Abstract
Accurate detection and quantification of human diabetic peripheral neuropathy are important to define at-risk patients, anticipate deterioration, and assess new therapies. Easily performed clinical techniques such as neurological examination, assessment of vibration perception or insensitivity to the 10 g monofilament only assess advanced neuropathy, i.e., the at-risk foot. Techniques that assess early neuropathy include neurophysiology (which assesses only large fibers) and quantitative sensory testing (which assesses small fibers), but they can be highly subjective while more objective techniques, such as skin biopsy for intra-epidermal nerve fiber density quantification, are invasive and not widely available. The emerging ophthalmic technique of corneal confocal microscopy allows quantification of corneal nerve morphology and enables clinicians to diagnose peripheral neuropathy in diabetes patients, quantify its severity, and potentially assess therapeutic benefit. The present review provides a detailed critique of the rationale, a practical approach to capture images, and a basis for analyzing and interpreting the images. We also critically evaluate the diagnostic ability of this new noninvasive ophthalmic test to diagnose diabetic and other peripheral neuropathies.
Keywords: corneal confocal microscopy, diabetic neuropathy
Diabetic neuropathy (DN) is a global problem affecting ~50% of the 26 million Americans and more than 366 million people worldwide with diabetes. It is the most common and costly complication of diabetes, leading to painful neuropathy (~21%) 1 and a 23.3-fold increased relative risk of foot ulceration and amputation.2 It has been previously shown that foot ulceration is much more common in patients with diabetic peripheral neuropathy (DPN) with the annual incidence rising from <1% in those without neuropathy to >7% in those with established neuropathic deficits.3 Furthermore, it has been shown to be an independent predictor for all-cause (hazard ratio = 4.4) and diabetes-related (hazard ratio = 11.82) mortality.4
Management is difficult, as even tight glycemic control, a cornerstone for the management of diabetes, has been shown, at best, to limit progression of neuropathy in patients with type 1 diabetes,5 but not type 2 diabetes.6–9 There is no Food and Drug Administration-approved therapy to prevent or reverse human DN. Moreover, the development of disease-modifying drugs for DPN has stalled completely. Of course, there are many potential reasons/excuses for the multiple failed trials. However, it is increasingly apparent that there are significant issues with the end points deployed in clinical trials of human DN. Indeed a two-step hierarchical cluster analysis has revealed that neurophysiological tests do not aggregate by typical “small,” “large,” or “autonomic” nerve fiber subtypes.10 Yet the latest recommendations continue to advocate a combination of symptoms and signs, quantitative sensory testing (QST), and electrophysiology for the diagnosis of DN.11 By default, rather than design, these same measures have been adopted as surrogate end points to establish the benefits of therapeutic intervention and yet have clearly failed in several major clinical trials.12,13
While symptoms and neurological deficits have direct relevance to patients, the assessment is excessively variable with poor reproducibility.14 Similarly, QST is subjective, is highly variable, and has limited reproducibility.15 Neurophysiology is objective and reproducible but does not assess small fibers, which are the earliest to be damaged and show repair.16 Small fibers can be assessed objectively by quantifying intra-epidermal nerve fiber density (IENFD) in skin biopsies; however, this is an invasive procedure that requires expert laboratory assessment and has considerable variability even among control.17,18 Therefore, effective treatments may have failed not because of a lack of efficacy, but because of an inability of the currently advocated end points to detect improvement in clinical trials of DN.19 A summary of the advantages and limitations of the present techniques to quantify nerve fiber damage in DN is presented in Table 1.
Table 1.
Summary of Advantages and Disadvantages of Tests to Assess Diabetic Neuropathy
| Method | Advantage | Disadvantage |
| Clinical/neurological examination | Simple, easy to perform, does not require special equipment | Not sensitive, not reproducible |
| Nerve conduction studies | Sensitive, objective, currently the gold standard for diagnosis | Assesses only large fibers, requires special equipment |
| QST | Evaluates both large and small nerve fibers, quantitative, relatively easy to perform | Subjective, moderate reproducibility, requires special equipment |
| Sympathetic skin response | Simple, fast, objective | Semiquantitative, low sensitivity |
| Quantitative sudomotor axon reflex test | Sensitive, objective, reproducible | Requires special equipment, time consuming |
| Autonomic testing | Objective, quantitative | Moderate sensitivity, requires special equipment |
| Neuropad™ (sudomotor function assessment) | Noninvasive, easy to perform, does not require special equipment | Subjective, moderate sensitivity, uncertain interpretation |
| Sural nerve/skin biopsy | Quantitative, sensitive, currently the gold standard to quantify small fibers | Invasive, costly, risk of infection at the site of biopsy, requires specialist histological technique to quantify IENFD |
| Noncontact Corneal Aesthesiometry | Noninvasive, quantitative | Subjective, moderate sensitivity |
| IVCCM | Reproducible, rapid, sensitive, noninvasive, reiterative, quantitative | Requires special equipment and expertise |
Hence, there is an urgent need for a noninvasive, sensitive surrogate marker in clinical trials of DN. There is strong evidence that the ophthalmic technique of in vivo corneal confocal microscopy (IVCCM) might be such an ideal surrogate end point for DPN.
Morphology of Human Corneal Innervation
The cornea is the most densely innervated tissue in the body.20 Corneal nerves are derived from the ophthalmic division of the trigeminal nerve and enter the cornea in the middle third of the stroma and run forward anteriorly in a radial fashion toward the center, where they lose their myelin sheath. The human cornea contains myelinated Aδ fibers, which are large-diameter (6 μm), straight nerves that respond primarily to mechanical stimuli, and unmyelinated C fibers, which are small-diameter (2–4 μm), beaded nerves that respond to thermal and chemical stimuli21 (Figure 1). Detailed knowledge of corneal nerve architecture and morphology has been provided by studies employing light22–24 and electron21,25 microscopy and, later, IVCCM.26
Figure 1.
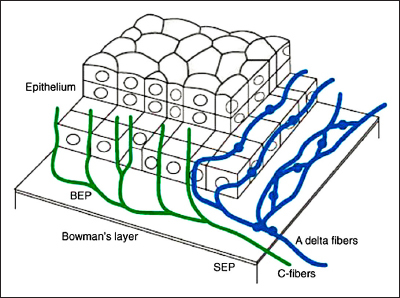
Three-dimensional representation of the innervation of the human corena. BEP, basal epithelial plexus; SEP, subepithelial plexus32
Corneal innervation plays an important role in regulating epithelial cell growth, proliferation, and differentiation in normal physiological states or in response to corneal disease, trauma, or surgery through the release of several growth factors, cytokines, and neurochemicals.33 Although there are numerous studies on the anatomy and physiology of corneal innervation, its complete and complex physiological role remains unclear. In vitro coculture studies suggest that neurons and epithelial cells provide each other trophic support through the release of soluble substances. Neurons release substance P that stimulates epithelial cell growth, proliferation, differentiation, and type VII collagen production.20 Thus patients with impaired corneal innervation may be at increased risk of ulceration due to impaired trophic support provided by the corneal nerves.34,35
In Vivo Corneal Confocal Microscopy
In vivo corneal confocal microscopy is an established technique, which has evolved rapidly from a predominantly research application to a diagnostic tool with a variety of clinical applications in ocular and neurological diseases. The noninvasive nature and rapid image acquisition time of the technique has made it an ideal method to extensively study all microstructures of the cornea, including the epithelial cell layer, Bowman’s membrane, sub-basal nerve plexus, stroma, and endothelium.
Image Acquisition
The type of IVCCM used can significantly affect the quality of images. As a result, studies using a laser-scanning confocal microscope (e.g., Heidelberg Retina Tomograph III Rostock Cornea Module, Heidelberg GmbH, Heidelberg, Germany) have reported higher sub-basal nerve densities compared with studies using a tandem-scanning confocal microscope or a slit-scanning confocal microscope (e.g., Nidek Confoscan 4, Nidek Technologies, Padova, Italy, and Tomey Confoscan P4, Tomey, Erlangen, Germany) due to differences in the light source, contrast, and resolution.32 Furthermore, studies have employed a range of scanning, image sampling, and quantification methodologies. There is no consensus regarding the minimum number of images required for representative quantitative analysis. The majority of published studies have used up to five images per layer per eye for analysis, and one study has suggested that 5–8 images are optimal, depending on the parameter being assessed.36
Image Quantification
The quality of the selected images is vital, and once image selection is complete, all images should be deidentified and randomized by an independent investigator prior to analysis to avoid observer bias. The majority of studies have defined sub-basal nerve density as the total number of nerves in each image, which allows quantification of the nerve density in an area (number/mm2).37–43 Others have presented the data as the number of nerves per image44 or the total length of the nerves within a frame45,46 but have nevertheless referred to the measure as a nerve density, which can be confusing to the nonexpert reader.
Adaptation of a global protocol to quantify corneal nerve morphology is of paramount importance, as it will enable a direct comparison of the results from different studies and allow multicenter studies. To date, most studies have employed semiautomated image analysis to assess sub-basal nerve alterations, which is a labor-intensive, subjective, and time consuming task. Studies from several different centers have assessed the impact of interobserver and intra-observer variability on the quantification of corneal nerve morphology using IVCCM and have reported excellent reproducibility among patients with diabetes and controls.47–49 Very good reproducibility has been shown using a clinically relevant “study-level” protocol of subject re-examination (intraobserver intraclass correlation coefficient, 0.72; interobserver intraclass correlation coefficient, 0.73).49 Inherent interobserver differences and experience were identified as the main causes of variation, especially for the parameter of nerve branch density, suggesting the need for a fully automated image analysis system to eliminate inconsistencies and expedite image analysis time. Such software has been developed.50–52
Stromal nerves have been studied less extensively with IVCCM. A few studies have quantified stromal nerves and quantified the density and the diameter of the nerves.26,53,54 However, a wide range of results have been reported that may be due to the inconsistency in capturing stromal nerves because of their orientation and sparse distribution.53 Stromal rather than sub-basal nerves appear more robust in surviving postmortem change,54 therefore in vitro studies should focus on stromal nerves, whereas in vivo studies using IVCCM should focus on sub-basal nerves.
Corneal Nerve Changes in Diabetic Neuropathy
There has been increasing research interest in modeling the relationship between corneal nerve fiber loss and neuropathy. An association between neurotrophic corneal ulcers and diabetes was reported as early as 1977.55 Subsequently, a reduction in corneal nerve density was demonstrated in experimental diabetes ex vivo.35 The cornea, due to the unique property of transparency, allows direct, noninvasive, in vivo imaging of the small unmyelinated nerve fiber bundles. The first study using noncontact corneal esthesiometry in diabetes was by Rosenberg and colleagues56 in 2000, showing sub-basal nerve alterations and a reduction in corneal sensitivity in patients with DN. However, since then, a burgeoning literature shows that IVCCM can quantify DN (Table 2).39–42,44,56,57
Table 2.
Summary of the Results of Quantitative Corneal Nerves Assessment with IVCCM in Diabetic Neuropathya
| Studies, first author | n | Age, years | Type of IVCCM | Acquisition method/images assessed per subject | Corneal nerve fiber density | Corneal nerve branch density | Corneal nerve fiber length | Study limitations |
| Sellers58 | 12 | 14.8 ± 2.1 | LSCM | Section/5 images | 24.1 ± 3.1 no/mm2 | 43.7 ± 13.7 no/mm2 | 18.2 ± 2.4 mm/mm2 | Small sample size |
| Zhivov59 | 18 | 68.8 ± 8.8 | LSCM | Section/not specified | 0.006 ± 0.002 mm/mm2 | 25.3 ± 28.6 no/frame | 6222 ± 2419 μm | Small sample size |
| Ahmed43 | 33 | 50 ± 14.3 | LSCM | Volume/2 images | 28.0 ± 9.0 no/mm2 | 17.0 ± 12.0 no/mm2 | 11.1 ± 3.6 mm/mm2 | Image selection criteria |
| Edwards38 | 88 | 58 ± 9 | LSCM | Section/8 images | — | Graphical | Graphical | Corneal nerve fiber density not presented |
| Nitoda60 | 139 | 63 ± 2 | LSCM | Sequence/3–5 images | 23.3 ± 0.8 no/mm2 | 31.8 ± 2.6 no/mm2 | 12.5 ± 2.6 mm/mm2 | — |
| Tavakoli61 | 25 | 52 ± 2 | SSCM | Section/3–5 images | 18.8 ± 2.1 no/mm2 | 6.9 ± 1.5 no/mm2 | 8.3 ± 0.9 mm/mm2 | Small sample size |
| Hertz49 | 26 | 43 ± 16.9 | LSCM | Volume/2 images | 32.5 ± 9.7 no/mm2 | 26.0 ± 17.1 no/mm2 | 13.6 ± 3.5 no/mm2 | Image selection criteria |
| Ishibashi62 | 38 | 46.7 ± 1.6 | LSCM | Not specified/4–5 images | 25.3 ± 1.0 no/mm2 | — | 9.8 ± 0.3 mm/mm2 | — |
| Tavakoli39 | 101 | 58.3 ± 2.2 | SSCM | Section/3–5 images | 24.1 ± 2.6 no/mm2 | 10.3 ± 1.7 no/mm2 | 4.9 ± 0.5 mm/mm2 | — |
| Messmer40 | 67 | 54 | LSCM | Volume and sequence/ 5 images | 16.5 no/mm2 | 17.5 no/m m 2 | 10.2 mm/mm2 | Sample demographics |
| De Cillà63 | 50 | 62.6 ± 6 | LSCM | Not specified/1 image | 2.4 ± 1.0 no/frame | — | — | Image selection and analysis criteria |
| Midena44 | 42 | — | SSCM | — | 2.2 ± 0.3 no/frame | 0.8 ± 0.1 (degree) | — | — |
| Chang41 | 42 | 63.8 ± 7.2 | SSCM | Not specified | 16.1 ± 5.7 no/mm2 | 24.9 ± 7.7 no/mm2 | — | Image selection and analysis criteria |
| Quattrini16 | 54 | 58 ± 10.9 | SSCM | Section/3–5 images | 23.7 ± 3.2 no/mm2 | 7.31 ± 1.98 no/mm2 | 3.94 ± 0.63 mm/mm2 | — |
| Mocan64 | 35 | 58.4 ± 10 | SSCM | Not specified/1 image | 28.3 ± 10.4 | 39.7 ± 13.2 no/mm2 | — | Image analysis criteria |
| Malik42 | 18 | 57 ± 12.8 | SSCM | Section/3–5 images | 27.8 ± 6.5 no/mm2 | 27.2 ± 13.2 no/mm2 | 7.5 ± 1.1 mm/mm2 | Sample size |
| Rosenberg56 | 23 | 46 ± 8.3 | TSCM | Section/2 images | 3.1 ± 1.2 no/frame | — | — | Type of IVCCM |
LSCM, laser-scanning confocal microscope; SSCM, slit-scanning confocal microscope; TSCM, tandem-scanning confocal microscope
We have demonstrated that IVCCM quantifies early small nerve fiber damage39, 42,65 with good sensitivity and specificity39 (Figure 2). Others have confirmed that IVCCM detects mild neuropathy,38 and corneal nerve fiber length in particular has a high sensitivity (91%) and specificity (93%) for identifying diabetic sensorimotor polyneuropathy.43
Figure 2.
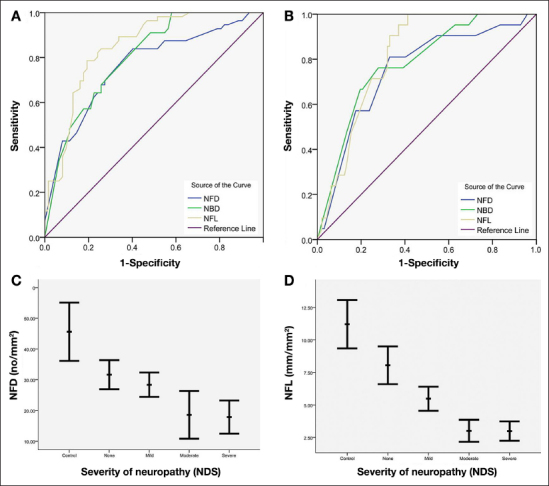
Receiver operating characteristic curves for the diagnostic validity of nerve fiber density, nerve branch density, and nerve fiber length for (A) NDS > 3 and (B) NDS > 6. Corneal nerve morphology in control subjects and diabetes patients with increasing neuropathic severity: (C) nerve fiber density (p < .0001) and (D) nerve fiber length (p < .0001). NFD, nerve fiber density; NBD, nerve branch density; NDS, neuropathy disability score; NFL, nerve fiber length.39
Furthermore, a reduction in corneal nerve fiber length has been related to elevated hemoglobin A1c even in normal subjects, suggesting that IVCCM may detect early subclinical prediabetic nerve injury.66 In a study of patients with idiopathic small fiber neuropathy, we have demonstrated significant corneal nerve damage, which was related to higher triglycerides.67 We have also shown that IVCCM can be performed in children with diabetes.58 Importantly, we have shown that corneal nerve damage assessed using IVCCM relates to the severity of intra-epidermal nerve fiber loss (gold standard for small fiber damage) in foot skin biopsies.16 Corneal nerve fiber length has been shown to correlate significantly with three independent measures of small fiber function: cold detection thresholds, laser Doppler imager fare, and heart rate variability.68 The further significant potential of IVCCM as a viable surrogate end point has been evidenced by demonstrating that IVCCM detects nerve fiber regeneration within 6 months of simultaneous pancreas kidney transplantation, while neurological deficits, QST, nerve conduction studies, and IENFD remain unchanged in diabetes patients (Figures 3 and 4).37,69
Figure 3.
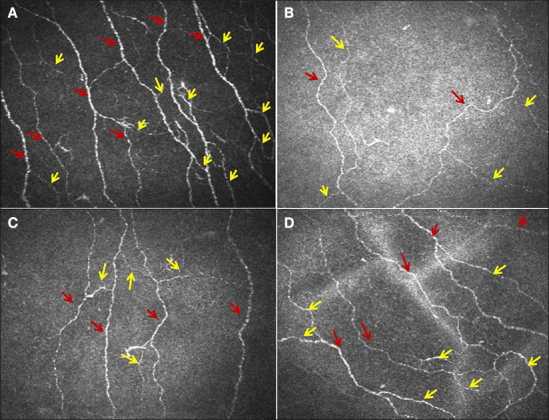
Sub-basal nerve images from the cornea of (A) a control subject and (B) a patient with type 1 diabetes at baseline and at (C) 6 and (D) 12 months after simultaneous pancreas kidney transplantation. The red arrows indicate main nerve fibers, and yellow arrows indicate branches.37
Figure 4.
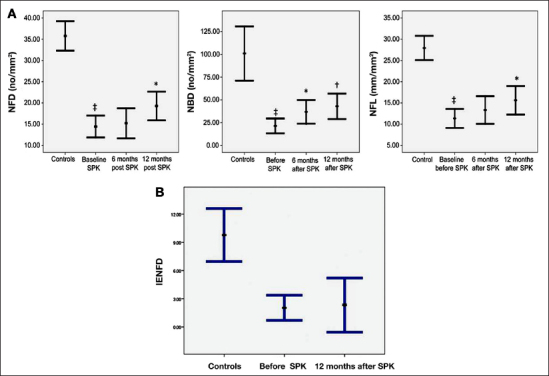
(A) Corneal nerve fiber density (left), corneal nerve branch density (middle), and corneal nerve fiber length (right) in diabetes patients at baseline and at 6 and 12 months after simultaneous pancreas kidney transplantation, where significant regeneration is recorded. (B) Intra-epidermal nerve fiber density in control subjects and in diabetes patients at baseline and 12 months after simultaneous pancreas kidney transplantation (SPK) showed no significant improvement. NFD, nerve fiber density; NBD, nerve branch density; NFL, nerve fiber length.37
Of immediate clinical relevance, we have also demonstrated an improvement in corneal nerve fiber density after improvement in glycemia, blood pressure, and lipids in diabetes patients.61 A potential limitation of IVCCM is the speed of analysis; however, automated image analysis has been developed for the rapid quantification of corneal nerve images.70,71 Indeed we have developed an automated image analysis system that shows high correlation with manually assessed corneal nerve fiber density and length.52,72 Our automated algorithm uses a dual model feature descriptor with a neural network classifier for dynamic detection and quantification whereas others approaches are based primarily on the refectivity of structures.60 This particular method has also been chosen from a number of possible combinations, following evaluation of its clinical effectiveness in a cohort of patients with DPN.51
Arguments against IVCCM have revolved around the relatively short nerves being studied and the fact that the cornea is avascular, which is in contrast with the long somatic nerves and the compelling evidence for a vascular basis of DN.73, 74 However, reassuringly, corneal nerve pathology has been found to correlate with IENFD loss in biopsies from the dorsum of the foot16 and a range of small fiber measures of DN.68 Furthermore, studies in animal models of DN using IVCCM have shown a significant reduction in blood flow in the posterior ciliary artery and corneal nerve fiber loss with an improvement in both blood flow and corneal innervation after intervention with a vasopeptidase inhibitor.75,76
In vivo corneal confocal microscopy has shown a decrease in the number and density of sub-basal nerves in a variety of abnormal ocular and systemic conditions, including dry eyes not related to Sjögren’s syndrome and dry eyes related to primary Sjögren’s syndrome.77–79 There is also a burgeoning literature on the use of IVCCM to quantify not only DN,39–42,44,56 but also idiopathic small fiber neuropathy,69 Fabry disease,80 hereditary sensory and autonomic neuropathy, 81 autoimmune neuropathy,82 Crohn’s disease,78 and neuropathy associated with chemotherapy.78,83
Summary
In conclusion, IVCCM appears to be an ideal noninvasive clinical technique that can assess alterations in corneal cellular pathology and, in particular, has been used to quantify small nerve fiber pathology in relation to DN. With the development of automated image analysis, we predict a rapid increase in the clinical utility of IVCCM in the assessment of DN and a range of peripheral neuropathies. In this review, we have summarized the potential of this powerful technique to undertake detailed morphological analysis of corneal nerves to act as a surrogate measure of peripheral neuropathy. It appears that the widest application of IVCCM may well be in the field of metabolic or neurological disease, particularly as it may provide a noninvasive means to identify patients with minimal neuropathy, quantify the severity of neuropathy, and follow progression of or assess therapeutic response, in not only DN, but also a range of other neuropathies. There is clearly a need to standardize the method of capturing, sampling, and analyzing the images in order to use IVCCM in longitudinal prospective or interventional multicenter studies. Finally, “prevention is better than cure.” Hence, preventing foot ulceration may well require a paradigm shift from identifying advanced neuropathy (monofilament)—which may be too late for intervention)—to minimal neuropathy, which may be amenable to intervention; hence keeping an “eye on the foot.”
Acknowledgments
This study was supported by the National Institute for Health Research/Wellcome Trust Clinical Research Facility at Central Manchester University Hospitals National Health Service Foundation Trust.
Glossary
- (DN)
diabetic neuropathy
- (DPN)
diabetic peripheral neuropathy
- (IENFD)
intra-epidermal nerve fiber density
- (IVCCM)
in vivo corneal confocal microscopy
- (QST)
quantitative sensory testing
Funding
This work was funded by JDRF International Grant #5-2002-185 and National Eye Institute Grant #1 R01 NS46259-01, and facilitated by the Manchester Biomedical Research Center and the Greater Manchester Comprehensive Local Research Network.
References
- 1.Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34(10):2220–2224. doi: 10.2337/dc11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holman N, Young RJ, Jefcoate WJ. Variation in the recorded incidence of amputation of the lower limb in England. Diabetologia. 2012;55(7):1919–1925. doi: 10.1007/s00125-012-2468-6. [DOI] [PubMed] [Google Scholar]
- 3.Abbott CA, Vileikyte L, Williamson S, Carrington AL, Boulton AJ. Multicenter study of the incidence of and predictive risk factors for diabetic neuropathic foot ulceration. Diabetes Care. 1998;21(7):1071–1075. doi: 10.2337/diacare.21.7.1071. [DOI] [PubMed] [Google Scholar]
- 4.Hsu WC, Chiu SY, Yen AM, Chen LS, Fann CY, Liao CS, Chen HH. Somatic neuropathy is an independent predictor of all- and diabetes-related mortality in type 2 diabetic patients: a population-based 5-year follow-up study (KCIS No 29) Eur J Neurol. 2012;19(9):1192–1198. doi: 10.1111/j.1468-1331.2011.03659.x. [DOI] [PubMed] [Google Scholar]
- 5.Albers JW, Herman WH. Pop-Busui R, Fieldman EL, Martin CL, Cleary PA, Waberski BH, Lachin JM. Diabetes Control and Complications Trial /Epidemiology of Diabetes Interventions and Complications Research Group. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010;33(5):1090–1096. doi: 10.2337/dc09-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumont IJ, Lepeut MS, Tsirtsikolou DM, Popielarz SM, Cordonnier MM, Fayard AJ, Devemy F, Fernandez E, Basuyaux O, Jefcoate WJ. A proof-of-concept study of the effectiveness of a removable device for ofoading in patients with neuropathic ulceration of the foot: the Ransart boot. Diabet Med. 2009;26(8):778–782. doi: 10.1111/j.1464-5491.2009.02772.x. [DOI] [PubMed] [Google Scholar]
- 7.Gaede P. Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 8.Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, Cuddihy R, Cushman WC, Genuth S, Grimm RH, Jr, Hamilton BP, Hoogwerf B, Karl D, Katz L, Krikorian A. O’Connor P. Pop-Busui R, Schubart U, Simmons D, Taylor H, Thomas A, Weiss D, Hramiak I. ACCORD trial group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–430. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoungas S, de Galan BE, Ninomiya T, Grobbee D, Hamet P, Heller S, MacMahon S, Marre M, Neal B, Patel A, Woodward M, Chalmers J. ADVANCE Collaborative Group Cass A, Glasziou P, Harrap S, Lisheng L, Mancia G, Pillai A, Poulter N, Perkovic V, Travert F. Combined efects of routine blood pressure lowering and intensive glucose control on macrovascular and microvascular outcomes in patients with type 2 diabetes: new results from the ADVANCE trial. Diabetes Care. 2009;32(11):2068–2074. doi: 10.2337/dc09-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibbons CH, Freeman R, Veves A. Diabetic neuropathy: a cross-sectional study of the relationships among tests of neurophysiology. Diabetes Care. 2010;33(12):2629–2634. doi: 10.2337/dc10-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L. Valensi P. Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on defnitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tesfaye S, Tandan R. Bastyr EJ., 3rd Kles KA, Skljarevski V. Price KL, Ruboxistaurin Study Group Factors that impact symptomatic diabetic peripheral neuropathy in placebo-administered patients from two 1-year clinical trials. Diabetes Care. 2007;30(10):2626–2632. doi: 10.2337/dc07-0608. [DOI] [PubMed] [Google Scholar]
- 13.Ziegler D, Low PA, Litchy WJ, Boulton AJ, Vinik AI, Freeman R, Samigullin R, Tritschler H, Munzel U, Maus J. Schütte K, Dyck PJ. Efficacy and safety of antioxidant treatment with α-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. 2011;34(9):2054–2060. doi: 10.2337/dc11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dyck PJ, Overland CJ, Low PA, Litchy WJ, Davies JL, Dyck PJ. O’Brien PC, Cl vs. NPhys Trial Investigators Albers JW, Andersen H, Bolton CF, England JD, Klein CJ, Llewelyn JG, Mauermann ML, Russell JW, Singer W, Smith AG, Tesfaye S, Vella A. Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathy: Cl vs NPhys trial. Muscle Nerve. 2010;42(2):157–164. doi: 10.1002/mus.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman R, Chase KP, Risk MR. Quantitative sensory testing cannot differentiate simulated sensory loss from sensory neuropathy. Neurology. 2003;60(3):465–470. doi: 10.1212/wnl.60.3.465. [DOI] [PubMed] [Google Scholar]
- 16.Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, Tesfaye S, Finnigan J, Marshall A, Boulton AJ, Efron N, Malik RA. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56(8):2148–2154. doi: 10.2337/db07-0285. [DOI] [PubMed] [Google Scholar]
- 17.Engelstad JK, Taylor SW, Witt LV, Hoebing BJ, Herrmann DN, Dyck PJ, Klein CJ, Johnson DM, Davies JL, Carter RE, Dyck PJ. Epidermal nerve fibers: confdence intervals and continuous measures with nerve conduction. Neurology. 2012;79(22):2187–2193. doi: 10.1212/WNL.0b013e3182759608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauria G, Bakkers M, Schmitz C, Lombardi R, Penza P, Devigili G, Smith AG, Hsieh ST, Mellgren SI, Umapathi T, Ziegler D, Faber CG, Merkies IS. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst. 2010;15(3):202–207. doi: 10.1111/j.1529-8027.2010.00271.x. [DOI] [PubMed] [Google Scholar]
- 19.Dyck PJ, Norell JE, Tritschler H, Schuette K, Samigullin R, Ziegler D, Bastyr EJ., 3rd Litchy WJ, O’Brien PC. Challenges in design of multicenter trials: end points assessed longitudinally for change and monotonicity. Diabetes Care. 2007;30(10):2619–2625. doi: 10.2337/dc06-2479. [DOI] [PubMed] [Google Scholar]
- 20.Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 21.Beckers H, Klooster J, Vrensen G, Lamers W. Sympathetic innervation of the rat’s eye and peripheral ganglia: an electron microscopic autoradiographic tracing study. Graefes Arch Clin Exp Ophthalmol. 1994;232(1):57–65. doi: 10.1007/BF00176438. [DOI] [PubMed] [Google Scholar]
- 22.Guthof RF, Wienss H, Hahnel C, Wree A. Epithelial innervation of human cornea: a three-dimensional study using confocal laser scanning fuorescence microscopy. Cornea. 2005;24(5):608–613. doi: 10.1097/01.ico.0000154384.05614.8f. [DOI] [PubMed] [Google Scholar]
- 23.Jones MA, Marfurt CF. Peptidergic innervation of the rat cornea. Exp Eye Res. 1998 Apr;66(4):421–435. doi: 10.1006/exer.1997.0446. [DOI] [PubMed] [Google Scholar]
- 24.Müller LJ, Vrensen GF, Pels L, Cardozo BN, Willekens B. Architecture of human corneal nerves. Invest Ophthalmol Vis Sci. 1997;38(5):985–994. [PubMed] [Google Scholar]
- 25.Marfurt CF, Cox J, Deek S, Dvorscak L. Anatomy of the human corneal innervation. Exp Eye Res. 2010;90(4):478–492. doi: 10.1016/j.exer.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira-Soto L, Efron N. Morphology of corneal nerves using confocal microscopy. Cornea. 2001;20(4):374–384. doi: 10.1097/00003226-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Patel DV, McGhee CN. Mapping of the normal human corneal sub-basal nerve plexus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2005;46(12):4485–4488. doi: 10.1167/iovs.05-0794. [DOI] [PubMed] [Google Scholar]
- 28.Møller-Pedersen T, Li HF, Petroll WM, Cavanagh HD, Jester JV. Confocal microscopic characterization of wound repair after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 1998;39(3):487–501. [PubMed] [Google Scholar]
- 29.Yokogawa H, Kobayashi A, Sugiyama K. Mapping of normal corneal K-structures by in vivo laser confocal microscopy. Cornea. 2008;27(8):879–883. doi: 10.1097/ICO.0b013e318170aed0. [DOI] [PubMed] [Google Scholar]
- 30.Mannion LS, Tromans C, O’Donnell C. Corneal nerve structure and function in keratoconus: a case report. Eye Contact Lens. 2007 Mar;33(2):106–108. doi: 10.1097/01.icl.0000235270.45379.9c. [DOI] [PubMed] [Google Scholar]
- 31.Patel DV, McGhee CN. Mapping the corneal sub-basal nerve plexus in keratoconus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2006;47(4):1348–1351. doi: 10.1167/iovs.05-1217. [DOI] [PubMed] [Google Scholar]
- 32.Tavakoli M, Petropoulos IN, Malik RA. Assessing corneal nerve structure and function in diabetic neuropathy. Clin Exp Optom. 2012;95(3):338–347. doi: 10.1111/j.1444-0938.2012.00743.x. [DOI] [PubMed] [Google Scholar]
- 33.Gallar J, Acosta MC, Moilanen JA, Holopainen JM, Belmonte C, Tervo TM. Recovery of corneal sensitivity to mechanical and chemical stimulation after laser in situ keratomileusis. J Refract Surg. 2004;20(3):229–235. doi: 10.3928/1081-597X-20040501-06. [DOI] [PubMed] [Google Scholar]
- 34.Allen VD, Malinovsky V. Management of neurotrophic keratopathy. Cont Lens Anterior Eye. 2003;26(3):161–165. doi: 10.1016/S1367-0484(03)00027-4. [DOI] [PubMed] [Google Scholar]
- 35.Yamada J, Dana MR, Sotozono C, Kinoshita S. Local suppression of IL-1 by receptor antagonist in the rat model of corneal alkali injury. Exp Eye Res. 2003;76(2):161–167. doi: 10.1016/s0014-4835(02)00293-2. [DOI] [PubMed] [Google Scholar]
- 36.Vagenas D, Pritchard N, Edwards K, Shahidi AM, Sampson GP, Russell AW, Malik RA, Efron N. Optimal image sample size for corneal nerve morphometry. Optom Vis Sci. 2012;89(5):812–817. doi: 10.1097/OPX.0b013e31824ee8c9. [DOI] [PubMed] [Google Scholar]
- 37.Tavakoli M, Mitu-Pretorian M, Petropoulos IN, Fadavi H, Asghar O, Alam U, Ponirakis G, Jeziorska M, Marshall A, Efron N, Boulton AJ, Augustine T, Malik RA. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes. 2013;62(1):254–260. doi: 10.2337/db12-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edwards K, Pritchard N, Vagenas D, Russell A, Malik RA, Efron N. Utility of corneal confocal microscopy for assessing mild diabetic neuropathy: baseline fndings of the LANDMark study. Clin Exp Optom. 2012;95(3):348–354. doi: 10.1111/j.1444-0938.2012.00740.x. [DOI] [PubMed] [Google Scholar]
- 39.Tavakoli M, Quattrini C, Abbott C, Kallinikos P, Marshall A, Finnigan J, Morgan P, Efron N, Boulton AJ, Malik RA. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. 2010;33(8):1792–1797. doi: 10.2337/dc10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Messmer EM. Schmid-Tannwald C, Zapp D, Kampik A. In vivo confocal microscopy of corneal small fiber damage in diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 2010;248(9):1307–1312. doi: 10.1007/s00417-010-1396-8. [DOI] [PubMed] [Google Scholar]
- 41.Chang PY, Carrel H, Huang JS, Wang IJ, Hou YC, Chen WL, Wang JY, Hu FR. Decreased density of corneal basal epithelium and subbasal corneal nerve bundle changes in patients with diabetic retinopathy. Am J Ophthalmol. 2006;142(3):488–490. doi: 10.1016/j.ajo.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 42.Malik RA, Kallinikos P, Abbott CA, van Schie CH, Morgan P, Efron N, Boulton AJ. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46(5):683–688. doi: 10.1007/s00125-003-1086-8. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed A, Bril V, Orszag A, Paulson J, Yeung E, Ngo M, Orlov S, Perkins BA. Detection of diabetic sensorimotor polyneuropathy by corneal confocal microscopy in type 1 diabetes: a concurrent validity study. Diabetes Care. 2012;35(4):821–828. doi: 10.2337/dc11-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Midena E, Brugin E, Ghirlando A, Sommavilla M, Avogaro A. Corneal diabetic neuropathy: a confocal microscopy study. J Refract Surg. 2006;22(9 Suppl):S1047–S1052. doi: 10.3928/1081-597X-20061102-08. [DOI] [PubMed] [Google Scholar]
- 45.Grupcheva CN, Wong T, Riley AF, McGhee CN. Assessing the sub-basal nerve plexus of the living healthy human cornea by in vivo confocal microscopy. Clin Experiment Ophthalmol. 2002;30(3):187–190. doi: 10.1046/j.1442-9071.2002.00507.x. [DOI] [PubMed] [Google Scholar]
- 46.Erie JC, McLaren JW, Hodge DO, Bourne WM. The efect of age on the corneal subbasal nerve plexus. Cornea. 2005;24(6):705–709. doi: 10.1097/01.ico.0000154387.51355.39. [DOI] [PubMed] [Google Scholar]
- 47.Petropoulos IN, Manzoor T, Morgan P, Fadavi H, Asghar O, Alam U, Ponirakis G, Dabbah MA, Chen X, Graham J, Tavakoli M, Malik RA. Repeatability of in vivo corneal confocal microscopy to quantify corneal nerve morphology. Cornea. 2013;32(5):e83–e89. doi: 10.1097/ICO.0b013e3182749419. [DOI] [PubMed] [Google Scholar]
- 48.Efron N, Edwards K, Roper N, Pritchard N, Sampson GP, Shahidi AM, Vagenas D, Russell A, Graham J, Dabbah MA, Malik RA. Repeatability of measuring corneal subbasal nerve fiber length in individuals with type 2 diabetes. Eye Contact Lens. 2010;36(5):245–248. doi: 10.1097/ICL.0b013e3181eea915. [DOI] [PubMed] [Google Scholar]
- 49.Hertz P, Bril V, Orszag A, Ahmed A, Ng E, Nwe P, Ngo M, Perkins BA. Reproducibility of in vivo corneal confocal microscopy as a novel screening test for early diabetic sensorimotor polyneuropathy. Diabet Med. 2011;28(10):1253–1260. doi: 10.1111/j.1464-5491.2011.03299.x. [DOI] [PubMed] [Google Scholar]
- 50.Scarpa F, Grisan E, Ruggeri A. Automatic recognition of corneal nerve structures in images from confocal microscopy. Invest Ophthalmol Vis Sci. 2008;49(11):4801–4807. doi: 10.1167/iovs.08-2061. [DOI] [PubMed] [Google Scholar]
- 51.Sindt CW, Lay B, Bouchard H, Kern JR. Rapid image evaluation system for corneal in vivo confocal microscopy. Cornea. 2013;32(4):460–465. doi: 10.1097/ICO.0b013e31825ab9e2. [DOI] [PubMed] [Google Scholar]
- 52.Dabbah MA, Graham J, Petropoulos IN, Tavakoli M, Malik RA. Automatic analysis of diabetic peripheral neuropathy using multi-scale quantitative morphology of nerve fibres in corneal confocal microscopy imaging. Med Image Anal. 2011;15(5):738–747. doi: 10.1016/j.media.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 53.Hoşal BM, Ornek N. Zilelioğlu G, Elhan AH. Morphology of corneal nerves and corneal sensation in dry eye: a preliminary study. Eye (Lond) 2005;19(12):1276–1279. doi: 10.1038/sj.eye.6701760. [DOI] [PubMed] [Google Scholar]
- 54.SimoMannion L, Tromans C, O’Donnell C. An evaluation of corneal nerve morphology and function in moderate keratoconus. Cont Lens Anterior Eye. 2005;28(4):185–192. doi: 10.1016/j.clae.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 55.Hyndiuk RA, Kazarian EL, Schultz RO, Seideman S. Neurotrophic corneal ulcers in diabetes mellitus. Arch Ophthalmol. 1977;95(12):2193–2196. doi: 10.1001/archopht.1977.04450120099012. [DOI] [PubMed] [Google Scholar]
- 56.Rosenberg ME, Tervo TM, Immonen IJ, Müller LJ. Grönhagen-Riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2000;41(10):2915–2921. [PubMed] [Google Scholar]
- 57.Smith AG, Kim G, Porzio M, Allen B, Koach M, Mifin M, Digre K, Keung BM, Singleton JR. Corneal confocal microscopy is efficient, well-tolerated, and reproducible. J Peripher Nerv Syst. 2013;18(1):54–58. doi: 10.1111/jns5.12008. [DOI] [PubMed] [Google Scholar]
- 58.Sellers EA, Clark I, Tavakoli M, Dean HJ, McGavock J, Malik RA. The acceptability and feasibility of corneal confocal microscopy to detect early diabetic neuropathy in children: a pilot study. Diabet Med. 2013;30(5):630–631. doi: 10.1111/dme.12125. [DOI] [PubMed] [Google Scholar]
- 59.Zhivov A, Winter K, Hovakimyan M, Peschel S, Harder V, Schober HC, Kundt G, Baltrusch S, Guthof RF, Stachs O. Imaging and quantification of subbasal nerve plexus in healthy volunteers and diabetic patients with or without retinopathy. PLoS One. 2013;8(1):e52157. doi: 10.1371/journal.pone.0052157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nitoda E, Kallinikos P, Pallikaris A, Moschandrea J, Amoiridis G, Ganotakis ES, Tsilimbaris M. Correlation of diabetic retinopathy and corneal neuropathy using confocal microscopy. Curr Eye Res. 2012;37(10):898–906. doi: 10.3109/02713683.2012.683507. [DOI] [PubMed] [Google Scholar]
- 61.Tavakoli M, Kallinikos P, Iqbal A, Herbert A, Fadavi H, Efron N, Boulton AJ, A Malik R. Corneal confocal microscopy detects improvement in corneal nerve morphology with an improvement in risk factors for diabetic neuropathy. Diabet Med. 2011;28(10):1261–1267. doi: 10.1111/j.1464-5491.2011.03372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishibashi F, Okino M, Ishibashi M, Kawasaki A, Endo N, Kosaka A, Uetake H. Corneal nerve fiber pathology in Japanese type 1 diabetic patients and its correlation with antecedent glycemic control and blood pressure. J Diabetes Invest. 2012;3(2):191–198. doi: 10.1111/j.2040-1124.2011.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Cillà S, Ranno S, Carini E, Fogagnolo P, Ceresara G, Orzalesi N, Rossetti LM. Corneal subbasal nerves changes in patients with diabetic retinopathy: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2009;50(11):5155–5158. doi: 10.1167/iovs.09-3384. [DOI] [PubMed] [Google Scholar]
- 64.Mocan MC, Durukan I, Irkec M, Orhan M. Morphologic alterations of both the stromal and subbasal nerves in the corneas of patients with diabetes. Cornea. 2006;25(7):769–773. doi: 10.1097/01.ico.0000224640.58848.54. [DOI] [PubMed] [Google Scholar]
- 65.Hossain P, Sachdev A, Malik RA. Early detection of diabetic peripheral neuropathy with corneal confocal microscopy. Lancet. 2005;366(9494):1340–1343. doi: 10.1016/S0140-6736(05)67546-0. [DOI] [PubMed] [Google Scholar]
- 66.Wu T, Ahmed A, Bril V, Orszag A, Ng E, Nwe P, Perkins BA. Variables associated with corneal confocal microscopy parameters in healthy volunteers: implications for diabetic neuropathy screening. Diabet Med. 2012;29(9):e297–303. doi: 10.1111/j.1464-5491.2012.03678.x. [DOI] [PubMed] [Google Scholar]
- 67.Tavakoli M, Marshall A, Pitceathly R, Fadavi H, Gow D, Roberts ME, Efron N, Boulton AJ, Malik RA. Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol. 2010;223(1):245–250. doi: 10.1016/j.expneurol.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sivaskandarajah GA, Halpern EM, Lovblom LE, Weisman A, Orlov S, Bril V, Perkins BA. Structure-function relationship between corneal nerves and conventional small-fiber tests in type 1 diabetes. Diabetes Care. 2013 doi: 10.2337/dc12-2075. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mehra S, Tavakoli M, Kallinikos PA, Efron N, Boulton AJ, Augustine T, Malik RA. Corneal confocal microscopy detects early nerve regeneration after pancreas transplantation in patients with type 1 diabetes. Diabetes Care. 2007;30(10):2608–2612. doi: 10.2337/dc07-0870. [DOI] [PubMed] [Google Scholar]
- 70.Patel DV, McGhee CN. Quantitative analysis of in vivo confocal microscopy images: a review. Surv Ophthalmol. 2013 doi: 10.1016/j.survophthal.2012.12.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 71.Petroll WM, Weaver M, Vaidya S, McCulley JP, Cavanagh HD. Quantitative 3-dimensional corneal imaging in vivo using a modified HRT-RCM confocal microscope. Cornea. 2013;32(4):e36–e43. doi: 10.1097/ICO.0b013e31825ec44e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dabbah MA, Graham J, Petropoulos I, Tavakoli M, Malik RA. Dual-model automatic detection of nerve-fibres in corneal confocal microscopy images. Med Image Comput Comput Assist Interv. 2010;13(Pt 1):300–307. doi: 10.1007/978-3-642-15705-9_37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malik RA, Newrick PG, Sharma AK, Jennings A. Ah-See AK, Mayhew TM, Jakubowski J, Boulton AJ, Ward JD. Microangiopathy in human diabetic neuropathy: relationship between capillary abnormalities and the severity of neuropathy. Diabetologia. 1989;32(2):92–102. doi: 10.1007/BF00505180. [DOI] [PubMed] [Google Scholar]
- 74.Malik RA, Veves A, Masson EA, Sharma AK, Ah-See AK, Schady W, Lye RH, Boulton AJ. Endoneurial capillary abnormalities in mild human diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1992;55(7):557–561. doi: 10.1136/jnnp.55.7.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davidson EP, Coppey LJ, Holmes A, Yorek MA. Changes in corneal innervation and sensitivity and acetylcholine-mediated vascular relaxation of the posterior ciliary artery in a type 2 diabetic rat. Invest Ophthalmol Vis Sci. 2012;53(3):1182–1187. doi: 10.1167/iovs.11-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davidson EP, Coppey LJ, Yorek MA. Early loss of innervation of cornea epithelium in streptozotocin-induced type 1 diabetic rats: improvement with ilepatril treatment. Invest Ophthalmol Vis Sci. 2012;53(13):8067–8074. doi: 10.1167/iovs.12-10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Benítez-Del-Castillo JM, Acosta MC, Wassf MA, Díaz-Valle D, Gegúndez JA, Fernandez C, García-Sánchez J. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007;48(1):173–181. doi: 10.1167/iovs.06-0127. [DOI] [PubMed] [Google Scholar]
- 78.Gemignani F, Ferrari G, Vitetta F, Giovanelli M, Macaluso C, Marbini A. Non-length-dependent small fibre neuropathy. Confocal microscopy study of the corneal innervation. J Neurol Neurosurg Psychiatry. 2010;81(7):731–733. doi: 10.1136/jnnp.2009.177303. [DOI] [PubMed] [Google Scholar]
- 79.Tuisku IS, Konttinen YT, Konttinen LM, Tervo TM. Alterations in corneal sensitivity and nerve morphology in patients with primary Sjogren’s syndrome. Exp Eye Res. 2008;86(6):879–885. doi: 10.1016/j.exer.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 80.Tavakoli M, Marshall A, Thompson L, Kenny M, Waldek S, Efron N, Malik RA. Corneal confocal microscopy: a novel noninvasive means to diagnose neuropathy in patients with Fabry disease. Muscle Nerve. 2009;40(6):976–984. doi: 10.1002/mus.21383. [DOI] [PubMed] [Google Scholar]
- 81.Mimura T, Amano S, Fukuoka S, Honda N, Arita R, Ochiai M, Yanagisawa M, Usui T, Ono K, Araki F, Yamagami S, Araie M, Awaya Y. In vivo confocal microscopy of hereditary sensory and autonomic neuropathy. Curr Eye Res. 2008;33(11):940–945. doi: 10.1080/02713680802450992. [DOI] [PubMed] [Google Scholar]
- 82.Lalive PH, Trufert A, Magistris MR, Landis T, Dosso A. Peripheral autoimmune neuropathy assessed using corneal in vivo confocal microscopy. Arch Neurol. 2009;66(3):403–405. doi: 10.1001/archneurol.2008.587. [DOI] [PubMed] [Google Scholar]
- 83.Ferrari G, Gemignani F, Macaluso C. Chemotherapy-associated peripheral sensory neuropathy assessed using in vivo corneal confocal microscopy. Arch Neurol. 2010;67(3):364–365. doi: 10.1001/archneurol.2010.17. [DOI] [PubMed] [Google Scholar]


