Abstract
Concise syntheses of two Leucetta-derived naphthimidazole alkaloids, kealiiquinone and 2-deoxy-2-aminokealiiquinone, are described based on a biosynthetic-guided hypothesis. Advanced intermediates containing the full naphthimidazole framework are constructed through Friedel-Crafts chemistry followed by oxidation of the electron rich C-ring with hydrogen peroxide. The cytotoxicity of these alkaloids in a breast cancer cell line along with several closely related marine-derived natural products kealiinines A-C and analogs are reported.
Keywords: Naphthimidazole, Biomimetic, Oxidation, MCF7, Keyword_5
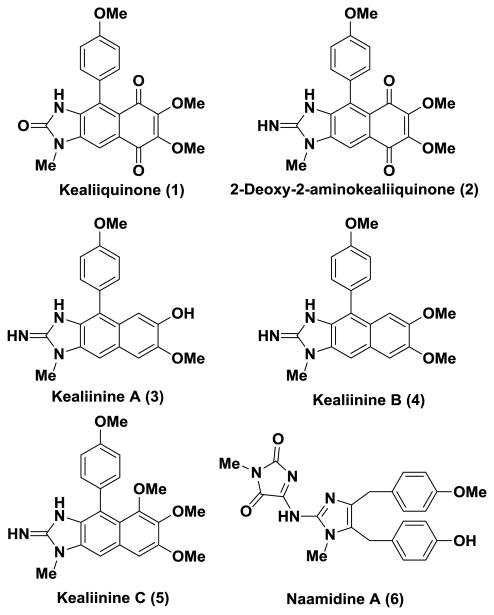
Marine sponges produce an array of structurally unique secondary metabolites that exhibit interesting biological activities and as such serve as lead compounds in drug discovery programs.1, 2 Sponges of the Leucetta and Clathrina families are known to produce various types of natural products, including several examples of imidazole-containing alkaloids.3, 4 In the course of their isolation bioactivity-guided fractionation schemes are used and as a result some biological information emerges. However, it is oftentimes limited in scope based on the screens available in a particular lab and because of the nature of the isolation process the purity of the isolated material can be questionable. While some limited structure-activity relationship information can be obtained in these efforts through the isolation of related molecules, it is restricted to (usually) modest structural changes. Such is the case in the Leucetta and Clathrina alkaloids, wherein a number of different groups of natural products have been isolated and their biological activities assessed (Figure 1), but few direct comparative studies have been performed and limited in depth investigations have been undertaken.4 A notable exception to this is naamidine A (6),5, 6 for which both SAR7 and mechanism of action data have been obtained.8-10 Our lab has developed a number of total syntheses of various family members of the Calcarous family of alkaloids11 and herein we report the synthesis and evaluation of synthetic versions of several naphthimidazole-natural products, precursors and analogs as potential anti-cancer molecules.
Figure 1. Various Leucetta-derived alkaloids.
Several years ago Clardy and Scheuer reported the isolation of kealiiquinone (1) from a Leucetta-derived sponge possessing a unique imidazobenzoquinone framework (Figure 1).12 At the time of this report, no bioactivity data were reported, but in a subsequent synthetic effort it was determined that it was cytotoxic, with potentially a unique mechanism of action based on the inhibition profile in a multi cell line screen.13 Schmitz reported the isolation of 2-deoxy-2-aminokealiiquinone (2), but no bioactivity was reported for this molecule.14 More recently, Proksch and coworkers reported the isolation of three related naphthimidazole derivatives for which only limited investigations were performed using the brine shrimp toxicity assay.15 Our group has developed concise synthetic methods for the total syntheses o all of these natural products.16, 17 The general strategy that we have adopted involves the elaboration of simple imidazoles and as such provides the opportunity to evaluate C2-deletion derivatives as well as to prepare other analogs.
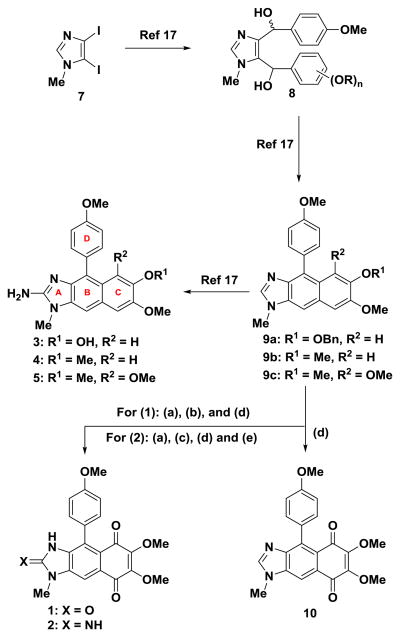
We have described recently the synthesis of kealiinines A-C (3-5) and isokealiinine C (11) from diiodoimidazole 7 utilizing a Friedel-Crafts/dehydration sequence to construct the B-ring, these are efforts are summarized in Scheme 1.16 the Ohta lab has published a total synthesis of kealiiquinone (1) via the oxidation of intermediate related to 9c.18, 19 Taking our lead from this latter report, we have streamlined this sequence substantially to provide access to both kealiiquinone (1) and the 2-amino congener 2, the latter for the first time in synthetic form. Specifically, for synthesis of kealiiquinone (1) the imidazole C2-position was oxidized by lithiation with n-BuLi followed by treatment with TMS-peroxide, according to the protocol of the Lipshutz group.20 Subsequent oxidation o the C-ring with hydrogen peroxide resulted in the formation of the corresponding benzoquinone and thus kealiiquinone (1) (Scheme 1).21 While in many respects our synthesis is broadly similar to the one reported by Ohta in terms of general strategy, it is substantially shorter and protecting group ftree.18, 19 In addition, we have employed extremely simple conditions for the introduction of the benzoquinone moiety via hydrogen peroxide mediate oxidation. For the preparation of the 2-amino congener, lithiation of the imidazole C2 position with n-BuLi and trapping with TrisN3 provided the corresponding 2-azido derivative, which was converted to the kealiiquinone derivative by oxidation with hydrogen peroxide.21 Reduction of the azide by catalytic hydrogenation then delivered 2-deoxy-2-aminokealiiquinone (2) (Scheme 1). The C2-deletion analog 10 was prepared from 9c by oxidation with hydrogen peroxide (Scheme 1).21
Scheme 1. Reagents and conditions.
(a) n-BuLi, THF, −78 °C. (b) (TMSO)2, 59%. (c) TrisN3, −78 °C to rt, 69%. (d) H2O2, MeOH (for 1 = 60%; for 10 = 67%). (e) Pd-C, H2, MeOH, 75% (two steps from 9c)
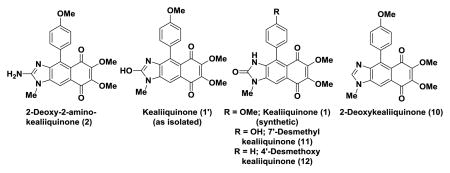
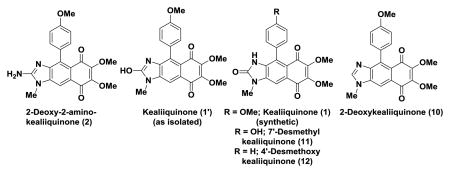
The spectroscopic data for 2-deoxy-2-aminokealiiquinone (2) matched exactly with that reported by Schmitz and coworkers (Table 1 and Table 2),14 however this proved not to be the case for the NMR data reported for the sponge-derived material for kealiiquinone (1). Our data were generally a good match with that obtained by Ohta with the exception of two signals in the 13C NMR spectrum (vide infra).18 The Ohta lab showed through X-ray crystallography that they had obtained the 2-oxo derivative rather than the 2-hydroxy derivative described for the natural product.18 Interestingly, in the isolation report an X-ray structure was obtained for the natural material that indicated that it was the 2-hydroxy form.12 Further support for this assignment was derived from the appearance of a peak in the 1H NMR spectrum at 8.14 ppm that was attributed to the enolic OH (2-hydroxy moiety, see 1′, Table 1).12 Our data are also consistent with the synthesis of the 2-oxo form, in particular an absorption at 154.8 ppm in the 13C NMR spectrum is characteristic for a 2-imdazolone.22 Careful comparison of the 13C NMR data obtained by us and the Ohta lab indicated that one signal at 145.2 ppm was missing and an extra absorbance appears at 126.5 ppm in the Ohta report. In the course of our studies towards the kealiiquinone group of molecules we have prepared two other kealiiquinone-like structures 11 and 12 via a completely different strategy involving an intramolecular Diels-Alder reaction and we find that the 13C NMR data are an excellent match for 1 (Table 2).19, 23 Based on these observations we conclude that the Ohta lab may have inadvertently misreported one signal, unfortunately we have been unable to obtain copies of the original spectroscopic data to confirm or refute this hypothesis. The preparation of the 2-oxo isomer through both approaches is presumably a reflection of its thermodynamic stability. Interestingly, examination of the spectroscopic data for amino and hydroxyl tautomers shows a correlation with the chemical shift of the N-methyl group being further downfield than for the corresponding 2-oxo derivative. Unfortunately we have been unable to secure a sample of the natural material to confirm the original structural assignment and to establish whether it can indeed be converted into the 2-oxo form.
Table 1.
Comparative 1H NMR data for natural and synthetic 2-deoxy-2-aminokealiiquinone (2) and kealiiquinone (1) and related compounds acquired at 500 MHz in DMSO-d6.
| |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 2-Deoxy-2-aminokealiiquinone | Kealiiquinone | 7′-Desmethyl kealiiquinone | 4′-Desmethoxy kealiiquinone | 2-Deoxykealiiquinonee | |||
| Synthetic | Natural | Synthetic (This work) | Natural | Synthetic (Ohta) | |||
| 11.02b | 11.03b | 10.95 b | 8.11b | ||||
| 8.14c | 9.41d | 8.24 | |||||
| 7.75 | 7.75 | 7.68 | 7.69 | 7.68 | 7.63 | 7.74 | 8.00 |
| 7.14 | 7.14 | 7.13 | 7.12 | 7.13 | 6.96 | 7.49-7.43 | 7.28 |
| 7.13 | 7.10 | 6.98 | 6.88 | 6.98 | 6.75 | 7.22-7.21 | 7.02 |
| 6.90a | 6.90a | ||||||
| 3.94 | 3.94 | 3.94 | 3.92 | 3.94 | 3.90 | 4.10 | |
| 3.85 | 3.85 | 3.86 | 3.83 | 3.85 | 4.07 | 4.01 | |
| 3.80 | 3.80 | 3.83 | 3.78 | 3.82 | 3.82 | 3.97 | 3.95 |
| 3.61 | 3.60 | 3.40 | 3.58 | 3.39 | 3.35 | 3.47 | 3.86 |
Two proton signal due to NH2.
One proton signal due to NH.
One proton due to OH.
Phenolic OH.
Recorded in CDCl3.
Table 2.
Comparative 13C NMR data for natural and synthetic 2-deoxy-2-aminokealiiquinone and kealiiquinone and related compounds acquired at 125 MHz in DMSO-d6.
| |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 2-Deoxy-2-aminokealiiquinone | Kealiiquinone | 7′-Desmethyl kealiiquinone | 4′-Desmethoxy kealiiquinone | 2-Deoxykealiiquinonea | |||
| Synthetic | Natural | Synthetic (This work) | Natural | Synthetic (Ohta) | |||
| 182.0 | 182.0 | 181.3 | 182.4 | 181.3 | 181.9 | 181.9 | 182.3 |
| 181.5 | 181.4 | 181.1 | 181.8 | 181.1 | 181.7 | 181.5 | 182.0 |
| 158.6 | 158.5 | 158.5 | 159.0 | 158.5 | 157.2 | 159.1 | |
| 157.9 | 157.8 | 154.8 | 158.3 | 154.8 | 155.3 | 154.2 | 148.9 |
| 147.9 | 147.8 | 147.8 | 148.2 | 147.8 | 148.4 | 147.6 | 147.4 |
| 147.5 | 147.4 | 145.2 | 147.8 | 145.6 | 146.0 | 146.5 | |
| 145.6 | 145.5 | 134.0 | 146.0 | 134.0 | 134.4 | 135.3 | 136.8 |
| 137.5 | 137.5 | 132.7 | 137.9 | 132.6 | 133.1 | 134.1 | 130.6 |
| 130.7 | 130.6 | 129.9 | 131.1 | 129.9 | 130.3 | 131.6 | 130.2 |
| 130.2 | 130.2 | 127.7 | 130.6 | 127.7 | 127.0 | 129.1 | 128.8 |
| 129.0 | 129.0 | 126.4 | 129.4 | 126.50 | 126.4 | 128.3 | 128.3 |
| 126.46 | 128.1 | ||||||
| 123.7 | 123.6 | 123.5 | 124.1 | 123.5 | 124.5 | 127.8 | 122.8 |
| 122.5 | 122.5 | 122.6 | 122.9 | 122.6 | 123.2 | 124.3 | 114.5 |
| 112.9 | 112.8 | 113.9 | 113.3 | 113.9 | 115.8 | 123.0 | 113.8 |
| 105.1 | 105.1 | 104.5 | 105.5 | 104.6 | 104.9 | 105.5 | 108.8 |
| 60.7 | 60.6 | 60.8 | 61.0 | 60.8 | 61.2 | 61.5 | 61.4 |
| 60.6 | 60.6 | 60.8 | 61.0 | 60.8 | 61.2 | 61.4 | 61.3 |
| 55.0 | 55.0 | 55.0 | 55.4 | 55.0 | 55.3 | ||
| 28.8 | 28.7 | 26.8 | 29.2 | 26.8 | 27.3 | 27.4 | 31.7 |
These data were acquired in CDCl3.
Each of the synthetic natural products 1-5 along with the pre-C2 functionalized intermediates 9a-c were evaluated against breast cancer cell line MCF7 using an MTT growth assay, using cisplatin as a positive control (IC50: 19.4 μM).24 In addition, isokealiinine C (14), isokealiinine C C2-deletion compound 15, and two precursors en route to kealiiquinone 10 and 13 were assayed. All of the natural products exhibit low micromolar cytotoxicity with the exception of kealiinine C (5), which is inactive up to 100 μM (Figure 2 and Table 3). The Looper lab has recently evaluated the bioactivity of synthetic versions of kealiinine B and C and obtained broadly similar results to ours; specifically kealiinine C was devoid of cytotoxicity.17 All of the substrates which were unsubstituted at C2 were less active, a trend that we have observed with other series in the Leucetta family of alkaloids, suggesting that there may be a key binding interaction with the C2-amino moiety or oxo group in the case of compound 13, but there is greater tolerance to substitutions elsewhere in the molecular framework. Notably, kealiinine B precursor 9b was an exception to this trend. Of particular note was the observation that the non-natural congener, isokealiinine C (14) was substantially more active than the corresponding natural isomer, in fact it is comparable to the two other kealiinine derivatives. Such activity patterns an observation reported by Ohta's group where isokealiiquinone is more active than kealiiquinone.13 As far as our studies with the benzoquinone group are concerned, kealiiquinone is the least active with the 2-amino derivative 2 and the C2-deletion analog 2-deoxykealiiquinone (10) being comparably active. At the present time no information is available regarding the mechanism of action of these compounds. In their report of the synthesis of kealiiquinone and isokealiiquinone, the Ohta lab suggest that these molecules act via a unique mechanism, based on the profile these compounds exhibit in a Japanese version of the NCI 60 cancer cell line panel.13 Likewise, the Looper group has ruled out some of the common pathways for cytotoxicity for kealiinine B(4).17
Figure 2. Cytotoxicity plots of selected compounds 1–5 and 9a–b and cisplatin.
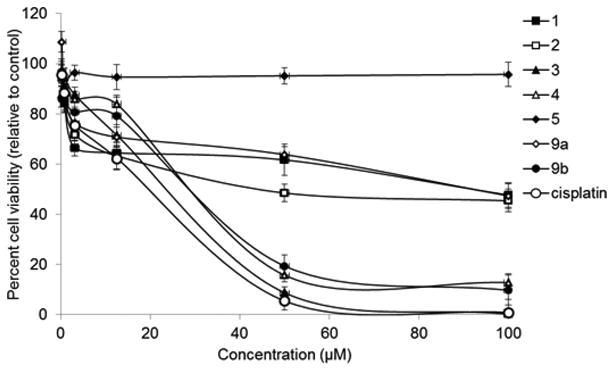
Table 3. IC50 values of test compounds derived from MMT growth assays in MCF7 cells.
| Compound | Structure | IC50 μM | SEM μM |
|---|---|---|---|
| 1 |
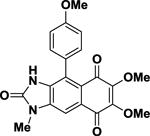 Kealiiquinone Kealiiquinone |
91.9 | ±0.5 |
| 2 |
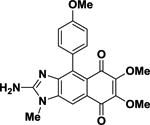 2-Deoxy-2-aminokealiiquinone 2-Deoxy-2-aminokealiiquinone |
43.8 | ±0.4 |
| 3 |
 Kealiinine A Kealiinine A |
23.4 | ±1.9 |
| 4 |
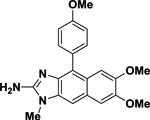 Kealiinine B Kealiinine B |
28.8 | ±1.2 |
| 5 |
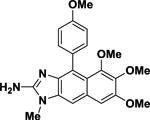 Kealiinine C Kealiinine C |
>100 | N/A |
| 9a |
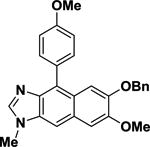
|
92.0 | ±2.2 |
| 9b |
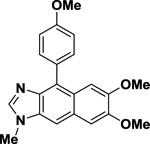
|
28.0 | ±1.3 |
| 9c |
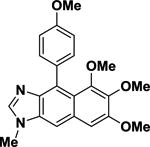
|
99.8 | ±0.9 |
| 10 |
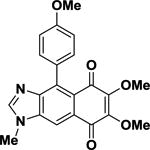 2-Deoxykealiiquinone 2-Deoxykealiiquinone |
41.1 | ±0.1 |
| 13 |
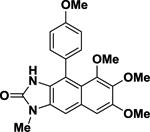
|
29.9 | ±0.3 |
| 14 |
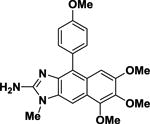 Isokealiinine C Isokealiinine C |
22.7 | ±1.0 |
| 15 |
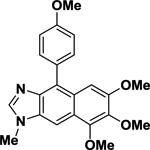
|
68.2 | ±1.6 |
| 16 |
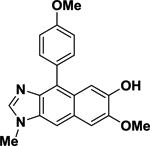
|
>100 | N/A |
The syntheses of kealiiquinone (1) and 2-deoxy-2-aminokealiiquinone (2) are described, the latter for the first time through a short sequence of reactions inspired by biosynthetic considerations. These two natural products, along with a small series of precursors and related naphthimidazole natural products were evaluated in cell growth assays against a hormone dependent breast cancer cell line. In general terms these derivatives showed modest levels of growth inhibition, with a 2-amino substituent necessary for enhanced levels of activity, but the substituents on the C-ring appear to be less critical.
Supplementary Material
Acknowledgments
This work was supported in part by the NIH (ES019129 (SM) and in part by GM065503 (CJL)) and the Robert A. Welch Foundation (Y-1362 (CJL)). Spectroscopic data were obtained on NMR instruments purchased through grants provided by the NSF (CHE-0234811 and CHE-0840509).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR. Marine Natural Products. Nat Prod Rep. 2012;29:144–222. doi: 10.1039/c2np00090c. [DOI] [PubMed] [Google Scholar]
- 2.Morris JC, Phillips AJ. Marine natural products. Synthetic aspects. Nat Prod Rep. 2011;28:269–289. doi: 10.1039/c0np00066c. [DOI] [PubMed] [Google Scholar]
- 3.Koswatta PB, Lovely CJ. Structure and synthesis of 2-aminoimidazole alkaloids from Leucetta and Clathrina sponges. Nat Prod Rep. 2011;28:511–528. doi: 10.1039/c0np00001a. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan JD, Giles RL, Looper RE. 2-Aminoimidazoles from Leucetta Sponges; Synthesis, Biology and the Emergence of a Privileged Pharmacophore. Curr Bioact Cpds. 2009;5:39–78. [Google Scholar]
- 5.Carmely S, Ilan M, Kashman Y. 2-Amino imidazole alkaloids from the marine sponge leucetta chagosensis. Tetrahedron. 1989;45:2193–2200. [Google Scholar]
- 6.Aberle NS, Lessene G, Watson KG. A concise total synthesis of naamidine A. Org Lett. 2006;8:419–421. doi: 10.1021/ol052568o. [DOI] [PubMed] [Google Scholar]
- 7.Aberle NS, Catimel J, Nice EC, Watson KG. Synthesis and biological evaluation of analogues of the anti-tumor alkaloid naamidine A. Bioorg Med Chem Lett. 2007;17:3741–3744. doi: 10.1016/j.bmcl.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Copp BR, Fairchild CR, Cornell L, C AM, Robinson S, Ireland CM. Naamidine A is an antagonist of the epidermal growth factor receptor and an in vivo active antitumor agent. J Med Chem. 1998;41:3909–3911. doi: 10.1021/jm980294n. [DOI] [PubMed] [Google Scholar]
- 9.LaBarbera DV, Modzelewska K, Glazar AI, Gray PD, Kaur M, Liu T, Grossman D, Harper MK, Kuwada SK, Moghal N, Ireland CM. The marine alkaloid naamidine A promotes caspase-dependent apoptosis in tumor cells. Anticancer Drugs. 2009;20:425–436. doi: 10.1097/CAD.0b013e32832ae55f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James RD, Jones DA, Aalbersberg W, Ireland CM. Naamidine A Intensifies the Phosphotransferase Activity of Extracellular Signal-regulated Kinases Causing A-431 Cells to Arrest in G1. Mol Cancer Ther. 2003;2:747. [PubMed] [Google Scholar]
- 11.Lovely CJ. Strategies and Tactics in Organic Synthesis. Vol. 8. Academic Press; 2012. pp. 199–224. [Google Scholar]
- 12.Akee RK, Carroll TR, Yoshida WY, Scheuer PJ, Stout TJ, Clardy J. Two imidazole alkaloids from a sponge. J Org Chem. 1990;55:1944–1946. [Google Scholar]
- 13.Nakamura S, Tsuno N, Yamashita M, Kawasaki I, Ohta S, Ohishi Y. Synthesis of a regio-isomer of kealiiquinone, a marine benzimidazole alkaloid. J Chem Soc Perkin Trans. 2001;1:429–436. [Google Scholar]
- 14.Fu X, Barnes JR, Do T, Schmitz FJ. New imidazole alkaloids from the sponge Leucetta chagosensis. J Nat Prod. 1997;60:497–498. [Google Scholar]
- 15.Hassan W, Edrada R, Ebel R, Wray V, Berg A, Van Soest R, Wiryowidagdo S, Proksch P. New imidazole alkaloids from the Indonesian sponge Leucetta chagosensis. J Nat Prod. 2004;67:817–822. doi: 10.1021/np0305223. [DOI] [PubMed] [Google Scholar]
- 16.Das J, Koswatta PB, Yousufuddin M, Jones JD, Lovely CJ. Total Syntheses of Kealiinines A-C. Org Lett. 2012;14:6210–6213. doi: 10.1021/ol302958e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.For an alternative approach to kealiinines B and C see: Gibbons JB, Gligorich KM, Welm BE, Looper RE. Synthesis of the Reported Structures for Kealiinines B and C. Org Lett. 2012;14:4734–4737. doi: 10.1021/ol3019242.
- 18.Kawasaki I, Taguchi N, Yamashita M, Ohta S. Total synthesis of kealiiquinone, an imidazole marine alkaloid. Chem Pharm Bull. 1997;45:1393–1398. [Google Scholar]
- 19.Lima HM, Sivappa R, Yousufuddin M, Lovely CJ. Total synthesis of 7′-desmethylkealiiquinone. Org Lett. 2012;14:2274–2277. doi: 10.1021/ol300704w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipshutz BH, Hagen W. Single-Flask Polyfunctionalization of the Imidazole Ring: A Streamlined Route to the Antitumor Agent Carmethizole. Tetrahedron Lett. 1992;33:5865–5868. [Google Scholar]
- 21.Orita H, Shimizu M, Hayakawa T, Takehira K. Bull Chem Soc Jpn. 1989;62:1652. [Google Scholar]
- 22.Lima HM, Lovely CJ. Synthesis of 2-Imidazolones and 2-Iminoimidazoles. Org Lett. 2011;13:5736–5739. doi: 10.1021/ol2022438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lima HM. PhD Dissertation. The University of Texas at Arlington; Arlington: 2011. Total Synthesis of Marine Derived Natural Products: Kealiiquinone and Ageliferin. [Google Scholar]
- 24.Dvorák Z, Štarha P, Šindelár Zk, Trávnícek Zk. Evaluation of in vitro cytotoxicity of one-dimensional chain [Fe(salen)(L)]n complexes against human cancer cell lines. Toxicol in Vitro. 2012;26:480–484. doi: 10.1016/j.tiv.2012.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.