Abstract
Hypoxia and adenosine are known to upregulate angiogenesis; however, the role of peroxisome proliferator-activated receptor alpha (PPARα) in angiogenesis is controversial. Using transgenic Tg(fli-1 :EGFP) zebrafish embryos, interaction of PPARα and adenosine receptors in angiogenesis were evaluated under hypoxic conditions. Epifluorescent microscopy was used to assess angiogenesis by counting the number of intersegmental (ISV) and dorsal longitudinal anastomotic vessels (DLAV) at 28 hours post-fertilization (hpf). Hypoxia (6h) stimulated angiogenesis as the number of ISV and DLAV increased by 18-fold (p<0.01) and 100±8 % (p<0.001), respectively, at 28 hpf. Under normoxic and hypoxic conditions, WY-14643 (10 µM), a PPARα activator, stimulated angiogenesis at 28 hpf, while MK-886 (0.5 µM), an antagonist of PPARα, attenuated these effects. Compared to normoxic condition, adenosine receptor activation with NECA (10 µM) promoted angiogenesis more effectively under hypoxic conditions. Involvement of A2B receptor was implied in hypoxia-induced angiogenesis as MRS-1706 (10 nM), a selective A2B antagonist attenuated NECA (10 µM)-induced angiogenesis. NECA- or WY-14643-induced angiogenesis was also inhibited by miconazole (0.1 µM), an inhibitor of epoxygenase dependent production of eicosatrienoic acid (EET) epoxide. Thus, we conclude that: activation of PPARα promoted angiogenesis just as activation of A2B receptors through an epoxide dependent mechanism.
Keywords: adenosine receptors, angiogenesis, eicosatrienoic acid, PPARα, zebrafish
1. Introduction
Angiogenesis is a process involving growth of new blood vessels from pre-existing ones. In physiological conditions it occurs during embryonic development, wound healing and in the female reproductive cycle. In pathological conditions, an uncontrolled angiogenesis has been implicated in many diseases including cancer, psoriasis and age-related macular degeneration (Liekens et al., 2001). Currently; many studies are focusing on angiogenesis as a potential target for treatment of many diseases (Maleck, et al., 2005). Inhibition of angiogenesis is a strategy that has been approved for the treatment of diseases associated with elevated angiogenesis, such as tumor, cancer, retinopathy, rheumatoid arthritis and psoriasis (Westra et al., 2010). Similarly, promotion of angiogenesis is another strategy to treat diseases associated with reduced angiogenesis, such as ischemic heart disease, cerebral ischemia, peripheral artery disease and wound healing (Sabti, 2007).
Peroxisome proliferator-activated receptor alpha (PPARα), a transcription factor, is a negative regulator of inflammation (Bordiji et al., 2000). However, its role in angiogenesis is still controversial as both pro- and anti-angiogenic effects have been reported. For example, PPARα promotes angiogenesis by suppressing thrombospondin-1 (TSP-1), an inhibitor of angiogenesis (Kaipainen et al., 2007), through prostacyclin-mediated activation of PPARγ angiopoietin-related gene (PGAR); which is a physiological target for both PPARα and PPARγ (Pola et al., 2004) and vascular endothelial growth factor (VEGF)-dependent mechanism (Biscetti et al., 2008). However, PPARα as well as PPARγ elicit anti-angiogenic effects through blockade of VEGFR2 (Meissner et al., 2004). Fenofibrate, an agonist of PPARα, inhibits angiogenesis through PPARα –dependent (Panigrahy et al., 2008) and independent pathways (Araki et al., 2009). Clofibric acid, another PPARα ligand, also caused PPARα-dependent reduction in VEGF expression in human ovarian cancer cell (Yokoyama et al., 2007). WY-14643, a selective PPARα ligand, was reported to inhibit tumor angiogenesis through transcriptional down regulation of cytochrome P450 2C (CYP2C) epoxygenase (Pozzi et al., 2007).
Hypoxia elevates extracellular adenosine by promoting ATP dephosphorylation and by inhibiting equilibrating nucleoside transporter 1 (ENT1) that translocates extracellular adenosine into the cell. The extracellular accumulation of adenosine protects cells from hypoxia-induced injury through four major ways: increased oxygen supply/demand ratio, preconditioning, anti-inflammatory effects and stimulation of angiogenesis. Adenosine exerts these effects through G-protein-coupled receptors: A1, A2A, A2B, A3. Endothelial cells predominantly express A2A and A2B receptors. Higher expression and activation of adenosine receptor subtype A2B under hypoxic conditions was reported in many studies (Feoktisov et al., 2002; Feoktisov et al., 2004; Kong et al., 2006L). Unlike adenosine receptor A2A which exerts cell-dependent pro- as well as anti-angiogenic phenotypes (Desai et al., 2005; Olah and Roudabush, 2000), available information indicates that A2B exhibits only pro-angiogenic effect and its stimulation was associated with enhanced expression of VEGF and IL-8 (Ryzhov et al., 2007).
PPARα-dependent up regulation of A2A (Araki et al., 2009) and A2B-mediated down regulation of PPARα is also reported (Peng et al., 2009). However, the interaction of adenosine receptors and PPARα in angiogenesis is not known. Thus, the purpose of this study was to explore the downstream angiogenic mediators of PPARα and adenosine receptors and their possible interaction in angiogenesis.
2. Materials and methods
2.1. Zebrafish
Two to three month old wild type (AB strain) and transgenic friendly leukemia integration 1 a enhanced green fluorescent protein Tg(fli1a:EGFP) zebrafish (Danio rerio) from Zebrafish International Resource Center (ZIRC), Eugene , Oregon, (USA).
2.2. Chemicals
WY-14643 (pirinixic acid), tricaine (ethyl 3-aminobenzoate salt), pronase (protease from Streptomyces griseus) and miconazole nitrate salt 1-(2,4-Dichloro-β-[(2,4-dichlorobenzyl)oxy]phenethyl)imidazole from Sigma-Aldrich Corp., St Louis, MO (USA). NECA (6-Amino-9H–purin-9-yl)-1 -deoxy-N-ethyl-b-D-ribofuranuronamide 5′-N- Ethylcarboxamidoadenosine) was obtained from Fischer Scientific, Pittsburg, PA, (USA). MRS-1706 ( N -(4-Acetylphenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H–purin-8-yl) phenoxy]acetamide and MK-886 (3-[3-tert-Butylthio-1-(4-chlorobenzyl)-5-isopropyl-1H–indol-2-yl]-2,2-dimethylpropionic acid, sodium salt hydrate) were purchased from Tocris Cooks Inc., St. Louis, MO (USA). Leukotriene B4 was purchased from Cayman Chemical, Ann Arbor, Michigan (USA).
Stock solutions of WY-14643 (50 mg/ml), MK-886 (25 mg/ml), NECA (50 mg/ml) MRS-1706 (5 mg/ml) and miconazole (10 mg/ml) were prepared in dimethyl sulfoxide (DMSO). All stock solutions were kept at 4°C.
2.3. Maintenance and generation of embryos
Fish were maintained at 28±0.5°C in 14:10h light: dark cycle and fed twice daily with TetraMin tropical flakes. Group mating of 10 pairs of male and female zebrafish was performed around 4:00 PM. Embryos were collected the next morning and examined for viability using a dissecting microscope. 30–50 embryos were incubated in 30 ml of fish water (0.06 g/l of Instant Ocean Salt in distilled water) with or without test compounds at 28±0.5°C. The fish water was replenished every day. For pilot studies, embryos (n=12– 14) 2–4 hour post-fertilization (hpf) were exposed to WY-14643 (1.0, 2.5, 10 and 100 µM), agonist of PPARα, with or without MK-886 (0.5–5.0 µM; IC50=0.5 –1.0 µM) (Tocris Cooks Inc., St. Louis, MO, USA) (Kehrer et al., 2001) an antagonist of PPARα, or NECA (1.0 –100 µM), a non selective adenosine receptor agonist with or without MRS-1706, a selective antagonist of A2B (10 nM; Ki values for adenosine receptors are 1.39, 157, 112 and 230 nM for A2B , A1, A2a and A3 receptors, respectively) (Tocris Cooks Inc., St. Louis, MO, USA) (Desai et al., 2005).
2.4. Epifluorescence microscopy
Transgenic Zebrafish (TG(Fli:EGFP)) expressing green fluorescent protein under the control of the VEGF receptor promoter were used. To monitor the effects of PPARα and adenosine receptor agonists and epoxygenases in hypoxia-induced angiogenesis, based on pilot study, embryos (2–4 hpf) (n=12–14) were exposed to WY-14643 (10 µM; n=14), a PPARα ligand, NECA (10 µM; n=12), a non selective adenosine receptor agonist or miconazole (0.1 µM; n=12–15), an inhibitor of epoxygenase (Dong et al., 2002). For combined administration two groups were made: NECA (10 µM) + miconazole (0.1 µM) (n=12) and WY-14643 (10 µM) + miconazole (0.1 µM) (n=12). All groups were kept under normoxic (20.9 % oxygen) condition for 22–24 h. Embryos (22– 24 hpf) were dechorionated by treating them with a dilute solution of pronase (2 mg/ml in embryo water) (Sigma Aldrich Corp., St Louis, MO, USA ) for 2–5 min and then incubated in the hypoxic (5% oxygen) or normoxic chamber at 28 °C for 6 h. Generation of hypoxia (5% oxygen) was accomplished by using an oxygen controller (Coy Laboratory Products, Grass Lake, Michigan, USA).
Embryos were anesthetized with tricaine solution (0.016%) (Sigma Aldrich Corp., St Louis, MO, USA). Blood vessels, namely; intersegmental vessel (ISV) and dorsal longitudinal anastomotic vessel (DLAV) were visualized at 28 hpf using epifluorescence microscopy and images were captured using a Nikon 4X objective with a 30 s Nikon camera exposure. Three parameters were used to assess angiogenesis: (i) total number of ISV (ii) total number of completely formed ISV and (iii) total number of completely formed DLAV. ISVs that reached to the dorsal periphery of the body and DLAVs that formed complete T shaped at the dorsal periphery were considered as completely formed ISV and DLAV, respectively. Angiogenesis was defined as the ratio of the number of completely formed ISV or DLAV to the total number of ISV in the trunk region.
2.6. Data analysis
Data were expressed as means ± SEM. A two way analysis of variance (ANOVA) followed by Bonferroni’s analysis as a post hoc test was performed to compare mean values from different groups. A value of p≤ 0.05 was considered significant.
3. Results
3.1. Effect of hypoxia (6 h) on ISV and DLAV
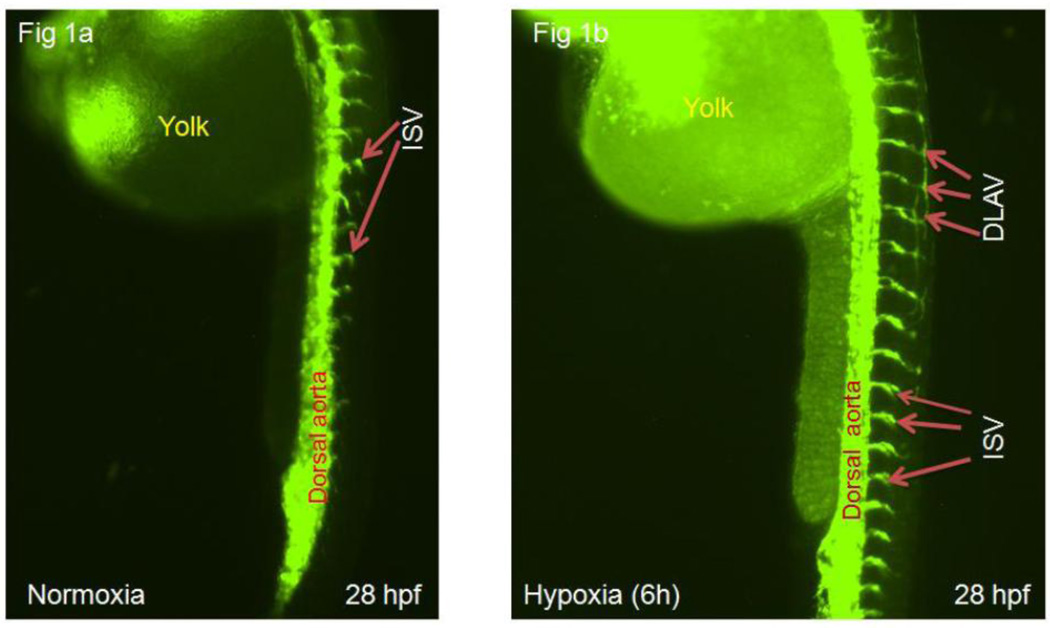
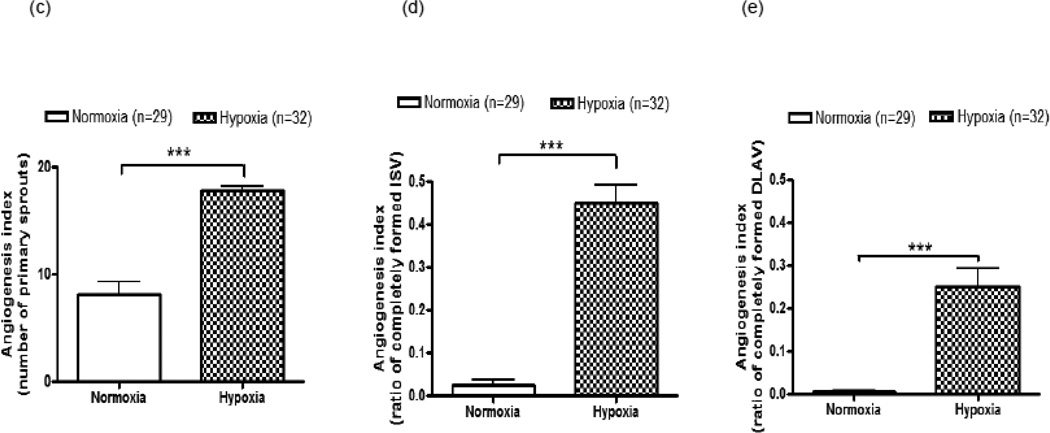
Compared to normoxia, hypoxia increased angiogenesis in terms of sprout number. The number of primary sprout increased by 119±8% (p<0.001) (fig 1a, b & c ) and there was an 18-fold (p<0.001) or 100±8% (p<0.001) increase in number of completely formed ISV (fig 1a, b and d ) or DLAV (fig 1a, b and e ), respectively, at 28 hpf.
Figures 1.
a–b: Representative epi-fluorescent images of Tg(fli:EGFP) zebrafish embryos showing ISV and DLAV at 28 hours post-fertilization (hpf) under normoxic (fig 1a) or hypoxic conditions (fig 1b). Intersegmental vessels are represented as ISV and dorsal longitudinal anastomotic vessels as DLAV.
c–e: Quantitative evaluation of angiogenesis index at 28 hpf: primary sprout (figure 1c), ISV (1d) and DLAV (figure 1e). Data are expressed as mean (±SEM) of the total number of primary sprout (c) or the ratio between completely formed ISV (d) or DLAV (e) to the total number of ISV. A two way analysis of variance (ANOVA) was used to compare mean of hypoxic group vs. normoxic control. *** p<0.001
3.2. Effects of PPARα ligand and antagonist on angiogenesis
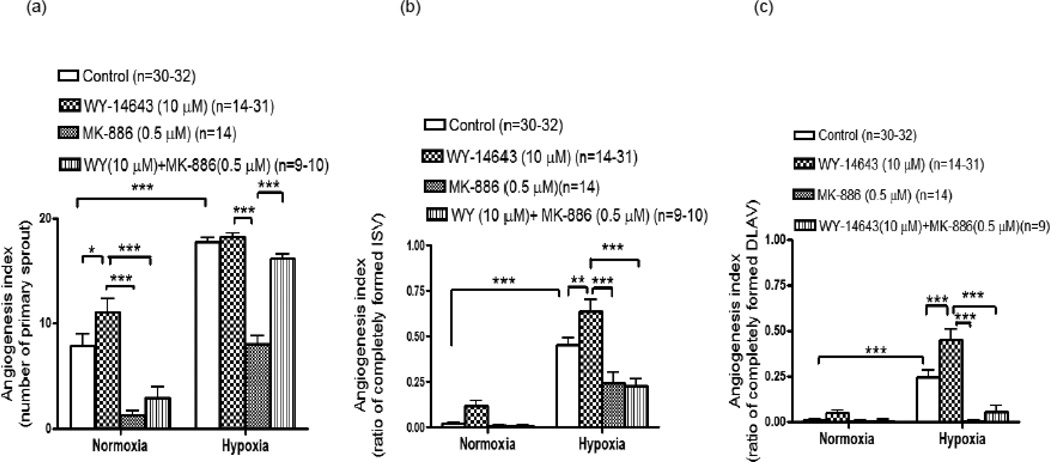
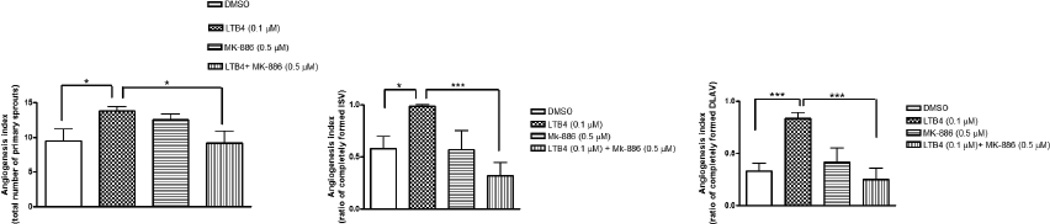
Lower concentrations of WY-14643; 1.0 and 2.5 µM elicited no effect on angiogenesis under normoxic condition (data not shown). However, WY-14643 (10 µM) increased angiogenesis (primary sprouts) by 41±13% (p<0.05) (fig 2a) but there was no change in angiogenesis in ISV or DLAV at 28 hpf. Under normoxic conditions, MK-886 (0.5 µM) alone decreased angiogenesis as manifested by the number of primary sprouts by 84±25% (p<0.001) and attenuated WY-14643 (10 µM)-induced increase in primary sprouts by 73±20% (p<0.001). MK-886 also inhibited LTB4-induced angiogenic effects on primary sprout by 34±11% (p<0.05) (fig 3a), ISV by 68± 20% (p<0.001) (fig 3b) and DLAV formation by 70±25% (p<0.001) (fig 3c).
Figures 2.
a–c: Quantitative evaluation of epi-fluorescent images of Tg(fli:EGFP) zebrafish embryos showing involvement of PPARα in angiogenesis under normoxic or hypoxic condition. Histograms represent the effects of WY-14643 (10 µM), a selective agonist of PPARα on emergence of primary sprout (ISV) (fig a), ISV development (fig b) and DLAV development (fig c) at 28 hpf in the presence of MK-886 (0.5 µM), an antagonist of PPARα. Data are expressed as mean (±SEM) of total number of primary sprout (fig a) or the ratio between completely formed ISV (fig b) or DLAV (fig c) to the total number of ISV. A two way analysis of variance (ANOVA) was used to compare means of each group. *** p<0.001; **p<0.01; *p<0.05
Figures 3.
(a–c); Quantitative evaluation of epi-fluorescent images of Tg(fli:EGFP) zebrafish embryos showing inhibitory effect of MK-886 on LTB4-induced angiogesis under normoxic condition. Histograms represent the angiogenic effects of LTB4 (0.1 µM), a selective agonist of PPARα on emergence of primary sprouts (fig a), ISV development (fig b) and DLAV development (fig c) at 28 hpf in the presence or absence of MK-886 (0.5 µM). Data are expressed as mean (±SEM) of total number of primary sprout (fig a) or the ratio between completely formed ISV (fig b) or DLAV (fig c) to the total number of ISV. A two way analysis of variance (ANOVA) was used to compare means of each group. *** p<0.001; *p<0.05
Under hypoxic conditions, WY-14643 (10 µM)-stimulated the number of primary sprouts, ISV and DLAV formation by 3±2% (p>0.05), 41±9% (p<0.01) and 82±15% (p<0.001) (fig 2a–c), respectively. Under hypoxic conditions, MK-886 (0.5 µM) alone inhibited angiogenesis in primary sprouts by 55±6% (p<0.001), in ISV by 47±17 (p<0.001) and in DLAV by 100±8% (p<0.001). MK-886 (0.5 µM) also blunted WY-14643-induced ISV and DLAV formation by 65±14% (p<0.001) and 87±37% (p<0.001), respectively.
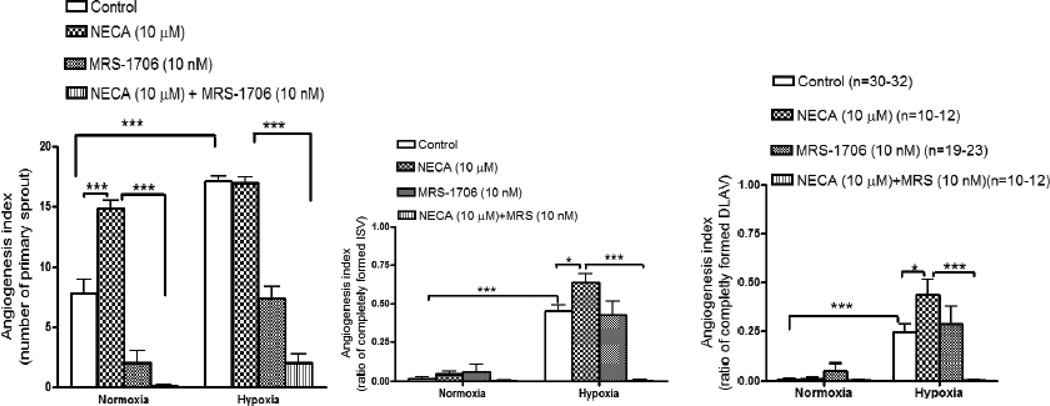
3.3. Effects of adenosine receptor agonist and antagonist on angiogenesis
Under normoxic conditions, NECA did not show any angiogenic effect on ISV or DLAV. However, NECA increased primary sprout density 1.9-fold (p<0.001) (fig 4a). After exposure to hypoxia (6 h), NECA (10 µM) increased angiogenesis in ISV (41 ±9%; p<0.05) (fig 4b) and DLAV (78±17%; p>0.05) (fig 4c) at 28 hpf.
Figures 4.
a–c: cQuantitative evaluation of epi-fluorescent images of Tg(fli:EGFP) zebrafish embryos showing involvement of adenosine receptor in angiogenesis under normoxic or hypoxic condition. Histograms represent the effect of NECA (10 µM), a non selective agonist of adenosine receptor on emergence of primary sprout (ISV) (fig a), ISV development (fig b) and DLAV development (fig c) at 28 hpf in the presence of MRS-1706 (10 nM), a selective antagonist of A2B adenosine receptor. Data are expressed as mean (±SEM) of total number of primary sprout (fig a) or the ratio between completely formed ISV (fig b) or DLAV (fig c) to the total number of ISV. A two way analysis of variance (ANOVA) was used to compare means of each group. *** p<0.001; **p<0.01; *p<0.05
In order to evaluate the specific type of adenosine receptor involved in angiogenesis, embryos were pretreated with MRS-1706, a selective antagonist of A2B receptor which selectively inhibited A2B-receptor- induced angiogenesis at 10 nM concentrations (Desai et al., 2005). At 28 hpf, MRS-1706, a selective A2B receptor antagonist, inhibited NECA-induced increase in the number of primary sprouts (fig 4a) by 99+31.8% (p<0.001) and 88±20% (p<0.001) under normoxic and hypoxic conditions, respectively. MRS-1706 also abolished NECA-induced increase in angiogenesis in ISV at 28 hpf by 100±4% (p<0.001) (fig 4b) and in DLAV by 99±8% (p<0.001) (fig 4c) under hypoxic but not under normoxic conditions. Compared to NECA (10 µM) alone, MRS-1706 (10 nM) attenuated NECA-induced increase in primary sprouts by 88±20% (p<0.05) (fig 4a) and abolished NECA-induced ISV (100±4%; p<0.001) (fig 4b).
3.4. Effect of epoxygenase inhibition on PPARα-mediated angiogenesis
In order to evaluate the involvement of epoxyeicosatrienoic acids (EETs) as a downstream mediator of PPARα-dependent angiogenesis, embryos (2–4 hpf) (n=12) were exposed to WY-14643 (50 µM) with or without miconazole (0.1 µM), an inhibitor of epoxygenase. At 28 hpf, miconazole (0.1 µM) reduced angiogenesis in primary sprout, ISV and DLAV by 5-fold, 2-fold and 6-fold, respectively (fig 5) under hypoxic conditions (fig 5). Compare to miconazole alone, WY-14643 (10 µM) mitigated inhibitory action of miconazole (0.1 µM) in primary sprout by 5-fold (p<0.05), in ISV by 4-fold (p<0.05) and in DLAV by 6-fold (p<0.01) (fig 5).
Figures 5.
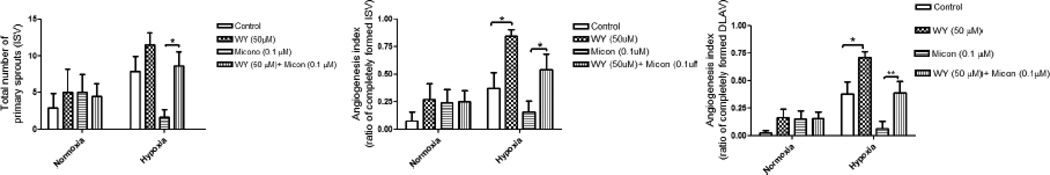
(a–c): Quantitative evaluation of epi-fluorescent images of Tg(fli:EGFP) zebrafish embryos showing the effect of epoxygenase inhibition on WY-14643-induced angiogenesis under normoxic or hypoxic condition. Histograms represent the effect of miconazole (0.1 µM), an inhibitor of epoxygenase, on WY-14643-induced (50 µM) emergence of primary sprout (ISV) (fig a), ISV development (fig b) and DLAV development (fig c) at 28 hours-post fertilization (hpf). Data are expressed as mean (±SEM) of the total number of primary sprout (fig a) or the ratio between completely formed ISV (fig b) or DLAV (fig c) to the total number of ISV. A two way analysis of variance (ANOVA) was used to compare means of each group. ** p<0.01; *p<0.05
3.5. Effect of epoxygenase inhibition on adenosine receptor-mediated angiogenesis
The role of epoxyeicosatrienoic acid (EET) in adenosine receptor-mediated angiogenesis was evaluated in embryos (2–4 hpf) (n=12) exposed to miconazole (0.1 µM) or combined miconazole (0.1 µM) + NECA (50 µM). Under hypoxic conditions NECA (50 µM) diminished miconazole (0.1 µM)-mediated antiangiogenic effect. Compared to miconazole (0.1 µM) alone, the combined treatment of miconazole (0.1 µM) with NECA (50 µM) showed about 5-fold (p<0.05) angiogenic recovery in spout number and 3-fold (p<0.05) angiogenic improvement in ISV and DLAV development, (p<0.05 (fig 6) under hypoxic condition.
Figures 6.
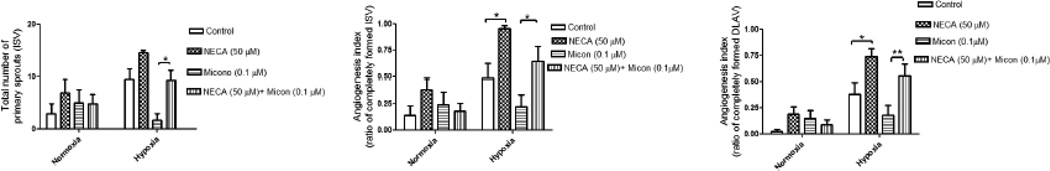
(a–c): Quantitative evaluation of epi-fluorescent images of Tg(fli:EGFP) zebrafish embryos showing the effect of epoxygenase inhibition on NECA-induced angiogenesis under normoxic or hypoxic condition. Histograms represent the effect of miconazole (0.1µM), an inhibitor of epoxygenase, on NECA-induced (50 µM) emergence of primary sprout (ISV) (fig a), ISV development (fig b) and DLAV development (fig c) at 28 hours-post fertilization (hpf) Data are expressed as mean (±SEM) of the total number of primary sprout (fig a) or the ratio between completely formed ISV (fig b) or DLAV (fig c) to the total number of ISV . A two way analysis of variance (ANOVA) was used to compare means of each group. ** p<0.01; *p<0.05
4 Discussion
Adenosine receptors are the known therapeutic targets in a wide range of conditions, including cerebral and cardiac ischemic diseases, sleep disorders, wound healing, immune and inflammatory disorders and cancer. Agonists of adenosine receptor have proven effective in the treatment of many cardiovascular disorders including, arrhythmia and cardiac ischemia (Jacobson and Gao, 2006). On the other hand, PPARα, an antiinflammatory transcription factor, is known to play important roles in lipid metabolism and glucose homeostasis and PPARα ligands are currently used for reducing coronary vascular events, decreasing mortality in heart disease (Bishop-Bailey, 2000) and inhibiting inflammation in certain diseases such as atherosclerosis.
Despite the fact that both adenosine receptor and PPARα have been recognized as therapeutic targets for common diseases such as cardiac ischemia, inflammation-related diseases and wound healing, their cross-talk in the normal physiological and pathological conditions is mostly unknown. There is scanty number of studies represented interactions of PPARα and adenosine receptor. The studies of Peng et al., (2009) demonstrated that activation of A2B receptor inhibited expression and activity of PPARα in pathogenesis of fatty liver. Soledade et al., (2007) also reported that A2A adenosine receptor activation stimulated PPARα expression and activity in THP-1 cells and mouse peritoneal macrophages and speculated a possible pathway for A2A-mediated anti-inflammatory mechanism. On other hand, Araki et al. (2009) reported that PPARα up regulated transcription of the A2A adenosine receptor gene in human umbilical vein endothelial cell (HUVEC). While the role of adenosine receptors in angiogenesis is well defined; the role of PPARα in angiogenesis is not definitive. Since both receptors are the therapeutic targets for the cardiovascular diseases, especially cardiac ischemia or peripheral artery disease where recovery depends in part on the ability to make collateral vessels, the interaction of PPARα and adenosine receptor in angiogenesis merits careful evaluation.
The present study evaluated the distinct roles of PPARα and adenosine receptors in angiogenesis and their possible interactions in hypoxia-induced angiogenesis. For this study we used zebrafish embryo that has been used as a preclinical model for assessment of angiogenesis (Parng, et al, 2002; Oh, et al., 2008; Lam et al., 2008). Furthermore, this model also showed for the presence of PPARα (Ibabe et al., 2005) and adenosine receptors A2A and A2B (Boehmler et al., 2009) during early stages of embryonic development.
The role of PPARα in angiogenesis is controversial as it acts as an inhibitor as well as an inducer of angiogenic process (Biscetti et al., 2009). Many studies reported an anti-angiogenic effect (Panigrahy et al., 2008; Grabacka et al., 2006) whereas others reported a pro-angiogenic role for PPARα (Kaipainen et al., 2007; Biscetti et al., 2008) Our epifluorescence microcopy data showed that WY-14643, a PPARα ligand, stimulated angiogenesis at 28 hpf. The involvement of PPARα activation in angiogenesis was further confirmed in experiments in which embryos (28 hpf) were exposed to MK-886 with or without WY-14643, at the 4–6 hpf stage. The greater inhibition of basal angiogenesis by MK-886 indicated that MK-886 antagonizes the effect of endogenous PPARα ligands more effectively compared to that of an exogenous activators like WY-14643. This effect likely reflects inhibition of leukotriene B4 (LTB4), a known endogenous ligand of PPARα (Narala, et al., 2010) and which synthesis is inhibited by MK-886.
Hypoxia is a known stimulator of angiogenesis. It mainly stimulates angiogenesis by preventing degradation of hypoxia inducible factor-1-α (HIF-1α), a transcription factor, which activates the transcription of many pro-angiogenic genes. However, studies also showed that hypoxia through HIF-1α, reduced PPARα expression and activity in intestinal epithelial cells (Narravula and Colgan, 2001) and cardiac myocytes (Huss et al., 2001; Belanger et al., 2007). Based on the consensus that hypoxia reduces PPARα expression/activity, we expected that PPARα ligands would attenuate hypoxia-induced angiogenesis when compared to normoxia, However, in our experimental setting, administration of PPARα ligands promoted angiogenesis to a similar extent in both normoxic and hypoxic conditions . Although we have not verified yet, however, PPARα-mediated angiogenesis under hypoxic conditions implies that the transcription factors other than HIF-1α such as HIF-2α may be involved to promote PPARα expression (Kelly, 2008), and thereby preserved PPARα-mediated angiogenesis under hypoxic condition.
A protective role for adenosine through its four known receptor subtypes (A1, A2A, A2B and A3) in ischemic and hypoxia-induced injury has been documented in many studies (Linden, 2005; Auchampach, 2007). The role of adenosine receptors in angiogenesis has been evaluated in many studies (Auchampach 2007; Clark et al., 2007). Among the four known receptor sub types of adenosine, A1, A2B and A3 have been shown to stimulate angiogenesis under physiological as well as pathological conditions (Desai et al., 2005; Linden 2005; Auchampach 2007), whereas A2A receptor activation either promotes (Desai et al., 2005; Montesinos et al., 2004) or inhibits angiogenesis (Olah and Roudabush, 2000). Our data demonstrated that adenosine receptor activation stimulated angiogenesis which was greater under hypoxic condition, indicating consistent with a greater generation of adenosine and adenosine receptor expression in hypoxia, (Kong et al., 2006) and greater adenosine receptor-mediated NECA-induced VEGF secretion under hypoxic conditions (Ryzhov et al., 2007)
Since many studies reported that adenosine receptor-mediated angiogenesis contributed up to 50–70% of hypoxia-induced angiogenesis (Adair, 2005) and for the higher expression/activity of A2B receptor under hypoxia-induced angiogenesis Kong et al., 2006; Ma et al., 2010) we used MRS-1706, a selective antagonist of A2B, to investigate the involvement of A2B in angiogenesis under hypoxic condition. Although specific involvement of A2B receptor has not been clearly defined, a role for A2B receptor activation was inferred as MRS-1706 abolished NECA-induced angiogenesis under hypoxic condition (fig 3a & b). These results are in agreement with the studies that demonstrated involvement of A2B receptor in hypoxia-induced angiogenesis in human microvascular endothelial cells (HMEC-1) (Kong et al., 2006).
EETs are epoxygenase-dependent eicosanoids which have demonstrated a pro-angiogenic effect. (Pozzi et al., 2005; Panigrahy et al., 2010; Michaelis et al., 2008). Indeed, a link between activation of adenosine receptors (A2A) and enhanced generation of CYP2C9-dependent EET in A2AAR-WT mice was reported by Nayeem et al., (2008). In this study, CGS-21680, a selective agonist of A2AR elicited vasorelaxation that was higher in A2A-wild type compared to A2A-knockout mice aortae, and was attenuated by CYP-epoxygenase inhibitor or EET antagonist. Given this background, we examined whether EETs play any role in A2B receptor mediated hypoxia-induced angiogenesis in the zebrafish embryo. Our data demonstrated that, miconazole, an inhibitor of epoxygenase, decreased NECA-induced angiogenesis under normoxic and hypoxic conditions, indicating that EET generation is the downstream mechanism responsible for A2B receptor-mediated hypoxia-induced angiogenesis.
PPARα acts as an inducer (Zhao et al., 2009) as well as inhibitor (Pozzi et al., 2007) of CYP2C expression, our data demonstrating that miconazole, an inhibitor of CYP2C attenuated PPARα-mediated angiogenesis under normoxic as well as hypoxic conditions, indicated that PPARα-activation also stimulates angiogenesis through EET-dependent mechanism.
5. Conclusions
Data from this study demonstrated that: (i) activation of PPARα and adenosine receptors independently promoted angiogenesis in the zebrafish embryo (ii) hypoxia-induced angiogenesis involved in activation of both PPARα and adenosine receptors (iii) both PPARα and A2B receptor promoted angiogenesis through an epoxide-dependent mechanism.
Acknowledgements
We thank Dr. X. Zhao and Mr. Whitney Jesse (The University of Texas Health Science Center at Houston Medical School), for providing zebrafish embryo. We are also thankful to Dr. K. Ranganna and Dr. M. Philip (Texas Southern University) assistance in fluorescence microscopy. This research was supported by NIH grant #HL03674.
Abbreviations
- CYP2C9
cytochrome P450 2C9
- DLAV
dorsal longitudinal anastomotic vessel
- EET
eicosatrienoic acid
- HIF-1α
hypoxia-inducible factor-1 alpha
- hpf
hour post-fertilization
- IL-8
interleukin-8
- ISV
intersegmental vessel
- NECA
5′-N-Ethylcarboxamidoadenosine
- PPARα
peroxisome proliferator-activated receptor alpha
- PPARγ
peroxisome
- PGAR
PPARγ angiopoietin-related gene
- TSP-1
thrombospondin-1
- Tg(fli-1:EGFP)
transgenic friendly leukemia integration-1 enhanced green fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adair TH. Growth regulation of the vascular system: an emerging role for adenosine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R283–R296. doi: 10.1152/ajpregu.00840.2004. [DOI] [PubMed] [Google Scholar]
- Araki H, Tamada Y, Imoto S, Dunmore B, Sanders D, Humphrey S, Nagasaki M, Doi A, Nakanishi Y, Yasuda K, Tomiyasu Y, Tashiro K, Cristin PD, Charnock-Jones S, Kuhara S, Miyano S. Analysis of PPARα-dependent and PPARα-independent transcript regulation following fenofibrate treatment of human endothelial cells. Angiogenesis. 2009;12:221–129. doi: 10.1007/s10456-009-9142-8. [DOI] [PubMed] [Google Scholar]
- Auchampach JA. Adenosine receptors and angiogenesis. Circ. Res. 2007;101:1075–1077. doi: 10.1161/CIRCRESAHA.107.165761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger AJ, Luo Z, Vincent KA, Akita G, Cheng SH, Gregory RJ, Jiang C. Hypoxia-inducible factor 1 mediates hypoxia-induced cardiomyocyte lipid accumulation by reducing the DNA binding activity of peroxisome proliferator-activated receptor α/retinoid X receptor. Biochem. Biophys. Res. Commun. 2007;364:567–572. doi: 10.1016/j.bbrc.2007.10.062. [DOI] [PubMed] [Google Scholar]
- Biscetti F, Gaetani E, Flex A, Aprahamian T, Hopkins T, Straface G, Pecorini G, Stigliano E, Smith RC, Angelini F, Castellot JJ, Pola R. Selective Activation of Peroxisome Proliferator-Activated Receptor (PPAR) α and PPARγ induces neoangiogenesis through a vascular endothelial growth factor-dependent mechanism. Diabetes. 2008;57:1394–1404. doi: 10.2337/db07-0765. [DOI] [PubMed] [Google Scholar]
- Biscetti F, Straface G, Pittocco D, Zaccardi F, Ghirlanda G, Flex A. Peroxisome proliferator-activated receptors and angiogenesis. Nutr. Metab. Cardiovasc. Dis. 2009;9:751–759. doi: 10.1016/j.numecd.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Bishop-Bailey D. Peroxisome proliferator-activated receptors in the cardiovascular system. Br. J. Pharmacol. 2000;129(5):823–834. doi: 10.1038/sj.bjp.0703149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmler W, Petko J, Woll M, Frey C, Thisse B, Thisse C, Canfield VA, Levenson R. Identification of zebrafish A2 adenosine receptors and expression in developing embryos. Gene Expr. Patterns. 2009;9:144–151. doi: 10.1016/j.gep.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordiji K, Grillasca J, Gouze J, Magdalou J, Schohns H, Kellers JM, Bianchis A, Dauca M, Netter P, Terlain B. Evidence for the Presence of Peroxisome Proliferator-activated Receptor (PPAR)α and γ and Retinoid Z Receptor in Cartilage. J. Biol. Chem. 2000;275:12243–12250. doi: 10.1074/jbc.275.16.12243. [DOI] [PubMed] [Google Scholar]
- Clark AN, Youkey R, Liu X, Jia L, Blatt R, Day Y, Sullivan GW, Linden J, Tucker AL. A1 Adenosine Receptor Activation Promotes Angiogenesis and Release of VEGF from Monocytes. Circ. Res. 2007;101:1130–1138. doi: 10.1161/CIRCRESAHA.107.150110. [DOI] [PubMed] [Google Scholar]
- Desai A, Victor-Vega C, Gadangi S, Montesinos MC, Chu CC, Cronstein BN. Adenosine A2A receptor stimulation increases angiogenesis by down-regulating production of the antiangiogénica matrix proteína thrombospondin1. Mol Pharmacol. 2005;67:1406–1413. doi: 10.1124/mol.104.007807. [DOI] [PubMed] [Google Scholar]
- Feoktisov I, Goldstein AE, Ryzhov S, Zeng D, Belardinelli L, Voyno-Yasenetskaya T, Biaggioni I. Differential expression of adenosine receptors in human endothelial cells: Role of A2B receptors in angiogenic factor regulation. Circ. Res. 2002;90:531–538. doi: 10.1161/01.res.0000012203.21416.14. [DOI] [PubMed] [Google Scholar]
- Feoktistov I, Ryzhov S, Zhong H, Goldstein AE, Matafonov A, Zeng D, Biaggioni I. Hypoxia modulates adenosine receptors in human endothelial and smooth muscle cells toward an A2B angiogenic phenotype. Hypertension. 2004;44:649–654. doi: 10.1161/01.HYP.0000144800.21037.a5. [DOI] [PubMed] [Google Scholar]
- Grabacka M, Plonka PM, Urbanska K, Reiss K. Peroxisome proliferator-activated receptor alpha activa decreases metastatic potential of melanoma cells in vitro via down-regulation of Akt. Clin. Cancer Res. 2006;12:3028–3036. doi: 10.1158/1078-0432.CCR-05-2556. [DOI] [PubMed] [Google Scholar]
- Huss JM, Levy FH, Kelly DP. Hypoxia inhibits the peroxisome proliferator-activated receptor α/retinoid X receptor gene regulatory pathway in cardiac myocytes. J. Biol. Chem. 2001;276:27605–27612. doi: 10.1074/jbc.M100277200. [DOI] [PubMed] [Google Scholar]
- Ibabe A, Bilbao E, Cajaraville MP. Expression of peroxisome proliferator-activated receptors in zebrafish (Danio rerio) depending on gender and developmental stage. Histochem. Cell. Biol. 2005;123:75–87. doi: 10.1007/s00418-004-0737-2. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Gao Z. Adenosine receptors as therapeutic targets. Nature Rev. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaipainen A, Kieran MW, Huang S, Butterfield C, Bielenberg D, Mostoslvsky G, Mulligan R, Folkman J, Panigrahy D. PPARα deficiency in inflammatory cells suppresses tumor growth. Plos One. 2007;e260:1–11. doi: 10.1371/journal.pone.0000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrer JP, Biswal ss, Eunhye AL, Thuillier P, Datta K, Fischer SM. Vanden Heuvel JP. Inhibition of peroxisome-proliferator-activated receptor (PPAR)α by MK886. Biochem. J. 2001;356:899–906. doi: 10.1042/0264-6021:3560899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DP. Hypoxic reprogramming. Nature Genetics. 2008;40:132–134. doi: 10.1038/ng0208-132. [DOI] [PubMed] [Google Scholar]
- Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB. J. 2006;20:2242–2250. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- Lam H, Lin H, Lao S, Gao J, Hong S, Leong C, Yue PY, Kwan Y, Leung AY, Wang Y, Lee SM. The angiogenic effects of Angelica sinensis extract on HUVEC in vitro and zebrafish in vivo . J. Cell. Biochem. 2008;103:195–211. doi: 10.1002/jcb.21403. [DOI] [PubMed] [Google Scholar]
- Liekens S, Clercq ED, Neyts J. Angiogenesis: regulators and clinical applications. Biochem. Pharmacol. 2001;61:253–270. doi: 10.1016/s0006-2952(00)00529-3. [DOI] [PubMed] [Google Scholar]
- Linden J. Adenosine in tissue protection and tissue regeneration. Mol. Pharmacol. 2005;67:1385–1387. doi: 10.1124/mol.105.011783. [DOI] [PubMed] [Google Scholar]
- Ma DF, Kondo T, Nakazawa T, Niu DF, Mochizuki K, Kawasaki T, Yamane T, Katoh R. Hypoxia-inducible adenosine A2B receptor modulates proliferation of colon carcinoma cells. Hum. Pathol. 2010;41:1550–1557. doi: 10.1016/j.humpath.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Maleck M, Kolsut P, Proczka R. Angiogenic and antiangiogenic gene therapy. Gene Therapy. 2005;12:S159–S169. doi: 10.1038/sj.gt.3302621. [DOI] [PubMed] [Google Scholar]
- Meissner M, Stein M, Urbich C, Reisinger K, Suske G, Staels B, Kaufmann R, Gille J. PPARα activators inhibits vascular endothelial growth factor receptor-2 expression by repressing Sp1-dependent DNA binding and transactivation. Circ. Res. 2004;94:324–332. doi: 10.1161/01.RES.0000113781.08139.81. [DOI] [PubMed] [Google Scholar]
- Michaelis UR, Xia N, Barbosa-Sicard E, Falck JR. Role of cytochrome P450 2C epoxygenases in hypoxia –induced cell migration and angiogenesis in retinal endothelial cells. 2008. Fleming. Invest. Ophthamol. Vis. Sci. 49:1242–1271. doi: 10.1167/iovs.07-1087. [DOI] [PubMed] [Google Scholar]
- Montesinos MC, Shaw JP, Yee H, Shamamian P, Cronstein BN. Adenosine A2A receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am. J. Pathol. 2004;164:1887–1892. doi: 10.1016/S0002-9440(10)63749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narala VR, Adapala RK, Suresh MV, Brock TG, Peters-Golden M, Reddy RC. Leukotriene B4 is a physiologically relevant endogenous peroxisome proliferator-activated receptor-α agonist. J. Biol. Chem. 2010;285(29):22067–22074. doi: 10.1074/jbc.M109.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narravula S, Colgan SP. Hypoxia-Inducible Factor 1-Mediated Inhibition of peroxisome proliferator-activated receptor {{alpha}} expression during hypoxia. J. Immunol. 2001;166:7543–7548. doi: 10.4049/jimmunol.166.12.7543. [DOI] [PubMed] [Google Scholar]
- Nayeem MA, Poloyac SM, Falck JR, Zeldin DC, Ledent C, Ponnoth DS, Ansari HR, Mustafa SJ. Role of CYP epoxygenases in A2AAR-mediated relaxation using A2AAR-null and wild-type mice. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2068–H2078. doi: 10.1152/ajpheart.01333.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Woo JK, Jin Q, Kang H, Jeong J, Kim K. Identification of novel antiangiogenic anticancer activities of deguelin targeting hypoxia-inducible factor-1 alpha. Int. J. Cancer. 2008;122:5–14. doi: 10.1002/ijc.23075. [DOI] [PubMed] [Google Scholar]
- Olah ME, Roudabush FL. Down-regulation of vascular endothelial growth factor expression after A(2A) adenosine receptor activation in PC12 pheochromocytoma cells. J. Pharmacol. Exp. Ther. 2000;293:779–787. [PubMed] [Google Scholar]
- Panigrahy D, Kaipainen A, Huang S, Butterfield CE, Barnés CM, Fannon M, Laforme MA, Chaponis DM, Folkman J, Kieran MW. PPARα agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. Proc. Natl. Acad. Sci.USA. 2008;105:985–990. doi: 10.1073/pnas.0711281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahy D, Kaipainen A, Greene ER, Huang S. Cytochrome P450-derived eicosanoids: the neglected pathway in cancer. Cancer Metastasis Rev. 2010;29:723–735. doi: 10.1007/s10555-010-9264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parng C, Seng WL, Semino c, McGrath P. Zebrafish: A preclinical model for drug screening. Assay& Drug Devlop. Tech. 2002;1:41–48. doi: 10.1089/154065802761001293. [DOI] [PubMed] [Google Scholar]
- Peng Z, Borea PA, Wilder T, Yee H, Chiriboga L, Blackburn MR, Azzena G, Resta G, Cronstein BN. Adenosine signaling contributes to ethanol induced fatty liver in mice. J. Clin. Invest. 2009;119:582–594. doi: 10.1172/JCI37409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pola R, Gaetani E, Flex A, Aprahamian TR, Bosch-Marce M, Losordo DW, Smith RC, Pola P. Comparative analysis of the in vivo angiogenic properties of stable prostacyclin analogs: a possible role for peroxisome proliferator-activated receptors. J. Mol. Cell Cardiol. 2004;36:363–370. doi: 10.1016/j.yjmcc.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Pozzi A, Macias-Perez I, Abair T, Wei S, Su Y, Zent R, Falck JR, Capdevila JH. Characterization of 5,6- and 8,9-epoxyeicosatrienoic acids (5,6- and 8,9-EET) as potent in vivo angiogenic lipid. J. Biol. Chem. 2005;280:27138–27146. doi: 10.1074/jbc.M501730200. [DOI] [PubMed] [Google Scholar]
- Pozzi A, Ibanez MR, Gatica AE, Yang S, Wei S, Mei S, Falck JR, Capdevila JH. Peroxisomal proliferator-activated receptor-α-dependent-inhibition of endothelial cell proliferation and tumorigenesis. J. Biol. Chem. 2007;282:17685–17695. doi: 10.1074/jbc.M701429200. [DOI] [PubMed] [Google Scholar]
- Ryzhov S, McCaleb JL, Goldstein AE, Biaggioni I, Feoktistov I. Role of adenosine receptors in the regulation of angiogenic factors andneovascularization in hypoxia. J. Pharmacol. Exp. Ther. 2007;320:565–572. doi: 10.1124/jpet.106.114850. [DOI] [PubMed] [Google Scholar]
- Sabti HA. Therapeutic angiogenesis in cardiovascular disease. J. Cardiothoracic Surgery. 2007;2:49–55. doi: 10.1186/1749-8090-2-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soledade CS, Franchini KG, Linden J, Huo Y. Stimulation of Adenosine A2A Receptor Regulates Peroxisome Proliferator Activated Receptors in Macrophages. FASEB J. 2007;21:816. 6. [Google Scholar]
- Vanalstine MA, Hough LB. Effects of acetylenic epoxygenase inhibitors on recombinant P450s.Drug Metab. Dispos. 2011;39:1221–1226. doi: 10.1124/dmd.110.037424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westra J, Molema G, Kallenberg CG. Hypoxia-inducible factor-1 as regulator of angiogenesis in rheumatoid arthritis-therapeutic implications. Curr. Med. Chem. 2010;17:254–263. doi: 10.2174/092986710790149783. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Xin B, Shigeto T, Umemoto M, Kasai-Sakamoto A, Futagami M, Tsuchida S, Al-Mulla F, Mizunuma H. Clofibric acid, a peroxisome proliferator-activated receptor alpha ligand, inhibits growth of human ovarian cancer. Mol Cancer Ther. 2007;4:1379–1386. doi: 10.1158/1535-7163.MCT-06-0722. [DOI] [PubMed] [Google Scholar]
- Zhao X, Quigley JE, Yuan J, Wang MH, Zhou Y, Imig JD. PPAR alpha activator fenofibrate increases renal CYP-derived eicosanoid synthesis and improves endothelial dilator function in obese Zucker rats. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2187–H2195. doi: 10.1152/ajpheart.00937.2005. [DOI] [PubMed] [Google Scholar]