Abstract
Background
Research concerning prevention of diabetic foot complications is critical. A novel in-shoe plantar sensory replacement unit (PSRU) has been developed that provides alert-based feedback derived from analyzing plantar pressure threshold measurements in real time. The purpose of this study was to compare the PSRU device to a gold standard pressure-sensing device (GS-PSD) to determine the correlation between concurrent measures of plantar pressure during walking.
Methods
The PSRU had an array of eight sensors with a range of 10–75 mm Hg and collected data at 4 Hz, whereas the GS-PSD had 99 sensors with a range of 1–112 mm Hg and collected data at 100 Hz. Based on an a priori power analysis, data were collected from 10 participants (3 female, 7 male) while walking over ground in both devices. The primary variable of interest was the number of data points recorded that were greater than 32 mm Hg (capillary arterial pressure—the minimum pressure reported to cause pressure ulcers) for each of the eight PSRU sensors and corresponding average recordings from the GS-PSD sensor clusters. Intraclass correlation coefficient (2,1) was used to compare data between the two devices.
Results
Compared with the GS-PSD, we found good-to-very-good correlations (r-value range 0.67–0.86; p-value range 0.01–0.05) for six of the PSRU’s eight sensors and poor correlation for only two sensors (r = 0.41, p = .15; r = 0.38, p = .18) when measuring the number of data points recorded that were greater than 32 mm Hg.
Conclusions
Based on the results of the present study, we conclude the PSRU provides analogous data when compared with a GS-PSD.
Keywords: correlation, diabetes, insole, neuropathy, orthotic, pressure
Introduction
With more than 194 million people suffering from diabetes worldwide, diabetes is now the fourth leading cause of death, contributing to approximately 18% of deaths over the age of 25.1–4 For up to 58% of diabetes patients, the disease is complicated by peripheral neuropathy,5,6 a symmetrical, distal sensory neuropathy that leads to a distal-to-proximal loss of sensation in the lower extremities. This lack of protective sensation, often combined with a biomechanical inability to accommodate foot deformities6,7 potentially exposes these patients to focal stresses, which may lead to ulceration.8,9
The sensory loss of peripheral neuropathy contributes to the development of foot ulcers in nearly 15% of all diabetes patients (a 2% annual incidence), which can progress to systemic infection and amputation. It has been reported that up to 85% of nontraumatic limb amputations are a direct result of neuropathic and ischemic complications, with at least 80% of amputations preceded by a foot ulceration.2,6,8,9 The morbidity, mortality, and economic costs associated with foot ulcerations in diabetes patients pose significant challenges, highlighting the importance of further research in both prevention and treatment aspects of this disease process.10–12
Prevention of diabetic foot ulceration is the most effective controllable parameter for the diabetes patient, and it has been estimated that, with education and preventative management, upwards of 50% of diabetic foot patients may escape amputation.12 The majority of neuropathic ulcer formation occurs under areas of high, localized plantar pressure (e.g., under the metatarsal heads), and it has been reported that there is a strong inverse relationship between the thickness of pressure-dissipating plantar tissue and dynamic foot pressure measurements.13–15 If given an aid that would enable awareness of sole pressure distribution, it is possible that diabetic neuropathic patients would be able to actively alter their foot biomechanics or stop their activity, thereby engaging in a system of self-directed pressure offloading as a result of real-time feedback.
In 2012, the International Scientific Consensus on Medical Plantar Pressure Measurement Devices16 outlined the need for “continuous research and technology development to design and validate innovative and valuable methods and instruments for PMD testing, both on the bench and in the field.” Further, Giacomozzi17,18 described two types of such pressure measurement device (PMDs), one of which was a “switch-like” mechanism, whereby monitoring is accomplished via resistive sensors that function as simple on–off contacts that monitor the absence or presence of significant pressure over time. Given the etiological role that even low-level, sustained plantar pressure plays in the development of diabetic neuropathic ulcers, the need for specific investigation into PMDs for this application is underscored.
Pressure redistribution via PMD insole is not a novel idea. Many researchers have focused on how orthotics can be designed to best redistribute pressure and how to determine which orthotic is best for each patient.19–23 For example, Zequera and Solomonidis20 performed a pressure measurement study for a number of orthotics designed for diabetes patients. These authors concluded that every diabetes patient requires an individual assessment and often a personalized insole. Systematic reviews regarding the use of insoles for prevention of ulcers in the neuropathic diabetic foot also supports their use.22,23 Thus, while the concept of pressure offloading via custom insole orthotics has been previously studied, it is important to note that these studies involved analysis of foot pressure distribution in a laboratory setting and did not use a tool capable of real-time feedback or technology designed for use outside of the laboratory. Despite the potential benefit, to the authors’ knowledge, there are no existing commercially available pressure-monitoring insoles for outpatient use.
Innovative technologies aimed at improving pressure awareness could reduce the incidence of diabetic foot and the associated costs and complications. This study aims to validate a novel pressure-sensing array made by Orpyx Medical Technologies Inc. (Calgary, Canada). This insole is a plantar sensory replacement unit (PSRU) designed to provide real-time feedback regarding foot pressure above a specific threshold and thus guidance to patients with peripheral neuropathy (Figure 1). We sought to compare the PSRU to a gold standard pressure-sensing device (GS-PSD) and determine the PSRU’s ability to detect plantar pressures greater than 32 mm Hg compared with the GS-PSD. Thus, the purpose of this study was to compare the PSRU to the GS-PSD and determine the correlation between concurrent measures of plantar pressure.
Figure 1.

The SurroSense Rx Insole manufactured by Orpyx Medical Technologies Inc.
Methods
Based on a priori power analyses [intraclass correlation coefficient (ICC): 2,1; r = 0.80; agreement aspiration, p = .05] 10 participants [3 females and 7 males; 27.91 (6.65) years old, 174.75 (7.37) cm, 78.56 (11.12) kg] volunteered. Exclusion criteria were
Diagnosis of diabetes
Diagnosis of peripheral neuropathy
Subjective loss of sensation in the feet
Abnormal Semmes–Weinstein monofilament testing (<10/10)
Nonpalpable dorsalis pedis, posterior tibialis, and popliteal pulses
Underlying severe vascular disease (absent peripheral pulses, ankle-brachial index <0.6, and cap refill time >5 s)
Dementia or visual or psychological impairment
Inner-ear pathology or other underlying balance dysfunction
Presence of active ulceration
Lower limb musculoskeletal fixed deformities (such as Charcot arthropathy)
Psychiatric illnesses or social situations that would limit compliance with study
Current participation in another clinical investigation of a medical device or a drug or has participated in such a study within 30 days prior to study enrollment
Significant cardiopulmonary or other systemic disease
Body mass index >30
Presence of Charcot arthropathy
All participants provided informed, written consent approved by the Conjoint Health Research Ethics Board of the University of Calgary.
Equipment
The PSRU was the SurroSense RxTM Insole (Orpyx Medical Technologies Inc., Calgary, Canada; Figure 1) and the GS-PSD was the Pedar X® (Novel, St. Paul, MN) pressure system. The PSRU has an array of eight pressure sensors that were designed to measure pressure 10–75 mm Hg. In contrast, the Pedar X has 99 sensors, each designed to measure pressure 1–112 mm Hg. Table 1 summarizes the main characteristics of the PSRU and GS-PSDs.18
Table 1.
Main Characteristics of the Plantar Sensory Replacement Unit and Gold Standard Pressure-Sensing Devices
| PSRU | GS-PSD | |
| Total sensors | 8 | 99 |
| Pressure range (mmHg) | 10–75 | 1–112 |
| Data collection frequency (Hz) | 4 | 100 |
| Hysteresis | <4.5% | <7% |
| Resolution | 8 bit | 2.5 kPa |
| Offset temperature drift (%) | <0.2 | <0.5 |
It has been reported24 that the minimum pressure to cause pressure ulcers is 32 mm Hg, and the PSRU pressure threshold was a priori designed to detect and provide real-time feedback for any plantar pressure measure greater than 32 mm Hg. Specifically, the PSRU measures pressure over time and alerts the wearer when the specified pressure threshold is exceeded. Each insole contains eight, flexible, resistive pressure sensors located at critical, discrete points along the plantar surface of the foot [the heel (1), the lateral foot (2), the first metatarsal head (1), the lateral metatarsal heads (2), the great toe (1), and the lateral toes (1)].6,13,15
Pressure measurements from these key locations are continuously recorded at a rate of 4 Hz, and these values are analyzed and catalogued by the device as being either “above” or “below” the aforementioned capillary pressure threshold. The insole takes the information over the past 15 min and calculates whether each area on the foot has seen “high,” “medium,” or “low” integrated pressure over that 15 min period. Pressure stratifications are based on the percentage of measurements taken over 15 min that exceed capillary pressure (“high” pressure corresponds to >95%, “medium” pressure corresponds to 50–89%, and “low” pressure corresponds to <50%). The PSRU device collects and relays integrated time-pressure data from the sole of the foot, via wireless protocol, and the collected data is sent to a display device (mobile app or wristwatch) to provide alerts when “high” pressure has been reached, as well as offloading guidance to the wearer. For the purpose of this study though, data were recorded and analyzed over a shorter period of time and according to the data collection procedures.
Procedures
For this study, the pressure-sensing elements for the PSRU were incorporated into custom foot orthoses for each of the participating subjects. For each subject, the orthoses were casted, designed, and fabricated by a single certified pedorthist. Casting was completed with the participant in a prone, non-weight-bearing position with plaster bandage using the subtalar neutral method. All orthoses were fabricated with the same combination of materials: the shell was 50-durometer ethyl vinyl acetate and the cover was a combination of 3 mm, 15-durometer P-CellTM and 3 mm Poron®. The electronic sensor array was incorporated between the ethyl vinyl acetate and the top cover. All orthoses were posted with neutral heel and forefoot angles and no offloading with metatarsal pads or cutouts were used.
Each participant walked along a 10 m walkway at a self-selected speed in standard laboratory shoes (Nike Air Pegasus, Nike Inc.), with the molded PSRU placed inside the shoe and the flat GS-PSD secured over-top of the PSRU using tape. Pilot testing allowed us to determine how the eight PSRU sensors corresponded to the 99 sensors of the Pedar X for each foot size. Specifically, both the PSRU and GS-PSD were placed over-top of each other, and a rigid object that was the same diameter as the PSRU sensors was placed directly over each of the PSRU sensors while measures were simultaneously recorded from both devices, which resulted in a corresponding array of 5–6 sensors from the GS-PSD. The average pressure from the corresponding GS-PSD sensor clusters was used for comparison to the PSRU. Data from the PSRU (4 Hz) and a GS-PSD (100 Hz) were collected simultaneously from the left foot, and the middle five consecutive footfalls were chosen for analysis.
Variables of Interest
The primary variable of interest was the number of data points recorded that were greater than the 32 mm Hg threshold for each of the eight PSRU sensors and corresponding average recordings from the GS-PSD sensor clusters. For the GS-PSD, the data were based on the average recording from the cluster of sensors then down sampled from 100 to 4 Hz. SPSS (version 20.0, SPSS Inc., Chicago, IL) was used to calculate the ICC (2,1) values between the two devices. We a priori defined an ICC r-value less than 60% as poor, 61–80% as good, 81–99% as very good, and 100% as perfect.25
Results
Table 2 summarizes the average number of data points measured above 32 mm Hg and r-values for the PSRU sensors and corresponding GS-PSD cluster locations. Sensors 1 and 8 correlated well (r = 0.86 and 0.84, respectively) and were considered very good as compared with the GS-PSD (Figures 2 and 3). Sensors 2, 3, 5, and 6 also correlated well (r-value range 0.67–0.73) and were considered good as compared with the GS-PSD. Sensors 4 and 7 did not correlate well (r = 0.41 and 0.38) and were considered poor as compared with the GS-PSD (Figures 2 and 3).
Table 2.
Summary of the Average (Standard Deviation) Number of Data Points Measured above 32 mm Hg along with Intraclass Correlation Coefficient R-Values and P-Values for the Plantar Sensory Replacement Unit Sensors and Corresponding Gold Standard Pressure-Sensing Device Cluster Locations
| Sensor 1 | Sensor 2 | Sensor 3 | Sensor 4 | Sensor 5 | Sensor 6 | Sensor 7 | Sensor 8 | |
| r-value | 0.86 | 0.67 | 0.69 | 0.41 | 0.72 | 0.73 | 0.38 | 0.84 |
| p-value | 0.01 | 0.05 | 0.05 | 0.15 | 0.04 | 0.04 | 0.18 | 0.01 |
| PSRU | 14.4 | 10.8 | 9.7 | 9.1 | 12.1 | 14.2 | 11.7 | 7.7 |
| (5.6) | (3.2) | (6.8) | (3.9) | (5.8) | (4.8) | (3.1) | (4.3) | |
| GS-PSD | 13.1 | 10.0 | 9.5 | 10.1 | 12.0 | 11.6 | 10.5 | 8.3 |
| (6.6) | (2.4) | (4.4) | (2.4) | (5.6) | (4.6) | (3.5) | (2.6) |
Figure 2.
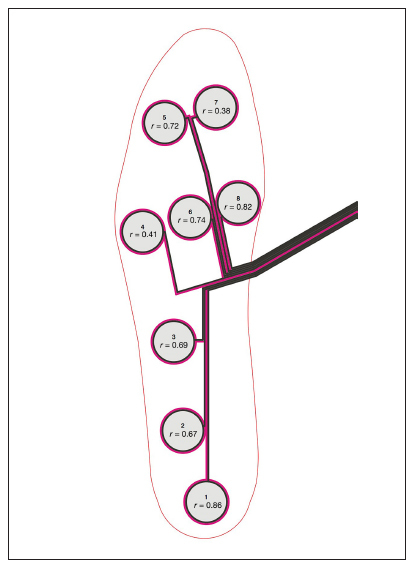
Location of each of the PSRU sensors and the correspondin r-values as compared with the cluster locations from the GS-PSD.
Figure 3.

Individual plots of each PSRU sensor for the 10 participants along with corresponding R2 values as compared with the cluster locations from the GS-PSD.
Discussion
This study aims to examine the validity of a novel PSRU in a group of healthy volunteer participants. The novelty of this device is the anticipated potential for prevention, treatment, and real-time monitoring of plantar pressures for neuropathy- and pressure-related diabetic foot disease (e.g., the propensity for tissue ulceration, infection, amputation, and balance and gait disturbance). We know of no other commercially available device capable of these aspects. Moreover, the PSRU has the ability to track compliance of prescribed orthotic insole therapy, contribute to significant long-term health care savings, and ultimately improve patient quality of life. The results of the present study suggest that the PSRU correlates well with GS-PSD technology.
Overall, the PSRU’s ability to accurately measure plantar pressure greater than 32 mm Hg correlated well with the gold standard device. It would also appear that the ability of the PSRU to detect pressure is related to the location, line of travel, and velocity of movement in the center of pressure (CoP) during a gait cycle.26 The sensors located near the hindfoot (sensor 1) and first metatarsal head (sensor 8) exhibited very good correlations and would correspond to the heel strike and toe-off events from a gait biomechanics perspective. We would expect the CoP to begin at or near sensor 1, reach peak pressure at approximately 13% of stance (approximately 100 ms), and track anteriorly along the middle of the midfoot toward
the first metatarsal head (sensor 8) over only the next 27% of stance duration (approximately 200 ms). Considering the 4 Hz data collection frequency of the PSRU, sensors 2, 3, and 4 exhibit only poor-to-good correlation primarily as a function of how quickly the CoP travels from the hindfoot to the medial aspect of the forefoot. Sensors 5, 6, and 8 exhibit good-to-very-good correlation considering that the CoP remains in this region for approximately 80–85% of stance whereas sensors 4 and 7 had poor correlation since the CoP only remains in these regions of the foot for 50% of stance. 26
The GS-PSD was collecting information at 100 Hz, which would be more sensitive and would thus have a much higher likelihood of recording these values, as opposed to potentially missing them with the 4 Hz PSRU. However, the PSRU is designed to collect data over long (>10 h) periods of time so missing data points, as a result of the relatively low collection frequency, would be collected over multiple footfalls. Thus, we attribute the poor-to-good correlation values for some of the PSRU sensors to the fast-moving trajectory of the CoP and the fact that data are being collected at 4 Hz over five footfalls, as compared with the GS-PSD.
Limitations of this study are acknowledged. First, we located clusters of GS-PSD sensors that corresponded to the location of single PSRU sensors, so it is possible that either the cluster could be too large or too small as compared with the PSRU sensor. However, we underwent a systematic process to determine the location of the GS-PSD sensor clusters prior to data collection and afterward to ensure accuracy of the data. Regardless, future studies incorporating kinematic and kinetic data and comparing the location of the CoP to the PSRU sensors would be beneficial. Second, we analyzed a total of five walking trials based on previous studies,27, 28 reporting three trials are sufficient to gain a representative sample of walking gait. Also, the GS-PSD has been designed to clinically measure plantar pressures for research purposes. The higher sensor resolution of the GS-PSD exceeds the requirements for a mobile, longitudinal, outpatient plantar pressure monitoring. Beyond this, the GS-PSD is not functionally designed for consumer use and would be a prohibitively costly solution. In contrast, and considering that the PSRU is designed for use outside the laboratory setting, validation and reliability studies over longer periods of time, similar to those of Hurkmans and coauthors29 are necessary. Third, differences in resolution and frequency of data collection may have affected results of the comparison between the two devices. Future validation studies involving GS-PSD that are more similar in sensor resolution and technological functionality, similar to the comparative study of different pressure-sensing devices conducted by Giacomozzi,18 are necessary. Finally, the PSRU is designed to measure downward pressure, otherwise known as the normal force, between the foot and shoe. However, it is well documented that shear stress also plays a significant role in the etiology of diabetic foot ulcers.30,31 Thus, while the PSRU correlates well to the gold standard with respect to downward pressure, caution must be taken when considering the pathomechanics of foot ulcer formation.
We acknowledge that other measures (i.e., shape and amplitude of the time curve of the instantaneous maximum pressure) have been reported to provide valuable information for understanding the pathomechanics leading to the development and progression of diabetic foot ulcers.32 Future studies and commercialized products that incorporate these data are needed to optimize patient care and might be an effective method for detecting diabetes patients who are at high risk of foot ulceration development.
The PSRU represents a novel method to monitor foot health proactively in an effort to reduce and prevent diabetic foot complications. Ulcers result from excessive pressure on focal areas of the foot; subsequently, pressure offloading is commonly used to treat pressure ulcers. Reduction or redistribution of pressure minimizes further complications in the ulcerated foot, including delayed healing, further progression of existing ulcers, or the development of additional ulceration. Additionally, this concept of pressure redistribution can be used as a preventative measure in order to minimize excessive load over time.
Conclusions
We compared a novel in-shoe pressure-sensing device to a gold standard device to determine the correlation between concurrent measures of plantar pressure. Overall, we found good-to-very good correlations compared with the gold
standard device when measuring the number of data points recorded that were greater than 32 mm Hg during walking. We conclude that the PSRU provides analogous data when compared with a gold standard pressure sensitive orthotic device.
Acknowledgments
We thank Sandy Connery for her assistance in casting and fabricating the orthoses used in this study.
Glossary
- (CoP)
center of pressure
- (ICC)
intraclass correlation coefficient
- (GS-PSD)
gold standard pressure-sensing device
- (PMD)
pressure measurement device
- (PSRU)
plantar sensory replacement unit
Funding
We thank Alberta Innovates - Health Solutions, Canadian Institutes of Health Research, National Research Council Canada - Industrial Research Assistance Program, and Mitacs-Accelerate for funding this project.
Disclosures
Orpyx Medical Technologies Inc. authors, Everett and Groenland, were involved in the design and development of the PSRU insole. Neither Orpyx Medical Technologies Inc. representative was involved in data processing, analysis, interpretation, or statistical analysis.
References
- 1.Bailes BK. Diabetes mellitus and its chronic complications. AORN J. 2002;76(2):266–282. doi: 10.1016/s0001-2092(06)61065-x. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi N. The burden of diabetes and its complications: trends and implications for intervention. Diabetes Res Clin Pract. 2007;76:S3–12. doi: 10.1016/j.diabres.2007.01.019. Suppl 1. [DOI] [PubMed] [Google Scholar]
- 3.Sicree R, Shaw J. Type 2 diabetes: an epidemic or not, and why it is happening. Diabetes Metabolic Syndrome Clin Res Rev. 2007;1(2):75–81. [Google Scholar]
- 4.Zhang P, Zhang X, Brown J, Vistisen D, Sicree R, Shaw J, Nichols G. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(3):293–301. doi: 10.1016/j.diabres.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 5.Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 6.Cavanagh PR, Simoneau GG, Ulbrecht JS. Ulceration, unsteadiness, and uncertainty: the biomechanical consequences of diabetes mellitus. J Biomech. 1993;26:23–40. doi: 10.1016/0021-9290(93)90077-r. Suppl 1. [DOI] [PubMed] [Google Scholar]
- 7.Paquette D, Falanga V. Leg ulcers. Clin Geriatr Med. 2002;18(1):77–88. doi: 10.1016/s0749-0690(03)00035-1. [DOI] [PubMed] [Google Scholar]
- 8.Veves A, Murray HJ, Young MJ, Boulton AJ. The risk of foot ulceration in diabetic patients with high foot pressure: a prospective study. Diabetologia. 1992;35(7):660–663. doi: 10.1007/BF00400259. [DOI] [PubMed] [Google Scholar]
- 9.Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A. Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care. 2000;23(5):606–611. doi: 10.2337/diacare.23.5.606. [DOI] [PubMed] [Google Scholar]
- 10.Ollendorf DA, Kotsanos JG, Wishner WJ, Friedman M, Cooper T, Bittoni M, Oster G. Potential economic benefits of lower-extremity amputation prevention strategies in diabetes. Diabetes Care. 1998;21(8):1240–1245. doi: 10.2337/diacare.21.8.1240. [DOI] [PubMed] [Google Scholar]
- 11.Vileikyte L. Diabetic foot ulcers: a quality of life issue. Diabetes Metab Res Rev. 2001;17(4):246–249. doi: 10.1002/dmrr.216. [DOI] [PubMed] [Google Scholar]
- 12.Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg. 2010;52:17S–22S. doi: 10.1016/j.jvs.2010.06.003. 3 Suppl. [DOI] [PubMed] [Google Scholar]
- 13.Actis RL, Ventura LB, Lott DJ, Smith KE, Commean PK, Hastings MK, Mueller MJ. Multi-plug insole design to reduce peak plantar pressure on the diabetic foot during walking. Med Biol Eng Comput. 2008;46(4):363–371. doi: 10.1007/s11517-008-0311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bus SA, Valk GD, van Deursen RW, Armstrong DG, Caravaggi C, Hlavácek P, Bakker K, Cavanagh PR. The Effectiveness of footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in diabetes: a systematic review. Diabetes Metab Res Rev. 2008;24:S162–80. doi: 10.1002/dmrr.850. Suppl 1. [DOI] [PubMed] [Google Scholar]
- 15.Abouaesha F, van Schie CH, Grifths GD, Young RJ, Boulton AJ. Plantar tissue thickness is related to peak plantar pressure in the high-risk diabetic foot. Diabetes Care. 2001;24(7):1270–1274. doi: 10.2337/diacare.24.7.1270. [DOI] [PubMed] [Google Scholar]
- 16.Giacomozzi C, Keijsers N, Pataky T, Rosenbaum D. International Scientific consensus on medical plantar pressure measurement devices: technical requirements and performance. Ann Ist Super Sanita. 2012;48(3):259–271. doi: 10.4415/ANN_12_03_06. [DOI] [PubMed] [Google Scholar]
- 17.Giacomozzi C. Hardware performance assessment recommendations and tools for baropodometric sensor systems. Ann Ist Super Sanita. 2010;46(2):158–167. doi: 10.4415/ANN_10_02_09. [DOI] [PubMed] [Google Scholar]
- 18.Giacomozzi C. Appropriateness of plantar pressure measurement devices: a comparative technical assessment. Gait Posture. 2010;32(1):141–144. doi: 10.1016/j.gaitpost.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Wu SC, Marston W, Armstrong D. Wound care: The role of advanced wound healing technologies. J Vasc Surg. 2010;52(3 Suppl):59S–66S. doi: 10.1016/j.jvs.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Zequera ML, Solomonidis S. Performance of insole in reducing plantar pressure on diabetic patients in the early stages of the disease. Conf Proc IEEE Eng Med Biol Soc. 2010. 2010:2982–2985. doi: 10.1109/IEMBS.2010.5626170. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong DG, Nguyen HC, Lavery LA, van Schie CH, Boulton AJ, Harkless LB. Of-loading the diabetic foot wound: a randomized clinical trial. Diabetes Care. 2001;24(6):1019–1022. doi: 10.2337/diacare.24.6.1019. [DOI] [PubMed] [Google Scholar]
- 22.Paton J, Bruce G, Jones R, Stenhouse E. Effectiveness of insoles used for the prevention of ulceration in the neuropathic diabetic foot: a systematic review. J Diabetes Complications. 2011;25(1):52–62. doi: 10.1016/j.jdiacomp.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Lewis J, Lipp A. Pressure-relieving interventions for treating diabetic foot ulcers. Cochrane Database Syst Rev. 2013;1:CD002302. doi: 10.1002/14651858.CD002302.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Husain T. An experimental study of some pressure Effects on tissues, with reference to the bed-sore problem. J Pathol Bacteriol. 1953;66(2):347–358. doi: 10.1002/path.1700660203. [DOI] [PubMed] [Google Scholar]
- 25.Ferber R, Kendall KD, McElroy L. Normative and critical criteria for iliotibial band and iliopsoas muscle flexibility. J Athl Train. 2010;45(4):344–348. doi: 10.4085/1062-6050-45.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu XC, Thometz JG, Tassone C, Barker B, Lyon R. Dynamic plantar pressure measurement for the normal subject: free-mapping model for the analysis of pediatric foot deformities. J Pediatr Orthop. 2005;25(1):103–106. doi: 10.1097/00004694-200501000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Holmes GB, Jr, Timmerman L, Willits NH. Practical considerations for the use of the pedobarograph. Foot Ankle. 1991;12(2):105–108. doi: 10.1177/107110079101200208. [DOI] [PubMed] [Google Scholar]
- 28.Imamura M, Imamura ST, Salomão O, Pereira CA, De Carvalho AE, Jr, Neto RB. Pedobarometric evaluation of the normal adult male foot. Foot Ankle Int. 2002;23(9):804–810. doi: 10.1177/107110070202300906. [DOI] [PubMed] [Google Scholar]
- 29.Hurkmans HL, Bussmann JB, Selles RW, Horemans HL, Benda E, Stam HJ, Verhaar JA. Validity of the Pedar Mobile system for vertical force measurement during a seven-hour period. J Biomech. 2006;39(1):110–118. doi: 10.1016/j.jbiomech.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 30.Sawacha Z, Guarneri G, Cristofferi G, Guiotto A, Avogaro A, Cobelli C. Integrated kinematics-kinetics-plantar pressure data analysis: a useful tool for characterizing diabetic foot biomechanics. Gait Posture. 2012;36(1):20–26. doi: 10.1016/j.gaitpost.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Merolli A, Uccioli L. Plantar pressure distribution in patients with neuropathic diabetic foot. J Appl Biomater Biomech. 2005;3(1):61–64. [PubMed] [Google Scholar]
- 32.Giacomozzi C, Martelli F. Peak pressure curve: an Effective parameter for early detection of foot functional impairments in diabetic patients. Gait Posture. 2006;23(4):464–470. doi: 10.1016/j.gaitpost.2005.06.006. [DOI] [PubMed] [Google Scholar]


