Abstract
Systemic lupus erythematosus is a complex autoimmune disease of multifactorial origins. All compartments of the immune system appear to be affected, at least in some way, and to contribute to disease pathogenesis. Due to an escape from negative selection autoreactive T and B cells accumulate in SLE patients leading to the production of autoantibodies mainly raised against nuclear components and their subsequent deposition into target organs. We recently showed that basophils, in an IgE and IL-4 dependent manner, contribute to SLE pathogenesis by amplifying autoantibody production. Here, we summarize what we have learned about the deleterious role of basophils in lupus both in a mouse model and in SLE patients. We discuss which possible pathways could be involved in basophil activation and recruitment to secondary lymphoid organs during SLE, and how basophils may amplify autoantibody production.
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease of multifactorial origin including genetic, hormonal and environmental factors. This disease can affect multiple organs including skin, joints, heart, central nervous system and kidneys. Involvement of the latter leads to lupus nephritis (LN) affecting 40 to 60% of SLE patients. It is considered as one of the most severe manifestations as it may evolve to end-stage renal failure [1]. It is widely admitted that SLE pathogenesis is associated with a break in tolerance of apoptotic bodies, particularly nuclear antigens, leading autoreactive T and B cells to expand. They produce autoreactive antibodies mainly raised against nuclear components such as double stranded DNA (dsDNA) or RNA-binding protein Smith (Sm), Ro (SS-A) and La (SS-B) antigens…[1]. These autoantibodies will form circulating immune complexes (CIC) when bound to their antigens and complement factors. Deposition of these CICs in the target organ promotes a chronic inflammatory response, leading ultimately to tissue damage and organ dysfunction [1]. It is well established that disease activity and especially lupus nephritis activity correlate with autoantibody titers [2]. Like for most other autoimmune diseases, specific or curative therapy for SLE do not exist, and flares of the disease are contained by aggressive immunosuppressive treatments [3].
Basophils as immune regulators
Representing less than 1% of circulating white blood cells, basophils have long been ignored apart from their involvement in allergic diseases and parasitic infections as they express the high affinity IgE receptor (FcεRI) in its tetrameric isoform (αβγ2) like mast cells. However, new insights about their role in immune regulation have emerged in the past few years [4]. Following stimulation with a number of stimuli, basophils can secrete large amounts of cytokines (mainly IL-4 and IL-6), upregulate particular molecules at their surface with activating functions (such as MHC class II, BAFF, APRIL…) to influence a wide array of immune cells [4]. Basophils can promote T-helper type 2 CD4+ cells (Th2) differentiation [5,6], inhibit Th1 differentiation [7], act as antigen presenting cells for CD8+ T cells via MHC class I [8] and for CD4+ T cells via the upregulation of MHC class II [9–12]. If the latter point is controversial in the literature [13,14], a recent report by Otsuka et al. demonstrated that basophils were required for the induction of Th2 immunity to haptens and peptide antigens [9]. Basophils have been also described as components involved in the B cell class switch towards Th2-type immunoglobulins (IgG1, IgG2b and IgE), plasma cell differentiation and survival, as well as to support the humoral memory response [15–17]. Basophils also interact with the myeloid compartment since they cooperate with dendritic cells in a papain-induced Th2 differentiation model [18]. They also promote monocyte differentiation into M2 macrophage [19] and inhibit inflammatory responses by inducing FcγRIIb expression on monocytes through dendritic cell (DC)-induced IL-33 stimulation [20]. Basophils have been shown to promote the recruitment of immune cells at the site of inflammation during IgE-mediated chronic allergic inflammation [21] and in acquired immunity against ticks [22]. Their immunomodulatory actions have led to the identification of basophils as deleterious contributors to some human non-atopic diseases such as certain autoinflammatory syndromes (Hyper IgD syndrome (HIDS), TNF receptor-associated periodic syndrome (TRAPS)…) [23] and autoimmune diseases (SLE) [15].
Immune dysregulations in SLE
As a complex systemic autoimmune disease, SLE has been extensively studied both in animal models and in patient samples. T cell involvement in lupus pathogenesis has been well documented and regulatory T cells, Th1, Th2, Th17 and T follicular helper cell (TFH) components of the disease have been, at least partially, described [24]. As a break in tolerance is clearly occurring, regulatory T cells have been studied [25]. In animal models, the Th1/Th2 balance has been shown to be central for disease development and the way it affects organs, especially the kidney [26]. The Th1 component of the disease has been associated with tissue damage and was also shown to participate to the chronic inflammatory environment in affected organs [1,27]. IL-17 levels correlate with disease activity and IL-17 producing T cell number are increased in the periphery and in the kidneys of SLE patients [28]. IL-17 participates in increasing the survival and the proliferation of B cells, and their differentiation into antibody secreting cells in germinal centers [28,29]. TFH-like cells are detected in the periphery of active SLE patients [30] and associated with SLE development in mouse models [31].
Plasmacytoid dendritic cells (pDCs) are well studied for their involvement in SLE, particularly for the type I interferon signature, which has been observed both in SLE patients and mouse models. CICs and TLRs stimulate type I interferon production by pDCs and promote autoantibody production [32–34]. When stimulated by nucleic acid-containing immune complexes (NA-ICs), neutrophils may play a central role in the amplification of the disease by extruding DNA webs associated with alarmin peptides when dying via a phenomenon called NETosis (Neutrophil Extracellular Traps, NET). These newly available NA-ICs activate pDCs to amplify type I interferon production by these cells [35,36].
With the help of autoreactive T cells and other humoral stimulators described above, autoreactive B cells mature as autoreactive plasma cells and subsequently produce autoantibodies [24]. This central humoral component of pathogenesis leads to the formation of CIC and their deleterious effects. Presence of overreactive and dysregulated B cells in SLE have led to the development of immunotherapies targeting this cell compartment (Rituximab, anti-CD20 depleting antibody, Belimumab, anti-Blys/BAFF blocking antibody…) [24]. However, clinical trials using these monoclonal antibodies showed very limited benefits for the patients, likely due to trial designs and regulations [24]. Together with elevated total IgE titers in SLE patient serum as compared to the general population (see below) [37], a Th2 component of the disease appears to be critical for full disease development. Detailed review of current knowledge concerning SLE pathogenesis is reviewed in reference [24].
Basophils and the Th2 environment in a spontaneous model of murine lupus
Lyn is a Src family kinase member that functions both as a positive and negative regulator in a wide variety of immune receptor signaling. Lyn is expressed in all hematopoietic cells with the exclusion of T cells [38]. Lyn deficient animals have a strong susceptibility to Th2 challenges and develop in their late life an SLE-like disease including its kidney component [39–41]. Lyn is a key negative regulator of BCR signaling. This feature is thought to be central for the loss of tolerance that occurs in aged mice [38]. We recently showed that these mice also develop a peripheral basophilia responsible for a profound Th2 bias in an IgE- and IL-4-dependent manner. Knocking-out the Igh-7 (IgE) gene, the Il-4 gene or depleting basophils in Lyn deficient animals could indeed reverse this Th2 bias [5]. Aged Lyn−/− mice acquire autoantibodies against nuclear antigens and CIC deposit in the kidney, leading to the development of end-stage renal disease and loss of kidney function [39,40]. We verified whether the early basophil-dependent Th2 bias of these mice was responsible for the autoimmune phenotype observed in aged animals. Our data showed that autoreactive IgE (anti-Nuclear Antigen (ANA) and anti-dsDNA) accumulated in CIC from Lyn−/− mice [15]. Furthermore, these IgE-containing immune complexes (IC) were able to strongly induce IL-4 production by basophils. Adding IgE or IL-4 deficiency to the Lyn−/− mice, despite a still present basophilia, led to the development of a much milder disease with decreased autoantibody titers and CIC deposition, rescuing the kidney function [15]. Basophils from aged Lyn−/− mice had an activated phenotype and were able to accumulate in secondary lymphoid organs (SLO). SLO-accumulated basophils expressed MHC class II molecule and a membrane-bound form of the B cell activating factor BAFF explaining how they could, in addition to their production of IL-4 and IL-6, interact with the T and B cell compartments [15]. Moreover, short-term antibody-mediated basophil depletion led to a dramatic decrease in kidney pro-inflammatory cytokine concentrations and in autoantibody titers. The latter effect was due to basophil-dependent support of (autoreactive) plasma cell survival in SLO [15]. Altogether, these data allowed us to conclude that basophils and the Th2 environment (through IgE- and IL-4-dependent mechanisms) were key contributors for lupus-like disease in the Lyn−/− mouse model. Basophil-deficient animal models have recently been reported and will allow to confirm basophil contribution in other spontaneous or inducible SLE mouse models [22,42].
Basophils and the Th2 environment in human disease
Evidences for a Th2 component in the human disease have been reported. Indeed, elevated levels of circulating IgE in SLE patients have been documented without being associated with a higher incidence of atopic diseases[37]. Autoreactive IgE raised against dsDNA could be detected in sera of SLE patients. Interestingly, their titers correlated with the SLE disease activity index (SLEDAI) and even more prominently in patients with active nephritis [15]. Such autoreactive IgE had already been described in the early 80’s and were known to be present at the surface of basophils [43]. However, no degranulated phenotype is observed in SLE patient basophils (unpublished data). These SLE patient basophils also had an activated phenotype exhibiting an upregulated surface expression of CD203c and HLA-DR molecules. Lyn, which is a negative regulator of mouse basophil proliferation [5], had normal expression levels in circulating basophils of SLE patient [15]. This explains why the basophilia observed in the Lyn−/− mouse model is not observed in SLE patients who show a marked basopenia that correlates with disease activity. These basophils had an upregulated CD62L (L-selectin) surface expression suggesting transendothelial migration capabilities for recruitment into SLO. This was supported by the observed accumulation of basophils in SLE patient SLO (spleen and lymph nodes) whereas control biopsies did not show any staining [15]. Since autoreactive IgE titers increase with disease activity, and especially with the activity of lupus nephritis, it is reasonable to assume that IgE concentration into CICs increases as well, suggesting that the Th2 component of the disease is particularly strengthened during flares. As in other renal pathologies, flares are thought to be associated with benign viral infections able to skew the immune system towards a Th2 humoral response [1]. This would correlate as well with the basopenia which is aggravated during active phases of the disease. However, CICs do not contain only IgE. Indeed, CICs contain autoantibodies from all other isotypes: IgM, IgG, IgA and IgD ([1] and unpublished data). Basophils are armed to bind several isotypes (IgG, IgE, IgA and IgD) which can induce positive or negative signals [23,44,45]. For instance, FcεRI triggers activating signals and FcγRIIb triggers inhibitory signals when co-engaged [44]. The extent of the CIC-mediated basophil activation therefore results from the integration of these different signals which, in all SLE cases we observed, does not lead to basophil degranulation (unpublished data). However, basophils are stimulated enough to produce cytokines and to upregulate stimulatory molecules on their surface. They can then provide a strong support to autoreactive B and T cells to amplify autoantibody and CIC production [15]. Chemotactic signals (ligands and receptors) responsible for the peripheral basopenia observed in SLE patients and for basophil addressing to SLO remain to be identified.
Basophil activation pathways in SLE
As indicated above, murine and human data led us to propose the following working hypothesis: basophils, due to their permanent, systemic and low intensity stimulation, amplify autoantibody production and CIC formation by supporting both autoreactive T and B cells in SLO [46]. This hypothesis supports the notion that basophils have a deleterious role in lupus pathogenesis. Whereas we demonstrated a clear correlation between autoreactive IgE titers in patients and the activation status of their basophils, together with the marked basopenia, it is possible that many other stimuli might impact on these cells in lupus pathogenesis. As summarized in Table 1, basophils are known to express a wide variety of receptors, the ligands of which have been described as being upregulated in SLE patient serum. By the same approach, chemokine receptors expressed by basophils, the ligands of which are dysregulated in SLE patients are listed in Table 1 together with their known effects on basophils. All these receptors and their respective ligands could participate to basophil recruitment into SLO and/or at the site of chronic inflammation during SLE and hence contribute to the deleterious role of basophils in SLE. Another example of possible basophil activation pathway in SLE concerns aberrant immune responses to dsDNA, RNA and other nuclear components which occur during SLE pathogenesis. An elegant study by Marichal et al. showed that the Th2 bias induced by alum-adjuvant based immunizations is in fact due to the DNA released by cell death [47]. Since basophils express TLR-9 [48], direct basophil stimulation by excessive self-antigen concentrations (dsDNA via NETosis and apoptotic bodies…) could occur. Similarly, TLR-2, which is expressed on basophils as well, could trigger an activation signal via low molecular weight hyaluronan derived from the inflamed site [49] and/or be engaged by a concomitant pathogenic infection. These events involving TLRs could amplify the deleterious role of basophils in SLE.
Table 1.
Basophil Receptors and their dysregulated ligands in SLE
| Molecule dysregulated in Human SLE | Basophil- expressed receptor involved | Effect on basophils | References for SLE | References for the effects on basophils |
|---|---|---|---|---|
| CCL2 (MCP-1) | CCR1 and/or CCR2 | Marginal IL-4 and LTC4 secretion, Histamine release, migration | [53] | [54–58] |
| CCL3 (MIP-1α) | CCR1 and/or CCR5 | Migration | [53,59,60] | [54,55,57] |
| CCL5 (RANTES) | CCR3 (CCR1 and CCR5) | Migration | [59,60] | [55,56,58] |
| CCL7 (MCP-3) | CCR1, CCR2, CCR3, CCR5 | Migration to SLO | [53] | [18] |
| CCL8 (MCP-2) | CCR1, CCR2, CCR3, CCR5 | Migration | [53] | [55] |
| CCL17 (TARC) | CCR4 | Skin recruitment | [53] | [61] |
| CCL19 (MIP-3β) | CCR7 | Migration | [53] | [62] |
| CCL21 (6Ckine) | CCR7 | Migration | [53] | [62] |
| CXCL1 (Gro-α) | CXCR2 | Activation and Migration | [59] | [57,63] |
| CXCL2 (Gro-β) | CXCR2 | Activation and Migration | [59] | [57,63] |
| CXCL8 (IL-8) | CXCR1, CXCR2 | Histamine release and migration | [53] | [57] |
| CXCL12 (SDF-1α) | CXCR4 | Migration | [64] | [56,57] |
| IL-18 | IL-18R | IL-4 and IL-13 secretion | [53,59] | [65] |
| IL-33 | T1/ST2 | IL-4, -8, -13 and LTC4 secretion, priming and adherence | [66,67] | [65,68,69] |
| Leptin | Leptin receptor | Survival, migration, degranulation, IL-4 and IL-13 secretion | [70] | [71] |
| VEGF | VEGFR2 | Migration | [72] | [73] |
| PGD2 | CRTH2 | Priming, activation and migration | [74] | [75,76] |
| PGD2 | DP1 | Inhibition | [74] | [75,76] |
| Low molecular weight Hyaluronan | TLR-2 | Priming, IL-4 and IL-13 secretion | [77] | [49,78] |
CCL: CC chemokine
CCR: CC chemokine receptor
CXCL: CXC chemokine
CXCR: CXC chemokine receptor
CRTH2: Chemoattractant receptor homologous molecule expressed on T helper type 2 cells, Prostaglandin D2 receptor 2, DP2
DP1: Prostaglandin D2 receptor 1
LTC4: leukotriene C4
MCP: Monocyte chemotactic protein
MIP: Macrophage inflammatory protein
PGD2: Prostaglandin D2
RANTES: Regulated on Activation, Normal T cell Expressed and Secreted
SDF: Stromal cell-derived factor
TARC: Thymus and activation regulated chemokine
TLR: Toll-like receptor
T1/ST2: IL-33 receptor
VEGF: Vascular endothelial growth factor
Putative pathways for additional deleterious function of basophils in SLE
As we demonstrated in the Lyn−/− mouse model, basophils act on T and B cell compartments via the production of IL-4, BAFF and MHC class II surface expression, promoting Th2 differentiation and autoantibody production [15]. However, as described above, basophils may act on the immune system in multiple ways. Some possible pathways by which basophils could contribute to the amplification of autoantibody production and to SLE pathogenesis in general are summarized in Figure 1. For instance, basophils have been shown to represent key players in the amplification of Th17 differentiation in vitro when co-cultured with T cells and lung or mucosal epithelial cells from patients with chronic inflammatory diseases [50,51]. By promoting Th17 differentiation and by the possibility of being indirectly activated by IL-17A, basophils could promote Th17 cell activity during SLE. On the other end, basophils can produce retinoic acid, promoting Th2 and inhibiting Th1 and Th17 differentiations [52]. Thus, activated basophils could influence in many ways the Th1 and Th17 components during SLE pathogenesis.
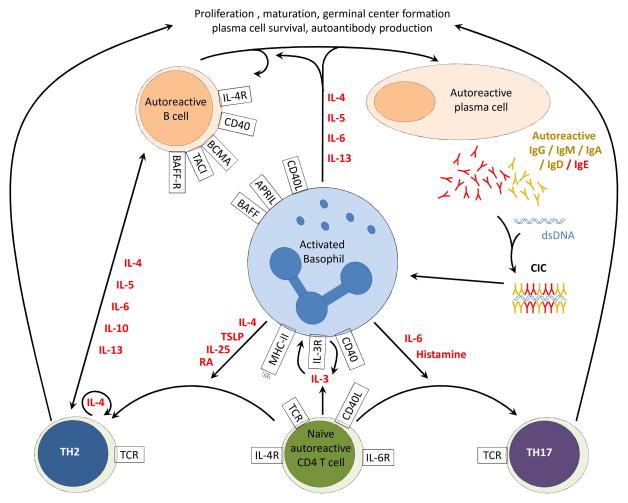
Figure 1. Deleterious interactions between basophils, B and T cells during SLE.
Autoreactive T and B cells trigger autoantibody production and CIC formation during SLE. Activated basophils migrate to lymph nodes where they can interact with both B and T cells. Through secretion of IL-4, IL-6, IL-5, IL-13 and membrane expression of CD40L, BAFF and APRIL, basophils interact with the B cell compartment. They induce autoreactive B cell proliferation, class switch (towards IgG, IgA and IgE), maturation, plasma cell survival and autoantibody production via relevant receptors expressed on B and plasma cells. Basophil-expressed CD40 and MHC class II can activate naïve T cells via CD40L and T cell receptor (TCR) respectively. Activated T Cells secrete IL-3 which induces basophils to secrete IL3 to sustain their own activation and survival. Basophils can drive the Th17 differentiation by secreting IL6 and histamine. Th17 cells promote germinal center formation and support autoantibody production. Through IL-4, Thymic stromal lymphopoietin (TSLP), IL-25 and retinoic acid secretion, together with MHC class II and CD40 surface expression, basophils induce Th2 differentiation of CD4+ T cells. Once primed by basophils, Th2 cells secrete large amounts of IL-4 to support their own generation. This IL-4, together with IL-5, IL-6, IL-10, IL-13 and cell-cell interactions drive autoantibody production by the B cell compartment.
Concluding remarks
In summary, research data obtained in recent years largely support a deleterious role of basophils in SLE pathogenesis. This involves autoreactive IgEs included in CICs that activate basophils, but also other factors dysregulated during SLE, which could influence the basophil activation status and their recruitment to SLO. Identifying these factors and analyzing basophil-mediated effects on the immune system during SLE pathogenesis should offer new diagnostic and prognostic tools. It has become clear that targeting basophil or involved basophil-activating factors represent valuable strategies to develop new therapeutic approaches in SLE. Will the targeting of autoreactive IgE allow to break the amplification loop of autoantibody production supported by basophils? Could immunotherapy allowing basophil depletion be an efficient new approach to prevent flares of the disease? These questions are likely to be central in our investigations in the coming years.
Highlights.
Basophils and Th2 environment contribute to systemic lupus erythematosus pathogenesis.
Basophil activation could occur through different stimuli during lupus.
Basophils amplify autoantibody production by supporting autoreactive B cell maturation and plasma cell survival.
Basophil-mediated T cell differentiation could be induced through various signals.
Acknowledgments
We are grateful to the patients and their referring physicians for consenting to participate to these studies. We apologize to colleagues whose work was not cited due to page limitations. We thank Dr Marc Benhamou and Dr Ulrich Blank (INSERM, France) for critical reading of the manuscript. We are indebted to Dr Juan Rivera (NIAMS, NIH, USA) who directed most of our original work described in this review. This work was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the U.S. National Institutes of Health (NIH), by the French Institut National de la Santé Et de la Recherche Médicale (INSERM), by the Assistance Publique – Hôpitaux de Paris (AP-HP) and by grants from the Mairie de Paris (Emergences 2010), ANR JCJC SVSE1 2011 BASILE and the French Kidney Foundation (Fondation du Rein). NC is under a translational research contract with the AP-HP (CHRT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the biannual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 2.Villegas-Zambrano N, Martinez-Taboada VM, Bolivar A, San Martin M, Alvarez L, Marin MJ, Lopez-Hoyos M. Correlation between clinical activity and serological markers in a wide cohort of patients with systemic lupus erythematosus: an eight-year prospective study. Ann N Y Acad Sci. 2009;1173:60–66. doi: 10.1111/j.1749-6632.2009.04650.x. [DOI] [PubMed] [Google Scholar]
- 3.Daugas E. Treatment of proliferative glomerulonephritis of systemic lupus erythematosus. Recent development and current recommendations. Rev Med Interne. 2008;29:710–717. doi: 10.1016/j.revmed.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 4••.Karasuyama H, Mukai K, Obata K, Tsujimura Y, Wada T. Nonredundant roles of basophils in immunity. Annu Rev Immunol. 2011;29:45–69. doi: 10.1146/annurev-immunol-031210-101257. This outstanding review summarizes the current knowledge on human and mouse basophils. [DOI] [PubMed] [Google Scholar]
- 5.Charles N, Watford WT, Ramos HL, Hellman L, Oettgen HC, Gomez G, Ryan JJ, O’Shea JJ, Rivera J. Lyn kinase controls basophil GATA-3 transcription factor expression and induction of Th2 cell differentiation. Immunity. 2009;30:533–543. doi: 10.1016/j.immuni.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez Gomez M, Talke Y, Hofmann C, Ketelsen I, Hermann F, Reich B, Goebel N, Schmidbauer K, Dunger N, Bruhl H, et al. Basophils control T-cell responses and limit disease activity in experimental murine colitis. Mucosal Immunol. 2013 doi: 10.1038/mi.2013.38. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Shen T, Min B. Basophils can directly present or cross-present antigen to CD8 lymphocytes and alter CD8 T cell differentiation into IL-10-producing phenotypes. J Immunol. 2009;183:3033–3039. doi: 10.4049/jimmunol.0900332. [DOI] [PubMed] [Google Scholar]
- 9•.Otsuka A, Nakajima S, Kubo M, Egawa G, Honda T, Kitoh A, Nomura T, Hanakawa S, Sagita Moniaga C, Kim B, et al. Basophils are required for the induction of Th2 immunity to haptens and peptide antigens. Nat Commun. 2013;4:1738. doi: 10.1038/ncomms2740. This article shows that basophils indeed act as antigen presenting cells via MHC class II molecules but are not able to efficiently process full proteins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, Nakanishi K. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 13.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, Lambrecht BN. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan BM, Liang HE, Bando JK, Wu D, Cheng LE, McKerrow JK, Allen CD, Locksley RM. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12:527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charles N, Hardwick D, Daugas E, Illei GG, Rivera J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med. 2010;16:701–707. doi: 10.1038/nm.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denzel A, Maus UA, Rodriguez Gomez M, Moll C, Niedermeier M, Winter C, Maus R, Hollingshead S, Briles DE, Kunz-Schughart LA, et al. Basophils enhance immunological memory responses. Nat Immunol. 2008;9:733–742. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez Gomez M, Talke Y, Goebel N, Hermann F, Reich B, Mack M. Basophils support the survival of plasma cells in mice. J Immunol. 2010;185:7180–7185. doi: 10.4049/jimmunol.1002319. [DOI] [PubMed] [Google Scholar]
- 18.Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, Murthy N, Kepler TB, Malissen B, Pulendran B. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11:608–617. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Egawa M, Mukai K, Yoshikawa S, Iki M, Mukaida N, Kawano Y, Minegishi Y, Karasuyama H. Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 phenotype via basophil-derived interleukin-4. Immunity. 2013;38:570–580. doi: 10.1016/j.immuni.2012.11.014. This study unravels the influence of basophils on monocyte differentiation in an IgE-dependent chronic inflammatory disease mouse model. [DOI] [PubMed] [Google Scholar]
- 20.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukai K, Matsuoka K, Taya C, Suzuki H, Yokozeki H, Nishioka K, Hirokawa K, Etori M, Yamashita M, Kubota T, et al. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity. 2005;23:191–202. doi: 10.1016/j.immuni.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Wada T, Ishiwata K, Koseki H, Ishikura T, Ugajin T, Ohnuma N, Obata K, Ishikawa R, Yoshikawa S, Mukai K, et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest. 2010;120:2867–2875. doi: 10.1172/JCI42680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen K, Xu W, Wilson M, He B, Miller NW, Bengten E, Edholm ES, Santini PA, Rath P, Chiu A, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10:889–898. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24••.Liu Z, Davidson A. Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat Med. 2012;18:871–882. doi: 10.1038/nm.2752. This outstanding review summarizes the current understanding of SLE pathogenesis from genetic basis to clinical trials via unravelling the current knowledge on lupus-related immune dysregulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheinecker C, Bonelli M, Smolen JS. Pathogenetic aspects of systemic lupus erythematosus with an emphasis on regulatory T cells. J Autoimmun. 2010;35:269–275. doi: 10.1016/j.jaut.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu S, Sugiyama N, Masutani K, Sadanaga A, Miyazaki Y, Inoue Y, Akahoshi M, Katafuchi R, Hirakata H, Harada M, et al. Membranous glomerulonephritis development with Th2-type immune deviations in MRL/lpr mice deficient for IL-27 receptor (WSX-1) J Immunol. 2005;175:7185–7192. doi: 10.4049/jimmunol.175.11.7185. [DOI] [PubMed] [Google Scholar]
- 27.Peng SL, Szabo SJ, Glimcher LH. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci U S A. 2002;99:5545–5550. doi: 10.1073/pnas.082114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alunno A, Bartoloni E, Bistoni O, Nocentini G, Ronchetti S, Caterbi S, Valentini V, Riccardi C, Gerli R. Balance between regulatory T and Th17 cells in systemic lupus erythematosus: the old and the new. Clin Dev Immunol. 2012;2012:823085. doi: 10.1155/2012/823085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 30.Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, Manku H, Vyse TJ, Roncador G, Huttley GA, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 31.Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 33.Mathian A, Weinberg A, Gallegos M, Banchereau J, Koutouzov S. IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black x New Zealand White) F1 but not in BALB/c mice. J Immunol. 2005;174:2499–2506. doi: 10.4049/jimmunol.174.5.2499. [DOI] [PubMed] [Google Scholar]
- 34.Obermoser G, Pascual V. The interferon-alpha signature of systemic lupus erythematosus. Lupus. 2010;19:1012–1019. doi: 10.1177/0961203310371161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atta AM, Sousa CP, Carvalho EM, Sousa-Atta ML. Immunoglobulin E and systemic lupus erythematosus. Braz J Med Biol Res. 2004;37:1497–1501. doi: 10.1590/s0100-879x2004001000008. [DOI] [PubMed] [Google Scholar]
- 38.Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity. 2005;22:9–18. doi: 10.1016/j.immuni.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 40.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 41.Odom S, Gomez G, Kovarova M, Furumoto Y, Ryan JJ, Wright HV, Gonzalez-Espinosa C, Hibbs ML, Harder KW, Rivera J. Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase. J Exp Med. 2004;199:1491–1502. doi: 10.1084/jem.20040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–374. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 43.Camussi G, Tetta C, Benveniste J. Detection of basophil sensitization by IgE antibodies to nuclear antigens in connective tissue diseases. Int Arch Allergy Appl Immunol. 1982;69:358–362. doi: 10.1159/000233200. [DOI] [PubMed] [Google Scholar]
- 44.Cassard L, Jonsson F, Arnaud S, Daeron M. Fcgamma receptors inhibit mouse and human basophil activation. J Immunol. 2012;189:2995–3006. doi: 10.4049/jimmunol.1200968. [DOI] [PubMed] [Google Scholar]
- 45.Iikura M, Yamaguchi M, Fujisawa T, Miyamasu M, Takaishi T, Morita Y, Iwase T, Moro I, Yamamoto K, Hirai K. Secretory IgA induces degranulation of IL-3-primed basophils. J Immunol. 1998;161:1510–1515. [PubMed] [Google Scholar]
- 46.Charles N, Rivera J. Basophils and autoreactive IgE in the pathogenesis of systemic lupus erythematosus. Curr Allergy Asthma Rep. 2011;11:378–387. doi: 10.1007/s11882-011-0216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marichal T, Ohata K, Bedoret D, Mesnil C, Sabatel C, Kobiyama K, Lekeux P, Coban C, Akira S, Ishii KJ, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17:996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 48.Komiya A, Nagase H, Okugawa S, Ota Y, Suzukawa M, Kawakami A, Sekiya T, Matsushima K, Ohta K, Hirai K, et al. Expression and function of toll-like receptors in human basophils. Int Arch Allergy Immunol. 2006;140 (Suppl 1):23–27. doi: 10.1159/000092707. [DOI] [PubMed] [Google Scholar]
- 49.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 50.Wakahara K, Baba N, Van VQ, Begin P, Rubio M, Ferraro P, Panzini B, Wassef R, Lahaie R, Caussignac Y, et al. Human basophils interact with memory T cells to augment Th17 responses. Blood. 2012;120:4761–4771. doi: 10.1182/blood-2012-04-424226. [DOI] [PubMed] [Google Scholar]
- 51.Wong CK, Cao J, Yin YB, Lam CW. Interleukin-17A activation on bronchial epithelium and basophils: a novel inflammatory mechanism. Eur Respir J. 2010;35:883–893. doi: 10.1183/09031936.00088309. [DOI] [PubMed] [Google Scholar]
- 52.Spiegl N, Didichenko S, McCaffery P, Langen H, Dahinden CA. Human basophils activated by mast cell-derived IL-3 express retinaldehyde dehydrogenase-II and produce the immunoregulatory mediator retinoic acid. Blood. 2008;112:3762–3771. doi: 10.1182/blood-2008-01-135251. [DOI] [PubMed] [Google Scholar]
- 53.Bauer JW, Baechler EC, Petri M, Batliwalla FM, Crawford D, Ortmann WA, Espe KJ, Li W, Patel DD, Gregersen PK, et al. Elevated serum levels of interferon-regulated chemokines are biomarkers for active human systemic lupus erythematosus. PLoS Med. 2006;3:e491. doi: 10.1371/journal.pmed.0030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gaga M, Ong YE, Benyahia F, Aizen M, Barkans J, Kay AB. Skin reactivity and local cell recruitment in human atopic and nonatopic subjects by CCL2/MCP-1 and CCL3/MIP-1alpha. Allergy. 2008;63:703–711. doi: 10.1111/j.1398-9995.2007.01578.x. [DOI] [PubMed] [Google Scholar]
- 55.Heinemann A, Hartnell A, Stubbs VE, Murakami K, Soler D, LaRosa G, Askenase PW, Williams TJ, Sabroe I. Basophil responses to chemokines are regulated by both sequential and cooperative receptor signaling. J Immunol. 2000;165:7224–7233. doi: 10.4049/jimmunol.165.12.7224. [DOI] [PubMed] [Google Scholar]
- 56.Iikura M, Ebisawa M, Yamaguchi M, Tachimoto H, Ohta K, Yamamoto K, Hirai K. Transendothelial migration of human basophils. J Immunol. 2004;173:5189–5195. doi: 10.4049/jimmunol.173.8.5189. [DOI] [PubMed] [Google Scholar]
- 57.Iikura M, Miyamasu M, Yamaguchi M, Kawasaki H, Matsushima K, Kitaura M, Morita Y, Yoshie O, Yamamoto K, Hirai K. Chemokine receptors in human basophils: inducible expression of functional CXCR4. J Leukoc Biol. 2001;70:113–120. [PubMed] [Google Scholar]
- 58.Ochensberger B, Tassera L, Bifrare D, Rihs S, Dahinden CA. Regulation of cytokine expression and leukotriene formation in human basophils by growth factors, chemokines and chemotactic agonists. Eur J Immunol. 1999;29:11–22. doi: 10.1002/(SICI)1521-4141(199901)29:01<11::AID-IMMU11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 59.Lit LC, Wong CK, Tam LS, Li EK, Lam CW. Raised plasma concentration and ex vivo production of inflammatory chemokines in patients with systemic lupus erythematosus. Ann Rheum Dis. 2006;65:209–215. doi: 10.1136/ard.2005.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vila LM, Molina MJ, Mayor AM, Cruz JJ, Rios-Olivares E, Rios Z. Association of serum MIP-1alpha, MIP-1beta, and RANTES with clinical manifestations, disease activity, and damage accrual in systemic lupus erythematosus. Clin Rheumatol. 2007;26:718–722. doi: 10.1007/s10067-006-0387-y. [DOI] [PubMed] [Google Scholar]
- 61.Gilet J, Chang Y, Chenivesse C, Legendre B, Vorng H, Duez C, Wallaert B, Porte H, Senechal S, Tsicopoulos A. Role of CCL17 in the generation of cutaneous inflammatory reactions in Hu-PBMC-SCID mice grafted with human skin. J Invest Dermatol. 2009;129:879–890. doi: 10.1038/jid.2008.333. [DOI] [PubMed] [Google Scholar]
- 62.Lim LH, Burdick MM, Hudson SA, Mustafa FB, Konstantopoulos K, Bochner BS. Stimulation of human endothelium with IL-3 induces selective basophil accumulation in vitro. J Immunol. 2006;176:5346–5353. doi: 10.4049/jimmunol.176.9.5346. [DOI] [PubMed] [Google Scholar]
- 63.Geiser T, Dewald B, Ehrengruber MU, Clark-Lewis I, Baggiolini M. The interleukin-8-related chemotactic cytokines GRO alpha, GRO beta, and GRO gamma activate human neutrophil and basophil leukocytes. J Biol Chem. 1993;268:15419–15424. [PubMed] [Google Scholar]
- 64.Wang A, Fairhurst AM, Tus K, Subramanian S, Liu Y, Lin F, Igarashi P, Zhou XJ, Batteux F, Wong D, et al. CXCR4/CXCL12 hyperexpression plays a pivotal role in the pathogenesis of lupus. J Immunol. 2009;182:4448–4458. doi: 10.4049/jimmunol.0801920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kroeger KM, Sullivan BM, Locksley RM. IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38alpha-dependent pathway. J Leukoc Biol. 2009;86:769–778. doi: 10.1189/jlb.0708452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mok MY, Huang FP, Ip WK, Lo Y, Wong FY, Chan EY, Lam KF, Xu D. Serum levels of IL-33 and soluble ST2 and their association with disease activity in systemic lupus erythematosus. Rheumatology (Oxford) 2010;49:520–527. doi: 10.1093/rheumatology/kep402. [DOI] [PubMed] [Google Scholar]
- 67.Yang Z, Liang Y, Xi W, Li C, Zhong R. Association of increased serum IL-33 levels with clinical and laboratory characteristics of systemic lupus erythematosus in Chinese population. Clin Exp Med. 2011;11:75–80. doi: 10.1007/s10238-010-0115-4. [DOI] [PubMed] [Google Scholar]
- 68.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suzukawa M, Iikura M, Koketsu R, Nagase H, Tamura C, Komiya A, Nakae S, Matsushima K, Ohta K, Yamamoto K, et al. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J Immunol. 2008;181:5981–5989. doi: 10.4049/jimmunol.181.9.5981. [DOI] [PubMed] [Google Scholar]
- 70.Garcia-Gonzalez A, Gonzalez-Lopez L, Valera-Gonzalez IC, Cardona-Munoz EG, Salazar-Paramo M, Gonzalez-Ortiz M, Martinez-Abundis E, Gamez-Nava JI. Serum leptin levels in women with systemic lupus erythematosus. Rheumatol Int. 2002;22:138–141. doi: 10.1007/s00296-002-0216-9. [DOI] [PubMed] [Google Scholar]
- 71.Suzukawa M, Nagase H, Ogahara I, Han K, Tashimo H, Shibui A, Koketsu R, Nakae S, Yamaguchi M, Ohta K. Leptin enhances survival and induces migration, degranulation, and cytokine synthesis of human basophils. J Immunol. 2011;186:5254–5260. doi: 10.4049/jimmunol.1004054. [DOI] [PubMed] [Google Scholar]
- 72.Moneib HA, Salem SA, Aly DG, Khedr HT, Wafaey HA, Hassan HE. Assessment of serum vascular endothelial growth factor and nail fold capillaroscopy changes in systemic lupus erythematosus with and without cutaneous manifestations. J Dermatol. 2012;39:52–57. doi: 10.1111/j.1346-8138.2011.01322.x. [DOI] [PubMed] [Google Scholar]
- 73.de Paulis A, Prevete N, Fiorentino I, Rossi FW, Staibano S, Montuori N, Ragno P, Longobardi A, Liccardo B, Genovese A, et al. Expression and functions of the vascular endothelial growth factors and their receptors in human basophils. J Immunol. 2006;177:7322–7331. doi: 10.4049/jimmunol.177.10.7322. [DOI] [PubMed] [Google Scholar]
- 74.Somparn P, Hirankarn N, Leelahavanichkul A, Khovidhunkit W, Thongboonkerd V, Avihingsanon Y. Urinary proteomics revealed prostaglandin H(2)D-isomerase, not Zn-alpha2-glycoprotein, as a biomarker for active lupus nephritis. J Proteomics. 2012;75:3240–3247. doi: 10.1016/j.jprot.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 75.Monneret G, Boumiza R, Gravel S, Cossette C, Bienvenu J, Rokach J, Powell WS. Effects of prostaglandin D(2) and 5-lipoxygenase products on the expression of CD203c and CD11b by basophils. J Pharmacol Exp Ther. 2005;312:627–634. doi: 10.1124/jpet.104.074823. [DOI] [PubMed] [Google Scholar]
- 76.Yoshimura-Uchiyama C, Iikura M, Yamaguchi M, Nagase H, Ishii A, Matsushima K, Yamamoto K, Shichijo M, Bacon KB, Hirai K. Differential modulation of human basophil functions through prostaglandin D2 receptors DP and chemoattractant receptor-homologous molecule expressed on Th2 cells/DP2. Clin Exp Allergy. 2004;34:1283–1290. doi: 10.1111/j.1365-2222.2004.02027.x. [DOI] [PubMed] [Google Scholar]
- 77.Fattal I, Shental N, Mevorach D, Anaya JM, Livneh A, Langevitz P, Zandman-Goddard G, Pauzner R, Lerner M, Blank M, et al. An antibody profile of systemic lupus erythematosus detected by antigen microarray. Immunology. 2010;130:337–343. doi: 10.1111/j.1365-2567.2010.03245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bieneman AP, Chichester KL, Chen YH, Schroeder JT. Toll-like receptor 2 ligands activate human basophils for both IgE-dependent and IgE-independent secretion. J Allergy Clin Immunol. 2005;115:295–301. doi: 10.1016/j.jaci.2004.10.018. [DOI] [PubMed] [Google Scholar]