Abstract
Schizencephaly is a rare malformation of cortical development characterized by congenital clefts extending from the pial surface to the lateral ventricle that are lined by heterotopic gray matter. The clinical presentation is variable and can include motor or cognitive impairment and epilepsy. The causes of schizencephaly are heterogeneous and can include teratogens, prenatal infection, or maternal trauma. Reported genetic causes include chromosomal aneuploidy, EMX2 mutations, and possible autosomal recessive familial cases based on recurrence in siblings. In an effort to identify risk factors for schizencephaly, we conducted a survey of 48 parents or primary caretakers of patients with schizencephaly born between 1983 and 2004. We discovered that young maternal age, lack of prenatal care, and alcohol use were all significantly associated with risk of schizencephaly. Our results suggest that there are important nongenetic, intrauterine events that predispose to schizencephaly.
Keywords: schizencephaly, magnetic resonance imaging (MRI), cortical dysplasia, genetics, prenatal care
Schizencephaly is a rare congenital structural brain abnormality characterized by a cleft of the cerebral mantle from the pial surface to the lateral ventricle. The term schizencephaly was coined by Yakovlev and Wadsworth in 1946 based on their neuropathologic studies of 5 patients with severe neurologic deficits and brain malformations. They described 2 types of signs: type I or closed-lip schizencephaly and type II or open-lip schizencephaly.1,2 The clinical presentations of schizencephaly range from normal cognition with seizure onset in adulthood, to hemiparesis and mild developmental delay to severe cognitive impairment with quadriparesis. Advances in neuroimaging have enhanced the recognition of schizencephaly in vivo. Based on magnetic resonance imaging (MRI) findings, schizencephalies are classified as unilateral or bilateral, open lip or closed lip, and the open clefts are further subdivided according to size and the presence or absence of associated brain malformations.3 Reported brain anomalies include polymicrogyria, absent septum pellucidum, optic nerve atrophy, agenesis or thinning of the corpus callosum, arachnoid cyst, calcification, hippocampal malformation, mega cisterna magna, and cerebellar abnormalities. Schizencephaly has been reported in rare syndromes as Vici syndrome (MIM 242849), characterized by agenesis of the corpus callosum, ocular albinism, immunodeficiency, and cardiomyopathy.4 Unilateral schizencephaly is more frequent (60%) than bilateral clefts; the most frequent anatomic localization is in the frontal and parietal lobes (in up to 70%). There is a consistent correlation between severity of outcome and the following features: open-lip cleft, large or medium cleft size, and bilateral clefts.3,5
There is debate about the role of acquired versus genetic factors in determining schizencephaly. Some authors support a common pathogenesis of schizencephaly and polymicrogyria, with the manifestations depending on the timing and severity of the prenatal event. Prenatal exposure to cytomegalovirus infection is associated with schizencephaly in humans6 and experimentally induced schizencephaly following exposure to a mumps virus has been reported in a hamster model.7 Heterozygous mutations in the homeobox gene EMX2 have been identified in a few cases8,9; however, EMX2 knockout mice and heterozygotes do not have schizencephaly, and EMX2 mutations are likely to be a rare cause of schizencephaly.10 In addition, heterozygous mutations in SIX3 and SHH have been associated in a small number of individuals affected with schizencephaly.11 In an ongoing series of more than 1000 patients with various developmental brain disorders, it was surprising that patients with schizencephaly seemed to be born to young mothers with poor prenatal care, and so we have performed a systematic analysis of potential acquired nongenetic risk factors for a large series of patients with schizencephaly.
Methods
Participants
Participants included parents or caretakers of 104 patients ascertained through a research advertisement in an online support group, physician referrals, or the laboratory website as well as 27 patients previously enrolled in a genetic research study of schizencephaly.
Each participant was sent an introduction letter by mail, medical record release form for MRI films and medical records, and a consent form for study. After written informed consent was provided and medical records obtained, the MRI films were obtained and reviewed by one of us (AJB or BSC) for confirmation of diagnosis. Following review of the MRI and medical records, the parent or caretaker was contacted for a structured telephone interview. The questionnaire included demographic info, prenatal history, family history, and medical history of the child.
Clinical Evaluation
Medical history was obtained from the interview and confirmed by review of medical records. The legal guardian gave written, informed consent for participation in the research study. MRI films were reviewed independently by 2 authors (BSC and AJB). Schizencephaly was positively confirmed on brain imaging by (1) the presence of a full-thickness cleft that extended from the ependymal lining of the lateral ventricles to the pial surface and (2) the presence of dysplastic-appearing gray matter lining both sides of the cleft, usually with a scalloped or irregular gray-white junction (Figure 1). Schizencephalic clefts in which the lips on either side of the cleft appeared fused together, without evident cerebrospinal fluid between them, were diagnosed as closed-lip, whereas those with evident cerebrospinal fluid signal between separated sides were diagnosed as open-lip. In summary, schizencephaly was classified as to whether it was unilateral or bilateral, closed- or open-lip cleft, and whether there were other CNS anomalies.
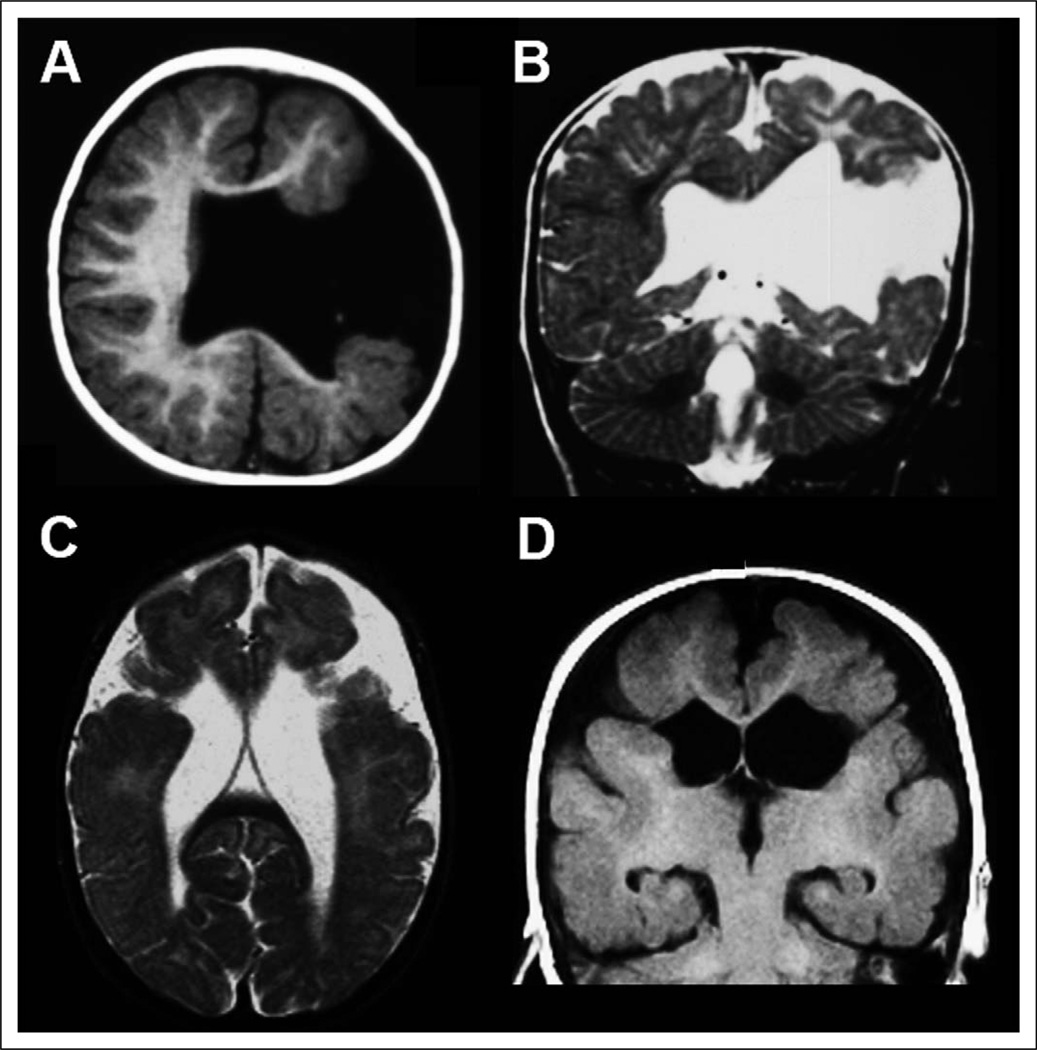
Figure 1.
Magnetic resonance imaging (MRI) appearance of schizencephaly. T1-weighted axial (A) and T2-weighted coronal (B) images from an 11-month-old boy with unilateral left hemisphere open-lip schizencephaly demonstrate a wide cleft lined by gray matter extending from the lateral ventricle to the pial surface. T2-weighted axial (C) and T1-weighted coronal (D) images from a 6-year-old girl with bilateral closed-lip schizencephaly demonstrate clefts extending from the lateral ventricles to the pial surface in both hemispheres; in this case, the regions of gray matter on either side of the clefts are closely apposed.
Statistical Analysis
We used 2 control groups as follows: the first control group consisted of more than 4 million U.S. births from the 2002 National Vital Statistics Report from the Centers for Disease Control and Prevention.12 These data are based on 100% of birth certificates registered in all 50 states and the District of Columbia. Recorded data include demographic information on maternal age, ethnicity, prenatal care, tobacco use, and educational status but not age-specific information on alcohol or substance abuse. The birth certificate question on alcohol use is considered not sensitive enough because alcohol use is substantially underreported on birth certificates compared with data collected in nationally representative surveys of pregnant women.13 Therefore, we used a second control group of 1104 pregnant women aged 15 to 44 years from the 2002 National Survey on Drug Use and Health. This survey is sponsored by the Substance Abuse and Mental Health Services Administration of the Department of Health and Human Services. The National Survey on Drug Use and Health conducts approximately 70 000 interviews each year using a computer-assisted interviewing methodology. The survey includes information about the prevalence and incidence of illicit drug, alcohol, and tobacco use in the civilian, non-institutionalized population age 12 or older in the United States in all 50 states and the District of Columbia. Persons excluded from the survey include homeless persons who do not use shelters, active-duty military personnel, and residents of prisons and long-term hospitals. The survey is available as Department of Health and Human Services publication no. SMA03-3836 National Survey on Drug Use and Health series H-22 or at www.DrugAbuseStatistics.samhsa.gov.
Risk factors including maternal age (<20 years old), maternal education (college or higher), ethnicity (non-Hispanic white), smoking, drinking, and prenatal care (first trimester) and drug use were analyzed as dichotomous variables. Crude associations between each risk factor and schizencephaly were examined using chi-square test of independence. Logistic regression was implemented to perform age and multivariable adjusted analysis.
Results
Of the 131 families invited to participate in the study, 59 did not send in MRI films for review. Among the remaining 72 patients with MRI films, 23 (32%) were excluded because of absence of schizencephaly on neuroimaging. Therefore, a total of 49 children with schizencephaly met the inclusion criteria and their caretakers were invited to participate in the study. However, 1 individual could not be reached, and 48 caretakers were interviewed. The questionnaire was administered to the biological or adoptive parent, or other primary caregiver (usually another family member). Two adopted infants were abandoned at birth; information on their mothers’ prenatal care, risk factors, and family history were unavailable. Twenty-nine percent of the children with schizencephaly included in this study were adopted.
Imaging Characteristics
Thirty-five patients had isolated schizencephaly (50% unilateral, 50% bilateral). The imaging and clinical characteristics of the cohort are presented in Table 1.
Table 1.
Clinical and Imaging Features.
| Schizencephaly type | n (%) (total = 48) |
Other anomalies |
Epilepsy (%) |
|---|---|---|---|
| Unilateral closed lip | 4 (8.3%) | 0 | 25 |
| Unilateral open lip | 20 (41.7%) | Heterotopia (5%) | 55 |
| Polymicrogyria (35%) | |||
| Septo-optic displasia (25%) | |||
| Bilateral closed lip | 3 (6.3%) | 0 | 33 |
| Bilateral open lip | 20 (41.7%) | 0 | 75 |
| Bilateral: L open lip, R closed lip | 1 (2%) | 0 | 0 |
Karyotypes
Karyotypes were analyzed in 7 (15%) patients and all of them had normal chromosomes.
Risk Factors for Schizencephaly
Maternal education and ethnicity were not significantly associated with schizencephaly. Significant risk factors for schizencephaly were maternal age, smoking, alcohol, and prenatal care. The crude association results are displayed in Table 2.
Table 2.
Crude Analysis of Maternal Risk Factors for Schizencephaly.
| Cases, % (n = 46)a |
NVSR controls, % (n = 4 021 726) |
P | |
|---|---|---|---|
| Ethnicity (% white) | 70.8 | 78.9 | .1684 |
| Maternal education (% college or higher) | 16.7 | 25.5 | .1590 |
| Maternal age (% <20 y) | 43.8 | 10.8 | <.0001*** |
| Smoking (% smoker)b | 22.9 | 11.4 | .0121 |
| Alcohol (% drinking)b | 20.8 | 0.8 | <.0001*** |
| Prenatal care (% with first trimester care) | 45.8 | 83.7 | <.0001*** |
| Drug use (any type)c | 11.6 | 5.6 | .1642 |
Abbreviation: NVSR, National Vital Statistics Report.
Cases exclude those for whom information on 2 mothers was unavailable.
Tobacco use and alcohol use excludes California births which did not require reporting on birth certificates.
The control sample for drug use was from 2002 National Survey on Drug Use and Health.
P < 0.0001.
In Table 3, we display age-adjusted OR for smoking and prenatal care. When adjusted for age, we found smoking was not significant. We found that among mothers of children with schizencephaly, significantly fewer began prenatal care in the first trimester compared with age-matched controls: 19% versus 68% in women aged <20 years and 67% versus 81% in women aged 20 to 29 years. Among mothers of children with schizencephaly, there was a significant association with receiving no prenatal care compared with beginning prenatal care after the first trimester (P < .0001), where the odds ratio was 13.2 (95% Wald confidence interval 6.472–27.036).
Table 3.
Logistic Regression for Schizencephaly, Adjusted for Maternal Age (<20 vs ≥20).
| Odds ratio |
95% Wald confidence limits |
P | |
|---|---|---|---|
| Smoking (% smoker) | 1.977 | 0.970–4.027 | .0605 |
| No prenatal care: Began prenatal care first trimester | 13.228 | 6.472–27.036 | <.0001 |
| Began prenatal care second trimester: Began prenatal care first trimester | 2.075 | 0.939–4.582 | .0710 |
| Began prenatal care third trimester: Began prenatal care first trimester | 3.334 | 0.987–11.262 | .0526 |
Note: The control sample was from the 2002 National Vital Statistics Report.
Results of multivariable logistic regression using the National Survey on Drug Use and Health pregnant controls are displayed in Table 4. There were significant associations with young maternal age (odds ratio = 2.933, P = .0016) and alcohol (odds ratio = 2.825, P = .0098) after controlling for the rest of the risk factors. With regard to smoking, we found that mothers of children with schizencephaly had 2.67 times the odds of smoking compared to the controls; however, when adjusted for age and other risk factors, maternal smoking was not significantly associated (odds ratio = 0.74, P = .448).
Table 4.
Results of Multiple Logistic Regression for Schizencephaly.
| Odds ratio | 95% Wald confidence limits |
P | |
|---|---|---|---|
| Maternal age (<20 y) | 2.933 | 1.504–5.72 | .0016 |
| Alcohol | 2.825 | 1.284–6.214 | .0098 |
| Drugs | 1.156 | 0.381–3.504 | .7979 |
| Smoking | 0.737 | 0.335–1.622 | .4480 |
| Ethnicity (non-Hispanic white) | 2.026 | 0.972–4.221 | .0594 |
Note: The control sample was from the 2002 National Survey on Drug Use and Health.
Discussion
Schizencephaly signs typically present with neurologic abnormalities that are recognized in the first year of life, although a delay in diagnosis is common.14 The etiology of schizencephaly is heterogeneous, and some environmental exposures have been implicated: teratogens such as alcohol, warfarin, or cocaine15–17; viral infection, especially cytomegalovirus6; as well as attempted abortion, amniocentesis or chorionic villus biopsy, or maternal trauma.18 Chromosomal aneuploidy,19,20 syndromic forms,4 and EMX2 mutations10 are rare genetic causes in sporadic or familial cases. Case reports of affected siblings support possible autosomal recessive inheritance in other families.21,22
In the present study, we found a strong correlation between young maternal age and schizencephaly (P < .0001). Nearly half (44%) of the mothers of our subjects were less than 20 years old. This is in contrast to population trends in the United States: from 1970 to 2000, the age distribution of women giving birth has changed, with relatively fewer mothers under age 20 years and more mothers 35 years and older. During this period, the mean age of mothers rose 2.6 years, from 24.6 years to 27.2 years, and the mean age for women having their first live birth rose 3.5 years in the same period.23 The birth rates for teenagers in the general population have shown a particularly marked decline: the birth rate for teenagers 15 to 19 years dropped 30% between 1991 and 2002, whereas the number of female teenagers increased by 18%. The birth rate for younger girls aged 10 to 14 years in 2002 was one-half the rate in 1994, whereas the number of girls aged 10 to 14 years in the population increased 16%.
Because our cases were ascertained by self-referral or by physician referral with many initially identified via computer-based research advertisement or the laboratory website, it is possible that computer literacy resulted in a bias toward younger cases. However, we consider this unlikely as our findings are consistent with a recent report on data from 6 congenital anomaly registries in the United Kingdom that demonstrated an association between young maternal age and schizencephaly.24 In addition, our finding of a parental age effect is similar to those of a regional population-based study of schizencephaly conducted through the California Birth Defects Monitoring Program. In that study, the prevalence of schizencephaly was reported as 1.54/100 000 births of a total of 4 million births.25 Their study of 63 schizencephaly cases identified an association of young parental age in isolated schizencephaly (relative risk = 3.9 mothers, relative risk = 5.8 fathers), which was also observed in mothers (relative risk = 3.2) but not fathers of cases with schizencephaly and one or more major malformations in another organ system. In more than half the nonisolated schizencephaly cases, the associated abnormality could be attributed to vascular disruption (gastroschisis, bowel atresia, amniotic band sequence). They suggested a vascular disruptive pathogenesis for many cases of schizencephaly, with smaller contributions from chromosomal aneuploidies, nonrandom associations, and rare syndromes. A limitation of the California Birth Defects Monitoring Program study was that the actual MRI or computed tomographic scans were not reviewed personally, but relied on a schizencephaly diagnosis by a general physician, pediatrician, or neuroradiologist. We did not evaluate paternal age as a risk factor because according to the National Vital Statistics Report (NVSR), paternal age is unknown in 13% of all births, but in 24% of births to mothers <25 years old and 38% of nonmarital births. Our results confirm that young maternal age is a highly significant risk factor for schizencephaly.
Besides young maternal age, we identified alcohol consumption and lack of early prenatal care as independent risk factors for development of schizencephaly after controlling for age, with the highest rates among the youngest mothers. Alcohol consumption was significant compared with either the National Vital Statistics Report or the National Survey on Drug Use and Health control groups, and remained so after multiple logistic regression. Fetal alcohol syndrome has been associated with microcephaly. A case of bilateral schizencephaly with hypoplasia of the corpus callosum was recently reported with fetal alcohol syndrome.26 In a murine model, ethanol induced premature transformation of radial glial cells into astrocytes and affected neocortical vertical column formation.27 A limitation of our study is that the alcohol use, like smoking and drug use in the mothers of schizencephaly subjects, was self-reported, and details on amount and timing of use during pregnancy are not available. Further investigation into specific risk factors known to be associated with young maternal age (sociodemographic, reproductive, and lifestyle) is important in pinpointing modifiable risk factors for prevention of schizencephaly.
Acknowledgment
We thank the parents, family members and caretakers who participated in the study. We also thank Judi Wright and the Schiz Kidz Buddies organization for their help in recruitment of study participants (http://schizkidzbuddies.com/).
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by the National Institute of Neurological Disorders and Stroke (NINDS) (R37-35129).
Footnotes
Author Contributions
Kira A. Dies: Study concept and design, clinical and radiographic data collection, interviews of study participants, writing-up of manuscript, intellectual content of manuscript. Adria Bodell: Study concept and design, clinical and radiographic data collection, interviews of study participants, writing-up of manuscript, intellectual content of manuscript. Fuki M. Hisama: Analysis and interpretation of data, writing-up of manuscript, intellectual content of manuscript. Chao-Yu Guo: Statistical analysis. Brenda Barry: Intellectual content and critical revision of manuscript. Bernard S. Chang: Analysis and interpretation of clinical and radiographic data, intellectual content and critical revision of manuscript. A. James Barkovich: Analysis and interpretation of radiographic data, critical revision of manuscript. Christopher A. Walsh: Analysis and interpretation of clinical and radiographic data, intellectual content and critical revision of manuscript, mentorship of the project
Declaration of Conflicting Interests
The authors declared the following potential conflict of interest with respect to the research, authorship, and/or publication of this article: CAW is an Investigator of the Howard Hughes Medical Institute.
Ethical Approval
The Beth Israel Deaconess Medical Center Committee on Clinical Investigation approved the study (Protocol no. 2003-P-000358).
References
- 1.Yakovlev P, Wadsworth RC. Schizencephalies: a study of the congenital clefts in the cerebral mantle, I: Clefts with fused lips. J Neuropathol Exp Neurol. 1946;5:116–130. doi: 10.1097/00005072-194604000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Yakovlev P, Wadsworth RC. Schizencephalies: a study of the congenital clefts in the cerebral mantle, II: Clefts with hydrocephalus and lips separated. J Neuropathol Exp Neurol. 1946;5:169–206. [PubMed] [Google Scholar]
- 3.Barkovich AJ, Kjos BO. Schizencephaly: correlation of clinical findings with MR characteristics. Am J Neuroradiol. 1992;13:85–94. [PMC free article] [PubMed] [Google Scholar]
- 4.del Campo M, Hall BD, Aeby A, et al. Albinism and agenesis of the corpus callosum with profound developmental delay: Vici syndrome, evidence for autosomal recessive inheritance. Am J Med Genet. 1999;85:479–485. doi: 10.1002/(sici)1096-8628(19990827)85:5<479::aid-ajmg9>3.3.co;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Granata T, Freri E, Caccia C, Setola V, Taroni F, Battaglia G. Schizencephaly: clinical spectrum, epilepsy, and pathogenesis. J Child Neurol. 2005;20:313–318. doi: 10.1177/08830738050200040801. [DOI] [PubMed] [Google Scholar]
- 6.Iannetti P, Nigro G, Spalice A, Faiella A, Boncinelli E. Cytomegalovirus infection and schizencephaly: case reports. Ann Neurol. 1998;43:123–127. doi: 10.1002/ana.410430122. [DOI] [PubMed] [Google Scholar]
- 7.Takano T, Takikita S, Shimada M. Experimental schizencephaly induced by Kilham strain of mumps virus: pathogenesis of cleft formation. Neuroreport. 1999;10:3149–3154. doi: 10.1097/00001756-199910190-00005. [DOI] [PubMed] [Google Scholar]
- 8.Brunelli S, Faiella A, Capra V, et al. Germline mutations in the homeobox gene EMX2 in patients with severe schizencephaly. Nat Genet. 1996;12:94–96. doi: 10.1038/ng0196-94. [DOI] [PubMed] [Google Scholar]
- 9.Merello E, Swanson E, De Marco P, et al. No major role for the EMX2 gene in schizencephaly. Am J Med Genet Part A. 2008;146A:1142–1150. doi: 10.1002/ajmg.a.32264. [DOI] [PubMed] [Google Scholar]
- 10.Tietjen I, Bodell A, Apse K, et al. Comprehensive EMX2 genotyping of a large schizencephaly case series. Am J Med Genet A. 2007;143:1313–1316. doi: 10.1002/ajmg.a.31767. [DOI] [PubMed] [Google Scholar]
- 11.Hehr U, Pineda-Alvarez DE, Uyanik G, et al. Heterozygous mutations in SIX3 and SHH are associated with schizencephaly and further expand the clinical spectrum of holoprosencephaly. Hum Genet. 2010;127:555–561. doi: 10.1007/s00439-010-0797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Births: final data for 2002. Natl Vital Stat Rep. 2003;52:1–113. [PubMed] [Google Scholar]
- 13.Sidhu JS, Floyd RL. Alcohol use among women of childbearing age—United States, 1991–1999. MMWR Morb Mortal Wkly Rep. 2002;51:273–276. [PubMed] [Google Scholar]
- 14.Denis D, Chateil JF, Brun M, et al. Schizencephaly: clinical and imaging features in 30 infantile cases. Brain Dev. 2000;22:475–483. doi: 10.1016/s0387-7604(00)00173-x. [DOI] [PubMed] [Google Scholar]
- 15.Roccella M, Testa D. Fetal alcohol syndrome in developmental age. Neuropsychiatric aspects. Minerva Pediatr. 2003;55:63–69. 69–74. [PubMed] [Google Scholar]
- 16.Dominguez R, Aguirre Vila-Coro A, Slopis JM, Bohan TP. Brain and ocular abnormalities in infants with in utero exposure to cocaine and other street drugs. Am J Dis Child. 1991;145:688–695. doi: 10.1001/archpedi.1991.02160060106030. [DOI] [PubMed] [Google Scholar]
- 17.Pati S, Helmbrecht GD. Congenital schizencephaly associated with in utero warfarin exposure. Reprod Toxicol. 1994;8:115–120. doi: 10.1016/0890-6238(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 18.Mancini J, Lethel V, Hugonenq C, Chabrol B. Brain injuries in early foetal life: consequences for brain development. Dev Med Child Neurol. 2001;43:52–55. doi: 10.1017/s0012162201000081. [DOI] [PubMed] [Google Scholar]
- 19.Ehara H, Eda I. Schizencephaly in triple-X syndrome. Pediatr Int. 2001;43:296–297. doi: 10.1046/j.1442-200x.2001.01382.x. [DOI] [PubMed] [Google Scholar]
- 20.Zimmerman RA, Bilaniuk LT, Grossman RI. Computed tomography in migratory disorders of human brain development. Neuroradiology. 1983;25:257–263. doi: 10.1007/BF00540237. [DOI] [PubMed] [Google Scholar]
- 21.Hilburger AC, Willis JK, Bouldin E, Henderson-Tilton A. Familial schizencephaly. Brain Dev. 1993;15:234–236. doi: 10.1016/0387-7604(93)90072-g. [DOI] [PubMed] [Google Scholar]
- 22.Robinson RO. Familial schizencephaly. Dev Med Child Neurol. 1991;33:1010–1012. doi: 10.1111/j.1469-8749.1991.tb14817.x. [DOI] [PubMed] [Google Scholar]
- 23.Mathews TJ, Hamilton BE. Mean age of mother, 1970–2000. Natl Vital Stat Rep. 2002;51:1–13. [PubMed] [Google Scholar]
- 24.Howe DT, Rankin J, Draper ES. Schizencephaly prevalence, prenatal diagnosis and clues to etiology: a register-based study. Ultrasound Obstet Gynecol. 2012;39:75–82. doi: 10.1002/uog.9069. [DOI] [PubMed] [Google Scholar]
- 25.Curry CJ, Lammer EJ, Nelson V, Shaw GM. Schizencephaly: heterogeneous etiologies in a population of 4 million California births. Am J Med Genet A. 2005;137:181–189. doi: 10.1002/ajmg.a.30862. [DOI] [PubMed] [Google Scholar]
- 26.Spalice A, Del Balzo F, Nicita F, et al. Teaching NeuroImages: schizencephaly in fetal alcohol syndrome. Neurology. 2011;77:e96. doi: 10.1212/WNL.0b013e3182343343. [DOI] [PubMed] [Google Scholar]
- 27.Gressens P, Lammens M, Picard JJ, Evrard P. Ethanol-induced disturbances of gliogenesis and neuronogenesis in the developing murine brain: an in vitro and in vivo immunohistochemical and ultrastructural study. Alcohol Alcohol. 1992;27:219–226. [PubMed] [Google Scholar]