Abstract
Background
Gait-related fall risk is the leading cause of mortality among patients with diabetes, especially those older than 65 years. Deterioration in balance and loss of protective sensation in lower extremities contribute significantly to fall risk in patients with diabetic peripheral neuropathy (DPN). This study aimed to explore the impact of neuropathy and foot ulcer on gait.
Methods
We recruited 39 participants (age, 56.9 ± 8.2 years; body mass index, 29.6.3 ± 4.7 kg/m2), including 15 DPN patients without foot ulcers, 16 DPN patients with foot ulcers, and 8 healthy aged-matched controls. Patients with active foot ulcers wore an offloading device during gait examination, including removable cast walker.
Results
Results suggest that neuropathy alters gait mainly by increasing gait initiation, gait variability (coefficient of variation of gait velocity), and double support (DS) time, while reducing knee range of motion and center of mass sway (p < .05). Interestingly, the presence of foot ulcer does not impact gait velocity (p > .1) but enhances some of the gait parameters such as gait variability and DS time.
Conclusions
This study demonstrates that neuropathy deteriorates gait, but the presence of foot ulcers does not alter gait parameters further than neuropathy. In addition, patients with foot ulcers demonstrated a better gait compared with DPN patients without ulcers. We speculate that offloading footwear may be enhancing the somatosensory feedback from sensate skin, thereby positively affecting gait parameters. A study with a larger sample is required to explore the effect of prescribed footwear in the DPN population in order to validate the findings of this research study.
Keywords: diabetes, foot ulcer, gait, offloading, wearable sensors
Introduction
Statistics from Centers for Disease Control and Prevention reported 2.3 million nonfatal fall injuries and over 650,000 patients being hospitalized from fall-related injury.1 Fall risk is a leading cause of mortality among diabetes patients, especially those aged over 65 years. Falls result in serious bodily injuries, including fractures in the elderly population.2 A number of studies have shown that gait changes in the elderly are related to high fall risk, including gait unsteadiness and gait variability, especially if they have history of falls,3–5 causing them to alter gait strategies in order to reduce fall risk.3,6 However, additional gait parameters, including variability in ground–foot clearance, have been linked to increased fall risk.7
There has been an increasing body of evidence highlighting the high rate of falls in diabetes patients.2,8–10According to a survey, diabetes is one of the strongest predictors of high fall risk among the elderly.11 Among several factors that contribute to high fall risk in patients with diabetes, deterioration in balance and loss of protective sensation in lower extremities are the most common.12,13 Loss of protective sensation [diabetic peripheral neuropathy (DPN)] is a common complication of diabetes, affecting more than 50% of elderly diabetes patients aged above 60 years.14,15Other factors such as reduced speed, increased double support (DS) time, higher reaction time, changes in joint stiffness, reduced ankle joint moment, strength, higher vibration perception thresholds, lower gait velocity, and reduced muscle strength have also been linked to gait variations.9,14,16,17 Patients with DPN exhibit such gait changes as listed here and also have reduced ankle strength and are therefore 15 times more likely to report falls.18–20 These changes in gait may also exist and differ significantly in patients with or without neuropathic pain.21 Based on retrospective reports, Lalli and coauthors21 also found a nonsignificant increasing trend of number of falls and fall-related injuries.
Additionally, DPN is a major risk factor for developing diabetic foot ulcers. Early studies that focused on exploring gait variation between different groups with diabetes found significant difference in some gait and kinematic parameters, especially gait velocity between DPN with history of ulcer and diabetes controls; however, no significant gait changes were found between DPN with and DPN without ulcer history.22 It has been reported that, among a population of diabetes patients with a history of foot ulcer, fall risk is significantly associated with comorbidity, insensitive feet, and body mass index (BMI).2
After careful review of the literature, we determined the lack of studies on specific differences in gait parameter between DPN with and DPN without active foot ulcers. Additionally, some of the drawbacks of previous studies assessing gait on elderly and diabetes populations include measurements being performed either on a treadmill or inside a gait laboratory. Both these settings are not the actual environment where this population performs their activities of daily living and may also cause individuals to modify their gait pattern to adjust to the new environment. The current study was aimed to compare objective gait parameters, including gait velocity, gait initiation, and gait variability, among DPN patients with and without ulcer using wearable sensors that can be mounted unobtrusively on different body segments, allowing measurements to be done outside the laboratory, for instance, in a clinical environment or at home. Since gait parameters can change as a function of distance, speed, and environment, wearable sensors are therefore better suited for the proposed real-world measurements.23–25 Previous studies have demonstrated changes in gait termination in diabetes patients with peripheral neuropathy;26 however, gait initiation is another aspect that needs attention and was addressed in this research. Gait initiation and termination are more complex procedures than steady-state walking and therefore may increase the risk of falls.27
Methods
Subject Recruitment and Protocol
We recruited 39 study participants, 15 DPN patients without foot ulcers (age, 54.2 ± 11.3 years; BMI, 31.2 ± 5.9 kg/m2), 16 DPN patients with active foot ulcers (age, 58.3 ± 4.4 years; BMI, 29.5 ± 3.7 kg/m2), and 8 healthy controls (age, 59.6 ± 6 years; BMI, 27 ± 3.2 kg/m2). Ethical approval was received by the institutional review board, and all participants signed an informed consent form prior to participating in the study. Peripheral neuropathy in diabetes patients was confirmed from patient records and additionally by using a 10 g monofilament test at multiple plantar foot locations per the recommended American Diabetes Association guidelines. Diabetes patients with major foot amputation(s) were excluded from the study. Participants with inability to walk 100 m without assistance were also excluded.
All study participants walked along a predefined path of approximately 200 ft. All diabetes subjects wore their prescribed footwear, including surgical sandals, removable cast walkers, and prescribed shoes. Out of 15 DPN patients without ulcer, 5 had a cast from diagnosed Charcot, 2 wore prescribed shoes, 6 wore regular shoes, and 2 wore offloading sandals. All 16 DPN patients with an active foot ulcer wore prescribed shoes, including cast, offloading sandals, and offloading removable cast. Healthy controls wore their habitual footwear.
A set of validated wearable sensors was used to collect gait data (LEGSys™, Biosensics LLC, MA). Gait was assessed by processing wearable sensor data, and various spatiotemporal parameters were extracted.28 The method for calculating spatiotemporal parameters of gait and its validity has been previously described in detail.24,29–31 Based on statistical intercycle fluctuation of gait velocity, the beginning of gait steady state was objectively identified as described in our previous publications.27,32 In summary, the standard deviation (SD) of velocity of six successive strides (three right and three left strides) was calculated from the first stride (for example, SD of strides 1, 2, 3, 4, 5, and 6 and 2, 3, 4, 5, 6, 7, . . .). The marker of the beginning of steady-state walking was the first stride of the group of six strides with an SD below the median SD of the all analyzed strides ±6%, related to the sensitivity of the sensors.29 Additionally, gait steadiness and gait initiation was also quantified.24,32 In summary, for the purpose of this study, we focused on six gait parameters hypothesized to be the most important independent gait parameters that may be affected by neuropathy: (1) stride velocity, (2) stride length, (3) gait cycle time, (4) double stance presented as a percentage of gait cycle time, (5) motion of center of mass (COM) during walking estimated by the area of trunk sway during walking in medial–lateral and anterior–posterior, and (6) knee range of motion. All parameters listed here were averaged during both gait initiation as well as at least 10 strides during gait steady state. In addition, coefficient of variation (CV) of stride velocity during gait steady state was estimated as an indicator of gait variability. The CV of stride velocity was defined as the SD of measured stride velocities during steady state divided by the average of stride velocities multiplied by 100. Finally, to further quantify gait initiation phase, the number of steps and distance required to achieve gait steady state walking were estimated as described in earlier publications.24,27
Results were analyzed in SPSS using multivariate analysis of variance analysis for comparing the three groups, and the statistical significance was set to p = .05. Between-group comparison was performed for gait parameters during gait initiation and gait steady-state phases.
Results
Tables 1 and 2 list the statistical comparison between groups based on multivariate analysis of variance and pairwise comparison for gait parameters, respectively, during gait steady state and during gait initiation phases. There was no significant difference for age between the groups (p = . 2 3).
Table 1.
Between-Group Comparison during Gait Initiation Phasea
| Parameter | Group | Mean | SD | p value | Groups | Pairwise | 95% confidence interval for difference | |
| Stride velocity, m/s | DPN no ulcer | 0.83 | 0.30 | 0.13 | 1 and 2 | 0.76 | -0.20 | 0.15 |
| DPN with ulcer | 0.84 | 0.18 | 1 and 3 | 0.05 | -0.43 | 0.00 | ||
| Healthy | 1.02 | 0.19 | 2 and 3 | 0.08 | -0.38 | 0.02 | ||
| Steps to steady state | DPN no ulcer | 6.07 | 2.15 | 0.009 | 1 and 2 | 0.80 | -1.42 | 1.80 |
| DPN with ulcer | 5.88 | 2.47 | 1 and 3 | 0.005 | 0.97 | 4.92 | ||
| Healthy | 3.13 | 0.83 | 2 and 3 | 0.006 | 0.86 | 4.64 | ||
| Distance to steady state | DPN no ulcer | 1.41 | 1.07 | 0.16 | 1 and 2 | 0.50 | -0.48 | 0.96 |
| DPN with ulcer | 1.10 | 0.85 | 1 and 3 | 0.19 | -1.46 | 0.30 | ||
| Healthy | 1.90 | 0.93 | 2 and 3 | 0.05 | -1.67 | 0.00 | ||
| Stride length, m | DPN no ulcer | 1.05 | 0.26 | 0.17 | 1 and 2 | 0.76 | -0.18 | 0.13 |
| DPN with ulcer | 1.06 | 0.16 | 1 and 3 | 0.076 | -0.36 | 0.01 | ||
| Healthy | 1.20 | 0.16 | 2 and 3 | 0.10 | -0.33 | 0.03 | ||
| Gait cycle time, s | DPN no ulcer | 1.38 | 0.26 | 0.5 | 1 and 2 | 0.42 | -0.10 | 0.24 |
| DPN with ulcer | 1.31 | 0.17 | 1 and 3 | 0.26 | -0.09 | 0.34 | ||
| Healthy | 1.26 | 0.29 | 2 and 3 | 0.61 | -0.15 | 0.25 | ||
| Double stance, % | DPN no ulcer | 26.83 | 6.90 | 0.13 | 1 and 2 | 0.05 | 0.00 | 8.98 |
| DPN with ulcer | 22.64 | 4.81 | 1 and 3 | 0.14 | -1.49 | 9.63 | ||
| Healthy | 23.09 | 6.31 | 2 and 3 | 0.88 | -5.70 | 4.96 | ||
| COM, degree2 | DPN no ulcer | 24.35 | 33.5 | 0.30 | 1 and 2 | 0.13 | -3.98 | 29.11 |
| DPN with ulcer | 14.1 | 6.58 | 1 and 3 | 0.67 | -16.00 | 24.52 | ||
| Healthy | 23.14 | 14.36 | 2 and 3 | 0.39 | -27.76 | 11.15 | ||
| CV (stride velocity), % | DPN no ulcer | 9.30 | 7.17 | 0.03 | 1 and 2 | 0.15 | -1.15 | 7.17 |
| DPN with ulcer | 6.91 | 4.96 | 1 and 3 | 0.01 | 1.78 | 11.98 | ||
| Healthy | 3.26 | 2.58 | 2 and 3 | 0.11 | -1.02 | 8.76 | ||
| Knee range of motion, degree | DPN no ulcer | 50.60 | 9.13 | 0.003 | 1 and 2 | 0.30 | -4.54 | 14.02 |
| DPN with ulcer | 45.98 | 8.99 | 1 and 3 | 0.01 | -26.26 | -3.52 | ||
| Healthy | 65.65 | 20.69 | 2 and 3 | <0.001 | -30.54 | -8.71 | ||
Group 1, DPN no ulcer; group 2, DPN with ulcer; group 3, healthy control. Boldface items indicate statistical significance.
Table 2.
Between-Group Comparison during Steady-State Gait Phasea
| Parameter | Group | Mean | SD | p value | Groups | Pairwise | 95% confidence interval for difference | |
| Stride velocity, m/s | DPN no ulcer | 0.86 | 0.29 | 0.24 | 1 and 2 | 0.82 | -0.20 | 0.16 |
| DPN with ulcer | 0.87 | 0.19 | 1 and 3 | 0.12 | -0.39 | 0.04 | ||
| Healthy | 1.02 | 0.19 | 2 and 3 | 0.15 | -0.36 | 0.06 | ||
| Stride length, m | DPN no ulcer | 1.08 | 0.26 | 0.28 | 1 and 2 | 0.84 | -0.17 | 0.14 |
| DPN with ulcer | 1.08 | 0.17 | 1 and 3 | 0.14 | -0.34 | 0.05 | ||
| Healthy | 1.20 | 0.16 | 2 and 3 | 0.17 | -0.31 | 0.06 | ||
| Gait cycle time, s | DPN no ulcer | 1.34 | 0.26 | 0.34 | 1 and 2 | 0.42 | -0.10 | 0.23 |
| DPN with ulcer | 1.27 | 0.16 | 1 and 3 | 0.15 | -0.05 | 0.34 | ||
| Healthy | 1.19 | 0.20 | 2 and 3 | 0.40 | -0.11 | 0.27 | ||
| DS, % | DPN no ulcer | 26.3 | 6.88 | 0.04 | 1 and 2 | 0.03 | 0.28 | 8.80 |
| DPN with ulcer | 22.1 | 4.59 | 1 and 3 | 0.02 | 0.66 | 11.09 | ||
| Healthy | 20.8 | 4.9 | 2 and 3 | 0.59 | -3.67 | 6.34 | ||
| COM, degree2 | DPN no ulcer | 15.88 | 7.23 | 0.02 | 1 and 2 | 0.39 | -3.79 | 9.42 |
| DPN with ulcer | 12.91 | 6.16 | 1 and 3 | 0.039 | -16.65 | -0.46 | ||
| Healthy | 24.23 | 14.26 | 2 and 3 | 0.005 | -19.14 | -3.95 | ||
| CV (stride velocity), % | DPN no ulcer | 6.0 | 5.6 | 0.06 | 1 and 2 | 0.20 | -1.19 | 5.34 |
| DPN with ulcer | 4.4 | 4.1 | 1 and 3 | 0.02 | 0.72 | 8.71 | ||
| Healthy | 1.9 | 1.0 | 2 and 3 | 0.17 | -1.19 | 6.48 | ||
| Knee range of motion, degree | DPN no ulcer | 51.7 | 8.8 | 0.003 | 1 and 2 | 0.34 | -4.87 | 13.70 |
| DPN with ulcer | 47.4 | 9.6 | 1 and 3 | 0.01 | -26.56 | -3.81 | ||
| Healthy | 67.0 | 20.4 | 2 and 3 | <0.01 | -30.52 | -8.69 | ||
Group 1, DPN no ulcer; group 2, DPN with ulcer; group 3, healthy control. Boldface items indicate statistical significance.
Gait Initiation Phase
Comparison of gait parameters during gait initiation phase revealed significant group differences for number of steps required to reach steady-state gait (p = .009), knee range of motion (p = .003), and CV for stride velocity (p = .03; Table 1).
We observed that the presence of neuropathy increases the number of steps required to reach steady state gait (i.e. gait initiation) by nearly 90% on average compared with healthy individuals (p = .009). Pairwise comparison revealed significant difference between both DPN without (p = .005) and DPN with foot ulcer (p = .006) compared with healthy individuals; however, no difference was found between the two DPN groups (Figure 1).
Figure 1.
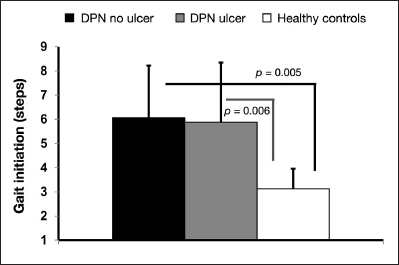
Between-group comparison of number of steps required to reach steady-state gait.
Knee range of motion was significantly reduced in DPN by 23% and further by 9% in DPN with ulcer compared with healthy individuals. Pairwise comparison revealed significant difference between the DPN with ulcer group and the healthy group with values of 45.98° ± 8.99° and 65.65° ± 20.69°, respectively.
Significant group differences were observed for changes in CV for stride velocity (p = .03). Again, we observed a lower value of CV in the DPN with ulcer group compared with the DPN without ulcer group, with values ranging from 6.91% ± 4.96% and 9.30% ± 7.17%, respectively (Figure 2). Between-group significance was only observed between the DPN group and the healthy group (p = .01). Other gait parameters did not reach the significance level (p > .15).
Figure 2.
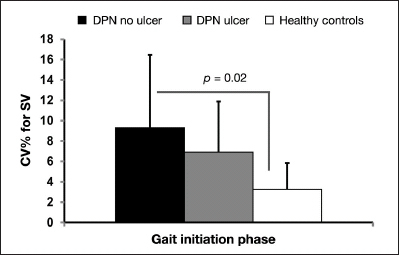
Coefficient of variation for stride velocity during gait initiation phase for the three groups. SV,stride velocity.
Steady-State Gait
Among various tested parameters, DS percentage (p = .04), COM area of sway (p = .02), CV in stride velocity (p = .06), and knee range of motion (p = .003) were significantly different between groups (Table 2).
Similar to the gait initiation phase, it seems that the presence of DPN reduces knee range of motion by 23% on average compared with healthy controls, and active ulcer causes a further reduction by 8.3%. Between-group difference achieved statistical significance level between DPN with active ulcer and healthy control (67.0° ± 20.4° in healthy versus 47.4°±9.6°; p < .01; 95% confidence interval -30.5° to -8.7°) despite nonsignificant difference for stride velocity (p = .15).
Double support in diabetes without foot ulcer was 26% higher than in healthy controls (26.3% ± 6.9% in DPN without ulcer versus 20.8% ± 4.9% in healthy controls; p = .02; 95% confidence interval 0.66% to 11.09%; Figure 3). Interestingly, DS was also longer in the DPN without foot ulcer group compared with those with active foot ulcer (p = .03). Although DS was 6.3% longer in the DPN with active ulcer group compared with healthy controls, the difference was not statistically significant in our sample (p = . 5 9) .
Figure 3.
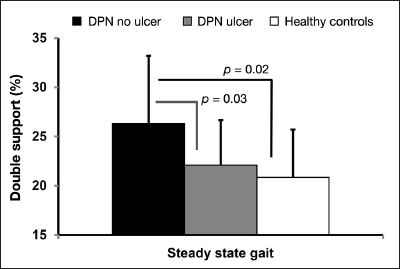
The DS period comparison between the three groups during steady-state gait.
Interestingly, COM sway quantified by range of motion of lower back in medial–lateral and anterior–posterior during walking was higher in healthy controls compared with DPN patients, irrespective of foot ulcer (p = .02), which may suggest more rigidity during walking due to DPN. Results suggest that COM sway during walking was reduced by 34% (p = .03) and 47% (p = .005) for both the DPN without and the DPN with ulcer groups, respectively, compared with healthy controls (Figure 4).
Figure 4.
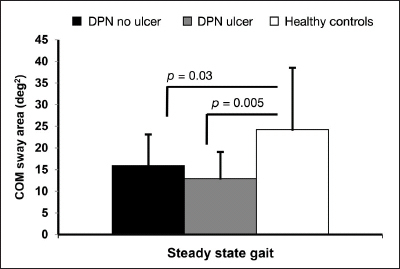
The COM sway area comparison between groups during steady-state gait.
We also observed significant group differences for changes in CV for stride velocity (p = .06). The CV was lowest for the healthy group; however, it was very interesting to observe that CV was lower for the group with foot ulcer compared with DPN without ulcer. The CV was 215% (pairwise p = .02) higher in the DPN group and 130% (p = 0.17) higher in the foot ulcer group compared with healthy individuals.
In comparing other parameters, including stride velocity, stride length, and gait cycle time, we observed no significant group differences (p > . 24).
Discussion
Patients with DPN have a significant deficit in the ability to perceive lower extremity proprioception.13 Additionally, DPN is one of the factors associated with fall risk.13,33 Other factors include changes in gait and balance.12,17,20,34,35The purpose of this study was to compare gait-related parameters in DPN with active foot ulcers, DPN without foot ulcers, and also a group of healthy controls. Only limited clinical studies have focused on or reported comparison of gait parameters between DPN and DPN with active foot ulcers. More importantly, no studies have explored the effect of offloading footwear on gait among diabetes patients,36 especially among different groups of diabetes patients. Studies have reported standing balance changes due to offloading devices, but it seems no studies so far have explored fall risk as a function of offloading devices. Previous studies on the elderly have demonstrated that there is no significant relation between regular footwear and falls,37 and no studies have explored the effect of offloading devices.
In the current study, when comparing the gait initiation phase, we observed significant group difference (p < .05) for parameters, including number of steps required to reach steady state gait, knee range of motion, and stride velocity CV. The results demonstrate how peripheral neuropathy may affect gait and subsequently impact foot ulcer on knee joint range of motion. However, based on the observed lower stride velocity CV in the ulcer group, we speculate that the prescribed footwear may be playing an important role. We further speculate that these footwear maybe compensating for the lost sensations of the foot. Adding to our speculations, we observed that the presence of ulcer did not significantly affect stride velocity among the ulcer group, as the values were close to those of the DPN group. Previous research studies have reported that, compared with healthy individuals, velocity is significantly lower in DPN with history of ulcer.22 Interestingly, our results also show that DPN with ulcer has similar velocity values as DPN without ulcer.
While comparing steady-state phase of gait, we observed significant group differences for other gait parameters, including DS and COM area of motion during walking in addition to knee range of motion and stride velocity CV. The DS period did not show any group difference during gait initiation period; however, there was apparent difference during the steady-state gait, where DPN groups were significantly higher than healthy controls, as reported previously.18However, it was observed that the DS period among DPN with active foot ulcer was lower than DPN without ulcer by 19%. The DS period observed in the current study is also comparable to previous research, which evaluated the effect of strut height on offloading capacity of removable cast contacts in moderate/high risk for ulcer patients.38
Since the DS period difference was not significantly different among the three groups during gait initiation period, it suggests that, during initial gait, all the three groups may be struggling; however, after gait initiation, only healthy controls managed to bring the DS period closer to 20%. We hypothesize that, since insensate feet are one of the major causes of gait deterioration among DPN patients, in our cohort of 15 DPN subjects with an active foot ulcer, 12 wore prescribed footwear, which may have improved their foot perception from contact with the sensate skin above the ankle. On the other hand, only 7 out of 15 DPN patients without ulcer wore prescribed footwear. Improved perception of lower extremities in DPN with an active foot ulcer may have caused better gait characteristics compared with DPN patients without ulcer.
The stride velocity CV maintained significant group difference both during gait initiation and during steady-state gait. A trend of increase in CV for stride velocity was found from healthy individuals to DPN with active foot ulcer followed by DPN without ulcer. This shows that the DPN with ulcer group was better able to reduce the variations in gait, again probably because of some somatosensory feedback that they received from the footwear to the sensate skin. All these findings indicate that offloading modalities are not only helping heal ulcers more rapidly, but may also be providing secondary feedback to intact skin.
Knee joint range in the sagittal plane also showed significant groups difference during steady-state gait. Several factors are associated with a reduction of range for diabetes, including peripheral neuropathy. Also, the offloading footwear would cause restriction and reduction of joint movement. Previous studies have found significant difference in maximum knee joint angle between DPN without foot ulcer and with history of foot ulcers.22 In comparison with other studies, it should be noted that this current study recruited participants with active foot ulcers and measured range of motion as opposed to absolute value. It is very interesting to note that, even though range of motion among the DPN with ulcer group is lower than the DPN without ulcer group, still the gait seems more stable in the DPN with ulcer group based on DS period and CV in gait speed data.
The COM sway area was significantly different between groups during steady-state gait. Both DPN groups, when compared with healthy individuals, had significant difference in sway, suggesting the rigid walking pattern from limited joint motion caused from diabetes complications. The COM sway differences were evident only during steady-state gait phase.
The current study had a few limitations, including that we excluded patients with peripheral artery disease; however, we did not do any additional tests for peripheral artery disease, therefore some of the patients recruited might have peripheral artery disease. Second, we did not standardize the type of footwear; however, since our intention was to measure gait alterations in real-world scenarios, we opted for patients to wear prescribed or habitual footwear. Based on the results of this study, it seems that further research is warranted to explore the effect of prescribed footwear in ulcer patients, and perhaps also daily activity monitoring to assess changes in quality of life from the provided ulcer treatment.
Conclusions
Overall comparison of various gait parameters suggests that there is significant group difference between the three groups: DPN without ulcer, DPN with ulcer, and healthy. Interestingly, DPN patients with active foot ulcers exhibit a slightly better gait than DPN patients without foot ulcer. We hypothesize that the type of footwear worn by DPN patients with active foot ulcers may provide a better gait and sensation from sensate skin. Previous studies have reported that, by using offloading devices, standing balance may be compromised;36,39 however, as far as gait related-parameters are concerned, the current study also found that variations in stride velocity and DS were lower in the DPN group with active foot ulcer wearing offloading device compared with the DPN without ulcer group. It should be pointed out that previous studies explored the effect of offloading footwear on balance among diabetes patients with foot ulcer only. Therefore, it would be beneficial to further elucidate the role of offloading devices in improving gait related-parameters, especially among the diabetes population. The possible explanation for improved gait could be enhancements in somatosensory feedback from cast via contact with sensate skin above and around the ankle joint. The results from this study also raise an interesting question to explore if the diabetes patient population wearing offloading devices is at risk of falling during gait from wearing the device.
Acknowledgments
The statements made herein are solely the responsibility of the authors.
Glossary
- (BMI)
body mass index
- (COM)
center of mass
- (CV)
coefficient of variation
- (DPN)
diabetic peripheral neuropathy
- (DS)
double support
- (SD)
standard deviation
Funding
This work was supported by a National Priorities Research Program grant from the Qatar National Research Fund (NPRP 4–1025–3–276).
References
- 1.Centers for Disease Control and Prevention; National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention. Falls among older adults: an overview. 2012. Sep 20, http://www.cdc.gov/HomeandRecreationalSafety/Falls/adultfalls.html.
- 2.Wallace C, Reiber GE, LeMaster J, Smith DG, Sullivan K, Hayes S, Vath C. Incidence of falls, risk factors for falls, and fall-related fractures in individuals with diabetes and a prior foot ulcer. Diabetes Care. 2002;25(11):1983–1986. doi: 10.2337/diacare.25.11.1983. [DOI] [PubMed] [Google Scholar]
- 3.Barak Y, Wagenaar RC, Holt KG. Gait characteristics of elderly people with a history of falls: a dynamic approach. Phys Ther. 2006;86(11):1501–1510. doi: 10.2522/ptj.20050387. [DOI] [PubMed] [Google Scholar]
- 4.Hausdorff JM, Edelberg HK, Mitchell SL, Goldberger AL, Wei JY. Increased gait unsteadiness in community-dwelling elderly fallers. Arch Phys Med Rehabil. 1997;78(3):278–283. doi: 10.1016/s0003-9993(97)90034-4. [DOI] [PubMed] [Google Scholar]
- 5.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82(8):1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 6.Judge JO, Davis RB, 3rd, Ounpuu S. Step length reductions in advanced age: the role of ankle and hip kinetics. J Gerontol A Biol Sci Med Sci. 1996;51(6):M303–M312. doi: 10.1093/gerona/51a.6.m303. [DOI] [PubMed] [Google Scholar]
- 7.Barrett RS, Mills PM, Begg RK. A systematic review of the effect of ageing and falls history on minimum foot clearance characteristics during level walking. Gait Posture. 2010;32(4):429–435. doi: 10.1016/j.gaitpost.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Maurer MS, Burcham J, Cheng H. Diabetes mellitus is associated with an increased risk of falls in elderly residents of a long-term care facility. J Gerontol A Biol Sci Med Sci. 2005;60(9):1157–1162. doi: 10.1093/gerona/60.9.1157. [DOI] [PubMed] [Google Scholar]
- 9.Macgilchrist C, Paul L, Ellis BM, Howe TE, Kennon B, Godwin J. Lower-limb risk factors for falls in people with diabetes mellitus. Diabet Med. 2010;27(2):162–168. doi: 10.1111/j.1464-5491.2009.02914.x. [DOI] [PubMed] [Google Scholar]
- 10.Najafi B, Bharara M, Talal T, Armstrong DG. Advances in balance assessment and balance training for diabetes. Diabetes Manag. 2012;2(4):293–308. [Google Scholar]
- 11.Hawkins K, Musich S, Ozminkowski RJ, Bai M, Migliori RJ, Yeh CS. The burden of falling on the quality of life of adults with Medicare supplement insurance. J Gerontol Nurs. 2011;37(8):36–47. doi: 10.3928/00989134-20110329-03. [DOI] [PubMed] [Google Scholar]
- 12.Conner-Kerr T, Templeton MS. Chronic fall risk among aged individuals with type 2 diabetes. Ostomy Wound Manage. 2002;48(3):28–35. [PubMed] [Google Scholar]
- 13.Grewal G, Sayeed R, Yeschek S, Menzies RA, Talal TK, Lavery LA, Armstrong DG, Najafi B. Virtualizing the assessment: a novel pragmatic paradigm to evaluate lower extremity joint perception in diabetes. Gerontology. 2012;58(5):463–471. doi: 10.1159/000338095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Schie CH. Neuropathy: mobility and quality of life. Diabetes Metab Res Rev. 2008;24(Suppl 1):S45–S51. doi: 10.1002/dmrr.856. [DOI] [PubMed] [Google Scholar]
- 15.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 16.Williams DS, 3rd, Brunt D, Tanenberg RJ. Diabetic neuropathy is related to joint stiffness during late stance phase. J Appl Biomech. 2007;23(4):251–260. doi: 10.1123/jab.23.4.251. [DOI] [PubMed] [Google Scholar]
- 17.Courtemanche R, Teasdale N, Boucher P, Fleury M, Lajoie Y, Bard C. Gait problems in diabetic neuropathic patients. Arch Phys Med Rehabil. 1996;77(9):849–855. doi: 10.1016/s0003-9993(96)90269-5. [DOI] [PubMed] [Google Scholar]
- 18.Mueller MJ, Minor SD, Sahrmann SA, Schaaf JA, Strube MJ. Differences in the gait characteristics of patients with diabetes and peripheral neuropathy compared with age-matched controls. Phys Ther. 1994;74(4):299–313. doi: 10.1093/ptj/74.4.299. [DOI] [PubMed] [Google Scholar]
- 19.Cavanagh PR, Derr JA, Ulbrecht JS, Maser RE, Orchard TJ. Problems with gait and posture in neuropathic patients with insulin-dependent diabetes mellitus. Diabet Med. 1992;9(5):469–474. doi: 10.1111/j.1464-5491.1992.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 20.Wrobel JS, Najafi B. Diabetic foot biomechanics and gait dysfunction. J Diabetes Sci Technol. 2010;4(4):833–845. doi: 10.1177/193229681000400411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalli P, Chan A, Garven A, Midha N, Chan C, Brady S, Block E, Hu B, Toth C. Increased gait variability in diabetes mellitus patients with neuropathic pain. J Diabetes Complications. 2013;27(3):248–254. doi: 10.1016/j.jdiacomp.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Katoulis EC, Ebdon-Parry M, Lanshammar H, Vileikyte L, Kulkarni J, Boulton AJ. Gait abnormalities in diabetic neuropathy. Diabetes Care. 1997;20(12):1904–1907. doi: 10.2337/diacare.20.12.1904. [DOI] [PubMed] [Google Scholar]
- 23.Allet L, Armand S, de Bie RA, Golay A, Monnin D, Aminian K, de Bruin ED. Reliability of diabetic patients’ gait parameters in a challenging environment. Gait Posture. 2008;28(4):680–686. doi: 10.1016/j.gaitpost.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Najafi B, Helbostad JL, Moe-Nilssen R, Zijlstra W, Aminian K. Does walking strategy in older people change as a function of walking distance? Gait Posture. 2009;29(2):261–266. doi: 10.1016/j.gaitpost.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 25.DeMott TK, Richardson JK, Thies SB, Ashton-Miller JA. Falls and gait characteristics among older persons with peripheral neuropathy. Am J Phys Med Rehabil. 2007;86(2):125–132. doi: 10.1097/PHM.0b013e31802ee1d1. [DOI] [PubMed] [Google Scholar]
- 26.Meier MR, Desrosiers J, Bourassa P, Blaszczyk J. Effect of type II diabetic peripheral neuropathy on gait termination in the elderly. Diabetologia. 2001;44(5):585–592. doi: 10.1007/s001250051664. [DOI] [PubMed] [Google Scholar]
- 27.Lindemann U, Najafi B, Zijlstra W, Hauer K, Muche R, Becker C, Aminian K. Distance to achieve steady state walking speed in frail elderly persons. Gait Posture. 2008;27(1):91–96. doi: 10.1016/j.gaitpost.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Najafi B, Khan T, Wrobel J. Laboratory in a box: wearable sensors and its advantages for gait analysis. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:6507–6510. doi: 10.1109/IEMBS.2011.6091605. [DOI] [PubMed] [Google Scholar]
- 29.Aminian K, Najafi B, Büla C, Leyvraz PF, Robert P. Spatio-temporal parameters of gait measured by an ambulatory system using miniature gyroscopes. J Biomech. 2002;35(5):689–699. doi: 10.1016/s0021-9290(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 30.Aminian K, Najafi B. Capturing human motion using body-fixed sensors: Outdoor measurement and clinical applications. Comput Animation Virtual Worlds. 2004;15(2):79–94. [Google Scholar]
- 31.Aminian K, Trevisan C, Najafi B, Dejnabadi H, Frigo C, Pavan E, Telonio A, Cerati F, Marinoni EC, Robert P, Leyvraz PF. Evaluation of an ambulatory system for gait analysis in hip osteoarthritis and after total hip replacement. Gait Posture. 2004;20(1):102–107. doi: 10.1016/S0966-6362(03)00093-6. [DOI] [PubMed] [Google Scholar]
- 32.Najafi B, Miller D, Jarrett BD, Wrobel JS. Does footwear type impact the number of steps required to reach gait steady state?: An innovative look at the impact of foot orthoses on gait initiation. Gait Posture. 2010;32(1):29–33. doi: 10.1016/j.gaitpost.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cavanagh PR, Derr JA, Ulbrecht JS, Maser RE, Orchard TJ. Problems with gait and posture in neuropathic patients with insulin-dependent diabetes mellitus. Diabet Med. 1992;9(5):469–474. doi: 10.1111/j.1464-5491.1992.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 34.Najafi B, Khan T, Fleischer A, Wrobel J. The impact of footwear and walking distance on gait stability in diabetic patients with peripheral neuropathy. J Am Podiatr Med Assoc. 2013;103(3):165–173. doi: 10.7547/1030165. [DOI] [PubMed] [Google Scholar]
- 35.Najafi B, Horn D, Marclay S, Crews RT, Wu S, Wrobel JS. Assessing postural control and postural control strategy in diabetes patients using innovative and wearable technology. J Diabetes Sci Technol. 2010;4(4):780–791. doi: 10.1177/193229681000400403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Deursen R. Footwear for the neuropathic patient: offloading and stability. Diabetes Metab Res Rev. 2008;24(Suppl 1):S96–S100. doi: 10.1002/dmrr.827. [DOI] [PubMed] [Google Scholar]
- 37.Menz HB, Morris ME, Lord SR. Footwear characteristics and risk of indoor and outdoor falls in older people. Gerontology. 2006;52(3):174–180. doi: 10.1159/000091827. [DOI] [PubMed] [Google Scholar]
- 38.Crews RT, Sayeed F, Najafi B. Impact of strut height on offloading capacity of removable cast walkers. Clin Biomech (Bristol, Avon) 2012;27(7):725–730. doi: 10.1016/j.clinbiomech.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lavery LA, Fleishli JG, Laughlin TJ, Vela SA, Lavery DC, Armstrong DG. Is postural instability exacerbated by off-loading devices in high risk diabetics with foot ulcers? Ostomy Wound Manage. 1998;44(1):26–32. 34. [PubMed] [Google Scholar]


