19,23,24 Chronic decreased sleep duration (less than 7 h) is associated with an increased risk for insulin resistance and glucose intolerance.2 Five to six hours of habitual sleep may project prevalence of impaired glucose tolerance and diabetes; due to its exacerbation of weight gain, abnormal glucose levels, and an increase of low-grade inflammation, chronic decreased sleep is often indicative of a prediabetic condition.1,17,25,26
Previous investigations in our own research showed significant differences in the sleep architecture of people diagnosed with diabetes and an SD compared with those who were diagnosed with an SD only. On average, those diagnosed with both diabetes and an SD had a significantly higher BMI, slept fewer hours, spent more time in stage N1 sleep, and spent less time in stages N3 and rapid eye movement (see Table 1).3
Table 1.
Sleep Architecture in Sleep Disorders and Diabetes
| Diabetes and SD | SD only | |
| Stage 1 (%)a | 18.34 | 13.50 |
| Stage 2 (%) | 65.88 | 65.23 |
| Stage 3 (%)b | 2.03 | 3.33 |
| Stage 4(%)b | 1.26 | 3.40 |
| Rapid eye movement (%)b | 11.91 | 14.53 |
| Total time asleep (min)a | 302.80 | 340.28 |
Significant at p < 0.001.
Significant at p < 0.01.
Diabetes and Obstructive Sleep Apnea
Among SDs, OSA is highly prevalent and has been studied in particular detail. It is estimated that 2–5% of the general population has been diagnosed with OSA or other breathing abnormalities.27–29 This prevalence is even higher in those diagnosed with diabetes.15 Still, the majority of people with type 2 diabetes may have undiagnosed OSA, which is associated with poorer glucose control.2,12,30 Sleep-disordered breathing often results in decreased quality of life due to sleep deprivation, excess daytime sleepiness, and neurocognitive impairment.31 The resultant decreased sleep duration and impaired quality of sleep from OSA indirectly influences metabolism, having an effect on the development or exacerbation of diabetes.2,5,12
By way of sleep fragmentation, OSA is associated with increases in glucose and insulin resistance levels, predisposing a person to diabetes.1,2,5,12,32 Indeed, people who have OSA but not diabetes have presented with evidence of insulin resistance.1,33,34 Hypoxemia, or reduced levels of oxygen in the circulating blood, is frequently observed in people with OSA and may indicate a relationship between OSA and dysfunctional glucose metabolism, as it is associated with abnormal sympathetic activity and increased glycogen breakdown.2,5,12 In addition, sleep-disordered breathing such as hypopneas, apneas, hypoxemia, and frequent arousals also contribute to metabolic and insulin resistance in diabetes.1
This link between OSA and diabetes is of concern, particularly because of their independent links to cardiovascular morbidity and mortality that can be amplified when experienced together. Indeed, OSA exacerbates the dangers and likelihood of cardiovascular disease and stroke in those with a high BMI and diabetes.32
Managing Diabetes and Sleep Disorders
It is evident the concurrence of diabetes and SDs may present unique challenges for daily self-management and treatment, as cognition, emotional well-being, and general health may be compromised, thereby decreasing overall quality of life (see Figure 1).3 Appropriate and timely treatment of both SDs and diabetes is vital for the successful reduction of sleep abnormalities, daytime sleepiness, cardiovascular risk, diabetes severity, obesity, and depressive symptoms.3
Figure 1.
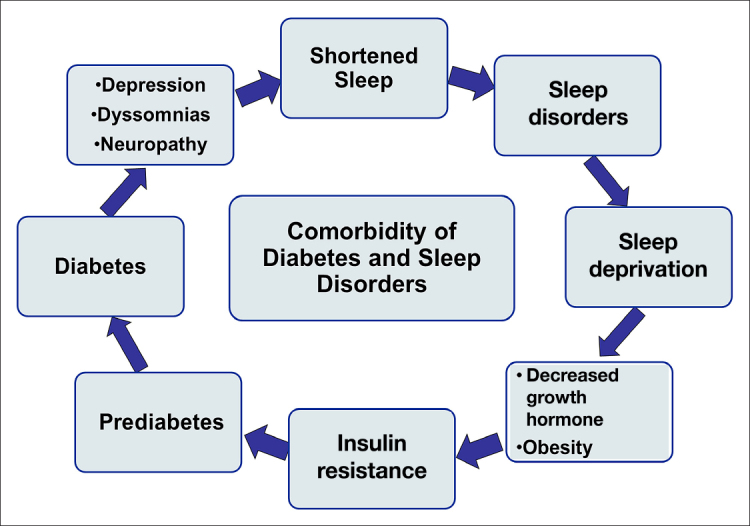
Pernicious cycle associated with diabetes and SDs.
Individuals with diabetes are compelled to make daily decisions involving treatment, along with lifestyle changes in diet, exercise, and coping strategies.35,36 This additional workload in day-to-day life can be daunting, particularly for individuals who are depressed, are in pain, have low motivation, and/or have a lack of social, emotional, and medical support. Poor patient–provider relationships and difficulty accessing adequate medical care may lead to poor diabetes self-management.35,37 Indeed, fewer than one-third of people with diabetes report being able to effectively manage their disease, while more than half of people with diabetes report significant distress related to their illness.35 The prevalence of inadequately managed diabetes is, in part, due to the potentially high cost of medical tests, treatments, and management.27 This is essential to understand, because diabetes can proliferate into myriad associated complications, such as hypertension and painful neuropathy.1,35 Thus, it is increasingly important to investigate both prevention and treatment of diabetes.3
Currently, continuous positive airway pressure (CPAP) is the most common tool used to treat SDs (specifically OSA). This noninvasive treatment operates by ventilating air through the mouth and nose during sleep, thereby decreasing sleep fragmentations and other sleep abnormalities.5 Utilizing CPAP intervention on a regular basis results in enhanced maintenance of normal glucose levels, improved insulin sensitivity, and better maintenance of appropriate leptin and ghrelin hormone levels.5,12 Previous research has demonstrated that those diagnosed both with diabetes and an SD who were complaining of fatigue and excessive daytime sleepiness were prescribed a CPAP for several months. After utilizing the CPAP, insulin sensitivity increased markedly.38 Further, compared with people diagnosed with an SD only, those diagnosed with diabetes and an SD required elevated average and optimal CPAP pressures.3 Nevertheless, many patients fail to use CPAP treatment consistently and attribute this to mask discomfort and a lack of understanding about the possible solutions to many common sleeping problems.27,31
Receiving adequate treatment for both conditions is challenging. It is important to note that overall costs related to diabetes and SDs can be decreased with effective education, medical assistance, and greater self-reliance. There has been a strong interest in implementing advanced technology such as telemedicine and telehealth to enhance patient care and provide a viable solution to these issues in a variety of health care domains.39–41
Emerging Technologies
Although telemedicine as an alternative mode of treatment is in its infancy, thus far it has showed both great potential and positive outcomes in an array of health-related applications.42–47 In fact, integration of telemedicine in the field of medicine has already shown to improve education, provide substantial diagnosis, and assist in efficient procedures to reduce medical expenditures.7,48–50 In practice, telemedicine serves to alleviate some health care costs by reducing the cost of scheduled office visits and allowing convenient and consistent interactions between patients and medical providers, particularly in the case of people in rural regions.42,51,52 Furthermore, telemedicine improves hospital efficiency and costs by providing the same quality of care with a lower amount of hospital employees and by reducing the need of hospital rooms for care.53
A rapidly expanding body of research has investigated the use of telemedicine in the treatment of diabetes specifically. As both the abilities of technology and the prevalence of diabetes rise, telemedicine is increasingly evaluated as an optimal treatment option.4 It is generally considered that telemedicine promotes consistent follow-ups, review of monitoring data, adherence to practice guidelines, and patient satisfaction.41,42,54 Implementation of telemedicine in treating diabetes specifically can lead to improved diet and exercise knowledge and behaviors, thus reducing the debilitating effects of diabetes.55 This is important, as compliance with recommended dietary and exercise modifications is often indicated as a primary cause for not achieving effective diabetes management. People who take ownership, who are involved in their own care, and possess the knowledge and skills to manage their disease are more likely to comply with lifestyle modifications and treatment regimens, which, in turn, improves clinical outcomes.10 These improvements to the general modality of treatment allow for improved at-home care and condition management while easing the financial strain for the affected individual.
As the field of telemedicine continues to emerge, we too have evaluated its efficacy in the realm of diabetes management. We examined in-home telemonitoring, electronic health records (EHRs), and education management programs for those afflicted with type 2 diabetes. We then compared the use of telemedicine to people receiving standard diabetes care with the goal of facilitating homogeneity. The telemonitoring devices, which were installed in the participant homes, were equipped with blood pressure cuffs, pulse meters, glucose meters, and a digital weight scale. The data obtained from the machine was then transmitted from participant homes to the EHR at the clinic.56
People who utilized the telemedicine technology reported an enhanced ability to make good health care choices and expressed overall support for the benefits of the telehealth approach. Additionally, people who used telemedicine reported that it allowed them to work more closely with a physician and felt that it contributed to health status. Feelings of enhanced education in regards to healthy diet, future lifestyle care, and recommendations of telehealth to others were also reported.56
While telemedicine has been implemented in health care for chronic conditions, it has been only recently implemented in sleep medicine. Sleep telemedicine has mainly been employed to evaluate the efficacy of telemedicine in promoting and reinforcing traditional methods of SD treatment, such as nasal CPAP therapy.42,57,58 It can be used in this realm for diagnostic and therapeutic strategies for sleep apnea syndromes, is cost-effective, and abets the reduction of patient travel.59 For those who experience difficulty with the long-term therapy associated with OSA, CPAP compliance is a struggle; problems with mask interface are the highest cause of CPAP noncompliance.57 In this realm, telemedicine may be applied to reinforce CPAP adherence.57 By addressing patient complaints early in treatment, this form of therapy may prove useful in patient motivation. Additionally, telemedicine provides sleep practitioners with insight into common CPAP-related issues through remote observation.57 Thus, sleep telemedicine has the potential to provide the same level of benefit and efficacy as standard care for CPAP therapy management, while improving CPAP adherence.8,42,57
Thus far, few studies have examined telemedicine in relation to SD diagnoses. This research received little enthusiasm as a result of low recording fidelity due to sensor disconnections during the night.39 However, advances in technology implementing a wireless PSG system resulted in positive feedback from patients, sleep technologists, and physicians alike and are encouraging for the future of at-home PSG systems.39 Utilization of telemedicine in this domain provides the potential for a greater sense of convenience and comfort in an environment more conducive to normal sleep than that of a sleep laboratory.39,60
To best facilitate telemedicine programs, challenges and limitations must be addressed. A common issue in telemedicine applications is the concern that person-to-person interaction is lost. Education may help both professionals and lay people to understand that telemedicine is a health care enhancement, rather than a replacement for traditional mechanisms.61 Furthermore, although technology is rapidly evolving to improve ease of access, people must be abetted to enable understanding.56
Consistency in the telemedicine modality is vital for users to become comfortable with using technology, which is potentially unfamiliar to them, in hospitals and in patient homes. Initial telemedicine encounters must be positive to find initial and inherent value in the programs, and both patients and physicians must be comfortable using this alternative care.40 Patients must be satisfied with the level of patient-to-provider interaction and should understand that telemedicine is not replacing in-person care; rather, it is providing additional treatment options. For the benefit of the physicians, the telemedicine programs should neither impair their ability to care for patients nor disrupt office workflow. Most importantly, programs must meet physician, patient, and hospital needs to maintain adherence at all positions. To accomplish this, telehealth technology should be utilized in a program that encompasses specific components to be applied as a broad heuristic.56 The versatility associated with telemedicine foreshadows the endless potential in telemedicine research that will further advance the medical field. These implications are applicable to the advancement of treatment and care for individuals affected by SD and diabetes, further promoting both traditional and future treatment and interventions (see Figure 2).
Figure 2.
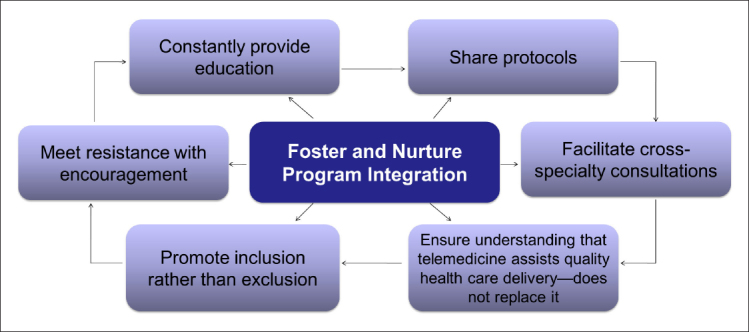
Factors contributing to a successful telemedicine program.
Conclusion
Although the causality of their comorbidity is still under investigation, the destructive relationship shared by abnormal sleep and diabetes poses a serious health threat. Enhancing medical knowledge regarding associations between these two conditions can lead to a better understanding and may also improve treatment for diabetes and SDs as both singular and concurrent conditions. Because of shared symptomology and risk factors such as hypoxemia, hypertension, and obesity, diabetes and SDs often engage in a harmful positive feedback loop in which the two conditions are exacerbated. By decreasing symptomology through treatment and lifestyle changes, the likelihood of harmful outcomes such as cardiovascular disease and mortality can be reduced, thus improving the quality of life and lessening the effects of these two conditions.
However, the ability to recognize this concurrency is multifaceted and complex. Simultaneous diagnosis is essential in order to ensure that each condition does not continue to exacerbate the other. Screening for diabetes concurrently with sleep studies, and assessing sleep in those who are at risk for or diagnosed with diabetes may serve to alleviate the prevalence of underdiagnoses of both diabetes and SDs.
Postdiagnosis, the challenge of managing diabetes is often rigorous and continues to diminish sleep quality and duration. In turn, abnormal sleep contributes to diabetes and obesity, specifically impacting sleep-stage cycling and possible hormonal alterations, and subsequently affects glucose intolerance. Both OSA and sleep deprivation contribute to the negative effects on metabolic and insulin resistance, predisposing individuals to type 2 diabetes.
Though viable treatments for diabetes and SDs exist, patient adherence and compliance often present barriers and challenges for the management of these conditions. Continuous positive airway pressure is considered a successful noninvasive treatment, providing positive results for individuals with both an SD and diabetes. Continuous positive airway pressure can improve glucose levels, insulin sensitivity, and healthy hormone levels; however, consistent utilization of CPAP does not always occur, and adherence to the device still remains a barrier to effective treatment. Similarly, lifestyle and diet changes associated with diabetes have poor adherence, and treatment is currently suboptimal for both condtions.35 This is equally costly and risky for affected individuals. Emerging telemedicine technologies provide exciting potential to combat this negligence by their ability to reduce medical cost, increase patient follow-ups, and enhance quality medical care treatment for SDs and diabetes. Based on the positive results of our own investigation of diabetes management via telemedicine and other positive testimonies in the treatment of SDs with telemedicine, we are encouraged to believe these positive results can be extended to those experiencing comorbid SDs and diabetes. By addressing the relationship between diabetes and SDs through further research and medical advancements using telehealth, preventative measures and disease management can increase the quality of care and ease of treatment in both current and future patients.
Glossary
- (BMI)
body mass index
- (CPAP)
continuous positive airway pressure
- (EHR)
electronic health record
- (OSA)
obstructive sleep apnea
- (PSG)
polysomnography
- (SD)
sleep disorder
References
- 1.Taub LF, Redeker NS. Sleep disorders, glucose regulation, and type 2 diabetes. Biol Res Nurs. 2008;9(3):231–243. doi: 10.1177/1099800407311016. [DOI] [PubMed] [Google Scholar]
- 2.Zizi F, Jean-Louis G, Brown CD, Ogedegbe G, Boutin-Foster C, McFarlane SI. Sleep duration and the risk of diabetes mellitus: epidemiologic evidence and pathophysiologic insights. Curr Diab Rep. 2010;10(1):43–47. doi: 10.1007/s11892-009-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seibert PS, Valerio J, Rafla Y, Grimsley FP. Diabetes and sleep disorders: the complex web of variables. Presented at: The 5th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD); February 2012; Barcelona, Spain. [Google Scholar]
- 4.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandey A, Demede M, Zizi F, Al Haija’a OA, Nwamaghinna F, Jean-Louis G, McFarlane SI. Sleep apnea and diabetes: insights into the emerging epidemic. Curr Diab Rep. 2011;11(1):35–40. doi: 10.1007/s11892-010-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vijan S. Type 2 diabetes. Ann Intern Med. 2010;152(5):ITC3–ITC16. doi: 10.7326/0003-4819-152-5-201003020-01003. [DOI] [PubMed] [Google Scholar]
- 7.Oh S, Kwon H, Varadan VK. Wireless telemedicine systems for diagnosing sleep disorders with Zigbee star network topology. Nanosys Eng Med. 2012:8548. [Google Scholar]
- 8.Fox N, Hirsch-Allen AJ, Goodfellow E, Wenner J, Fleetham J, Ryan CF, Kwiatkowska M, Ayas NT. The impact of a telemedicine monitoring system on positive airway pressure adherence in patients with obstructive sleep apnea: a randomized controlled trial. Sleep. 2012;35(4):477–481. doi: 10.5665/sleep.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seibert PS, Whitmore T, Reddy T, Valerio J, DeHaas C. The use of telemedicine to train perioperative nurses in rural settings. J Telemed Telecare. doi: 10.1177/1357633X13501777. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 10.Gellis ZD, Kenaley B, McGinty J, Bardelli E, Davitt J, Ten Have T. Outcomes of a telehealth intervention for homebound older adults with heart or chronic respiratory failure: a randomized controlled trial. Gerontologist. 2012;52(4):541–552. doi: 10.1093/geront/gnr134. [DOI] [PubMed] [Google Scholar]
- 11.Touma C, Pannain S. Does lack of sleep cause diabetes? Cleve Clin J Med. 2011;78(8):549–558. doi: 10.3949/ccjm.78a.10165. [DOI] [PubMed] [Google Scholar]
- 12.Bopparaju S, Surani S. Sleep and diabetes. Int J Endocrinol. 2010;2010:759509. doi: 10.1155/2010/759509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hicks D. Obstructive sleep apnoea: its link with diabetes. Nurs Times. 2011;107(40):31–32. [PubMed] [Google Scholar]
- 14.Ruder K. A good night’s sleep. Sleep loss and disorders can affect not only your quality of life, but your health as well. Diabetes Forecast. 2006;59(10):56–59. [PubMed] [Google Scholar]
- 15.Harada Y, Oga T, Chin K, Takegami M, Takahashi K, Sumi K, Nakamura T, Nakayama-Ashida Y, Minami I, Horita S, Oka Y, Wakamura T, Fukuhara S, Mishima M, Kadotani H. Differences in relationships among sleep apnoea, glucose level, sleep duration and sleepiness between persons with and without type 2 diabetes. J Sleep Res. 2012;21(4):410–418. doi: 10.1111/j.1365-2869.2012.00997.x. [DOI] [PubMed] [Google Scholar]
- 16.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and type 2 diabetes. J Appl Physiol (1985) 2005;99(5):2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 17.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gangwisch JE, Malaspina D, Babiss LA, Opler MG, Posner K, Shen S, Turner JB, Zammit GK, Ginsberg HN. Short sleep duration as a risk factor for hypercholesterolemia: analyses of the National Longitudinal Study of Adolescent Health. Sleep. 2010;33(7):956–961. doi: 10.1093/sleep/33.7.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 20.Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279(1):H234–H237. doi: 10.1152/ajpheart.2000.279.1.H234. [DOI] [PubMed] [Google Scholar]
- 21.National Center for Health Statistics. QuickStats: percentage of adults who reported an average of ≤ 6 hours of sleep per 24-hour period, by sex and age group - United States, 1985 and 2004. MMWR Morb Mortal Wkly Rep. 2005;54:933. [Google Scholar]
- 22.Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers GJ, van Kralingen KW, Corssmit EP, Romijn JA. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab. 2010;95(6):2963–2968. doi: 10.1210/jc.2009-2430. [DOI] [PubMed] [Google Scholar]
- 23.Keckeis M, Lattova Z, Maurovich-Horvat E, Beitinger PA, Birkmann S, Lauer CJ, Wetter TC, Wilde-Frenz J, Pollmächer T. Impaired glucose tolerance in sleep disorders. PLoS One. 2010;5(3):e9444. doi: 10.1371/journal.pone.0009444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105(3):1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grandner MA, Jackson NJ, Pak VM, Gehrman PR. Sleep disturbance is associated with cardiovascular and metabolic disorders. J Sleep Res. 2012;21(4):427–433. doi: 10.1111/j.1365-2869.2011.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bromley LE, Booth JN 3rd, Kilkus JM, Imperial JG, Penev PD. Sleep restriction decreases the physical activity of adults at risk for type 2 diabetes. Sleep. 2012;35(7):977–984. doi: 10.5665/sleep.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristo D, Eliasson AH, Netzer NC, Bigott T. Application of telemedicine to sleep medicine. Sleep Breath. 2001;5(2):97–99. doi: 10.1007/s11325-001-0097-2. [DOI] [PubMed] [Google Scholar]
- 28.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013 doi: 10.1093/aje/kws342. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Netzer N, Eliasson AH, Netzer C, Kristo DA. Overnight pulse oximetry for sleep-disordered breathing in adults: a review. Chest. 2001;120(2):625–633. doi: 10.1378/chest.120.2.625. [DOI] [PubMed] [Google Scholar]
- 30.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181(5):507–513. doi: 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isetta V, Montserrat JM, Leon C, Fonollosa D, Roca J, Farré R. Multicenter support network for CPAP therapy follow-up in sleep apnea. The Fourth International Conference on eHealth, Telemedicine, and Social Medicine; January 30–February 4, 2012; Valencia, Spain. [Google Scholar]
- 32.Rice TB, Foster GD, Sanders MH, Unruh M, Reboussin D, Kuna ST, Millman R, Zammit G, Wing RR, Wadden TA, Kelley D, Pi-Sunyer X, Newman AB. Sleep AHEAD Research Group. The relationship between obstructive sleep apnea and self-reported stroke or coronary heart disease in overweight and obese adults with type 2 diabetes mellitus. Sleep. 2012;35(9):1293–1298. doi: 10.5665/sleep.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuomilehto H, Peltonen M, Partinen M, Lavigne G, Eriksson JG, Herder C, Aunola S, Keinänen-Kiukaanniemi S, Ilanne-Parikka P, Uusitupa M, Tuomilehto J, Lindström J. Finnish Diabetes Prevention Study Group.Sleep duration, lifestyle intervention, and incidence of type 2 diabetes in impaired glucose tolerance: The Finnish Diabetes Prevention Study. Diabetes Care. 2009;32(11):1965–1971. doi: 10.2337/dc08-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165(5):670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 35.Peeples M, Seley JJ. Diabetes care: the need for change. Am J Nurs. 2007;107(6 Suppl):13–19. doi: 10.1097/01.NAJ.0000277818.69732.0f. [DOI] [PubMed] [Google Scholar]
- 36.Mulcahy K, Maryniuk M, Peeples M, Peyrot M, Tomky D, Weaver T, Yarborough P. Diabetes self-management education core outcomes measures. Diabetes Educ. 2003;29(5):768–770. 773–784, 787–788. doi: 10.1177/014572170302900509. [DOI] [PubMed] [Google Scholar]
- 37.Van den Arend IJ, Stolk RP, Krans HM, Grobbee DE, Schrijvers AJ. Management of type 2 diabetes: a challenge for patient and physician. Patient Educ Couns. 2000;40(2):187–194. doi: 10.1016/s0738-3991(99)00067-1. [DOI] [PubMed] [Google Scholar]
- 38.Brooks B, Cistulli PA, Borkman M, Ross G, McGhee S, Grunstein RR, Sullivan CE, Yue DK. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994;79(6):1681–1685. doi: 10.1210/jcem.79.6.7989475. [DOI] [PubMed] [Google Scholar]
- 39.Kayyali HA, Weimer S, Frederick C, Martin C, Basa D, Juguilon JA, Jugilioni F. Remotely attended home monitoring of sleep disorders. Telemed J E Health. 2008;14(4):371–374. doi: 10.1089/tmj.2007.0058. [DOI] [PubMed] [Google Scholar]
- 40.Dixon RF, Stahl JE. Virtual visits in a general medicine practice: a pilot study. Telemed J E Health. 2008;14(6):525–530. doi: 10.1089/tmj.2007.0101. [DOI] [PubMed] [Google Scholar]
- 41.Morgan GJ, Craig B, Grant B, Sands A, Doherty N, Casey F. Home videoconferencing for patients with severe congential heart disease following discharge. Congenit Heart Dis. 2008;3(5):317–324. doi: 10.1111/j.1747-0803.2008.00205.x. [DOI] [PubMed] [Google Scholar]
- 42.Parikh R, TouVelle MN, Wang H, Zallek SN. Sleep telemedicine: Patient satisfaction and treatment adherence. Telemed J E Health. 2011;17(8):609–614. doi: 10.1089/tmj.2011.0025. [DOI] [PubMed] [Google Scholar]
- 43.Marrone S, Mitchell JE, Crosby R, Wonderlich S, Jollie-Trottier T. Predictors of response to cognitive behavioral treatment for bulimia nervosa delivered via telemedicine versus face-to-face. Int J Eat Disord. 2009;42(3):222–227. doi: 10.1002/eat.20603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King VL, Stoller KB, Kidorf M, Kindbom K, Hursh S, Brady T, Brooner RK. Assessing the effectiveness of an Internet-based videoconferencing platform for delivering intensified substance abuse counseling. J Subst Abuse Treat. 2009;36(3):331–338. doi: 10.1016/j.jsat.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 45.Salvador CH, Ruiz-Sanchez A, González de Mingo MA, Carmona Rodríguez M, Carrasco MP, Sagredo PG, Fragua JA, Caballero-Martinez F, García-López F, Márquez-Montes JN, Monteagudo JL. Evaluation of a telemedicine-based service for the follow-up and monitoring of patients treated with oral anticoagulant therapy. IEEE Trans Inf Technol Biomed. 2008;12(6):696–706. doi: 10.1109/TITB.2008.910750. [DOI] [PubMed] [Google Scholar]
- 46.Breslow MJ, Rosenfeld BA, Doerfler M, Burke G, Yates G, Stone DJ, Tomaszewicz P, Hochman R, Plocher DW. Effect of a multiple-site intensive care unit telemedicine program on clinical and economic outcomes: an alternative paradigm for intensivist staffing. Crit Care Med. 2004;32(1):31–38. doi: 10.1097/01.CCM.0000104204.61296.41. [DOI] [PubMed] [Google Scholar]
- 47.Waite K, Silver F, Jaigobin C, Black S, Lee L, Murray B, Danyliuk P, Brown EM. Telestroke: a multi-site, emergency-based telemedicine service in Ontario. J Telemed Telecare. 2006;12(3):141–145. doi: 10.1258/135763306776738611. [DOI] [PubMed] [Google Scholar]
- 48.Klonoff DC, True MW. The missing element of telemedicine for diabetes: decision support software. J Diabetes Sci Technol. 2009;3(5):996–1001. doi: 10.1177/193229680900300501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bellazzi R. Telemedicine and diabetes management: current challenges and future research directions. J Diabetes Sci Technol. 2008;2(1):98–104. doi: 10.1177/193229680800200114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Po YM. Telemedicine to improve patients’ self-efficacy in managing diabetes. J Telemed Telecare. 2000;6(5):263–267. doi: 10.1258/1357633001935888. [DOI] [PubMed] [Google Scholar]
- 51.Swinton JJ, Robinson WD, Bischoff RJ. Telehealth and rural depression: physician and patient perspectives. Fam Syst Health. 2009;27(2):172–182. doi: 10.1037/a0016014. [DOI] [PubMed] [Google Scholar]
- 52.Campbell JD, Harris KD, Hodge R. Introducing telemedicine technology to rural physicians and settings. J Fam Pract. 2001;50(5):419–424. [PubMed] [Google Scholar]
- 53.Sparrow D, Gottlieb DJ, Demolles D, Fielding RA. Increases in muscle strength and balance using a resistance training program administered via a telecommunications system in older adults. J Gerontol A Biol Sci Med Sci. 2011;66(11):1251–1257. doi: 10.1093/gerona/glr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Timmerberg BD, Wurst J, Patterson J, Spaulding RJ, Belz NE. Feasibility of using videoconferencing to provide diabetes education: a pilot study. J Telemed Telecare. 2009;15(2):95–97. doi: 10.1258/jtt.2008.080813. [DOI] [PubMed] [Google Scholar]
- 55.Weinstock RS, Teresi JA, Goland R, Izquierdo R, Palmas W, Eimicke JP, Ebner S, Shea S. IDEATel Consortium. Glycemic control and health disparities in older ethnically diverse underserved adults with diabetes: five-year results from the Informatics for Diabetes Education and Telemedicine (IDEATel) study. Diabetes Care. 2011;34(2):274–279. doi: 10.2337/dc10-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seibert PS, Rafla Y, Valerio J, Whitmore T. Advanced diabetes self-management and professional care through telemedicine. Presented at: The 5th International Conference on Advanced Technologies and Treatments for Diabetes (ATTD); February 2012; Barcelona, Spain. [Google Scholar]
- 57.Taylor Y, Eliasson A, Andrada T, Kristo D, Howard R. The role of telemedicine in CPAP compliance for patients with obstructive sleep apnea syndrome. Sleep Breath. 2006;10(3):132–138. doi: 10.1007/s11325-006-0059-9. [DOI] [PubMed] [Google Scholar]
- 58.Oki Y, Shiomi T, Sasanabe R, Maekawa M, Hirota I, Usui K, Hasegawa R, Kobayashi T. Multiple cardiovascular risk factors in obstructive sleep apnea syndrome patients and an attempt at lifestyle modification using telemedicine-based education. Psychiatry Clin Neurosci. 1999;53(2):311–313. doi: 10.1046/j.1440-1819.1999.00514.x. [DOI] [PubMed] [Google Scholar]
- 59.Coma-Del-Corral MJ, Alonso-Álvarez ML, Allende M, Cordero J, Ordax E, Masa F, Terán-Santos J. Reliability of telemedicine in the diagnosis and treatment of sleep apnea syndrome. Telemed J E Health. 2013;19(1):7–12. doi: 10.1089/tmj.2012.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spaulding R, Stevens D, Velasquez SE. Experience with telehealth for sleep monitoring and sleep laboratory management. J Telemed Telecare. 2011;17(7):346–349. doi: 10.1258/jtt.2011.110202. [DOI] [PubMed] [Google Scholar]
- 61.Seibert PS, Whitmore TA, Parker PD, Grimsley FP, Payne K, O’Donnell JE. The emerging role of telemedicine in diagnosing and treating sleep disorders. J Telemed Telecare. 2006;12(8):379–381. doi: 10.1258/135763306779378681. [DOI] [PubMed] [Google Scholar]


