Abstract
Telomerase, comprising a reverse transcriptase protein (TERT) and an RNA template, plays a critical role during senescence and carcinogenesis; however, the mechanisms by which telomerase is regulated remain to be elucidated. Several signaling pathways are involved in the activation of TERT at multistep levels. The JAK-STAT pathway is indispensable for mediating signals through growth factor and cytokine receptors during the development of hematopoietic cells, and its activity is frequently upregulated in hematological malignancies. Here, we review the role of the JAK-STAT pathway and related signaling cascades in the regulation of telomerase in hematological malignancies.
Keywords: telomerase, JAK, STAT, signal transduction, hematologic malignancy
Introduction
Over the past decade, significant progress toward understanding the biological importance of telomerase to aging and cancer has been made (Fig. 1). It is now clear that telomerase undergoes complex regulation by numerous transcription factors involved in multiple intracellular and extracellular signaling pathways.1,2 Several kinase cascades, including phosphatidylinositol-3-kinase (PI3K), Akt, mechanical (mammalian) target of rapamycin (mTOR), and mitogen-activated protein kinase (MAPK), as well as members of the Smad family, play a prevalent role in telomerase regulation.3-5 Elucidation of the signal transduction mechanisms underlying this regulation will shed light on the relevance of telomerase to aging and cancer and will aid the development of methods to manipulate telomerase for prophylactics and therapeutic applications. Although there have been several reports that growth factors and cytokines stimulate human telomerase reverse transcriptase (TERT) expression in solid human tumor cells in a manner that is dependent on signal transducer and activator of transcription 3 (STAT3),6,7 only a few such observations have been made in hematological malignancies. This review describes the role of the Janus kinase (JAK)-STAT pathway and related signals in telomerase regulation in hematologic malignancies.
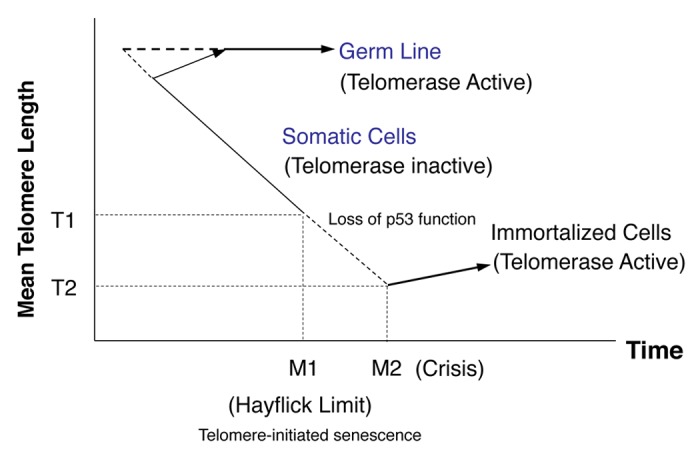
Figure 1. Schema describing the telomere hypothesis of cell aging and transformation in human cells. At the beginning of the time axis (corresponding to early embryonic development), telomerase in germ line tissues is either constitutively active (horizontal dotted line) or becomes activated at a slightly later time (diagonal dotted lines). In somatic cells, telomerase activity is repressed, and the telomeres shorten until they reach a length that signals a halt to cell division (the Hayflick Limit, which results in senescence). This barrier, called M1, can be bypassed by the administration of transforming agents, which allow telomere loss to continue until a crisis point, called M2, at which essentially all the TTAGGG sequences (and some proximal sequences) are lost. The result is cell death. Only rare cells, which have undergone a specific mutational event(s), emerge from M2 to become immortal: only these cells have stable telomeres and express telomerase (modified from ref. 113).
Telomere Replication and Telomerase
Human telomeric DNA consists of 2–15 kb region containing tandem-repeats of the sequence, (TTAGGG)n, which run 5′ to 3′ toward the end of the chromosome.8 This repetitive DNA sequence is evolutionary conserved, which implies its essentiality for crucial cellular functions.9 Telomeres protect chromosomes against degradation, fusion, and rearrangement during DNA replication. In addition, telomeres ensure that the chromosomes are positioned correctly within the nucleus prior to replication.10-12
Because DNA polymerase cannot completely replicate linear DNA molecules, it was previously hypothesized that terminal sequences are lost from the chromosomes during each round of replication.13,14 This event ultimately leads to cell death and may therefore play a role in senescence by limiting the proliferation of somatic cells.15 In many tumor cells and immature somatic cells, telomere length must be conserved to avoid exit from the cell cycle and entry into a senescent state; therefore, these cells express the enzyme telomerase. Telomerase is a large ribonucleoprotein complex, comprising a reverse transcriptase protein (TERT) and an RNA template (TERC),16,17 both of which are essential for its enzymatic activity. A region within the RNA component of telomerase is utilized as a template for synthesis of repeat sequences (Fig. 2). The catalytic subunit of the telomerase holoenzyme, TERT, is a polypeptide of 1132 amino acids.18,19 Whereas TERC expression is relatively ubiquitous throughout embryonic and somatic tissues, TERT expression is tightly regulated and undetectable in most somatic cells.20 Therefore, TERT expression is thought to be the rate-limiting step in telomerase activity.
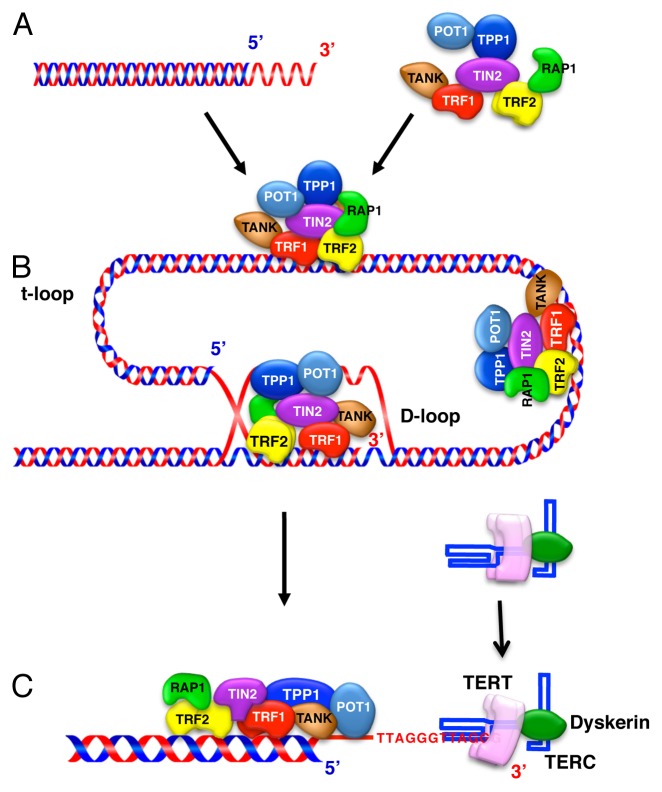
Figure 2. Schematic representation of the telomeric structure in mammalian cells. (A) Human telomeres of the chromosomal-end are composed of TTAGGG DAN repeats with the single-stranded overhang of the telomere end. (B) Telomeres are folded into a larger t-loop structure. The 3′ overhang engages in strand invasion of the adjacent duplex telomeric repeat array, forming a D-loop.114 Telomere-binding proteins, including TRF1, TRF2, RAP1, TIN2, TPP1, and POT1, form the shelterin complex, which stabilizes the t-loop structure and protects the access of telomerase to telomeric DNA.115 TRF1 can bend, loop, and pair telomeric DNA in vitro and could potentially fold the telomere. The shelterin component TRF2 mediates t-loop formation in vitro by forming a complex with RAP1, which protects telomere DNA against DNA double-strand break repair mechanisms such as non-homologous end joining, and POT1 binds both to shelterin along the duplex telomeric DNA and to the single-stranded overhang DNA. Furthermore, tankylase 1 (TANK) associates with and poly ADP ribosylates TRF1, resulting in TRF1 release from telomeric DNA and increased telomerase access to the telomere. (C) The unfolded state of the t-loop allows telomerase to access and act on the single-stranded overhang DNA. The telomerase complex extends the telomere end by the addition of TTAGGG DNA repeats using TERC as a template for the telomere repeats.
Multistep Regulation of Telomerase
Expression of TERT is regulated at the levels of transcription, mRNA splicing, and translation. The protein is also subjected to posttranslational modification and regulation of its subcellular location.21 Transcriptional regulation of TERT has been extensively explored.5 The TERT core promoter is located 330 bp upstream of the transcriptional start site; this region lacks TATA and CAAT boxes but contains binding sequences for various transcriptional activators and repressors, which relay the effects of growth factors and signaling molecules produced in response to diverse stimuli.5 The transcriptional activators that target the TERT promoter include c-Myc, Sp1, Ets-1, nuclear factor of activated T-cells, nuclear factor kappa B (NFκB), cAMP response element binding protein, hypoxia inducible factor-1, and STAT3; the transcriptional repressors include Wilms tumor 1, myeloid zinc finger 2, p53, AP-1, AP-2, AP-4, menin, Smad3, and CCCTC binding factor. Notably, transcriptional activation by Sp1 depends on its co-operation with c-Myc, which binds to an E-box motif on the TERT promoter and activates transcription.1 Epigenetic modifications for DNA and histones also regulate TERT transcription. The TERT promoter contains CpG islands and is therefore susceptible to regulation by changes in the DNA methylation status22; hypermethylation of CpG sites prevents the interaction of CCCTC binding factor with the promoter, resulting in transcriptional activation.23 Moreover, histone acetylation and deacetylation may contribute to the regulation of TERT mRNA expression. Histone deacetylase inhibitors activate the TERT promoter by recruiting SP124 and mimic the induction of TERT mRNA expression by T-cell antigen receptor stimulation through maintaining H3/H4 acetylation.25 TERT expression also regulated by an mRNA splicing mechanism; TERT mRNA transcripts contain at least six splice sites and α splicing variants exert a dominant negative effect on normal transcripts.26
MicroRNAs (miRNAs) are also involved in the control of TERT gene expression and translation. It is estimated that more than 400 miRNAs regulate at least one-third of all protein-coding human genes,27 and that some of these miRNA play a critical role in tumorigenesis.28 In NK/T-cell lymphomas, which display high levels of telomerase activity, miRNA-150 levels are diminished and transfection of these cells with exogenous miRNA-150 reduces telomerase activity by downregulating Akt kinase expression.29 On the contrary, miRNA-21 upregulates STAT3-mediated activation of TERT expression in glioblastoma.30
The enzymatic activity of TERT is controlled at the posttranslational level via phosphorylation and protein folding. The TERT protein contains multiple serine residues that are putative sites for phosphorylation and activation by Akt kinase.31 Protein kinase C (PKCα) also phosphorylates and activates TERT.32 Finally, TERT function can be controlled by modifying the subcellular location of the protein. Following T-cell activation, TERT is phosphorylated and translocated from the cytoplasm to the nucleus, where its enzymatic activity is principally exerted.33 The 14-3-3 signaling protein, which functions as a regulator or connecter of multiple molecules, binds to TERT and inhibits its nuclear export.34 TERT is also distributed in mitochondria, where it plays a role in protecting cells against oxidative stress.35
The following sections discuss the involvement of multiple signaling molecules, including STATs, in the transcriptional and post-transcriptional control of TERT expression.
The Implication of Signaling Molecules Other than the JAK-STAT Pathway in Regulating Telomerase in Hematologic Malignancies
The expression levels and enzymatic activity of TERT are regulated by multiple signaling molecules and pathways, including the RAS/RAF/MEK/MAPK pathway, the PI3K/Akt/mTOR pathway, the IKK/NFκB pathway, the transforming growth factor β/Smads pathway, PKC, and the JAK-STAT pathway.
RAS, a member of the family of small GTP binding proteins, plays a pivotal role in diverse physiological reactions and is mutated during oncogenic transformation; oncogenic mutations in the NRAS and KRAS genes are found in up to 25% of malignancies.36 The canonical RAS effectors include RAF, RalGDS, and PI3K, although RAF is the best characterized. Upon growth factor stimulation, GTP-bound RAS induces cellular proliferation and survival by activating RAF, MEK, and various MAPKs, including extracellular-regulated kinases (ERKs) 1 and 2. MAPKs also mediate the epidermal growth factor-induced stimulation of TERT mRNA expression via activation of Ets-1, Sp1, and c-Myc.3,37 A previous study demonstrated that phosphorylation of serine10 on histone H3 by MAPKs such as ERK1 and ERK2 induces TERT expression in concanavalin A-stimulated normal T cells and is required for constitutive activation of the enzyme in Jurkat cells.38 MAPKs also mediate TERT activation induced by viral latent membrane protein 1 in Epstein–Barr virus-infected B cells.39 In addition, TERT and transforming growth factor β synergistically activate ERK1 and ERK2 in human fibroblasts.40 Conversely, transforming growth factor β suppresses TERT mRNA expression by Smad3 binding to c-Myc in a variety of cell types.41
The PI3K/Akt/mTOR kinase cascade is another major pathway that controls cell proliferation, growth, survival, metabolism, and autophagy; this pathway also plays a pivotal role in the tumorigenesis of hematopoietic cells.4 PI3K, a heterodimeric lipid kinase composed of a catalytic subunit and a regulatory subunit, generates PI-3,4,5-triphosphate (PIP3), which binds to PI-dependent kinase 1 and promotes phosphorylation of Akt kinase at Thr308. This event accelerates the subsequent phosphorylation of Akt kinase at Ser473 by mTOR complex 2 (mTORC2). Fully activated Akt kinase then phosphorylates multiple downstream targets, including mTOR kinase, which forms two complexes, mTORC1 and mTORC2, containing raptor or rictor proteins, respectively. There is convincing evidence that the PI3K/Akt axis regulates TERT mRNA expression and post-transcriptional modification. E2 estradiol activates the TERT-promoter via a PI3K/Akt/NFκB cascade and accelerates the nuclear translocation of phosphorylated TERT in an Akt-dependent manner.42 Furthermore, IL-2 upregulates TERT mRNA expression in Tax-negative adult T-cell leukemia (ATL) cells via a PI3K-dependent mechanism that involves PI3K mediates cytoplasmic sequestration of Wilms tumor 1 protein, a strong repressor of the TERT promoter.43,44 Our group previously showed that IL-2-induced upregulation of TERT mRNA is also mediated by mTORC1 in ATL cells,43 which is consistent with another report that mTOR regulates TERT gene transcription in endometrial cancer cells.45 Akt kinase is also involved in the posttranslational regulation of TERT in a variety of cell types.42 The TERT protein contains putative Akt kinase phosphorylation motifs at amino acid positions 220–229 (220GARRRGGSAS229, 817AVRIRGKSYV826); therefore, it is likely that its activity is controlled through Akt-dependent phosphorylation at these sites, particularly Ser227 and Ser824.31 In support of this theory, an TERT synthetic peptide containing the Ser824 residue has been shown to be a substrate for activated recombinant Akt kinase protein.31 Several lines of evidence suggest that Akt kinase phosphorylates and activates TERT in hematological malignancies. For example, inhibitors that suppress the activities of PI3K and Akt kinase, such as LY294002, wortmanin, and tyrosine kinase inhibitors, abrogate growth factor receptor-regulated telomerase activity and phosphorylation of the Akt and TERT proteins in various hematopoietic tumor cells derived from leukemias and myelomas.46-51 In addition, Akt kinase is required for nuclear shuttling of TERT in various cells, including those from human leukemias.48-50,52,53 The TERT protein is functionally associated with heat shock protein 90 (HSP90), which is a chaperone for a variety of client proteins.54 Our group and others have previously demonstrated that activated TERT forms a complex with Akt, HSP90, mTOR, and S6K in IL-2-dependent NK lymphoma and prostate cancer cells, which suggests a unique regulation mechanism of the TERT protein.49,55
Other signaling molecules, such as PKC and NFκB, are also known to mediate telomerase activation. The PKC family of serine/threonine kinases consists of at least ten isoforms; conventional PKCs such as PKCα are activated by diacylglycerol and Ca2+ and are involved in the PLC signaling pathway. PKCα phosphorylates TERT and enhances telomerase activity both in vitro and in intact cancer cells.32 In T and B lymphocytes, telomerase activation is induced by treatment of the cells with phorbol ester, an activator of PKC, and ionomycin, a Ca2+ ionophore.56,57 PKCζ also regulates telomerase activity via transcriptional and post-transcriptional mechanisms.58 In cancer cells, the PKCα, β, δ, ε, and ζ isoforms regulate TERT phosphorylation and thus association of the holoenzyme with HSP90, leading to telomerase activation.59 We also previously demonstrated that IL-2-induced telomerase activation is blocked by treatment of NK cell tumors with the PKC inhibitor staurosporine (unpublished data). Intriguingly, PKC induces a megakaryocytic differentiation in human myeloid leukemia cells that is accompanied by transient telomerase upregulation.60
NFκB is regulated by the suppressor IκB and the upstream activator IKK and is involved in the control of TERT transcription in hematological tumors. NFκB is activated by PKCθ and regulates TERT expression through the T-cell antigen receptor signaling pathway.61 Tax protein, a product of human T-cell leukemia virus I (HTLV-I), plays a crucial role in leukemogenesis of HTLV-I-infected T-cells and upregulates the transcriptional activity of TERT via the NFκB signaling pathway.62 Moreover, tumor necrosis factor α induces nuclear translocation of TERT in multiple myeloma cells by forming a complex between TERT and NFκB.63
The Role of the JAK-STAT Signaling Pathway in Telomerase Regulation in Hematologic Malignancies
The JAK-STAT signaling pathway has been implicated in the regulation of hTERT. Cytokine plays a critical role in regulating the development, proliferation, and differentiation of multiple cell types, particularly immune cells and hematopoietic cells, by binding to type I and type II cytokine receptors, which lack intrinsic tyrosine kinase activity. Members of the JAK family of enzymes, which includes JAK1–3 and TYK2, constitutively associate with the intracellular portion of cytokine receptors.64 Once activated by cytokines, JAKs phosphorylate tyrosine residues of both the receptors and the STAT family of transcription factors, including STAT1–4, STAT5A, STAT5B, and STAT6. Phosphorylated STAT proteins dimerize and translocate into the nucleus, where they function as transcriptional activators for diverse target genes containing STAT-binding motifs, leading to the enhancement of cell proliferation, survival, and differentiation. Activation of the JAK-STAT pathway due to aberrant regulation or mutation of components of the pathway or upstream receptors leads to neoplastic transformation of hematopoietic cells. For example, a V617F mutation in JAK2 causes chronic myeloproliferative neoplasms such as polycythemia vera, essential thrombocythemia, and primary myelofibrosis.65 By contrast, an internal tandem duplication mutation in fms-like tyrosine kinase 3 (FLT3) that occurs in acute myeloid leukemia (AML) and a chimeric BCR-ABL protein generated by the translocation of t(9;22)(q34;q11) in chronic myeloid leukemia (CML) activate the JAK-STAT pathway.66 Intriguingly, we recently identified the BCR-ABL mutation in stem cells derived from a single myelofibrosis clone carrying the JAK2 V617F mutation,67 which is consistent with the concept that mutation of JAK2 induces genetic instability in stem cells.68 Furthermore, genetic gain of JAK2 due to 9p24 trisomy, which leads to phosphorylation of JAK2, STAT3, and STAT5, has been detected in B-cell, T-cell, and Hodgkin lymphomas.69 In particular, STAT3 and STAT5 phosphorylation has been frequently observed in AML, acute lymphoblastic leukemia, CML, and ATL, in which all JAK proteins can be constitutively phosphorylated at tyrosine residues.43,64,66 Furthermore, STAT5 activation induces self-renewal in both normal hematopoietic stem cells and leukemic stem cells, particularly during the co-culture with stromal cells.70 Therefore, the JAK-STAT pathway plays a central role in the pathophysiological process of hematopoietic neoplasms. Several studies show that STAT3 induces TERT expression by binding to a specific site in the TERT promoter in various tumor cells, including glioblastoma, breast cancer, gastric cancer, and prostate cancer cells as well as primary fibroblasts.6,7 However, evidence of a regulatory interaction between telomerase and the JAK-STAT signaling pathway is limited. The following sections describe the involvement of this pathway in the regulation of TERT in hematologic malignancies, with reference to our own published data.
Regulation of telomerase during differentiation of leukemic cells
Telomerase is activated in immature somatic cells and inactivated in differentiated cells.18 However, the mechanism by which telomerase activity is regulated during cell differentiation remains unclear. Our work has focused on the mechanism of regulation of telomerase activity during leukemic cell differentiation.60,71 In leukemic cell lines such as HL60, telomerase activity is commonly downregulated during monocytic or granulocytic differentiation induced by vitamin D3, all-trans retinoic acid, and Am80.72 Intriguingly, in the K562 CML cell line, megakaryocytic differentiation induced by the tumor promoter TPA is associated with transient upregulation of nuclear telomerase activity and a gradual decrease in telomerase mRNA expression; pre-treatment of cells with PKC inhibitors protects against both megakaryocytic differentiation and transient telomerase activation.71 STAT3 and STAT5, but not Sp1, dissociate from the TERT promoter during megakaryocytic differentiation and erythroid differentiation, indicating that these STAT proteins act as transcription factors that regulate telomerase and help to maintain the leukemic phenotype. In other cell systems, telomerase deficiency and telomere shortening is reported to impair osteoblast differentiation through increased p53/p21 expression,73 suggesting that telomerase activity is involved in the regulation of cell differentiation. On the other hand, despite its lifespan extension effect, ectopic expression of TERT does not appear to affect TPA-induced megakaryocytic differentiation in K562 leukemic cells, because biological phenotypes such as CD41 expression and ERK phosphorylation are retained in TERT-transfected K562 cells. These observations indicate that repression of telomerase activity may be a consequence rather than a prerequisite of leukemic cell differentiation.74
The role of telomerase in leukemic cell drug resistance
CML is a hematological stem cell disorder characterized by the presence of the BCR-ABL fusion gene, which produces the p190, p210, and p230 chimeric proteins.75 These proteins exhibit increased protein tyrosine kinase activities and play an essential role in leukemogenesis. Indeed, protein tyrosine kinase inhibitors such as imatinib are useful therapeutic agents for CML.76 Overexpression of TERT in K562 cells confers a survival advantage and leads to apoptosis resistance, likely as a result of increased telomerase activity.74,77 Our group and others have shown that treatment of K562 cells and primary leukemic cells derived from CML patients with protein tyrosine kinase inhibitors inhibits telomerase activity.50,78 However, the level of suppression of telomerase activity in cells derived from patients in the blast crisis phase of CML is less than that in cells derived from patients in the chronic phase.78
P-glycoprotein (P-gp) is the protein product of the multidrug resistance gene 1 and is responsible for ATP-dependent efflux of a variety of compounds across the plasma membrane; therefore, overexpression of P-gp induces drug resistance. Induction of P-gp by adriamycin treatment of K562 cells overexpressing the protein is associated with enhanced phosphorylation of BCR-ABL and STAT5, as well as enhanced telomerase protein expression. Intriguingly, STAT5 binds directly to the TERT and multidrug resistance gene 1 promoter regions in these cells.79 Conversely, siRNA-mediated silencing of endogenous STAT5 significantly represses both P-gp expression and telomerase activity, and results in the recovery of imatinib sensitivity, indicating crucial roles of STAT5 in the induction of P-gp and the modulation of telomerase activity in drug-resistant CML cells. In addition, increased levels of phosphorylated STAT5, P-gp, and TERT were also observed in primary cells derived from patients in the blast crisis phase of CML.
Upregulation of telomerase is mediated through the JAK-STAT pathway in hematologic tumor cells stimulated by cytokines
Cytokines such as erythropoietin (EPO), thrombopoietin, colony-stimulating factors, FLT3 ligand, and interleukins are essential for hematopoietic cell proliferation, survival, and differentiation. For example, IL-2 or IL-7 is required for the development of lymphocyte progenitor cells, including B cells and T cells, and for immunoglobulin gene rearrangement in B cells, during which STAT5 plays a crucial role in mediating the effect of the cytokines.80 Moreover, IL-21 and its receptor are expressed on resting and activated B cells, T cells, NK cells, macrophage, and dendritic cells; binding of IL-21 to its receptor activates the JAK-STAT pathway, resulting in either proliferation or apoptosis of lymphoid malignancies, depending on the specific isoforms of JAK and STAT involved.81 Therefore, the activation of the JAK-STAT pathway through cytokine receptors is implicated in the tumorigenesis of both lymphoid and myeloid malignancies.
FLT3, also known as cluster of differentiation antigen 135, is a cytokine receptor that belongs to receptor-type tyrosine kinase class III. The FLT3 gene is frequently mutated in AML, which results in constitutive activation of the kinase and its downstream targets, including STATs. In fact, high levels of STAT3 and STAT5 activation occur in 20–80% of AML cases.82 FLT3 mutations, including internal tandem duplications, are also related to poor prognosis.83 In our recent study, we found that blasts from de novo AML patients had high levels of P-gp, TERT, FLT3, STAT3, and STAT5 expression, as well as elevated phosphorylation of the FLT3, STAT3, and STAT5 proteins.84 The phosphorylation of STAT5 was associated with P-gp and TERT expression, suggesting that STAT5 is involved in the control of these genes in primary AML cells and is implicated in drug resistance, as noted for CML cells. Moreover, erythropoietin induces telomerase activation and concomitant phosphorylation of STAT5, ERK, and Akt kinases in erythroleukemia cells.85,86 In these cells, the erythropoietin-induced upregulation of telomerase mRNA is mediated by the STAT5-c-Myc axis, indicating a critical role of STAT5 in the pathogenesis of myeloid leukemias. Therefore, the STAT5 signaling pathway is an attractive target for therapeutic intervention, particularly in cases of drug resistance, and strategies designed to inhibit STAT5 activation and STAT5-mediated gene transcription may hold promise for leukemia therapy.87
ATL is an aggressive lymphoproliferative disorder that occurs in individuals infected with HTLV-1.88,89 ATL is classified into four subtypes (smoldering, chronic, lymphoma, and acute) according to its clinical manifestations, and each subtype may arise via distinct molecular mechanisms.90 Treating aggressive ATL is extremely difficult and effective therapies have yet to be developed, even for indolent ATL, which is estimated to progress into acute ATL in more than 50% of cases. Therefore, the development of novel therapies targeting ATL cells during the indolent phase is important. In some patients, such as those with chronic ATL, IL-2 is often required for ATL cell proliferation and survival, regardless of Tax protein expression. In addition, ATL cells display a high level of telomerase activity, which plays a pivotal role in tumorigenesis and is associated with progression of the disease. Binding of IL-2 to its receptor induces activation of JAK1–3 and accelerates nuclear translocation of the STATs in T cells. Constitutive phosphorylation of a tyrosine residue in JAKs and STATs, which is associated with the proliferation and survival of tumor cells, has been identified in IL-2-independent ATL cell lines and primary cells.91 Moreover, IL-2 increases telomerase activity in NK lymphoma cells and is associated with a PI3K/Akt-dependent elevation of telomerase activity in HTLV-1-transformed T-cells.49,92 Our group recently demonstrated that IL-2 induces telomerase activity and TERT expression, in both primary cells and cell lines derived from chronic ATL patients, and these responses are accompanied by an increase in tyrosine phosphorylation of JAK1–3 and STAT5, as well as an association between JAK1/2 and STAT5.43 We also showed that challenging these cells with IL-2 induces an association of STAT5 with the TERT promoter, and siRNA-mediated knockdown of STAT5 results in the functional silencing of telomerase activity. These data indicate that STAT5 is one of the transcription factors that regulate TERT expression in IL-2-stimulated ATL cells. TERT also interacts directly with the PI3K/Akt/HSP90/mTORC1 pathway in an IL-2-dependent fashion. Notably, we found that IL-2 enhances the association between the p85 regulatory subunit of PI3K with JAK2, suggesting that JAK2 propagates telomerase activation signals to the STAT5 and PI3K/Akt/mTORC1 pathways.43 These signaling proteins represent novel and promising molecular therapeutic targets for IL-2-dependent ATL.
Regulation of telomerase expression/activity by cross-talk between the JAK-STAT pathway and other signaling pathways
The interaction of major pathways, including JAK-STAT, RAS/RAF/MAPK, and PI3K/Akt/mTOR, in the regulation of cell responses has been reported previously. The PI3K/Akt pathway activates RAF and prevents apoptosis by regulating Bcl-2 family members.93 Inversely, RAF/MEK1 activation induces cellular arrest via PI3K/Akt inhibition.94 TGF-β prevents Fas-induced apoptosis of pre-B cell lines by inhibiting the PI3K/Akt pathway.95 The JAK-STAT pathway also interacts with these signaling pathways in hematopoietic and tumor cells. For example, interplay between the JAK-STAT pathway and the Ras-ERK or PI3K-Akt signaling pathway has been reported in Jurkat T cells and CD34+ erythroid progenitor cells, respectively.96,97 STAT5 interacts with the p85 subunit of PI3K via the scaffolding adaptor Gab2 and activates Akt kinase, resulting in the growth of myeloid leukemia cells.98 Furthermore, JAK2-STAT5 in association with the cytokine receptor-like factor 2 (CRLF2), a receptor for thymic stromal lymphopoietin (TSLP) that plays a pivotal role in normal lymphopoiesis, interconnects with the PI3K/Akt/mTOR pathway and causes the proliferation of B-precursor ALL cells.99 TSLP-induced activation of the JAK-STAT and PI3K/Akt/mTOR pathways is negated by treatment with the JAK1/2 inhibitor ruxolitinib. In addition, transformation of BaF3 cells, a mouse pro-B cell line, by the constitutively active form of leukocyte tyrosine kinase induces activation of JAK1/2-STAT5 and ERK1/2 downstream of JAKs.100 Because c-Myc and Sp1 transcription factors play a critical role in regulating telomerase activity, the signaling pathways that impinge on these factors can control telomerase expression. B-cell antigen receptor stimulation elicits c-Myc expression via ERK1/2 phosphorylation in B-cell chronic lymphocytic leukemia, in which ERK-dependent induction of c-Myc regulates leukemic cell proliferation.101 Thrombopoietin, a major regulator of megakaryocyte proliferation and differentiation, stimulates c-Myc expression in both hematopoietic cell lines and primary megakaryocytes via a PI3K- and MAPK-dependent mechanism.102 Furthermore, c-Myc is expressed in response to cytokines, such as IL-6, that induce B-cell proliferation and differentiation. IL-6/gp130-triggered activation of STAT3 induces c-Myc expression, which leads to cell cycle progression in B cells.103 JAK2-STAT3 is activated in diffuse large B-cell lymphoma and may play a role in IL-10-mediated tumor cell proliferation, suggesting that tumor cell apoptosis induced by JAK inhibition could be mediated via the repression of c-Myc expression.104 In addition, STAT5 decoy oligodeoxynucleotides downregulate c-Myc mRNA expression and induces leukemic cell apoptosis in CML.105 These observations indicate an important role for c-Myc as a target of the JAK-STAT pathway in the pathogenesis of hematologic malignancies. Similarly, ERK1/2 stimulates the expression of IL-6 mRNA, an autocrine growth factor for multiple myeloma cells, by increasing the binding of Sp1 to the IL-6 promoter in myeloma cells,106 and the PI3K/Akt pathway regulates miR-29b microRNA expression by recruiting Sp-1 to the miR-29b microRNA promoter, resulting in the protection of myeloma cells from apoptosis.107 Thus, converging signals from these various signaling pathways to c-Myc or Sp1 may lead to telomerase transcription and activation in tumors, including hematologic malignant tumors. Epidermal growth factor and vascular endothelial growth factor upregulate the TERT promoter by activating c-Myc and/or Sp1 transcription factors in an ERK1/2-dependent fashion in ovarian cancer cells.108,109 DJ-1, a regulator of PTEN, stimulates the PI3K/Akt/c-Myc pathway and upregulates telomerase transcription in renal cell carcinoma.110 In hematologic malignancies, cross-talk between these major signaling pathways plays an important role in the regulation of telomerase as described elsewhere in this paper. NFκB stimulated by Tax protein activates telomerase transcription via the binding of c-Myc and Sp1 to the TERT promoter in ATL tumor cells.62 Furthermore, JAK2-STAT5 mediates TERT gene expression in EPO-stimulated erythroleukemia cells85 and activates TERT mRNA transcription in cooperation with the PI3K/Akt/mTOR pathway in ATL cells.43 Multiple signaling molecules and transcription factors interact with each other to form a complex network that regulates telomerase expression and activity. Thus, several different types of signaling molecules lead to telomerase activation and must be targeted for the effective therapy of hematologic malignancies.
Conclusions and Perspectives
It is beginning to emerge that telomerase may play an additional role to that of telomere maintenance in hematopoietic cells. The expanding development of specific inhibitors for the treatment of hematological malignancies necessitates the urgent clarification of telomerase function in hematopoietic cells. In hematologic malignancies such as leukemia and lymphoma, the JAK-STAT pathway and other related signaling cascades, such as the PI3K/Akt/mTOR pathway, play critical roles in the telomerase activation, which is associated with cell proliferation, survival, differentiation, and tumor cell resistance (Fig. 3). Therefore, development of agents that target the JAK-STAT pathway is a promising therapeutic approach to hematological tumors. Additional therapeutic candidates are also emerging from the rapidly evolving field of non-coding RNA. Telomerase, which contains both structural and mRNAs, is a potential target for short RNA sequences such as miRNAs, as reported in the fields of thyroid cancer and hepatoma research.111,112 Similarly, long non-coding telomeric repeat-containing RNA transcripts are involved in regulating telomere length and telomeres as well as chromatin stability. Currently, information regarding the control of telomerase by these non-coding RNAs is limited. In this context, the molecular mechanisms that underlie the regulation of telomerase activity at telomere ends may not be the end, but just the beginning of a new round of investigations into this fundamental area of cell biology.
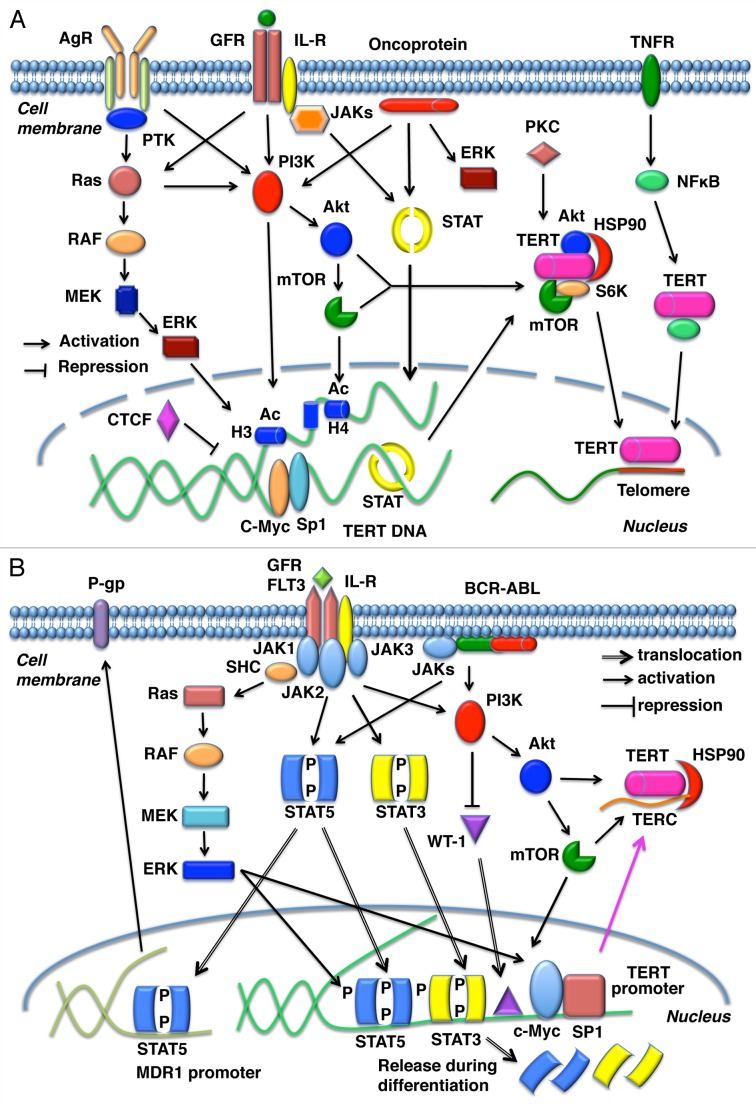
Figure 3. Schematic representation of signal transduction during regulation of TERT expression. (A) Growth factors, cytokine receptors, and oncoproteins such as BCR-ABL propagate signals to the nucleus through the JAK-STAT, PI3K/Akt/mTOR, NFκB, RAS/RAF/MEK/ERK1/2, and PKC pathways. These signaling cascades upregulate TERT expression in a variety of cells, including both hematopoietic and tumor cells. TERT expression is regulated at the transcriptional and posttranslational levels. AgR, antigen receptors; GFR, growth factor receptors; IL-R, interleukin receptors; TNFR, tumor necrosis factor receptor; PTK, protein tyrosine kinases; H, histone; Ac acetylation. (B) Ligation of receptors such as FLT3, IL-2, and the erythropoietin receptor or constitutive activation of oncoproteins such as the chimeric protein BCR-ABL activates JAK1–3 (or directly activates STAT5). This leads to tyrosine phosphorylation of STAT3 or STAT5 and the concomitant activation of other signaling pathways such as Ras/RAF/MEK/ERK1/2 and the PI3K/Akt/mTOR pathways in acute myeloid leukemia, chronic myeloid leukemia, and lymphoid tumors. These signals facilitate the translocation of several transcription factors such as STATs, c-Myc, and Sp1 to the nucleus, where these transcription factors bind to the TERT promoter region. STAT5 also binds to the MDR1 promoter and upregulates P-gp expression in some leukemia cells. Both STAT3 and STAT5 are released from the TERT promoter during the differentiation of myeloid leukemia cells.
Acknowledgments
The authors thank Tomohiro Watanabe for technical support.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/25256
References
- 1.Kyo S, Takakura M, Taira T, Kanaya T, Itoh H, Yutsudo M, et al. Sp1 cooperates with c-Myc to activate transcription of the human telomerase reverse transcriptase gene (hTERT) Nucleic Acids Res. 2000;28:669–77. doi: 10.1093/nar/28.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takakura M, Kyo S, Inoue M, Wright WE, Shay JW. Function of AP-1 in transcription of the telomerase reverse transcriptase gene (TERT) in human and mouse cells. Mol Cell Biol. 2005;25:8037–43. doi: 10.1128/MCB.25.18.8037-8043.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maida Y, Kyo S, Kanaya T, Wang Z, Yatabe N, Tanaka M, et al. Direct activation of telomerase by EGF through Ets-mediated transactivation of TERT via MAP kinase signaling pathway. Oncogene. 2002;21:4071–9. doi: 10.1038/sj.onc.1205509. [DOI] [PubMed] [Google Scholar]
- 4.Kawauchi K, Ogasawara T, Yasuyama M, Otsuka K, Yamada O. Regulation and importance of the PI3K/Akt/mTOR signaling pathway in hematologic malignancies. Anticancer Agents Med Chem. 2009;9:1024–38. doi: 10.2174/187152009789377772. [DOI] [PubMed] [Google Scholar]
- 5.Daniel M, Peek GW, Tollefsbol TO. Regulation of the human catalytic subunit of telomerase (hTERT) Gene. 2012;498:135–46. doi: 10.1016/j.gene.2012.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konnikova L, Simeone MC, Kruger MM, Kotecki M, Cochran BH. Signal transducer and activator of transcription 3 (STAT3) regulates human telomerase reverse transcriptase (hTERT) expression in human cancer and primary cells. Cancer Res. 2005;65:6516–20. doi: 10.1158/0008-5472.CAN-05-0924. [DOI] [PubMed] [Google Scholar]
- 7.Chau MN, El Touny LH, Jagadeesh S, Banerjee PP. Physiologically achievable concentrations of genistein enhance telomerase activity in prostate cancer cells via the activation of STAT3. Carcinogenesis. 2007;28:2282–90. doi: 10.1093/carcin/bgm148. [DOI] [PubMed] [Google Scholar]
- 8.Moyzis RK. The human telomere. Sci Am. 1991;265:48–55. doi: 10.1038/scientificamerican0891-48. [DOI] [PubMed] [Google Scholar]
- 9.Yu GL, Bradley JD, Attardi LD, Blackburn EH. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature. 1990;344:126–32. doi: 10.1038/344126a0. [DOI] [PubMed] [Google Scholar]
- 10.de Lange T. Human telomeres are attached to the nuclear matrix. EMBO J. 1992;11:717–24. doi: 10.1002/j.1460-2075.1992.tb05104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giraldo R, Rhodes D. The yeast telomere-binding protein RAP1 binds to and promotes the formation of DNA quadruplexes in telomeric DNA. EMBO J. 1994;13:2411–20. doi: 10.1002/j.1460-2075.1994.tb06526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chikashige Y, Ding DQ, Funabiki H, Haraguchi T, Mashiko S, Yanagida M, et al. Telomere-led premeiotic chromosome movement in fission yeast. Science. 1994;264:270–3. doi: 10.1126/science.8146661. [DOI] [PubMed] [Google Scholar]
- 13.Olovnikov AM. [Principle of marginotomy in template synthesis of polynucleotides] Dokl Akad Nauk SSSR. 1971;201:1496–9. [PubMed] [Google Scholar]
- 14.Watson JD. Origin of concatemeric T7 DNA. Nat New Biol. 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 15.Olovnikov AM. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973;41:181–90. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- 16.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–13. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 17.Morin GB. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989;59:521–9. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- 18.Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–95. doi: 10.1016/S0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, et al. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–9. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 20.Kolquist KA, Ellisen LW, Counter CM, Meyerson M, Tan LK, Weinberg RA, et al. Expression of TERT in early premalignant lesions and a subset of cells in normal tissues. Nat Genet. 1998;19:182–6. doi: 10.1038/554. [DOI] [PubMed] [Google Scholar]
- 21.Dolcetti R, De Rossi A. Telomere/telomerase interplay in virus-driven and virus-independent lymphomagenesis: pathogenic and clinical implications. Med Res Rev. 2012;32:233–53. doi: 10.1002/med.20211. [DOI] [PubMed] [Google Scholar]
- 22.Kyo S, Takakura M, Fujiwara T, Inoue M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 2008;99:1528–38. doi: 10.1111/j.1349-7006.2008.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renaud S, Loukinov D, Abdullaev Z, Guilleret I, Bosman FT, Lobanenkov V, et al. Dual role of DNA methylation inside and outside of CTCF-binding regions in the transcriptional regulation of the telomerase hTERT gene. Nucleic Acids Res. 2007;35:1245–56. doi: 10.1093/nar/gkl1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doetzlhofer A, Rotheneder H, Lagger G, Koranda M, Kurtev V, Brosch G, et al. Histone deacetylase 1 can repress transcription by binding to Sp1. Mol Cell Biol. 1999;19:5504–11. doi: 10.1128/mcb.19.8.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou M, Wang X, Popov N, Zhang A, Zhao X, Zhou R, et al. The histone deacetylase inhibitor trichostatin A derepresses the telomerase reverse transcriptase (hTERT) gene in human cells. Exp Cell Res. 2002;274:25–34. doi: 10.1006/excr.2001.5462. [DOI] [PubMed] [Google Scholar]
- 26.Colgin LM, Wilkinson C, Englezou A, Kilian A, Robinson MO, Reddel RR. The hTERTalpha splice variant is a dominant negative inhibitor of telomerase activity. Neoplasia. 2000;2:426–32. doi: 10.1038/sj.neo.7900112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alberts B, Bray D, Hopkin K, Johnson A, Lewis J, Raff M, et al. Control of gene expression. In: Alberts B, Bray D, Hopkin K, Johnson A, ed. Essential Cell Bioloigy. Third edition. New York and London: Garland Science, 2010:269-96. [Google Scholar]
- 28.Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J Pathol. 2011;223:102–15. doi: 10.1002/path.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe A, Tagawa H, Yamashita J, Teshima K, Nara M, Iwamoto K, et al. The role of microRNA-150 as a tumor suppressor in malignant lymphoma. Leukemia. 2011;25:1324–34. doi: 10.1038/leu.2011.81. [DOI] [PubMed] [Google Scholar]
- 30.Wang YY, Sun G, Luo H, Wang XF, Lan FM, Yue X, et al. MiR-21 modulates hTERT through a STAT3-dependent manner on glioblastoma cell growth. CNS Neurosci Ther. 2012;18:722–8. doi: 10.1111/j.1755-5949.2012.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang SS, Kwon TR, Kwon DY, Do SI. Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J Biol Chem. 1999;274:13085–90. doi: 10.1074/jbc.274.19.13085. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Zhao L, Yang Z, Funder JW, Liu JP. Telomerase is controlled by protein kinase Calpha in human breast cancer cells. J Biol Chem. 1998;273:33436–42. doi: 10.1074/jbc.273.50.33436. [DOI] [PubMed] [Google Scholar]
- 33.Liu K, Hodes RJ, Weng Np Cutting edge: telomerase activation in human T lymphocytes does not require increase in telomerase reverse transcriptase (hTERT) protein but is associated with hTERT phosphorylation and nuclear translocation. J Immunol. 2001;166:4826–30. doi: 10.4049/jimmunol.166.8.4826. [DOI] [PubMed] [Google Scholar]
- 34.Seimiya H, Sawada H, Muramatsu Y, Shimizu M, Ohko K, Yamane K, et al. Involvement of 14-3-3 proteins in nuclear localization of telomerase. EMBO J. 2000;19:2652–61. doi: 10.1093/emboj/19.11.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Indran IR, Hande MP, Pervaiz S. hTERT overexpression alleviates intracellular ROS production, improves mitochondrial function, and inhibits ROS-mediated apoptosis in cancer cells. Cancer Res. 2011;71:266–76. doi: 10.1158/0008-5472.CAN-10-1588. [DOI] [PubMed] [Google Scholar]
- 36.Ward AF, Braun BS, Shannon KM. Targeting oncogenic Ras signaling in hematologic malignancies. Blood. 2012;120:3397–406. doi: 10.1182/blood-2012-05-378596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bermudez Y, Yang H, Cheng JQ, Kruk PA. Pyk2/ERK 1/2 mediate Sp1- and c-Myc-dependent induction of telomerase activity by epidermal growth factor. Growth Factors. 2008;26:1–11. doi: 10.1080/08977190802001389. [DOI] [PubMed] [Google Scholar]
- 38.Ge Z, Liu C, Björkholm M, Gruber A, Xu D. Mitogen-activated protein kinase cascade-mediated histone H3 phosphorylation is critical for telomerase reverse transcriptase expression/telomerase activation induced by proliferation. Mol Cell Biol. 2006;26:230–7. doi: 10.1128/MCB.26.1.230-237.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terrin L, Dal Col J, Rampazzo E, Zancai P, Pedrotti M, Ammirabile G, et al. Latent membrane protein 1 of Epstein-Barr virus activates the hTERT promoter and enhances telomerase activity in B lymphocytes. J Virol. 2008;82:10175–87. doi: 10.1128/JVI.00321-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu WR, Lu L, Rosenberg DS, Procaccini PS, Mustoe TA. Synergistic activation of extracellular signal-regulated kinase in human dermal fibroblasts by human telomerase reverse transcriptase and transforming growth factor-beta1. J Surg Res. 2007;143:415–21. doi: 10.1016/j.jss.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 41.Li H, Xu D, Li J, Berndt MC, Liu JP. Transforming growth factor β suppresses human telomerase reverse transcriptase (hTERT) by Smad3 interactions with c-Myc and the hTERT gene. J Biol Chem. 2006;281:25588–600. doi: 10.1074/jbc.M602381200. [DOI] [PubMed] [Google Scholar]
- 42.Kimura A, Ohmichi M, Kawagoe J, Kyo S, Mabuchi S, Takahashi T, et al. Induction of hTERT expression and phosphorylation by estrogen via Akt cascade in human ovarian cancer cell lines. Oncogene. 2004;23:4505–15. doi: 10.1038/sj.onc.1207582. [DOI] [PubMed] [Google Scholar]
- 43.Yamada O, Ozaki K, Akiyama M, Kawauchi K. JAK-STAT and JAK-PI3K-mTORC1 pathways regulate telomerase transcriptionally and posttranslationally in ATL cells. Mol Cancer Ther. 2012;11:1112–21. doi: 10.1158/1535-7163.MCT-11-0850. [DOI] [PubMed] [Google Scholar]
- 44.Bellon M, Nicot C. Central role of PI3K in transcriptional activation of hTERT in HTLV-I-infected cells. Blood. 2008;112:2946–55. doi: 10.1182/blood-2008-01-134692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou C, Gehrig PA, Whang YE, Boggess JF. Rapamycin inhibits telomerase activity by decreasing the hTERT mRNA level in endometrial cancer cells. Mol Cancer Ther. 2003;2:789–95. [PubMed] [Google Scholar]
- 46.Akiyama M, Hideshima T, Hayashi T, Tai Y-T, Mitsiades CS, Mitsiades N, et al. Cytokines modulate telomerase activity in a human multiple myeloma cell line. Cancer Res. 2002;62:3876–82. [PubMed] [Google Scholar]
- 47.Akiyama M, Kawano T, Mikami-Terao Y, Agawa-Ohta M, Yamada O, Ida H, et al. Erythropoietin activates telomerase through transcriptional and posttranscriptional regulation in human erythroleukemic JAS-REN-A cells. Leuk Res. 2011;35:416–8. doi: 10.1016/j.leukres.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Moon DO, Kim MO, Lee JD, Choi YH, Kim GY. Butein suppresses c-Myc-dependent transcription and Akt-dependent phosphorylation of hTERT in human leukemia cells. Cancer Lett. 2009;286:172–9. doi: 10.1016/j.canlet.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 49.Kawauchi K, Ihjima K, Yamada O. IL-2 increases human telomerase reverse transcriptase activity transcriptionally and posttranslationally through phosphatidylinositol 3′-kinase/Akt, heat shock protein 90, and mammalian target of rapamycin in transformed NK cells. J Immunol. 2005;174:5261–9. doi: 10.4049/jimmunol.174.9.5261. [DOI] [PubMed] [Google Scholar]
- 50.Shapira S, Granot G, Mor-Tzuntz R, Raanani P, Uziel O, Lahav M, et al. Second-generation tyrosine kinase inhibitors reduce telomerase activity in K562 cells. Cancer Lett. 2012;323:223–31. doi: 10.1016/j.canlet.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 51.Damle RN, Temburni S, Banapour T, Paul S, Mongini PKA, Allen SL, et al. T-cell independent, B-cell receptor-mediated induction of telomerase activity differs among IGHV mutation-based subgroups of chronic lymphocytic leukemia patients. Blood. 2012;120:2438–49. doi: 10.1182/blood-2012-02-409110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ram R, Uziel O, Eldan O, Fenig E, Beery E, Lichtenberg S, et al. Ionizing radiation up-regulates telomerase activity in cancer cell lines by post-translational mechanism via ras/phosphatidylinositol 3-kinase/Akt pathway. Clin Cancer Res. 2009;15:914–23. doi: 10.1158/1078-0432.CCR-08-0792. [DOI] [PubMed] [Google Scholar]
- 53.Chung J, Khadka P, Chung IK. Nuclear import of hTERT requires a bipartite nuclear localization signal and Akt-mediated phosphorylation. J Cell Sci. 2012;125:2684–97. doi: 10.1242/jcs.099267. [DOI] [PubMed] [Google Scholar]
- 54.Forsythe HL, Jarvis JL, Turner JW, Elmore LW, Holt SE. Stable association of hsp90 and p23, but Not hsp70, with active human telomerase. J Biol Chem. 2001;276:15571–4. doi: 10.1074/jbc.C100055200. [DOI] [PubMed] [Google Scholar]
- 55.Sundin T, Peffley DM, Hentosh P. Disruption of an hTERT-mTOR-RAPTOR protein complex by a phytochemical perillyl alcohol and rapamycin. Mol Cell Biochem. 2013;375:97–104. doi: 10.1007/s11010-012-1532-3. [DOI] [PubMed] [Google Scholar]
- 56.Hiyama K, Hirai Y, Kyoizumi S, Akiyama M, Hiyama E, Piatyszek MA, et al. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J Immunol. 1995;155:3711–5. [PubMed] [Google Scholar]
- 57.Igarashi H, Sakaguchi N. Telomerase activity is induced in human peripheral B lymphocytes by the stimulation to antigen receptor. Blood. 1997;89:1299–307. [PubMed] [Google Scholar]
- 58.Yu C-C, Lo SC, Wang TC. Telomerase is regulated by protein kinase C-zeta in human nasopharyngeal cancer cells. Biochem J. 2001;355:459–64. doi: 10.1042/0264-6021:3550459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang JT, Lu Y-C, Chen YJ, Tseng CP, Chen YL, Fang CW, et al. hTERT phosphorylation by PKC is essential for telomerase holoprotein integrity and enzyme activity in head neck cancer cells. Br J Cancer. 2006;94:870–8. doi: 10.1038/sj.bjc.6603008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakatake M, Kakiuchi Y, Sasaki N, Murakami-Murofushi K, Yamada O. STAT3 and PKC differentially regulate telomerase activity during megakaryocytic differentiation of K562 cells. Cell Cycle. 2007;6:1496–501. doi: 10.4161/cc.6.12.4304. [DOI] [PubMed] [Google Scholar]
- 61.Sheng WY, Chen YR, Wang TC. A major role of PKC theta and NFkappaB in the regulation of hTERT in human T lymphocytes. FEBS Lett. 2006;580:6819–24. doi: 10.1016/j.febslet.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 62.Sinha-Datta U, Horikawa I, Michishita E, Datta A, Sigler-Nicot JC, Brown M, et al. Transcriptional activation of hTERT through the NF-kappaB pathway in HTLV-I-transformed cells. Blood. 2004;104:2523–31. doi: 10.1182/blood-2003-12-4251. [DOI] [PubMed] [Google Scholar]
- 63.Akiyama M, Hideshima T, Hayashi T, Tai Y-T, Mitsiades CS, Mitsiades N, et al. Nuclear factor-kappaB p65 mediates tumor necrosis factor α-induced nuclear translocation of telomerase reverse transcriptase protein. Cancer Res. 2003;63:18–21. [PubMed] [Google Scholar]
- 64.Chen E, Staudt LM, Green AR. Janus kinase deregulation in leukemia and lymphoma. Immunity. 2012;36:529–41. doi: 10.1016/j.immuni.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khwaja A. The role of Janus kinases in haemopoiesis and haematological malignancy. Br J Haematol. 2006;134:366–84. doi: 10.1111/j.1365-2141.2006.06206.x. [DOI] [PubMed] [Google Scholar]
- 66.Bar-Natan M, Nelson EA, Xiang M, Frank DA. STAT signaling in the pathogenesis and treatment of myeloid malignancies. JAK-STAT. 2012;1:55–64. doi: 10.4161/jkst.20006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamada O, Mahfoudhi E, Plo I, Nakatake M, Ozaki K, Akiyama M, et al. Emergence of a BCR-ABL translocation in a patient with the JAK2V617F mutation: evidence for secondary acquisition of BCR-ABL in the JAK2V617F clone. J Clin Oncol. 2013 doi: 10.1200/JCO.2012.47.8669. In press. [DOI] [PubMed] [Google Scholar]
- 68.Plo I, Nakatake M, Malivert L, de Villartay J-P, Giraudier S, Villeval JL, et al. JAK2 stimulates homologous recombination and genetic instability: potential implication in the heterogeneity of myeloproliferative disorders. Blood. 2008;112:1402–12. doi: 10.1182/blood-2008-01-134114. [DOI] [PubMed] [Google Scholar]
- 69.Meier C, Hoeller S, Bourgau C, Hirschmann P, Schwaller J, Went P, et al. Recurrent numerical aberrations of JAK2 and deregulation of the JAK2-STAT cascade in lymphomas. Mod Pathol. 2009;22:476–87. doi: 10.1038/modpathol.2008.207. [DOI] [PubMed] [Google Scholar]
- 70.Schepers H, Wierenga ATJ, Vellenga E, Schuringa JJ. STAT5-mediated self-renewal of normal hematopoietic and leukemic stem cells. JAK-STAT. 2012;1:13–22. doi: 10.4161/jkst.19316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakatake M, Sasaki N, Murakami-Murofushi K, Yamada O. Transient posttranslational up-regulation of telomerase activity during megakaryocytic differentiation of K562 cells. Biochem Biophys Res Commun. 2004;314:1080–5. doi: 10.1016/j.bbrc.2003.12.199. [DOI] [PubMed] [Google Scholar]
- 72.Yamada O, Ozaki K, Nakatake M, Akiyama M, Kawauchi K, Matsuoka R. Multistep regulation of telomerase during differentiation of HL60 cells. J Leukoc Biol. 2008;83:1240–8. doi: 10.1189/jlb.1207848. [DOI] [PubMed] [Google Scholar]
- 73.Wang H, Chen Q, Lee S-H, Choi Y, Johnson FB, Pignolo RJ. Impairment of osteoblast differentiation due to proliferation-independent telomere dysfunction in mouse models of accelerated aging. Aging Cell. 2012;11:704–13. doi: 10.1111/j.1474-9726.2012.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamada O, Akiyama M, Kawauchi K, Adachi T, Yamada H, Kanda N, et al. Overexpression of telomerase confers a survival advantage through suppression of TRF1 gene expression while maintaining differentiation characteristics in K562 cells. Cell Transplant. 2003;12:365–77. doi: 10.3727/000000003108746911. [DOI] [PubMed] [Google Scholar]
- 75.Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–56. [PubMed] [Google Scholar]
- 76.Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–42. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 77.Akiyama M, Yamada O, Kanda N, Akita S, Kawano T, Ohno T, et al. Telomerase overexpression in K562 leukemia cells protects against apoptosis by serum deprivation and double-stranded DNA break inducing agents, but not against DNA synthesis inhibitors. Cancer Lett. 2002;178:187–97. doi: 10.1016/S0304-3835(01)00838-2. [DOI] [PubMed] [Google Scholar]
- 78.Yamada O, Kawauchi K, Akiyama M, Ozaki K, Motoji T, Adachi T, et al. Leukemic cells with increased telomerase activity exhibit resistance to imatinib. Leuk Lymphoma. 2008;49:1168–77. doi: 10.1080/10428190802043861. [DOI] [PubMed] [Google Scholar]
- 79.Yamada O, Ozaki K, Furukawa T, Machida M, Wang YH, Motoji T, et al. Activation of STAT5 confers imatinib resistance on leukemic cells through the transcription of TERT and MDR1. Cell Signal. 2011;23:1119–27. doi: 10.1016/j.cellsig.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 80.Heltemes-Harris LM, Farrar MA. The role of STAT5 in lymphocyte development and transformation. Curr Opin Immunol. 2012;24:146–52. doi: 10.1016/j.coi.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma J, Ma D, Ji C, M J The role of IL-21 in hematological malignancies. Cytokine. 2011;56:133–9. doi: 10.1016/j.cyto.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 82.Ghoshal Gupta S, Baumann H, Wetzler M. Epigenetic regulation of signal transducer and activator of transcription 3 in acute myeloid leukemia. Leuk Res. 2008;32:1005–14. doi: 10.1016/j.leukres.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Swords R, Freeman C, Giles F. Targeting the FMS-like tyrosine kinase 3 in acute myeloid leukemia. Leukemia. 2012;26:2176–85. doi: 10.1038/leu.2012.114. [DOI] [PubMed] [Google Scholar]
- 84.Katsumi S, Kawauchi K, Ozaki K, Shimizu S, Kimura T, Motoji T, et al. Analysis of the molecular mechanism involved in the development of acute myeloid leukemia. Jpn J Cancer Chemother. 2013;40:471–7. [PubMed] [Google Scholar]
- 85.Akiyama M, Kawano T, Mikami-Terao Y, Agawa-Ohta M, Yamada O, Ida H, et al. Erythropoietin activates telomerase through transcriptional and posttranscriptional regulation in human erythroleukemic JAS-REN-A cells. Leuk Res. 2011;35:416–8. doi: 10.1016/j.leukres.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 86.Prade-Houdellier N, Frébet E, Demur C, Gautier EF, Delhommeau F, Bennaceur-Griscelli AL, et al. Human telomerase is regulated by erythropoietin and transforming growth factor-beta in human erythroid progenitor cells. Leukemia. 2007;21:2304–10. doi: 10.1038/sj.leu.2404874. [DOI] [PubMed] [Google Scholar]
- 87.Brady A, Gibson S, Rybicki L, Hsi E, Saunthararajah Y, Sekeres MA, et al. Expression of phosphorylated signal transducer and activator of transcription 5 is associated with an increased risk of death in acute myeloid leukemia. Eur J Haematol. 2012;89:288–93. doi: 10.1111/j.1600-0609.2012.01825.x. [DOI] [PubMed] [Google Scholar]
- 88.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415–9. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita KI, et al. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981;78:6476–80. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984-87) Br J Haematol. 1991;79:428–37. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 91.Ellery JM, Nicholls PJ. Alternate signalling pathways from the interleukin-2 receptor. Cytokine Growth Factor Rev. 2002;13:27–40. doi: 10.1016/S1359-6101(01)00023-5. [DOI] [PubMed] [Google Scholar]
- 92.Takemoto S, Mulloy JC, Cereseto A, Migone TS, Patel BK, Matsuoka M, et al. Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc Natl Acad Sci U S A. 1997;94:13897–902. doi: 10.1073/pnas.94.25.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Steelman LS, Abrams SL, Whelan J, Bertrand FE, Ludwig DE, Bäsecke J, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia. 2008;22:686–707. doi: 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- 94.Menges CW, McCance DJ. Constitutive activation of the Raf-MAPK pathway causes negative feedback inhibition of Ras-PI3K-AKT and cellular arrest through the EphA2 receptor. Oncogene. 2008;27:2934–40. doi: 10.1038/sj.onc.1210957. [DOI] [PubMed] [Google Scholar]
- 95.Lanvin O, Guglielmi P, Fuentes V, Gouilleux-Gruart V, Mazière C, Bissac E, et al. TGF-beta1 modulates Fas (APO-1/CD95)-mediated apoptosis of human pre-B cell lines. Eur J Immunol. 2003;33:1372–81. doi: 10.1002/eji.200323761. [DOI] [PubMed] [Google Scholar]
- 96.So E-Y, Oh J, Jang J-Y, Kim J-H, Lee C-E. Ras/Erk pathway positively regulates Jak1/STAT6 activity and IL-4 gene expression in Jurkat T cells. Mol Immunol. 2007;44:3416–26. doi: 10.1016/j.molimm.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 97.Cokic VP, Bhattacharya B, Beleslin-Cokic BB, Noguchi CT, Puri RK, Schechter AN. JAK-STAT and AKT pathway-coupled genes in erythroid progenitor cells through ontogeny. J Transl Med. 2012;10:116–27. doi: 10.1186/1479-5876-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harir N, Pecquet C, Kerenyi M, Sonneck K, Kovacic B, Nyga R, et al. Constitutive activation of Stat5 promotes its cytoplasmic localization and association with PI3-kinase in myeloid leukemias. Blood. 2007;109:1678–86. doi: 10.1182/blood-2006-01-029918. [DOI] [PubMed] [Google Scholar]
- 99.Tasian SK, Doral MY, Borowitz MJ, Wood BL, Chen I-M, Harvey RC, et al. Aberrant STAT5 and PI3K/mTOR pathway signaling occurs in human CRLF2-rearranged B-precursor acute lymphoblastic leukemia. Blood. 2012;120:833–42. doi: 10.1182/blood-2011-12-389932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roll JD, Reuther GW. ALK-activating homologous mutations in LTK induce cellular transformation. PLoS One. 2012;7:e31733. doi: 10.1371/journal.pone.0031733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krysov S, Dias S, Paterson A, Mockridge CI, Potter KN, Smith K-A, et al. Surface IgM stimulation induces MEK1/2-dependent MYC expression in chronic lymphocytic leukemia cells. Blood. 2012;119:170–9. doi: 10.1182/blood-2011-07-370403. [DOI] [PubMed] [Google Scholar]
- 102.Chanprasert S, Geddis AE, Barroga C, Fox NE, Kaushansky K. Thrombopoietin (TPO) induces c-myc expression through a PI3K- and MAPK-dependent pathway that is not mediated by Akt, PKCzeta or mTOR in TPO-dependent cell lines and primary megakaryocytes. Cell Signal. 2006;18:1212–8. doi: 10.1016/j.cellsig.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 103.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–56. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 104.Gupta M, Han JJ, Stenson M, Maurer M, Wellik L, Hu G, et al. Elevated serum IL-10 levels in diffuse large B-cell lymphoma: a mechanism of aberrant JAK2 activation. Blood. 2012;119:2844–53. doi: 10.1182/blood-2011-10-388538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang X, Zeng JH, Shi M, Zhao S, Bai W, Cao W, et al. Targeted blockage of signal transducer and activator of transcription 5 signaling pathway with decoy oligodeoxynucleotides suppresses leukemic K562 cell growth. DNA Cell Biol. 2011;30:71–8. doi: 10.1089/dna.2010.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gerlo S, Haegeman G, Vanden Berghe W. Transcriptional regulation of autocrine IL-6 expression in multiple myeloma cells. Cell Signal. 2008;20:1489–96. doi: 10.1016/j.cellsig.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 107.Amodio N, Di Martino MT, Foresta U, Leone E, Lionetti M, Leotta M, et al. miR-29b sensitizes multiple myeloma cells to bortezomib-induced apoptosis through the activation of a feedback loop with the transcription factor Sp1. Cell Death Dis. 2012;3:e436. doi: 10.1038/cddis.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bermudez Y, Yang H, Cheng JQ, Kruk PA. Pyk2/ERK 1/2 mediate Sp1- and c-Myc-dependent induction of telomerase activity by epidermal growth factor. Growth Factors. 2008;26:1–11. doi: 10.1080/08977190802001389. [DOI] [PubMed] [Google Scholar]
- 109.Bermudez Y, Yang H, Saunders BO, Cheng JQ, Nicosia SV, Kruk PA. VEGF- and LPA-induced telomerase in human ovarian cancer cells is Sp1-dependent. Gynecol Oncol. 2007;106:526–37. doi: 10.1016/j.ygyno.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 110.Sitaram RT, Cairney CJ, Grabowski P, Keith WN, Hallberg B, Ljungberg B, et al. The PTEN regulator DJ-1 is associated with hTERT expression in clear cell renal cell carcinoma. Int J Cancer. 2009;125:783–90. doi: 10.1002/ijc.24335. [DOI] [PubMed] [Google Scholar]
- 111.Mitomo S, Maesawa C, Ogasawara S, Iwaya T, Shibazaki M, Yashima-Abo A, et al. Downregulation of miR-138 is associated with overexpression of human telomerase reverse transcriptase protein in human anaplastic thyroid carcinoma cell lines. Cancer Sci. 2008;99:280–6. doi: 10.1111/j.1349-7006.2007.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miura N, Sato R, Tsukamoto T, Shimizu M, Kabashima H, Takeda M, et al. A noncoding RNA gene on chromosome 10p15.3 may function upstream of hTERT. BMC Mol Biol. 2009;10:5–21. doi: 10.1186/1471-2199-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Harley CB. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991;256:271–82. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- 114.Greider CW. Telomeres do D-loop-T-loop. Cell. 1999;97:419–22. doi: 10.1016/S0092-8674(00)80750-3. [DOI] [PubMed] [Google Scholar]
- 115.Bianchi A, Shore D. How telomerase reaches its end: mechanism of telomerase regulation by the telomeric complex. Mol Cell. 2008;31:153–65. doi: 10.1016/j.molcel.2008.06.013. [DOI] [PubMed] [Google Scholar]