Abstract
Donors after cardiac death (DCD) could increase the organ pool. Data supports good long-term renal graft survival. However, DCDs are <10% of deceased donors in the United States, due to delayed graft function, and primary nonfunction. These complications are minimized by extracorporeal support after cardiac death (ECS-DCD). This study assesses immediate and acute renal function from different donor types. DCDs kidneys were recovered by conventional rapid recovery or by ECS, and transplanted into nephrectomized healthy swine. Warm ischemia of 10 and 30 min were evaluated. Swine living donors were controls (LVD). ECS-DCDs were treated with 90 min of perfusion until organ recovery. After procurement, kidneys were cold storage 4-6 h. Renal vascular resistance (RVR), urine output (UO), urine protein concentration (UrPr) and creatinine clearance (CrCl), were collected during 4 h posttransplantation. All grafts functioned with adequate renal blood flow for 4 h. RVR at 4 h posttransplant returned to baseline only in the LVD group (0.36 mmHg/mL/min ± 0.03). RVR was higher in all DCDs (0.66 mmHg/mL/min ± 0.13), without differences between them. UO was >50 mL/h in all DCDs, except in DCD-30 (6.8 mL/h ± 1.7). DCD-30 had lower CrCl (0.9 mL/min ± 0.2) and higher UrPr >200 mg/dL, compared to other DCDs >10 mL/min and <160 mg/dL, respectively. Normothermic ECS can resuscitate kidneys to transplantable status after 30 min of cardiac arrest/WI.
Keywords: donor pool, donor preconditioning, early graft function, experimental models, extracorporeal membrane oxygenation, graft function, injury and preservation, ischemia time, kidney graft function, kidney, large animal model, nonbeating heart donor, normothermic recirculation, organ and tissue procurement, organ storage
End-stage renal disease is treated successfully with kidney transplantation; however this therapeutic option is limited due the shortage of organs available for transplantation. The waiting list for kidney transplants in the US reached more than 82,500 patients in 2008, despite a 30% increase in the number of renal transplants performed in 2007 (16,628) compared to 10 years ago (11,703). In 2007, the vast majority of transplanted renal grafts were from donors after neurologic determination of death (DND), or brain dead donors, with a significant portion (6,041) from living donors. (1)
The use of organs recovered from donors following circulatory determination of death (DCD)--previously known as non-heart beating donors--has the potential to increase the donor pool; but currently accounts for less than 8% of all organ transplants. (2) The reason is that organs taken by “rapid recovery” from DCD donors do not function as well as organs from DND, especially initially. Poor immediate graft function can be tolerated for renal transplants because of renal replacement therapies like dialysis, so almost all organs from DCD are kidneys. The incidence of delayed graft function and primary graft non-function for kidneys from DCD is significantly higher than kidneys from DND, in some series more than double. (3, 4) This is presumed to be due to the warm ischemic and hypoxic injury that inevitably occurs following withdrawal of life support until the organs are cold perfused.
The use of normothermic veno-arterial extracorporeal membrane oxygenation support (hereafter called ECS) after cardiac arrest restores circulation of warm oxygenated blood to the abdominal organs. It can be initiated immediately following declaration of death. Experimental data from our lab has shown that kidney and liver function returns and is maintained while on ECS (5). In animals, perfusing with warm oxygenated blood has been shown experimentally to increase the energy charge (ADP) and antioxidant levels in the recovered organs. (6, 7) By restarting circulation after cardiac arrest, the agonal, hypoxic and ischemic events surrounding death and subsequent reperfusion can be turned into an ischemic preconditioning phenomenon. (8)
In our clinical experience the donor pool was expanded by 33% by establishing normothermic, oxygenated blood perfusion of abdominal organs using ECS shortly after circulatory determination of death. Moreover it allowed for controlled, unhurried organ procurement; delayed graft function was developed in only two grafts (8%). (9)
The use of DCD grafts is encouraged in all the US transplant programs, but there is hesitation among clinical personnel due to uncertainty about the immediate and long term outcomes of these grafts, especially when the organs come from DCD donors that also fit criteria for expanded criteria donors (ECD). These donors are over 60 years of age or over 50 years of age with a history of hypertension, poor renal function at the time of recover, or death due to cerebrovascular accident. While ECS-DCD offers potentially improved results, it is also perceived as costly. However, if ECS-DCD can improve initial graft function, the cost of ECS is offset by the reduced hospital stay and need for dialysis if the kidneys function immediately. We developed an animal study to assess the immediate renal graft function from DCD donors under various warm ischemia times. This study was designed to evaluate ECS compared to rapid recovery in a swine model of renal transplantation after DCD.
Material and Methods
This study was approved by the University of Michigan University Committee on Use and Care of Animals (UCUCA). All pigs received humane in compliance with the Guide for the Care and Use of Laboratory Animals.
Experimental design
Using a standard swine model of cardiac death and warm ischemia, kidneys were removed by conventional rapid recovery or ECS, stored for 4-5 hours cold ischemia, transplanted into nephrectomized swine recipients. Warm ischemia times of 10 and 30 minutes were compared. Kidneys transplanted from living donors (LVD) served as a control group. 5 groups of 5 animals each were compared as shown in Table 1.
Table 1.
Donor Type and Characteristics
| Donor Type | Group Name | Warm Ischemia | Procurement Technique | Cold Ischenria | (n) |
|---|---|---|---|---|---|
| Living Donor | LVD | < 1 min | Standard | 4-5 hours | 5 |
| DCD | DCD 10min | 10 min | Rapid recovery | 4-5 hours | 5 |
| DCD 30mm | 30 mm | Rapid recovery | 4-5 hours | 5 | |
| ECS-DCD | ECS-DCD 10min | 10 mm | 90min ECS | 4-5 hours | 5 |
| ECS-DCD 30min | 30 min | 90min ECS | 4-5 hours | 5 |
LVD: Living donors; DCD: Donors after cardiac death; ECMO: Extracorporeal Membrane Oxygenation
Animal model
The following model was used in all experiments. Female swine (weighing 25-30 kg), were sedated with an intramuscular (i.m) mix of 5mg/kg Tiletamine HCl and Zolepam HCl (Telazol® Wyeth Holdings Corporation; Carolina, Puerto Rico) and 3mg/kg Xylazine (TranquiVed™ Vedco Inc; St. Joseph, MO). Swine were intubated and mechanical ventilated (MV) with 100% O2 and 1-3% Isofluorane (Hospira, INC; Lake Forest, IL). Initial MV settings were adjusted to maintain pCO2 between 35-45mmHg, and peak inspiratory pressures <25cmH2O. The right carotid artery and right internal jugular vein were catheterized to monitor arterial blood pressure and heart rate and to collect blood samples. A CCOmbo-CCO/SvO2/VIP pulmonary artery catheter (Edwards Lifesciences LLC; Irvine, CA) was placed via the internal jugular vein for administration of fluids and monitoring of cardiac output and central venous pressure. At the end of surgical instrumentation and prior to baseline data collection, a 20 minute acclimation period was allowed to all animals.
Donor models
(LVD) model: 100U/kg Heparin Sodium (APP Pharmaceuticals, LLC; Schaumburg, IL) was administered 5min before proximal ligation of the renal artery. The kidneys were subsequently resected, flushed with 300-500mL of Custodiol® (Methapharm Inc, BRANTFORD, ON. Canada), and stored cold for 4-5 hours before transplantation.
- DCD model: Anesthetized pigs were paralyzed using Pancuronium Bromide (Hospira, INC; Lake Forest, IL) and cardiac death was achieved by apnea. The agonal period was 17±1.8min, simulating at some extent clinical reality. Circulatory death was defined as: asystole or pulse-less arrhythmia with a pulse pressure less than 15mmHg. After death, warm ischemia (WI)/asystole times of 10 and 30 minutes were examined. These times are two and six times longer than the 5 minute period that is frequently used in the US as a “no touch” period. To achieve anticoagulation, heparin (100U/kg) was administered one minute after withdrawal of respiratory support. Kidneys were then recovered in one of the following ways.
- Rapid recovery/Conventional: After death, a midline laparotomy was performed to obtain access to the kidneys. The vessels were identified and surrounding soft tissue was dissected. After a period of 10 or 30 minutes of warm ischemia, the pig was cooled via ice in the abdominal cavity, followed by rapid removal of the renal grafts. Grafts were managed as in the LVD protocol with immediate cold perfusion and storage.
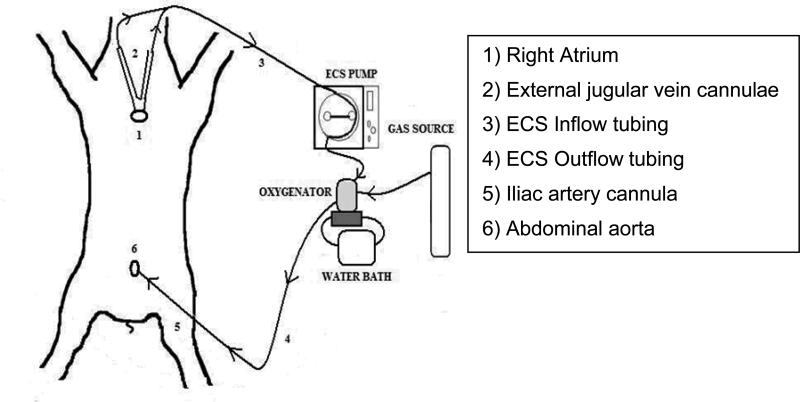
- ECS procurement: After death, both external jugular veins were cannulated with two 20-23Fr venous cannulae (to obtain access to the RA), and a 14-16Fr arterial cannula was advanced into the abdominal aorta via right iliac or femoral artery. The V-A ECS circuit animal model is represented in Figure 1, include: a roller pump (Cobe Cardiovascular®; Lakewood, CO), an external heat-exchanger (Seabrook Medical System Inc; Cincinnati, OH), and a membrane oxygenator (Affinity® NT, Medtronic Inc.; Minneapolis, MN; Rochester, NY) and then stepped up to 3/8” tubing to connect them to the oxygenator outlet. Pump flows were continuously monitored using a T208 monitor (Transonic System Inc; Ithaca, NY). The membrane oxygenator was primed with saline and 50mEq of HCO3 and maintained at 38°C. After 10 or 30 minutes of WI veno-arterial perfusion was begun and maintained at 50cc/kg/min for 90-100 minutes, aiming to maintain a CVP between 7-16cmH2O during ECS, avoiding cavitation or hemodilution (targeting hematocrit value >23%). The kidneys were removed while warm perfusion continued, and managed as in the LVD protocol with immediate cold perfusion and similar storage time.
Figure 1.
ECS-DCD Laboratory Model
Recipient Model
Healthy swine underwent surgical instrumentation as described above plus bilateral nephrectomies. After removal of one kidney, a perivascular renal (Transonic System Inc; Ithaca, NY) flow probe was placed to obtain measurement of normal renal blood flow to the contralateral kidney for baseline. A blood sample was collected, and urine output was quantified and collected for determination of creatinine and protein concentration. The donor kidney was implanted and the remaining kidney was removed. The inferior vena cava and infrarenal abdominal aorta were prepared for side-to-end vascular anastomosis of the experimental renal graft. Heparin (100U/kg) and 125mg of methylprednisolone (Solumedrol®, Pharmacia & Upjohn Co; New York, NY) were administered 5 minutes before surgical anastomosis. A catheter was advanced in the ureter for urine output collection. The pig remained under anesthesia for the entire experiment. For all recipients, maintenance i.v fluid was 120-150ml/hr, plus 1:1 replacement of urine output with NSS, maintaining CVP between 12±3cmH2O.
Data acquisition
Data collected during the experiment are summarized in Table 2. Baseline (BL) data was collected on the single recipient kidney after surgical instrumentation but before implantation of renal grafts.
Table 2.
Data Acquisition
| Variable Type | Freqaeacy | Description |
|---|---|---|
| Renal Hemodynamics | Recorded every 30min after transplantation | MAP: mean arterial pressure; CVF: central venous pressure; RAF: renal artery flow; RVR: renal vascular resistance |
| Renal Function | Baseline and every 1hr after transplantation | Urine output (UO), Urine Protein concentration (UrPr) |
| Venous & Arterial Blood Gases | Baseline, end of CA, every 1hr after transplantation | Blood pH, pCO2, pO2 hemoglobin, hematocrit, oxyhemoglobin saturation, electrolytes (Na, K, Ca, CI and HCO3)** |
| Chemistry panel | Baseline; immediately after reperfusion, and 4hr after transplantation. | Plasma creatinine, BUN, and ADL; urine creatinine, BUN, and protein. *** |
*BIOPAC Systems Inc; Goleta, CA;
Raliometer A/S; Copenhagen, NV Denmark
Animal Diagnostic Lab of the U.
Data analysis
Renal vascular resistance (RVR) was calculated using the following equation: RVR= (MAP-CVP) / RAF. Creatinine clearance (mL/min) was calculated using the following equation: CrCl= ([U] × (UO/60)) / [P], in which [U] = urine concentration of creatinine, [P] = plasma concentration of creatinine, and UO = urine output in mL/hr. Urine was collected continuously from the ureter, during the total length of the study (4hr). This measurement is not affected by serum concentration of creatinine because it did not change significantly during the collection period. A mixed model analysis was performed within SPSS 17.0 (Chicago, IL) to examine the effect of procurement technique on all acquired data. The pig/experiment number is the repeated measure variable, and the independent variables were the experimental group, and the experimental time. The dependant variables were recipient hemodynamics (MAP, CVP), renal hemodynamics (flows, resistance), UO, CrCl and urine protein concentration. Lastly, post-hoc analysis using a Bonferroni-corrected confidence interval was used to determine differences between experimental groups. Values of p<0.05 were considered statistically significant. Results are expressed as mean values with errors bars representing standard error. At the end of the study, the entire kidney was removed for histopathology; two, 2cm × 2cm tissue samples were placed in formalin. The histo-slides were processed by the University of Michigan Unit for Laboratory Animal Medicine, and read by a blinded pathologist.
Results
All grafts were successfully transplanted into each healthy but nephrectomized swine recipient. Average cold storage times per group and ECS-DCD perfusion characteristics are summarized in Table 3.
Table 3.
Graft Cold Ischemin Time and DCD-ECS Run Characteristics
| Average Graft Cold Ischemin Time per Donor Group m inutes | ||||
|---|---|---|---|---|
| LVD | DCD 10min | DCD 30min | ECS-DCD 10min | ECS-DCD 30min |
| 288.7±11.8 | 266.0±18.9 | 262.6±119 | 263.0±11.9 | 265.8±10.7 |
| DCD-ECS run Characteristics (n=3) | |||||
|---|---|---|---|---|---|
| ECS-DCD group | Baselie | 30min ECS | 60min ECS | 90min ECS | |
| MAP (mmHg) | 10min | 67.3±5.7 | 48.3±62 | 70.3±13.6 | 76.3±95 |
| 30min | 76.3±5.0 | 41.6±8.4 | 46.0 ±7.5 | 51.0±10.6 | |
| ECS flows (L/min) | 10min | N/A | 1.6±0.5 | 1.6±0.5 | 1.7±0.4 |
| 30min | N/A | 1.6±0.1 | 1.75±0.01 | 1.7±02 | |
| CVP (cmH2O) | 10min | 13.0±1.8 | 12.7±2.8 | 9.2±3.6 | 9.1±33 |
| 30min | 10.4±0.5 | 10.1±4.4 | 11.1±32 | 8.3±2.0 | |
| Hematocrit (%) | 10min | 31.6±1.5 | 28.4±2.0 | 32.4±1.8 | 34.8±3.3 |
| 30min | 33.8±0.6 | 30.1±0.6 | 24.6±15 | 28.4±2.2 | |
Recipients Systemic Hemodynamics
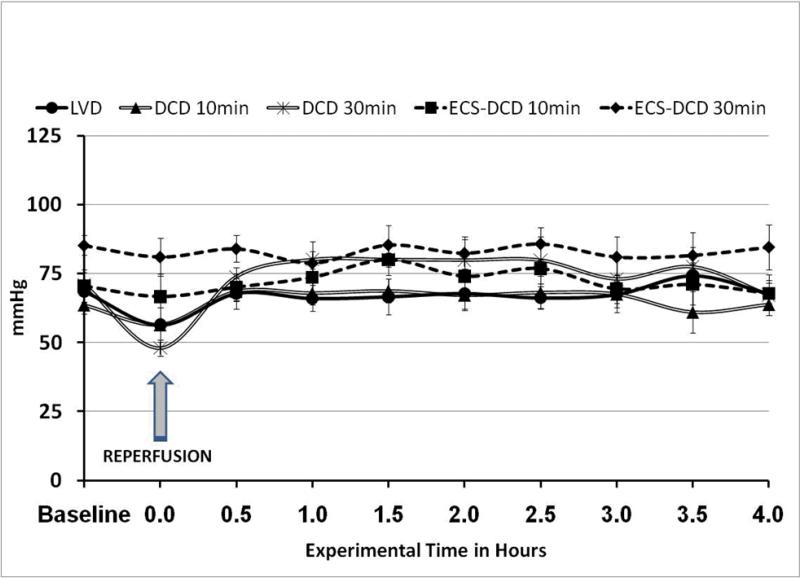
In all experimental groups, mean arterial pressure (MAP) was kept between 60-90mmHg as shown in Figure 2; and CVP was maintained between 12±3cmH2O during the whole experimental time in all groups. In most cases, MAP was maintained at baseline levels. However, MAP decreased significantly (p <0.05) immediately after reperfusion in the DCD 30min group. This decrease in MAP resolved after approximately five minutes of reperfusion and returned to baseline values by the 30min data point. After this point there were not significant differences between groups.
Figure 2.
Mean arterial pressure during the length of the study was in all groups between 60-90mmHg, normal values for a healthy swine under anesthesia.
Renal Hemodynamics and Function
Renal artery flow (RAF) following transplantation was higher at the end of the experiment in the group that received grafts from LVD (p<0.05) compared to all DCD groups (Figure 3). RAF was lower only in the DCD-10min group through all the experimental time, compared to other DCD groups.
Figure 3.
Renal artery blood flow (RAF). All grafts had adequate renal flows after reperfusion. RAF returned back to normal values in the control (LVD) group (p<0.05).
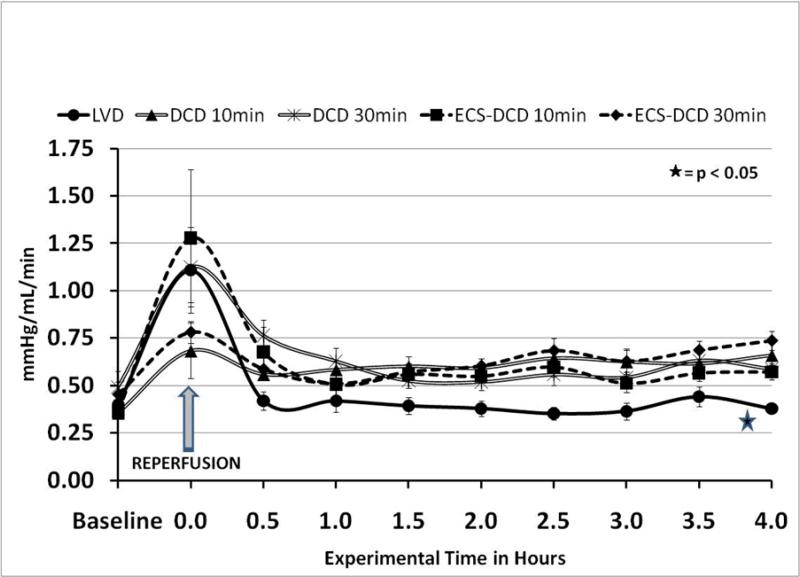
As expected renal vascular resistance (RVR) Figure 4, correlated with RAF. It increased significantly immediately after reperfusion in all groups due to cold preservation, and returned to normal only in the LVD group (p<0.05). In the other four groups, RVR was slightly higher than normal, baseline values during the whole experiment, without significant differences between them.
Figure 4.
Renal vascular resistance (RVR). RVR were back to normal in the control (LVD) group. All DCD's had significant higher RVR, correlating with the extent of warm ischemia time, but no significant differences between themwere observed.
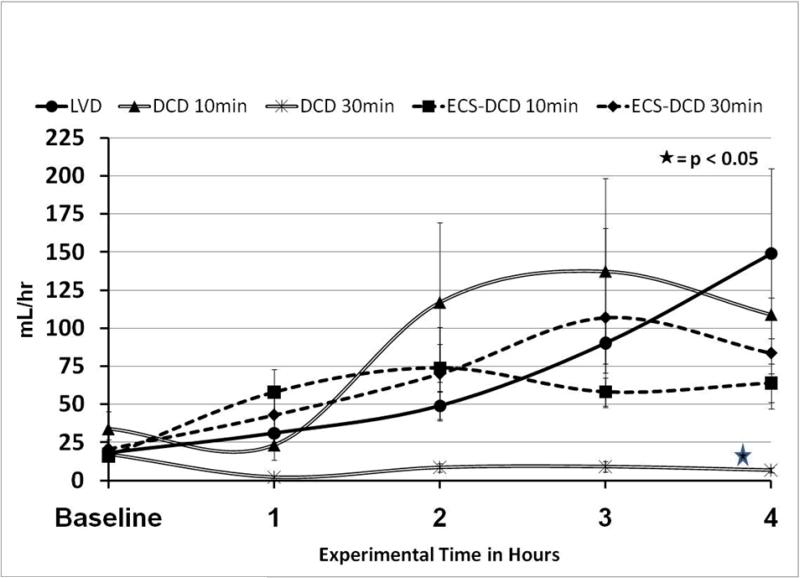
Urine Output is represented in Figure 4. Although all groups had some urine output, the amount produced in the DCD-30min group was minimal (6.8±1.7 mL/hr), and significantly less than in the other groups which all had urine output of more than 50mL/hr by the fourth hour.
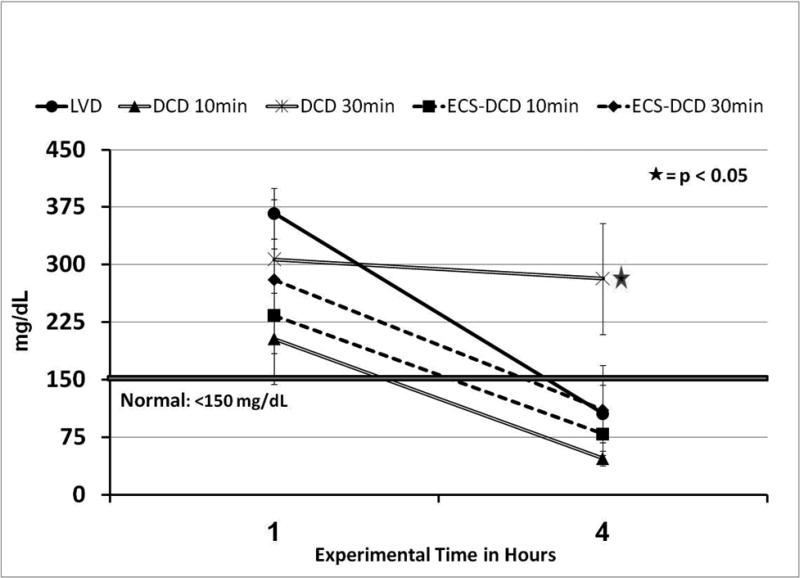
Creatinine Clearance (CrCl) is represented in Figure 6: CrCl was significantly lower (p<0.05) in the DCD-30 group 0.9±0.2 mL/min, compared to the other groups after 1hr post-transplant. Only grafts from the LVD group returned back to normal values (~ 25ml/min) after 4hr of reperfusion. The DCD-30min group had significantly lower CrCl at 1 and 4 hr post-transplantation, compared to all the groups. In particular, when ECS support was used, after 30min of warm ischemia (ECS-DCD 30min), the CrCl was similar to those DCD that sustained only 10min of warm ischemia.
Figure 6.
Measurement of Creatinine Clearance (CrCl). CrCl was calculated using the following equation: CrCl= ([U] × (UO/60)) / [P], in which [U] = urine concentration of creatinine, [P] = plasma concentration of creatinine, and UO = urine output in mL/hr. CrCl was significant lower in the DCD-30min group 0.9±0.2 mL/min, compared to the other groups after 1hr post transplant. Only grafts from the control (LVD) group returned back to normal values. Despite same warm ischemia time (30min) the ECS-DCD-30min group had higher CrCl than the one in which ECS was not used, and the values were similar to the 10min WI group.
Urine protein concentration (UrPr) at one and four hours following reperfusion is represented in Figure 7. UrPr was significant higher (p<0.05) in the DCD 30min group compared to all other groups where normal values for healthy swine of this size were evident at the end of the experimental time.
Figure 7.
Urine Protein Concentration (UrPr). UrPr was significant higher (p<0.05) in the DCD-30min group compared to all other groups where normal values for healthy swine of this size were evident at the end of the experimental time.
Renal Pathology
A summary of the pathologic findings from renal tissue collected at the end of the experiment can be found in Figure 8. Grafts from DCD 30min shown some signs of reversible acute tubular necrosis. No infarcts were seen.
Discussion
The increasing gap between organ demand and organ sources has led the medical community to go back to the way that grafts were procured in the early days of transplantation through the use of grafts from DCD. However renal grafts from DCD are associated with higher rates of delayed-graft function, primary graft-non-function and, in some series, long-term graft survival. (10-12)
After procurement with conventional techniques (rapid recovery) renal grafts had different outcomes directly related to the extent of warm ischemia time. In 1995, Chang et al (13),reported 26% of PGNF (excluding rejection) when organs from non-heart beating donors (NHBD) were used. More recently the rates of PGNF in renal grafts are: LVD 2%, brain dead/heart beating donors 3%, and NHBD 7%. The rate of DGF after rapid recovery is 40-50%. The rates of DGF in kidneys from heart-beating donors is 25-30%, significant lower compared to DCD organs. (4, 11, 14, 15) Despite higher rates of DGF reported when kidneys from DCD are used the long term survival rates some reports show graft survival that is similar to heart-beating donors at 2, 4 and 6 years. (16-24)
Due to the poor outcomes when DCD grafts were used, ex-vivo perfusion with a cold acellular solution is often used to measure renal vascular resistance in the donor kidney. This method allows transplant centers to identify grafts with elevated resistance and these kidneys were simply discarded. When implemented, the rates of PGNF were reduced to as little as 5%.(25) The use of this technique became standard in many DCD programs. (26-28)
Hypothermic extracorporeal support has been used in association with rapid recovery. Koyoma et al used cardiopulmonary bypass at 18°C and reported high rate of DGF in kidneys subjected to long periods of warm ischemia (29 out of 32 kidneys) but similar long term survival rates compared to other type of donors. (29) The transplant group from The National Taiwan University Hospital, used VA perfusion with oxygenation (ECS) at 4°C. They reported 66% of immediate graft function, and 33% incidence of DGF that resolved with short term (1-2weeks) hemodialysis therapy.(30-32). A group from Japan used rapid cooling techniques to procure organs from DCD obtaining similar results. (12, 20, 32, 33)
A transplant group in Spain has reported using normothermic ECMO perfusion to support the potential donor until organ procurement. (34) In 2000, Valero et al, reported significantly lower rates of DGF (13%) and no PGNF in kidneys from DCD when ECMO was used during organ donation in DCD (21) Our group at the University of Michigan Hospital, uses normothermic ECMO in DCD Maastricht Type II and IV donors and reported low rates of DGF (8%), no PGNF, and an increment of the organ pool at our institution by 33%. (9, 21, 35)
Almost all the studies on DCD kidneys are based on 5min of warm ischemia after cardiac arrest. This study evaluated the effectiveness of ECS during organ procurement after longer periods of cardiac arrest/warm ischemia time. The results indicate that implementation of ECS in the donor during organ procurement can resuscitate kidneys to transplantable state with immediate graft function after 30min of warm ischemia. The use of standard rapid recovery technique which is the most common method to procured DCD organs effectively resuscitated renal grafts after 10min of warm ischemia, but not after 30mins. ECS may be useful in situations where prolonged ischemia is probable including Maastricht Type 1 and 2 donors (“uncontrolled”) and situations where local practice involves withdrawal of support in a setting other than in the operating suite. We used 37°C (normothermic) perfusion in these experiments because the best clinical results were achieved at normothermia.
In this study, 90-100min of ECS was used to perfuse the donors because it represents our standard clinical and laboratory practice. (5, 9) Current studies in our laboratory assess the effects of longer ECS runs during the procurement of abdominal organs. Despite the difference of warm ischemia (10min and 30min) in the two groups in which ECS was implemented for organ recovery there were no significant differences between ECS perfusion flows between them. Post-reperfusion renal arterial flow was achieved in all transplanted kidneys, indicating no complications during vascular anastomosis, but normal values were only achieved in the LVD group. Renal flow and function, as measured by urine output, urine protein and creatinine clearance, was adequate during the first 4 hours post transplant after 10min of warn ischemia with or without ECS, indicating kidneys could probably be recovered from clinical DCD donors after 10 minutes of warm ischemic arrest. However, in the groups that sustained longer cardiac arrest times (30min), kidney function was established only when ECS was used during organ procurement. ECS helps in the correction of the acidosis before cold ischemia, restores ATP levels, regulates calcium homeostasis, and removes locally (renal) formed free radicals, in the donor and before cold storage. It's possible that ECS plays an important role in the preconditioning of organs before cold storage; this may explains why ECS resuscitated kidneys after 30 minutes of arrest/ischemia.(36) This observation, suggests that ECS has a protective role following a moderately severe ischemic insult, and may allow organs to recover from prolonged warm ischemia injury during donor reperfusion prior to cold preservation/storage.
Limitations of this study are: 1) the swine model of cardiac arrest does not exactly mimic the clinical situation. The model is both an asset and a limitation of this study. Cardiac arrest by apnea in the swine is a very reproducible model which permits evaluation of the variables in a standardized fashion. However many potential uncontrolled DCD subjects will have a prolonged period of attempted resuscitation by CPR and ventilation including many resuscitative drugs. We acknowledge this limitation, but we argue that the details of ECS must be characterized in the apneic arrest model before adding the variables of CPR and donor treatment; 2) The ECS cannulae used for blood inflow into the circuit were placed into the EJV, due to the limited size of the femoral vein in these size pigs; but we believe that the insertion site of the cannulae did not affect the results. Also, an intra-thoracic balloon was not used in these studies, but it will be implemented in our DCD model for further studies with the goal of isolating the brain from the circulation; 3) Grafts function were not evaluated beyond 4 hours. The swine model simulates the agonal period in “controlled” DCD, but is certainly less variable than observed in clinical practice. We did not evaluate graft function beyond 4 hours to avoid the problems of animal recovery and graft rejection. Kidneys which did not function immediately might recover with longer time (as in DGF). Conversely, kidneys which function immediately after transplant will function indefinitely depending on prevention of rejection; 4) CrCl was measured using a 4hr timed collection, not the clinical standard based on a 24hr urine sample collection, but Bloor et al, proposed that when healthy subjects with normal renal function pre-operative (such as the scenario of our study) are used a 4hr CrCl prediction method correlates with CrCl measurement. (37)
In a different model we have reported that room temperature perfusion is equivalent to perfusion at 37 degrees. We have also reported that heparin given 5 minutes after arrest (with CPR) is as effective as heparin before arrest. (38) We also evaluated if pulmonary congestion occurs during ECS with the heart arrested and we described an in-vivo method to assess if lungs are suitable for transplantation from DCD donors following ECS resuscitation. ECS does not cause pulmonary congestion, and lungs retain adequate function for transplantation, and compliance correlated with lung function.(39) Our results indicated that ECS resuscitation of DCD kidneys is feasible and allows for assessment of function prior to procurement by quantification of UO and urine components in the donor. Future studies include the identification of the maximal cardiac arrest/ warm ischemia time in which organs from DCD can be successfully resuscitated with ECS, as well as the optimization of the ECS perfusion (temperature, diuretics, n-acetylcysteine, thrombolytic agents, etc) with the goal to minimize the ischemic reperfusion injury of DCD abdominal grafts, and/or the addition of leukocyte depletion filters to the ECS circuit. Finally, this model also creates the opportunity to improve DCD-ECS organ recovery runs with the goal to increase the ratio of functional organs per donor, including liver, lung and pancreas donation in DCD.
Conclusions
1. Kidneys may be successfully recovered from DCD donors after 10 minutes of arrest/warm ischemia (but not after 30 minutes); 2. The use of normothermic veno-arterial perfusion of oxygenated blood (ECS) can resuscitate kidneys to transplantable status after 30 minutes of warm ischemia in a large animal model of DCD organ donation; 3. This study adds supporting physiologic data from a large animal model to the clinical data in human DCD donors that ECS improves post-transplant outcomes.
Figure 5.
Urine Output (UO). Renal grafts from the DCD-30min group produce almost no urine (6.8±1.7mL/hr) through the whole experimental time, significantly less than all other groups (>50mL/hr).
Acknowledgment
Supported by the Division of Transplantation at the University of Michigan.
Research Supported by NIH: RO1HL 069420
References
- 1.UNOS TOPaTNa [2008 June 12th];OPTN. Donors Recovered in the U.S. by Donor Type. Available at http://www.optn.org/latestData/rptData.asp. 2008; Available from.
- 2.(IOM) IOM . Organ Donation: Opportunities for Action. 2006 ed. The National Academies Press; Washington, D.C: 2006. [Google Scholar]
- 3.Alonso A, Buitron JG, Gomez M, Fernandez Garcia A, Fernandez Rivera C, Oliver J, et al. Short- and long-term results with kidneys from non-heart-beating donors. Transplant Proc. 1997;29(1-2):1378–1380. doi: 10.1016/s0041-1345(96)00714-2. [DOI] [PubMed] [Google Scholar]
- 4.Alonso A, Fernandez-Rivera C, Villaverde P, Oliver J, Cillero S, Lorenzo D, et al. Renal transplantation from non-heart-beating donors: a single-center 10-year experience. Transplant Proc. 2005;37(9):3658–3660. doi: 10.1016/j.transproceed.2005.09.104. [DOI] [PubMed] [Google Scholar]
- 5.Rojas A, Chen L, Bartlett RH, Arenas JD. Assessment of liver function during extracorporeal membrane oxygenation in the non-heart beating donor swine. Transplant Proc. 2004;36(5):1268–1270. doi: 10.1016/j.transproceed.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Valdecasas JC, Tabet J, Valero R, Taura P, Rull R, Garcia F, et al. Liver conditioning after cardiac arrest: the use of normothermic recirculation in an experimental animal model. Transpl Int. 1998;11(6):424–432. doi: 10.1007/s001470050169. [DOI] [PubMed] [Google Scholar]
- 7.Aguilar A, Alvarez-Vijande R, Capdevila S, Alcoberro J, Alcaraz A. Antioxidant patterns (superoxide dismutase, glutathione reductase, and glutathione peroxidase) in kidneys from non-heart beating-donors: experimental study. Transplant Proc. 2007;39(1):249–252. doi: 10.1016/j.transproceed.2006.10.212. [DOI] [PubMed] [Google Scholar]
- 8.Net M, Valero R, Almenara R, Barros P, Capdevila L, Lopez-Boado MA, et al. The effect of normothermic recirculation is mediated by ischemic preconditioning in NHBD liver transplantation. Am J Transplant. 2005;5(10):2385–2392. doi: 10.1111/j.1600-6143.2005.01052.x. [DOI] [PubMed] [Google Scholar]
- 9.Magliocca JF, Magee JC, Rowe SA, Gravel MT, Chenault RH, 2nd, Merion RM, et al. Extracorporeal support for organ donation after cardiac death effectively expands the donor pool. J Trauma. 2005;58(6):1095–1101. doi: 10.1097/01.ta.0000169949.82778.df. discussion 1101-1092. [DOI] [PubMed] [Google Scholar]
- 10.Koffman G, Gambaro G. Renal transplantation from non-heart- beating donors: a review of the European experience. J Nephrol. 2003;16(3):334–341. [PubMed] [Google Scholar]
- 11.Chapman J, Bock A, Dussol B, Fritsche L, Kliem V, Lebranchu Y, et al. Follow-up after renal transplantation with organs from donors after cardiac death. Transpl Int. 2006;19(9):715–719. doi: 10.1111/j.1432-2277.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- 12.Keizer KM, de Fijter JW, Haase-Kromwijk BJ, Weimar W. Non-heart-beating donor kidneys in the Netherlands: allocation and outcome of transplantation. Transplantation. 2005;79(9):1195–1199. doi: 10.1097/01.tp.0000160765.66962.0b. [DOI] [PubMed] [Google Scholar]
- 13.Chang RW. Transplantation of non-heart-beating donor kidneys. Lancet. 1995;346(8970):322. doi: 10.1016/s0140-6736(95)92215-6. [DOI] [PubMed] [Google Scholar]
- 14.Sudhindran S, Pettigrew GJ, Drain A, Shrotri M, Watson CJ, Jamieson NV, et al. Outcome of transplantation using kidneys from controlled (Maastricht category 3) non-heart-beating donors. Clin Transplant. 2003;17(2):93–100. doi: 10.1034/j.1399-0012.2003.00014.x. [DOI] [PubMed] [Google Scholar]
- 15.Sanni AO, Wilson CH, Wyrley-Birch H, Vijayanand D, Navarro A, Gok MA, et al. Non-heart beating kidney transplantation: 6-year outcomes. Transplant Proc. 2006;38(10):3396–3397. doi: 10.1016/j.transproceed.2006.10.108. [DOI] [PubMed] [Google Scholar]
- 16.Brook NR, White SA, Waller JR, Veitch PS, Nicholson ML. Non-heart beating donor kidneys with delayed graft function have superior graft survival compared with conventional heart-beating donor kidneys that develop delayed graft function. Am J Transplant. 2003;3(5):614–618. doi: 10.1034/j.1600-6143.2003.00113.x. [DOI] [PubMed] [Google Scholar]
- 17.Daemen JH, de Wit RJ, Bronkhorst MW, Yin M, Heineman E, Kootstra G. Non-heart-beating donor program contributes 40% of kidneys for transplantation. Transplant Proc. 1996;28(1):105–106. [PubMed] [Google Scholar]
- 18.Cho YW, Terasaki PI, Cecka JM. High kidney graft survival rates using non-heart-beating trauma donors. Transplant Proc. 1998;30(7):3795–3796. doi: 10.1016/s0041-1345(98)01240-8. [DOI] [PubMed] [Google Scholar]
- 19.Butterworth PC, Taub N, Doughman TM, Horsburgh T, Veitch PS, Bell PR, et al. Are kidneys from non-heart-beating donors second class organs? Transplant Proc. 1997;29(8):3567–3568. doi: 10.1016/s0041-1345(97)01143-3. [DOI] [PubMed] [Google Scholar]
- 20.Matsuno N, Sakurai E, Kubota K, Kozaki K, Uchiyama M, Nemoto T, et al. Evaluation of the factors related to early graft function in 90 kidney transplants from non-heart-beating donors. Transplant Proc. 1997;29(8):3569–3570. doi: 10.1016/s0041-1345(97)01029-4. [DOI] [PubMed] [Google Scholar]
- 21.Valero R, Cabrer C, Oppenheimer F, Trias E, Sanchez-Ibanez J, De Cabo FM, et al. Normothermic recirculation reduces primary graft dysfunction of kidneys obtained from non-heart beating donors. Transpl Int. 2000;13(4):303–310. doi: 10.1007/s001470050706. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson ML, Metcalfe MS, White SA, Waller JR, Doughman TM, Horsburgh T, et al. A comparison of the results of renal transplantation from non-heart-beating, conventional cadaveric, and living donors. Kidney Int. 2000;58(6):2585–2591. doi: 10.1046/j.1523-1755.2000.00445.x. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Fructuoso A, Prats Sanchez D, Marques Vidas M, Lopez De Novales E, Barrientos Guzman A. Non-heart beating donors. Nephrol Dial Transplant. 2004;19(Suppl 3):iii26–31. doi: 10.1093/ndt/gfh1011. [DOI] [PubMed] [Google Scholar]
- 24.Cho YW, Terasaki PI, Cecka JM, Gjertson DW. Transplantation of kidneys from donors whose hearts have stopped beating. N Engl J Med. 1998;338(4):221–225. doi: 10.1056/NEJM199801223380403. [DOI] [PubMed] [Google Scholar]
- 25.Kievit JK, Oomen AP, de Vries B, Heineman E, Kootstra G. Update on the results of non- heart-beating donor kidney transplants. Transplant Proc. 1997;29(7):2989–2991. doi: 10.1016/s0041-1345(97)00755-0. [DOI] [PubMed] [Google Scholar]
- 26.Matsuno N, Kozaki K, Degawa H, Narumi Y, Suzuki N, Kikuchi K, et al. A useful predictor in machine perfusion preservation for kidney transplantation from non-heart-beating donors. Transplant Proc. 2000;32(1):173–174. doi: 10.1016/s0041-1345(99)00919-7. [DOI] [PubMed] [Google Scholar]
- 27.Moustafellos P, Hadjianastassiou V, Roy D, Muktadir A, Contractor H, Vaidya A, et al. The influence of pulsatile preservation in kidney transplantation from non-heart-beating donors. Transplant Proc. 2007;39(5):1323–1325. doi: 10.1016/j.transproceed.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 28.Asher J, Wilson C, Gok M, Balupuri S, Bhatti AA, Soomro N, et al. Factors predicting duration of delayed graft function in non-heart-beating donor kidney transplantation. Transplant Proc. 2005;37(1):348–349. doi: 10.1016/j.transproceed.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 29.Koyama I, Shinozuka N, Watanabe T, Ogawa N, Nagashima N, Asami H, et al. Utilization of kidneys from non-heart-beating donors by portable cardiopulmonary bypass. Transplant Proc. 1997;29(8):3550–3551. doi: 10.1016/s0041-1345(97)01017-8. [DOI] [PubMed] [Google Scholar]
- 30.Chen KH, Tsai MK, Ko WJ, Chen YS, Chueh SC, Lai MK, et al. Renal transplantation from non-heart-beating donors with extracorporeal membrane oxygenation: preliminary results. Transplant Proc. 2000;32(7):1743–1744. doi: 10.1016/s0041-1345(00)01426-3. [DOI] [PubMed] [Google Scholar]
- 31.Ko WJ, Chen YS, Chen RJ, Lai MK, Lee PH. Non-heart-beating donors under extracorporeal membrane oxygenation support. Transplant Proc. 2002;34(7):2600–2601. doi: 10.1016/s0041-1345(02)03440-1. [DOI] [PubMed] [Google Scholar]
- 32.Lee CY, Tsai MK, Ko WJ, Chang CJ, Hu RH, Chueh SC, et al. Expanding the donor pool: use of renal transplants from non-heart-beating donors supported with extracorporeal membrane oxygenation. Clin Transplant. 2005;19(3):383–390. doi: 10.1111/j.1399-0012.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- 33.Nishikido M, Noguchi M, Koga S, Kanetake H, Matsuya F, Hayashi M, et al. Kidney transplantation from non-heart-beating donors: analysis of organ procurement and outcome. Transplant Proc. 2004;36(7):1888–1890. doi: 10.1016/j.transproceed.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez-Fructuoso AI, de Miguel Marques M, Prats D, Barrientos A. Non-heart-beating donors: experience from the Hospital Clinico of Madrid. J Nephrol. 2003;16(3):387–392. [PubMed] [Google Scholar]
- 35.Gravel MT, Arenas JD, Chenault R, 2nd, Magee JC, Rudich S, Maraschio M, et al. Kidney transplantation from organ donors following cardiopulmonary death using extracorporeal membrane oxygenation support. Ann Transplant. 2004;9(1):57–58. [PubMed] [Google Scholar]
- 36.de Groot H, Rauen U. Ischemia-reperfusion injury: processes in pathogenetic networks: a review. Transplant Proc. 2007;39(2):481–484. doi: 10.1016/j.transproceed.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 37.Bloor GK, Welsh KR, Goodall S, Shah MV. Comparison of predicted with measured creatinine clearance in cardiac surgical patients. J Cardiothorac Vasc Anesth. 1996;10(7):899–902. doi: 10.1016/s1053-0770(96)80053-x. [DOI] [PubMed] [Google Scholar]
- 38.Rojas A GG, Cook KE, Bartlett RH, Punch JD, Arenas JD. Role and Timing of Heparin During Procurement of Organs with Extracorporeal Life Support (ECLS).. 8th Congress of the International Society for Organ Donation and Procurement (ISODP).; Gramado, Brasil. December 4-7, 2005; 2005. 2005. [Google Scholar]
- 39.Reoma JL, Rojas A, Krause EM, Obeid NR, Lafayette NG, Pohlmann JR, et al. Lung physiology during ECS resuscitation of DCD donors followed by In Situ assessment of lung function. ASAIO J. 2009;55(4):388–394. doi: 10.1097/MAT.0b013e3181a8fd98. [DOI] [PMC free article] [PubMed] [Google Scholar]