Abstract
Introduction:
We review the current evidence for the role of low-dose rate brachytherapy (PB) in patients with low- or intermediate-risk prostate cancer using a systematic review of the literature.
Methods:
We searched MEDLINE and EMBASE (from January 1996 to October 2011), the Cochrane Library, relevant guideline web-sites, and websites for meetings specific for genitourinary diseases.
Results:
Ten systematic reviews and 55 single-study papers met the pre-planned study selection criteria. In the end, 36 articles were abstracted and analyzed for this systematic review. There is no evidence for a difference in efficacy between PB and external beam radiation therapy (EBRT), or between PB and radical prostatectomy (RP). During the 6 months to 3 years after treatment, PB was associated with less urinary incontinence and sexual impotency than RP, and RP was associated with less urinary irritation and rectal morbidity than PB. However, these differences diminished over time. PB conferred less risk of impotency and rectal morbidity in the three years after treatment than EBRT. Iodine-125 and alladium-103 did not differ with respect to biochemical relapse-free survival and patient-reported outcomes.
Conclusions:
PB alone is a treatment option with equal efficacy to EBRT or RP alone in patients with newly diagnosed low- or intermediate-risk prostate cancer who require or choose active treatment.
Introduction
Prostate cancer is a common cause of cancer-related mortality in North America and Europe.1,2 In Canada in 2013, 23 600 new prostate cancer cases were predicted, as are approximately 4000 deaths related to prostate cancer.1 In 2001, the Cancer Care Ontario (CCO) Genitourinary Cancer Disease Site Group (DSG) and the Program in Evidence-Based Care (PEBC) developed a guideline on PB for patients with low-risk prostate cancer (literature search from 1988 to 1999).3 Since studies with stronger quality of evidence (e.g., randomized controlled trials [RCTs]) on investigating low- and even intermediate-risk patient populations have been published during the last decade, it is necessary to update this systematic review.
Based on the clinical practice guidelines of the Genitourinary Radiation Oncologists of Canada4 and the National Comprehensive Cancer Network (NCCN),5 prostate cancer patients are classified into 3 groups: low-risk (defined as having a prostate-specific antigen [PSA] <10 ng/mL and clinical stage T1c-T2a and Gleason score <7), intermediate-risk (PSA ≥10 ng/mL, but <20 ng/mL or clinical stage T2b-T2c or Gleason score =7), and high-risk (PSA ≥20 ng/mL or clinical stage >T2c or Gleason score >7). To date, 3 isotopes have been available for PB in patients with prostate cancer: iodine-125 (I-125), palladium-103 (Pd-103), and cesium-131 (Cs-131), each of which has a different half-life (59.4 days, 17.0 days, and 9.7 days, respectively6). It is unclear which isotope maximizes clinical outcomes.
Our goal in this updated systematic review is to answer the following 3 questions in patients with newly diagnosed low- or intermediate-risk prostate cancer who are eligible for treatment with PB:
What is the efficacy of PB alone for clinical outcomes (specifically: prostate cancer specific mortality [PCSM], overall survival [OS], biochemical relapse-free survival [bRFS], negative biopsy rate, salvage treatment rate, toxicity, and patient-reported outcomes, including quality of life [QOL]), when compared with EBRT alone, or RP alone?
What is the efficacy of PB and EBRT in combination, for the above clinical outcomes, when compared with PB alone, EBRT alone, or RP alone?
Among the 3 isotopes used for PB, which one maximizes clinical outcomes?
Methods
Literature search strategy
A literature search with a priori defined study selection criteria was performed using MEDLINE and EMBASE through the Ovid search engine; also, the Cochrane Clinical Trial Register was searched for the period of January 1, 1996 to October 27, 2011. Alternative terms for brachytherapy including I-125, Pd-103, or Cs-131; alternative terms for prostate cancer were used to search literatures respectively, and then “AND” was used to combine results of these 2.7 The relevant guideline websites (e.g., National Guideline Clearinghouse), websites for meetings specific for genitourinary diseases (e.g., American Society for Radiation Oncology), and the Standards and Guidelines Evidence Inventory of Cancer Guidelines8 were checked for existing relevant systematic reviews and guidelines.
Study selection criteria
Inclusion criteria
Systematic reviews, RCTs, and prospective comparative studies with analyzed sample size ≥30 for each comparative subgroup, or retrospective comparative studies with total sample size ≥500.
Papers on studies comparing PB with EBRT or RP alone; PB plus EBRT with PB, EBRT, or RP alone; different doses of PB alone or PB plus EBRT; or any 2 of the 3 isotopes.
Papers reporting on at least one of the target clinical outcomes.
Papers published in English.
Exclusion criteria
Studies reporting outcomes on mixed risk group populations where >20% of the patients were from the high-risk category or studies in which outcomes were not stratified by risk group.
Studies in which treatments were selected according to known risk factors (e.g., patients with PSA <10 ng/mL received PB and patients with PSA ≥10 ng/mL received EBRT).
Synthesizing the evidence
The evidence from included papers was reviewed by 4 clinician members of the genitourinary cancer Disease Site Group (DSG), and 1 methodologist from the Program in Evidence-Based Care (PEBC). All data were audited by a second, independent auditor. Data for any subgroup with less than 30 patients were not used given the large confidence intervals implicit in the analysis of small numbers of patients. Significance testing was reported as per the source study.
Assessing quality of the studies
The quality of the RCTs included in this review was assessed using the modified Cochrane Collaboration’s tool.9 The quality of non-randomized studies was assessed using the modified Newcastle-Ottawa Scale.10
Results
Literature search results
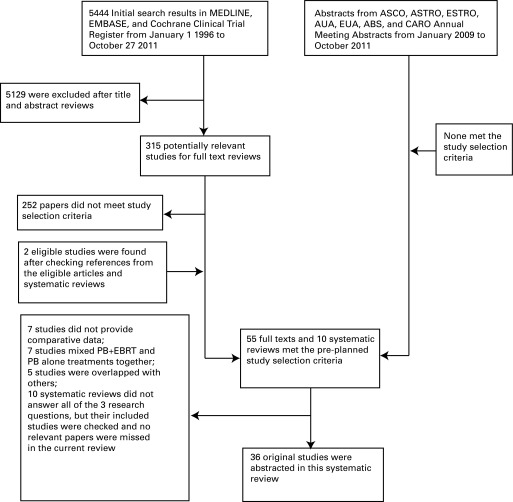
After searching the MEDLINE, EMBASE, and the Cochrane Clinical Trial Register, 5444 citations were identified (Fig. 1). Ultimately, a total of 10 systematic reviews11–20 and 55 unique studies21–75 met the study selection criteria. After careful consideration (Fig. 1), we finally chose 36 articles for this systematic review. Among them, 29 articles related to the first research question,21–23,25–27,29–31,33,34,37,38,41–46,49,51,52,56,61,65,66,72–74 6 related to the second question,35,36,58,63,69,72 and 3 related to the third research question.36,39,47
Fig. 1.
Flow of studies considered for this systematic review. ASCO: American Society of Clinical Oncology; ASTRO: American Society for Radiation Oncology; ESTRO: European Society for Radiotherapy and Oncology; AUA: American Urological Association; EAU: European Association of Urology; ABS: American Brachytherapy Society; CARO: Canadian Association of Radiation Oncologists; PB: brachytherapy; EBRT: external beam radiation therapy.
We identified the non-RCTs that met our pre-planned study selection criteria; we tallied them and included them for completeness (Table 1, http://journals.sfu.ca/cuaj/index.php/journal/article/view/1482/1457). They were not interpreted owing to the potential likelihood of significant bias influencing the study findings: studies that did not control for differences in baseline patient characteristics by using multivariate analysis; studies where overall survival (OS)/prostate cancer specific mortality (PCSM) were <10 years given that the relative 10-year and 15-year prostate-cancer survival rates are 98% and 91%, respectively, for all prostate cancer patients with any treatment (it is unlikely that between-group differences in survival due to treatment selection would manifest at <10 years);76 studies not reporting biochemical relapse-free survival (bRFS) rates ≥5 years (since “benign PSA bounce”77,78 can occur within 5 years following treatment with PB or EBRT); and studies including unclear proportion of high-risk patients.
Assessment of study quality
Among the 36 articles, 6 reported targeted outcomes from 3 RCTs.36,37,39,47,58,69 Only 1 of the 5 papers39 from the RCTs by Merrick and colleagues and Wallner and colleagues reached the minimal patient requirement to achieve a study power of 80% at an alpha of 0.05 for survival outcomes. These 5 papers reported the selected outcomes without pre-planned interim analysis. The randomization method and allocation concealment were clearly reported and by necessity, patients and clinicians were unblinded in both RCTs. The 2009 Giberti and colleagues’ trial was the sixth RCT and demonstrated good quality on the randomization method and allocation concealment;37 outcome assessors were blinded to patient treatment; the follow-up rate was 87% over 5 years. However, there was no expected effect size or sample size calculation, no intention-to-treat analysis, and no information about funding resources. Overall, the quality of evidence from these RCTs was considered to be poor to moderate.
Fourteen articles reported prospective studies and 16 were retrospective studies. The baseline patient characteristics or the proportion of different risk patients among the treatment groups were reported as significantly different in 21 studies.21,22,26,27,29,30,33,38,43–46,49,51,52,61,63,66,72–74 There was no statistical comparison of baseline patient characteristics among intervention groups in 6 studies.23,34,35,42,56,65 Only the study by Kirschner-Hermann and colleagues reported a blinded assessment of outcome.41 Although the follow-up rates in most studies were over 80%, only 10 studies had at least 1 group with at least 5 years of median or mean follow-up time.21–23,26,29,43,63,66,72,74 Overall, the study quality from the non-RCTs was poor to moderate.
Outcomes
Patient characteristics, treatment dose, and treatment effect outcomes are shown in Table 1 (http://journals.sfu.ca/cuaj/index.php/journal/article/view/1482/1457). Among the eligible papers, when PB was used alone, the dose was 140–160 Gy for I-125 or 108–125 Gy for Pd-103; when PB was combined with EBRT, the dose was 41.4–45 Gy EBRT followed by 100–120 Gy I-125, or 20–50.4 Gy EBRT followed by 90–115 Gy Pd-103.
Meta-analyses of the trial results were considered, but were deemed not feasible because patient characteristics, interventions, intervention doses, PSA failure definitions, toxicity assessment criteria, and assessment tools for patient-reported outcomes among the included studies were too heterogeneous.
Outcomes relevant to research question 1: What is the efficacy of PB alone for clinical outcomes when compared with EBRT alone, or RP alone?
PCSM, OS, and bRFS
When PB was compared with RP for PCSM or OS, 1 retrospective study with 41 395 mixed low-risk and intermediate-risk patients reported 10-year survival outcomes.65 In this study, there was no statistical difference between PB and RP groups, regardless of age (Table 1, http://journals.sfu.ca/cuaj/index.php/journal/article/view/1482/1457). Four additional studies compared PB with RP for bRFS. One RCT with 200 low-risk patients37 and 1 retrospective study with 927 low-risk patients31 showed no statistical difference between the 2 groups; 2 retrospective studies showed that PB led to a higher bRFS rate than did RP in 674 intermediate-risk patients66 and in 437 mixed low-, intermediate- and ≤20% of high-risk patients,26 respectively.
When PB was compared with EBRT, 3 retrospective studies with 1529 patients showed there were no significant differences between the 2 groups with respect to bRFS.26,31,66
Toxicity
We tallied the studies that reported comparisons of ≥grade 2 toxicities between treatments (Table 2, http://journals.sfu.ca/cuaj/index.php/journal/article/view/1482/1457). With regard to bladder and bowel function, a retrospective study with 729 low-risk patients reported that PB led to more late grade 2 genitourinary and gastrointestinal toxicities, but less impotence than did EBRT. There were no differences for the late grade 3 genitourinary and gastrointestinal toxicities between the 2 groups;74 however, this paper did not give the time definition of “late toxicity.” For second primary cancers, another retrospective study reported that PB led to fewer second primaries up to 5.3 years compared with EBRT in 58 623 patients (mixed risk categories, but ≤20% of high-risk patients),21 but the EBRT group had more patients ≥65 years old (82% vs. 63%) and more high-risk patients (19.6% vs. 5.4%) than those in the PB group.
Patient-reported outcomes
Three RCTs (4 papers36,37,39,58) and 16 non-RCTs23,25,27,29,33,34,38,41,44–46,49,51,52,61,73 reported data on patient-reported outcomes using 20 different instruments, but only 10 articles that had clear categories for low-, and/or intermediate-, and/or mixed ≤20% of high-risk patients. The data indicated no statistically significant differences at baseline for QOL among intervention groups; also, the data did not adjust for baseline imbalance when reporting QOL outcomes (Table 3, http://journals.sfu.ca/cuaj/index.php/journal/article/view/1482/1457). Given the heterogeneity of the study cohorts and the instruments used, estimates of the magnitude of impact of treatment on patient-reported outcomes could not be compared between studies.
When PB was compared with EBRT, 2 prospective studies with 792 patients showed no difference between the 2 groups for urinary domains. PB led to less sexual and rectal problems than did EBRT in patients with mixed low-, intermediate-, and mixed ≤20% of high-risk patients.33,51
When PB was compared with RP, 3 prospective studies with 913 patients in mixed low-, intermediate-, and ≤20% of high-risk showed that urinary incontinence and sexual potency favoured PB, while other urinary problems favoured RP.27,38,51 For rectal morbidity, 1 study favoured RP,27 but the 3 other studies found no difference.33,38,51 In an RCT with 200 low-risk patients, results were consistent with the above observational studies at 1 year, but these differences were not sustained at 5 years.37
Outcomes relevant to research question 2: What is the efficacy of PB and EBRT in combination, for the above clinical outcomes, when compared with PB alone, EBRT alone, or RP alone?
No study compared the combination of EBRT plus PB with PB, EBRT, or RP alone for treatment benefit outcomes in the target patients in (Table 1, http://journals.sfu.ca/cuaj/index.php/journal/article/view/1482/1457).
One retrospective study of 825 patients investigated gastrointestinal toxicity between PB plus EBRT and PB alone and found no difference between arms at 8 and 12 months in Table 2 (http://journals.sfu.ca/cuaj/index.php/journal/article/view/1482/1457).35
For patient-reported outcomes, when PB plus EBRT was compared with PB alone, 1 retrospective study73 demonstrated that PB led to a better urinary function and bother outcomes. There were no statistical differences for sexual, bowel function and bother, or overall QOL between 3 groups in 510 patients with mixed low-, intermediate-, and mixed ≤20% of high-risk in Table 3 (http://journals.sfu.ca/cuaj/index.php/journal/article/view/1482/1457).
Outcomes relevant to research question 3: Among the three isotopes used for PB, which one maximizes clinical outcomes?
In Table 1 (http://journals.sfu.ca/cuaj/index.php/journal/article/view/1482/1457), 1 RCT with 263 low-risk patients showed no significant difference for bRFS rate between the I-125 and Pd-103 groups (96.8% vs. 99.2%, p = 0.149).47
Up to the search date, there was no evidence to compare the toxicity of different isotopes used for PB in the target patients.
For patient-reported outcomes, when I-125 was compared with Pd-103, 1 RCT with 314 low-risk patients reported that Pd-103 resulted in worse overall QOL than did I-125 at 1 month, and I-125 resulted in worse overall QOL than did Pd-103 at 6 months. However, there was no difference between the 2 groups at 1 and 2 years.39
Ongoing trials
The National Cancer Institute Clinical Trials database was searched on September 12, 2012 and the Radiation Therapy Oncology Group website was searched on March 20, 2012 for potential trials meeting our selection criteria. Six ongoing RCTs were found (the summarized details can be obtained by contacting authors).
Discussion
There are many challenges inherent in conducting an RCT in prostate cancer patients, including the natural history of the disease, strong patient treatment preferences, or concerns regarding receiving non-standard of care approaches.79 For example, an American College of Surgeons Oncology Group phase III trial (SPIRIT) comparing RP and PB for patients with low-risk prostate cancer opened in 2002, but was closed in 2004 due to poor accrual.29
Due to the relative dearth of RCTs, comparative observational studies were therefore included in this review if they met the a priori selection criteria. Despite the fact that 36 original studies were abstracted in this review, there remains insufficient evidence to answer all 3 research questions with certainty.
Firstly, each of the eligible studies has substantial potential for bias and thus considerable uncertainty regarding its findings. Secondly, it is recognized that bRFS rate is a surrogate endpoint of treatment efficacy, and few studies reported survival outcomes (PCSM or OS). Thirdly, the studies reporting bRFS used different definitions for PSA failure, making between-study comparisons difficult. Most publications before 2006 used the 1996 ASTRO definition (3 consecutive rising PSA values each obtained at least 3 months apart);80 after 2006, most publications followed the 2006 ASTRO definition for failure following PB (PSA should be higher than nadir plus 2 ng/mL) in recognition of the “benign PSA bounce.”81 For RP, definitions for PSA failure included PSA value ≥0.2 ng/mL, >0.3 ng/mL, or ≥0.4 ng/mL. When PB was compared with RP, using the ASTRO 2006 definition for PSA failure created a bias favouring PB, but adequate follow-up reduced this bias.81 Therefore, only bRFS at ≥5 years was analyzed and interpreted in this review. Fourthly, about one-half of the included studies used neo-adjuvant hormone suppressive therapy for some patients, often with the intent to reduce prostate size before treatments. Again, without the protection of randomization, it is difficult to factor this element into between-study comparisons of bRFS outcomes. Fifthly, recommended doses have changed over time. Before 2000, most reports on EBRT alone included doses of 63–70 Gy; after 2000, most publications includes 70–81 Gy. Determining the appropriate dose for EBRT alone is beyond the scope of this review; however, the use of low-dose EBRT before 2000 may have underestimated the effect of EBRT. Likewise, the predominant use of the 1996 ASTRO definition for PSA failure prior to 2001 may have underestimated the effectiveness of PB, again preventing meaningful synthesis of the data among studies. Sixthly, it is recognized that PB and RP quality varies significantly between centres and may be an additional source of potential bias when comparing results. However, PB or RP quality was poorly reported for most studies so comparisons of high quality PB versus high quality RP were not possible. In addition, as RP and PB are widely performed, the authors of this systematic review wanted to assess the comparative effectiveness of the different procedures (i.e., what the results are across a large spectrum of centres).
Although the quality of evidence from included studies was low-to-moderate in nature within this systematic review, the evidence across the eligible studies consistently supports the conclusion that there is no difference in treatment efficacy between PB, EBRT, and RP in patients with low-, or intermediate-risk prostate cancer.
It is worthy to note that although the 2010 study by Pickles and colleagues is a retrospective study and does not meet the pre-planned study inclusion criteria (i.e., the sample size from a retrospective study should be >500), it is a relevant piece of work that fits within the Canadian context of practice.82 In total, 108 patients with low-risk and 31 with intermediate-risk prostate cancer in the PB group were matched with patients in the EBRT group. The median followed-up time was 5.5 years. The 5-year bRFS rates in the PB group and the EBRT group were 95% versus 85% (p < 0.001) for all patients, 94% versus 88% (p < 0.001) for low-risk patients, and 100% versus 78% (p = 0.016) for intermediate-risk patients, respectively. At 7 years, the bRFS rate was 95% versus 75% (p < 0.001) for all patients, respectively. Late urinary toxicity was worse in patients treated with PB, and late rectal toxicity was worse in patients treated with EBRT. Thus, this study also supports the conclusion of this systematic review.
After our search for this review, another similar systematic review had been done by Grimm and colleagues.83 They came to different conclusions and reported that “brachytherapy provides superior outcome in patients with low-risk disease.” There are several reasons for the difference. Firstly, this Grimm review included single-arm studies. We considered it inappropriate to investigate the treatment effect of one option since without direct comparison between 2 treatment options as many potential confounders can bias the treatment results. Secondly, the Grimm 2012 review combined all the comparative studies that met its inclusion criteria in a single analysis. However, for comparative studies (non-RCTs), if the patient characteristics were significantly different between 2 treatment groups at baseline, it would be impossible to know whether their final results for treatment effect were real or affected by uncontrolled for the potential confounders at baseline. Actually, many of the 10 systematic reviews that met the pre-planned selection criteria11,19 did not consider this important point.
Conclusions
To date, consistent evidence exists to support PB alone as a treatment option with equal efficacy to EBRT alone or RP alone for patients with newly diagnosed low- or intermediate-risk prostate cancer who require or choose active treatment; benefits and toxicities must also be balanced. There is insufficient evidence to recommend the use of combination of EBRT plus BT in the target patients. I-125 and Pd-103 are not different for bRFS and QOL in target patients. There is no efficacy evidence for Cs-131. In the absence of large RCTs and faced with small sample sizes in existing non-RCT studies, any possible difference that may exist between the treatment options cannot be conclusively identified. Hence, large sample-size, well-designed, and good-quality RCTs and/or prospective comparative studies are required to investigate novel or targeted approaches in patients with low- or intermediate-risk prostate cancer.
Acknowledgments
The authors would like to thank Harkanwal Randhawa for his contribution toward the data audit, and the Genitourinary Cancer Disease Site Group members in Ontario (Glenn Bauman, Jack Barkin, Rodney Breau, Christina Canil, Charles Catton, Urban Emmenegger, John Hastie, Sébastien Hotte, Himu Lukka, Scott Morgan, Bobby Shayegan, Tom Short, John Srigley, Padraig Warde, Eric Winquist) for their comments on the early draft of this project.
Footnotes
Competing interests: The Program in Evidence-based Care (PEBC) is supported by the Ontario Ministry of Health and Long-Term Care through Cancer Care Ontario. All work produced by the PEBC is editorially independent from its funding source. The lead author, George Rodrigues, declared that he published a commentary on intensity-modulated radiotherapy for prostate cancer (Rodrigues G. Is intensity-modulated radiotherapy for prostate cancer ready for prime-time? Can J Urol 2012;19:6381–2). Dr. Loblaw has consulted with, and has received honorarium from GE Healthcare. The other 3 authors declared none.
This paper has been peer-reviewed.
References
- 1.Canadian Cancer Society’s Steering Committee on Cancer Statistics . Canadian Cancer Statistics 2013. Toronto, ON: Canadian Cancer Society; 2013. http://www.cancer.ca/Ontario/About%20cancer/Cancer%20statistics/Stats%20at%20a%20glance/Prostate%20cancer.aspx?sc_lang=en&r=1. Accessed November 14, 2013. [Google Scholar]
- 2.Cancer Research UK UK Cancer Incidence (2010) and Mortality (2010) Summary. http://publications.cancerresearchuk.org/downloads/Product/CS_DT_CASESDEATHS.pdf. Accessed November 14, 2013.
- 3.Crook JLH, Klotz L, Bestic N, et al. Genitourinary Cancer Disease Site Group of the Cancer Care Ontario Practice Guidelines Initiative. Systematic overview of th e evidence for brachytherapy in clinically localized prostate cancer. Can Med Assoc J. 2001;164:975–81. [PMC free article] [PubMed] [Google Scholar]
- 4.Lukka H. Prostate cancer: risk categories and role of hormones and radiotherapy. Can J Urol. 2002;9(Suppl 1):26–9. [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network (NCCN) Clinical Practice Guideline in Oncology Prostate Cancer. 2012. Version 1; http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed November 14, 2013.
- 6.Yang RWJ, Zhang H. Dosimetric study of Cs-131, I-125, and Pd-103 seeds for permanent prostate brachytherapy. Cancer Biother Radiopharm. 2009;24:701–5. doi: 10.1089/cbr.2009.0648. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues G, Yao X, Loblaw A, et al. Genitourinary Cancer Disease Site Group . Low-dose rate brachytherapy for patients with low- or intermediate-risk prostate cancer. Toronto, ON: Cancer Care Ontario; 2012. Program in Evidence-based Care Evidence-Based Series No.: 3–10 Version 2. https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=254196. Accessed November 14, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer guidelines resource centre. Hamilton, ON: 2010. Canadian Partnership Against Cancer. http://shar.es/8hgVl. Accessed November 14, 2013. [Google Scholar]
- 9.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. The Collaboration; 2011. www.cochrane-handbook.org. Accessed November 14, 2013.
- 10.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed November 14, 2013. [Google Scholar]
- 11.Bannuru RR, Dvorak T, Obadan N, et al. Comparative evaluation of radiation treatments for clinically localized prostate cancer: an updated systematic review. Ann Intern Med. 2011;155:171–8. doi: 10.7326/0003-4819-155-3-201108020-00347. [DOI] [PubMed] [Google Scholar]
- 12.Bhatnagar V, Stewart ST, Huynh V, et al. Estimating the risk of long-term erectile, urinary and bowel symptoms resulting from prostate cancer treatment. Prostate Cancer Prostatic Dis. 2006;9:136–46. doi: 10.1038/sj.pcan.4500855. [DOI] [PubMed] [Google Scholar]
- 13.Katz A. Quality of life for men with prostate cancer. Cancer Nurs. 2007;30:302–8. doi: 10.1097/01.NCC.0000281726.87490.f2. [DOI] [PubMed] [Google Scholar]
- 14.Koukourakis GKN, Armonis V, Kouloulias V. Brachytherapy for prostate cancer: a systematic review. Adv Urol. 2009:327945. doi: 10.1155/2009/327945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peinemann F, Grouven U, Bartel C, et al. Permanent interstitial low-dose-rate brachytherapy for patients with localised prostate cancer: A systematic review of randomised and nonrandomised controlled clinical trials. Eur Urol. 2011;60:881–93. doi: 10.1016/j.eururo.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Peinemann F, Grouven U, Hemkens LG, et al. Low-dose rate brachytherapy for men with localized prostate cancer. Cochrane Database Syst Rev. 2011:CD008871. doi: 10.1002/14651858.CD008871.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Pieters BR, de Back DZ, Koning CCE, et al. Comparison of three radiotherapy modalities on biochemical control and overall survival for the treatment of prostate cancer: a systematic review. Radiother Oncol. 2009;93:168–73. doi: 10.1016/j.radonc.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 18.Sahgal A, Roach M., 3rd Permanent prostate seed brachytherapy: a current perspective on the evolution of the technique and its application. Nat Clin Pract Urol. 2007;4:658–70. doi: 10.1038/ncpuro0971. [DOI] [PubMed] [Google Scholar]
- 19.Wilt TJ, MacDonald R, Rutks I, et al. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148:435–48. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- 20.Zhang LL, Ma L, Tian JH, et al. 125I versus 103Pd brachytherapy for low risk prostate cancer: A systematic review. Chin J Cancer. 2009;28:872–8. doi: 10.5732/cjc.008.10378. [DOI] [PubMed] [Google Scholar]
- 21.Abdel-Wahab M, Reis IM, Hamilton K. Second primary cancer after radiotherapy for prostate cancer--a seer analysis of brachytherapy versus external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:58–68. doi: 10.1016/j.ijrobp.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 22.Arvold ND, Chen M-H, Moul JW, et al. Risk of death from prostate cancer after radical prostatectomy or brachytherapy in men with low or intermediate risk disease. J Urol. 2011;186:91–6. doi: 10.1016/j.juro.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Bacon CG, Giovannucci E, Testa M, et al. The impact of cancer treatment on quality of life outcomes for patients with localized prostate cancer. J Urol. 2001;166:1804–10. doi: 10.1016/S0022-5347(05)65679-0. [DOI] [PubMed] [Google Scholar]
- 24.Blasko JC, Grimm PD, Sylsvester JE, et al. The role of external beam radiotherapy with I-125/Pd-103 brachytherapy for prostate carcinoma. Radiother Oncol. 2000;57:273–8. doi: 10.1016/S0167-8140(00)00288-7. [DOI] [PubMed] [Google Scholar]
- 25.Borchers H, Kirschner-Hermanns R, Brehmer B, et al. Permanent 125I-seed brachytherapy or radical prostatectomy: A prospective comparison considering oncological and quality of life results. BJU Int. 2004;94:805–11. doi: 10.1111/j.1464-410X.2004.05037.x. [DOI] [PubMed] [Google Scholar]
- 26.Burdick MJ, Reddy C, Ulchaker J, et al. Comparison of biochemical relapse-free survival between primary Gleason score 3 and primary Gleason score 4 for biopsy Gleason score 7 prostate cancer. Int J Radiat Oncol Biol Phys. 2009;73:1439–45. doi: 10.1016/j.ijrobp.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 27.Buron C, Le Vu B, Cosset J-M, et al. Brachytherapy versus prostatectomy in localized prostate cancer: results of a French multicenter prospective medico-economic study. Int J Radiat Oncol Biol Phys. 2007;67:812–22. doi: 10.1016/j.ijrobp.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Colberg JW, Decker RH, Khan AM, et al. Surgery versus implant for early prostate cancer: results from a single institution, 1992–2005. Cancer J. 2007;13:229–32. doi: 10.1097/PPO.0b013e318046f14e. [DOI] [PubMed] [Google Scholar]
- 29.Crook JM, Gomez-Iturriaga A, Wallace K, et al. Comparison of health-related quality of life 5 years after SPIRIT: Surgical Prostatectomy Versus Interstitial Radiation Intervention Trial. J Clin Oncol. 2011;29:362–8. doi: 10.1200/JCO.2010.31.7305. [DOI] [PubMed] [Google Scholar]
- 30.D’Amico AV, Tempany CM, Schultz D, et al. Comparing PSA outcome after radical prostatectomy or magnetic resonance imaging-guided partial prostatic irradiation in select patients with clinically localized adenocarcinoma of the prostate. Urology. 2003;62:1063–7. doi: 10.1016/S0090-4295(03)00772-6. [DOI] [PubMed] [Google Scholar]
- 31.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 32.Eller LS, Lev EL, Gejerman G, et al. Prospective study of quality of life of patients receiving treatment for prostate cancer. Nurs Res. 2006;55:S28–36. doi: 10.1097/00006199-200603001-00006. [DOI] [PubMed] [Google Scholar]
- 33.Ferrer M, Suarez JF, Guedea F, et al. Health-related quality of life 2 years after treatment with radical prostatectomy, prostate brachytherapy, or external beam radiotherapy in patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72:421–32. doi: 10.1016/j.ijrobp.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 34.Frank SJ, Pisters LL, Davis J, et al. An assessment of quality of life following radical prostatectomy, high dose external beam radiation therapy and brachytherapy iodine implantation as monotherapies for localized prostate cancer. J Urol. 2007;177:2151–6. doi: 10.1016/j.juro.2007.01.134. [DOI] [PubMed] [Google Scholar]
- 35.Gelblum DY, Potters L. Rectal complications associated with transperineal interstitial brachytherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2000;48:119–24. doi: 10.1016/S0360-3016(00)00632-5. [DOI] [PubMed] [Google Scholar]
- 36.Ghaly M, Wallner K, Merrick G, et al. The effect of supplemental beam radiation on prostate brachytherapy-related morbidity: morbidity outcomes from two prospective randomized multicenter trials. Int J Radiat Oncol Biol Phys. 2003;55:1288–93. doi: 10.1016/S0360-3016(02)04527-3. [DOI] [PubMed] [Google Scholar]
- 37.Giberti C, Chiono L, Gallo F, et al. Radical retropubic prostatectomy versus brachytherapy for low-risk prostatic cancer: a prospective study. World J Urol. 2009;27:607–12. doi: 10.1007/s00345-009-0418-9. [DOI] [PubMed] [Google Scholar]
- 38.Guedea F, Ferrer M, Pera J, et al. Quality of life two years after radical prostatectomy, prostate brachytherapy or external beam radiotherapy for clinically localised prostate cancer: the Catalan Institute of Oncology/Bellvitge Hospital experience. Clin Trans Oncol. 2009;11:470–8. doi: 10.1007/s12094-009-0387-x. [DOI] [PubMed] [Google Scholar]
- 39.Herstein A, Wallner K, Merrick G, et al. I-125 versus Pd-103 for low-risk prostate cancer: long-term morbidity outcomes from a prospective randomized multicenter controlled trial. Cancer J. 2005;11:385–9. doi: 10.1097/00130404-200509000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Jabbari S, Weinberg VK, Shinohara K, et al. Equivalent biochemical control and improved prostate-specific antigen nadir after permanent prostate seed implant brachytherapy versus high-dose three-dimensional conformal radiotherapy and high-dose conformal proton beam radiotherapy boost. Int J Radiat Oncol Biol Phys. 2010;76:36–42. doi: 10.1016/j.ijrobp.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 41.Kirschner-Hermanns R, Brehmer B, Borchers H, et al. Do patients with urodynamically proven infravesical obstruction and detrusor overactivity have a higher risk for long-term bothersome symptoms after brachytherapy in comparison to patients treated with radical prostatectomy for localized prostate cancer? Curr Urol. 2008;2:135–41. doi: 10.1159/000189654. [DOI] [Google Scholar]
- 42.Klein EA, Ciezki J, Kupelian PA, et al. Outcomes for intermediate risk prostate cancer: are there advantages for surgery, external radiation, or brachytherapy? Urol Oncol. 2009;27:67–71. doi: 10.1016/j.urolonc.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Kupelian PA, Potters L, Khuntia D, et al. Radical prostatectomy, external beam radiotherapy <72 Gy, external beam radiotherapy > or =72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1–T2 prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58:25–33. doi: 10.1016/S0360-3016(03)00784-3. [DOI] [PubMed] [Google Scholar]
- 44.Lev EL, Eller LS, Gejerman G, et al. Quality of life of men treated for localized prostate cancer: outcomes at 6 and 12 months. Support Care Cancer. 2009;17:509–17. doi: 10.1007/s00520-008-0493-2. [DOI] [PubMed] [Google Scholar]
- 45.Litwin MS, Sadetsky N, Pasta DJ, et al. Bowel function and bother after treatment for early stage prostate cancer: a longitudinal quality of life analysis from CaPSURE. J Urol. 2004;172:515–9. doi: 10.1097/01.ju.0000129236.56712.e7. [DOI] [PubMed] [Google Scholar]
- 46.Malcolm JB, Fabrizio MD, Barone BB, et al. Quality of life after open or robotic prostatectomy, cryoablation or brachytherapy for localized prostate cancer. J Urol. 2010;183:1822–8. doi: 10.1016/j.juro.2009.12.102. [DOI] [PubMed] [Google Scholar]
- 47.Merrick GS, Butler WM, Wallner KE, et al. Dosimetry of an extracapsular anulus following permanent prostate brachytherapy. Am J Clin Oncol. 2007;30:228–33. doi: 10.1097/01.coc.0000258110.11024.c4. [DOI] [PubMed] [Google Scholar]
- 48.Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23:2772–80. doi: 10.1200/JCO.2005.07.116. [DOI] [PubMed] [Google Scholar]
- 49.Namiki S, Satoh T, Baba S, et al. Quality of life after brachytherapy or radical prostatectomy for localized prostate cancer: a prospective longitudinal study. Urology. 2006;68:1230–6. doi: 10.1016/j.urology.2006.08.1093. [DOI] [PubMed] [Google Scholar]
- 50.Ojha RP, Fischbach LA, Zhou Y, et al. Acute myeloid leukemia incidence following radiation therapy for localized or locally advanced prostate adenocarcinoma. Cancer Epidemiol. 2010;34:274–8. doi: 10.1016/j.canep.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 51.Pardo Y, Guedea F, Aguilo F, et al. Quality-of-life impact of primary treatments for localized prostate cancer in patients without hormonal treatment. J Clin Oncol. 2010;28:4687–96. doi: 10.1200/JCO.2009.25.3245. [DOI] [PubMed] [Google Scholar]
- 52.Pinkawa M, Asadpour B, Piroth MD, et al. Health-related quality of life after permanent I-125 brachytherapy and conformal external beam radiotherapy for prostate cancer--a matched-pair comparison. Radiother Oncol. 2009;91:225–31. doi: 10.1016/j.radonc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Potters L, Klein EA, Kattan MW, et al. Monotherapy for stage T1–T2 prostate cancer: radical prostatectomy, external beam radiotherapy, or permanent seed implantation. Radiother Oncol. 2004;71:29–33. doi: 10.1016/j.radonc.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 55.Schover LR, Fouladi RT, Warneke CL, et al. Defining sexual outcomes after treatment for localized prostate carcinoma. Cancer. 2002;95:1773–85. doi: 10.1002/cncr.10848. [DOI] [PubMed] [Google Scholar]
- 56.Sharkey J, Cantor A, Solc Z, et al. Brachytherapy versus radical prostatectomy in patients with clinically localized prostate cancer. Curr Urol Rep. 2002;3:250–7. doi: 10.1007/BF03200421. [DOI] [PubMed] [Google Scholar]
- 57.Sharkey J, Cantor A, Solc Z, et al. 103Pd brachytherapy versus radical prostatectomy in patients with clinically localized prostate cancer: a 12-year experience from a single group practice. Brachytherapy. 2005;4:34–44. doi: 10.1016/j.brachy.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 58.Sherertz T, Wallner K, Merrick G, et al. Factors predictive of rectal bleeding after 103Pd and supplemental beam radiation for prostate cancer. Brachytherapy. 2004;3:130–5. doi: 10.1016/j.brachy.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Smith DP, King MT, Egger S, et al. Quality of life three years after diagnosis of localised prostate cancer: population based cohort study. BMJ. 2009;339:b4817. doi: 10.1136/bmj.b4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soderdahl DW, Davis JW, Schellhammer PF, et al. Prospective longitudinal comparative study of health-related quality of life in patients undergoing invasive treatments for localized prostate cancer. J Endourol. 2005;19:318–26. doi: 10.1089/end.2005.19.318. [DOI] [PubMed] [Google Scholar]
- 61.Speight JL, Elkin EP, Pasta DJ, et al. Longitudinal assessment of changes in sexual function and bother in patients treated with external beam radiotherapy or brachytherapy, with and without neoadjuvant androgen ablation: data from CaPSURE. Int J Radiat Oncol Biol Phys. 2004;60:1066–75. doi: 10.1016/j.ijrobp.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 62.Steenland K, Goodman M, Liff J, et al. Quality of life among men with prostate cancer in rural Georgia. Urology. 2011;77:927–33. doi: 10.1016/j.urology.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 63.Stock RG, Klein TJ, Cesaretti JA, et al. Prognostic significance of 5-year PSA value for predicting prostate cancer recurrence after brachytherapy alone and combined with hormonal therapy and/or external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2009;74:753–8. doi: 10.1016/j.ijrobp.2008.08.049. [DOI] [PubMed] [Google Scholar]
- 64.Talcott JA, Manola J, Clark JA, et al. Time course and predictors of symptoms after primary prostate cancer therapy. J Clin Oncol. 2003;21:3979–86. doi: 10.1200/JCO.2003.01.199. [DOI] [PubMed] [Google Scholar]
- 65.Tward JD, Lee CM, Pappas LM, et al. Survival of men with clinically localized prostate cancer treated with prostatectomy, brachytherapy, or no definitive treatment: Impact of age at diagnosis. Cancer. 2006;107:2392–400. doi: 10.1002/cncr.22261. [DOI] [PubMed] [Google Scholar]
- 66.Vassil AD, Murphy ES, Reddy CA, et al. Five year biochemical recurrence free survival for intermediate risk prostate cancer after radical prostatectomy, external beam radiation therapy or permanent seed implantation. Urology. 2010;76:1251–7. doi: 10.1016/j.urology.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 67.Wallner K, Merrick G, True L, et al. I-125 versus Pd-103 for low-risk prostate cancer: morbidity outcomes from a prospective randomized multicenter trial. Cancer J. 2002;8:67–73. doi: 10.1097/00130404-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 68.Wallner K, Merrick G, True L, et al. Iodine-125 vs palladium-103 for low-risk prostate cancer: Preliminary urinary functional outcomes from a prospective randomized multicenter trial. J Brachytherapy Int. 2000;16:151–5. [Google Scholar]
- 69.Wallner K, Merrick G, True L, et al. 20 Gy versus 44 Gy supplemental beam radiation with Pd-103 prostate brachytherapy: preliminary biochemical outcomes from a prospective randomized multi-center trial. Radiother Oncol. 2005;75:307–10. doi: 10.1016/j.radonc.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 70.Wallner K, Merrick G, True L, et al. 125I versus 103Pd for low-risk prostate cancer: Preliminary PSA outcomes from a prospective randomized multicenter trial. Int J Radiat Oncol Biol Phys. 2003;57:1297–303. doi: 10.1016/S0360-3016(03)01448-2. [DOI] [PubMed] [Google Scholar]
- 71.Wei JT, Dunn RL, Sandler HM, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20:557–66. doi: 10.1200/JCO.20.2.557. [DOI] [PubMed] [Google Scholar]
- 72.Wong WW, Vora SA, Schild SE, et al. Radiation dose escalation for localized prostate cancer: intensity-modulated radiotherapy versus permanent transperineal brachytherapy. Cancer. 2009;115:5596–606. doi: 10.1002/cncr.24558. [DOI] [PubMed] [Google Scholar]
- 73.Wu AK, Cooperberg MR, Sadetsky N, et al. Health related quality of life in patients treated with multimodal therapy for prostate cancer. J Urol. 2008;180:2415–22. doi: 10.1016/j.juro.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 74.Zelefsky MJ, Yamada Y, Pei X, et al. Comparison of tumour control and toxicity outcomes of high-dose intensity-modulated radiotherapy and brachytherapy for patients with favorable risk prostate cancer. Urology. 2011;77:986–90. doi: 10.1016/j.urology.2010.07.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou EH, Ellis RJ, Cherullo E, et al. Radiotherapy and Survival in Prostate Cancer Patients: A Population-Based Study. Int J Radiat Oncol Biol Phys. 2009;73:15–23. doi: 10.1016/j.ijrobp.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Survival rates for prostate cancer. American Cancer Society; 2013. http://www.cancer.org/Cancer/ProstateCancer/OverviewGuide/prostate-cancer-overview-survival-rates. Accessed November 13, 2013.
- 77.Pickett B, Ten Haken RK, Kurhanewicz J, et al. Time to metabolic atrophy after permanent prostate seed implantation based on magnetic resonance spectroscopic imaging. Int J Radiat Oncol Biol Phys. 2004;59:665–73. doi: 10.1016/j.ijrobp.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 78.Thompson A, Keyes M, Pickles T, et al. Evaluating the Phoenix definition of biochemical failure after (125) I prostate brachytherapy: Can PSA kinetics distinguish PSA failures from PSA bounces? Int J Radiat Oncol Biol Phys. 2010;78:415–21. doi: 10.1016/j.ijrobp.2009.07.1724. [DOI] [PubMed] [Google Scholar]
- 79.Wilt TJ. Can randomized treatment trials in early stage prostate cancer be completed? Clin Oncol. 1998;10:141–3. doi: 10.1016/S0936-6555(98)80052-6. [DOI] [PubMed] [Google Scholar]
- 80.Consensus statement: guidelines for PSA following radiation therapy. American Society for Therapeutic Radiology and Oncology Consensus Panel. Int J Radiat Oncol Biol Phys. 1997;37:1035–41. [PubMed] [Google Scholar]
- 81.Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–74. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 82.Pickles T, Keyes M, Morris WJ. Brachytherapy or conformal external radiotherapy for prostate cancer: a single-institution matched-pair analysis. Int J Radiat Oncol Biol Phys. 2010;76:43–9. doi: 10.1016/j.ijrobp.2009.01.081. [DOI] [PubMed] [Google Scholar]
- 83.Grimm P, Billiet I, Bostwick D, et al. Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int. 2012;109(Suppl 1):22–9. doi: 10.1111/j.1464-410X.2011.10827.x. [DOI] [PubMed] [Google Scholar]