Abstract
Objective
To update the 2007 Partin tables in a contemporary patient population.
Patients and Methods
The study population consisted of 5,629 consecutive men who underwent RP and staging lymphadenectomy at the Johns Hopkins Hospital between January 1, 2006 and July 30, 2011 and met inclusion criteria.
Polychotomous logistic regression analysis was used to predict the probability of each pathologic stage category: organ-confined disease (OC), extraprostatic extension (EPE), seminal vesicle involvement (SV+), or lymph node involvement (LN+) based on preoperative criteria.
Preoperative variables included biopsy Gleason score (6, 3+4, 4+3, 8, and 9–10), serum PSA (0–2.5, 2.6–4.0, 4.1–6.0, 6.1–10.0, greater than 10.0 ng/mL), and clinical stage (T1c, T2c, and T2b/T2c).
Bootstrap re-sampling with 1000 replications was performed to estimate 95% confidence intervals for predicted probabilities of each pathologic state.
Results
The median PSA was 4.9 ng/mL, 63% had Gleason 6 disease, and 78% of men had T1c disease.
73% of patients had OC disease, 23% had EPE, 3% had SV+ but not LN+, and 1% had LN+ disease. Compared to the previous Partin nomogram, there was no change in the distribution of pathologic state.
The risk of LN+ disease was significantly higher for tumours with biopsy Gleason 9–10 than Gleason 8 (O.R. 3.2, 95% CI 1.3–7.6).
The c-indexes for EPE vs. OC, SV+ vs. OC, and LN+ vs. OC were 0.702, 0.853, and 0.917, respectively.
Men with biopsy Gleason 4+3 and Gleason 8 had similar predicted probabilities for all pathologic stages.
Most men presenting with Gleason 6 disease or Gleason 3+4 disease have <2% risk of harboring LN+ disease and may have lymphadenectomy omitted at RP.
Conclusions
The distribution of pathologic stages did not change at our institution between 2000–2005 and 2006–2011.
The updated Partin nomogram takes into account the updated Gleason scoring system and may be more accurate for contemporary patients diagnosed with prostate cancer.
Keywords: prostate cancer, prostatectomy, prostage-specific antigen, nomograms, staging
Introduction
The ‘Partin tables’ use commonly available preoperative data – serum PSA level, clinical stage and biopsy Gleason score – to predict pathological stage at radical prostatectomy (RP). The original Partin tables used preoperative data from men who were treated between 1982 and 1991; so most were diagnosed in the pre-PSA era [1]. Updates to the Partin tables reflected the changing nature of prostate cancer diagnosis in the USA [2-6] and abroad [7-12].
With the advent of PSA screening, the incidence of prostate cancer in the USA rose dramatically over the subsequent two decades, resulting in considerable changes in the clinical and pathological stage of men diagnosed with prostate cancer [13]. Contemporary men present with lower PSA, lower clinical stage and higher likelihood of harbouring organ-confined tumours than men diagnosed with prostate cancer in the pre-PSA era [4,14,15].
Over the past 5 years, men presenting with prostate cancer are more likely to have a PSA level <4.0 ng/mL and less likely to present with PSA > 10.0 ng/mL. In addition, an update to the Gleason scoring system was established at the 2005 International Society of Urological Pathology Consensus Conference [16]. The updated recommendations tend to narrow the scope of Gleason pattern 3 and widen the scope of Gleason pattern 4 [17]. Previous versions of the Partin tables were designed using a different patient population. The current work reflects the Partin nomogram in a contemporary cohort of men who underwent RP at our institution between 2006 and 2011.
Methods
This study was approved by the Institutional Review Board at Johns Hopkins. In all, 6289 men underwent RP and staging lymphadenectomy at The Johns Hopkins Hospital between 1 January 2006 and 30 July 2011. All men had diagnostic prostate biopsies reviewed and Gleason score assigned by pathologists at our institution, and were determined by the attending surgeon to have clinically localized tumours (T1c, T2a/b/c), using criteria from the American Joint Committee on Cancer TNM Staging, 1992/2002. Men who received any form of neoadjuvant treatment or those taking medications that affected androgen or PSA levels (e.g. 5α-reductase inhibitors) were excluded (n = 304). An additional 356 men were excluded because of missing values for any of the preoperative predictor variables, leaving a final analysis cohort of 5629 men (89.5% of initial cohort).
For pathological assessment, all pelvic lymph nodes removed at surgery were sectioned and examined for the presence of cancer. The surgical specimen, comprising the prostate and seminal vesicles, was totally embedded and analysed, and the pathological stage was determined as organ confined (OC) if all cancer was confined within the prostate, extraprostatic extension (EPE) if cancer was evident outside the prostate and the seminal vesicles and the pelvic lymph nodes were free of disease, positive seminal vesicle involvement (SV+) if tumour invaded the muscular wall of the seminal vesicle without lymph node involvement, and lymph node involvement (LN+) if the pelvic lymph nodes showed the presence of prostate cancer [4]. Staging categories were mutually exclusive (i.e. SV+ patients, although by definition all had EPE, were not also counted as positive in the EPE category). The grade used for the biopsy was the Gleason score of the core with the highest grade in cases with multiple cores having different grades. The grade of the RP specimen was based on the dominant nodule. The 2005 International Society of Urological Pathology modified Gleason grading system was used, with the additional update that all cribriform cancer was considered Gleason pattern 4 [18].
A polychotomous logistic regression based on preoperative variables was used to develop a model to predict the probability of each of the four (non-ordered) pathological stage categories. Predictor variables included PSA, clinical stage and biopsy Gleason score, all categorized as in our previous version of the Partin tables [4], with the exception of Gleason score, which was categorized 6, 3+4, 4+3, 8, 9-10. Bootstrap re-sampling with 1000 replications was used to derive 95% CI for each pathological stage; intervals were derived using the centile method. Model discrimination ability was assessed with the concordance index I, calculated separately for each non-OC stage vs OC [19]. The concordance index is analogous to the area under the receiver operating characteristic curve. Agreement between predicted and actual probability of each pathological stage was assessed graphically with calibration plots of a Loess curve fit to the data. The curve is compared to the ideal fit (45° line) where predicted values equal the actual values. All analyses were performed using SAS v9.3 (SAS Institute, Cary, NC, USA).
Results
Table 1 describes characteristics of the analysis cohort. The median age was 59 years, 81% of patients were Caucasian, median PSA value was 4.9 ng/mL, and the distribution of final pathological stage was OC 73%, EPE 23%, SV+ 3% and LN+ 1%. Compared with the cohort used in our previous version of the Partin tables [4], men in the current cohort were more likely to have PSA ≤ 4 ng/mL (30% vs 25%), more likely to have Gleason score >6 on both biopsy (37% vs. 23%) and prostatectomy (50% vs. 36%), and were virtually identical with respect to the distributions of clinical stage and pathologic stage.
Table 1.
Characteristics of men undergoing radical prostatectomy at Johns Hopkins Hospital, 2006–2011 compared with the 2000–2005 cohort used in the 2007 publication.
| Characteristic | Current cohort | 2000–2005 cohort |
|---|---|---|
| Age | ||
| Mean (sd) | 58.5 (6.66) | 57.4 (6.36) |
| Median (range) | 59 (34–77) | 58 (34–77) |
| Ethnicity, n (%) | ||
| White | 4579 (81)* | 5081 (89) |
| African-American | 665 (12) | 372 (7) |
| Hispanic | 113 (2) | 56(1) |
| Asian | 59(1) | 58 (1) |
| Other | 213 (4) | 158 (3) |
| PSA (ng/mL), n (%) | ||
| 0–2.5 | 518 (9) | 452 (8) |
| 2.6–4.0 | 1161 (21) | 946 (17) |
| 4.1–6.0 | 2266 (40) | 1994 (35) |
| 6.1–10.0 | 1219 (22) | 1671 (29) |
| >10.0 | 465 (8) | 667 (12) |
| Mean (sd) | 5.8 (4.35) | 6.5 (4.56) |
| Median (range) | 4.9 (0.2–67.0) | 5.5 (0.1–64.8) |
| Biopsy Gleason score, n (%) | ||
| 6 | 3538 (63) | 4402 (77) |
| 3+4 | 1268 (22) | 816 (14) |
| 4+3 | 495 (9) | 348 (6) |
| 8 | 218 (4 | 164 (3)f |
| 9–10 | 110 (2) | |
| Clinical stage, n (%) | ||
| T1c | 4380 (78) | 4419 (77) |
| T2a | 897 (16) | 998 (17) |
| T2b | 307 (5) | 279 (5) |
| T2c | 45 (1) | 34(1) |
| Prostatectomy Gleason Score, n (%) | ||
| 6 | 2834 (50) | 3693 (64) |
| 3+4 | 1689 (30) | 1304 (23) |
| 4+3 | 691 (12) | 425 (7) |
| 8 | 204 (4) | 308 (5)† |
| 9–10 | 211 (4) | |
| Pathological stage, n (%) | ||
| Organ confined | 4082 (73) | 4204 (73) |
| Extraprostatic extension | 1277 (23) | 1276 (22) |
| Seminal vesicle involvement | 191 (3) | 180 (3) |
| Lymph node involvement | 79(1) | 70(1) |
PSA, prostate-specific antigen.
Number of patients (percentage).
Gieason score includes Gleason 8–10.
As in our previous tables, we combined clinical stage T2b and T2c, given the small fraction of T2c patients (1%) and the similar association with pathological stage (data not shown). Because of the possibility that the 2005 revision to the Gleason scoring system altered the prognostic information for specific categories, we compared models with Gleason 8–10 combined with a model that assigned separate categories to Gleason 8 and Gleason 9–10. The latter model showed higher risk for Gleason 9–10. For example, the odds ratios and 95% CIs for Gleason 9-10 vs 8 were 3.2 (1.3–7.6) for risk of LN+ vs OC, 1.8 (0.9–3.9) for SV+ vs OC, and 1.2 (0.7–2.1) for EPE vs OC. Hence, we developed the tables based on the model that separated Gleason 8 from 9–10.
Table 2 presents the predicted probabilities from the polychotomous logistic regression model and the bootstrapped 95% CIs. Each cell shows the predicted probabilities for each of the pathological stage categories for specific clinical stage, PSA level and Gleason score. For example, for men with T1c tumours, the probability of OC tumour ranges from 23% (for men with biopsy Gleason score 9–10 and PSA > 10 ng/mL) to 93% (for men with Gleason score 6 and PSA ≤ 2.5 ng/mL). In contrast, the predicted risk of LN+ is no more than 3% for T1c tumours with biopsy Gleason score <9 regardless of PSA. The c-indexes for EPE vs OC, SV+ vs OC and LN+ vs OC were 0.702, 0.853 and 0.917, respectively. Although discrimination is quite good for SV+ and LN+ it is not surprising that EPE exhibits lower discrimination, because a significant fraction of EPE cases exhibited only focal areas of extension and probably have a prognosis similar to OC [20].
Table 2.
Predicted probability (95% confidence interval) of pathological stage according to clinical stage (TNM), PSA level, and biopsy Gleason score (Johns Hopkins RP patients 2006–2011).
| PSA | Pathological stage |
Biopsy Gleason Score |
||||
|---|---|---|---|---|---|---|
| 6 | 3+4 | 4+3 | 8 | 9–10 | ||
| Clinical stage T1c (n = 4380) 0–2.5 |
OC (n = 289) | 93 (91–95) | 83 (78–87) | 80 (74–85) | 79 (72–85) | 74 (61–83) |
| EPE (n = 21) | 7 (5–8) | 15 (11–20) | 17 (12–22) | 18 (12–24) | 20 (12–29) | |
| SV+ (n = 4) | 0 (0–1) | 2 (0–3) | 3 (1–6) | 3 (1–6) | 5 (1–12) | |
| LN+ (n = 0) | 0 (0–0) | 0 (0–1) | 0 (0–2) | 0 (0–2) | 2 (0–6) | |
| 2.6–4.0 | OC (n = 751) | 87 (85–89) | 71(67–75) | 66 (60–71) | 65 (57–72) | 56 (44–67) |
| EPE (n = 133) | 12 (10–14) | 25 (22–29) | 27 (22–32) | 28 (22–34) | 29 (20–40) | |
| SV+ (n = 10) | 0 (0–1) | 2 (1–4) | 4 (2–7) | 4 (2–8) | 7(3–12) | |
| LN+ (n = 4) | 0 (0–0) | 1 (0–2) | 3 (1–5) | 3 (1–6) | 8 (3–16) | |
| 4.1–6.0 | OC (n = 1439) | 84 (83–86) | 66 (63–69) | 60 (55–65) | 59 (51–66) | 50 (38–60) |
| EPE (n = 371) | 15 (13–16) | 29 (26–33) | 31 (26–36) | 32 (25–38) | 32 (23–42) | |
| SV+ (n = 37) | 1 (0–1) | 4(2–5) | 6 (4–9) | 6 (4–10) | 10 (5–16) | |
| LN+ (n = 11) | 0 (0–0) | 1 (0–2) | 3 (2–5) | 3 (1–6) | 8 (4–15) | |
| 6.1–10.0 | OC (n = 686) | 80 (78–82) | 59 (55–63) | 53 (47–58) | 52 (44–59) | 42 (31–52) |
| EPE (n = 226) | 18 (16–20) | 34 (30–38) | 35 (30–40) | 36 (29–43) | 36 (26–46) | |
| SV+ (n = 36) | 1 (1–2) | 6 (4–8) | 9 (6–13) | 9(5–14) | 14 (8–21) | |
| LN+ (n = 8) | 0 (0–0) | 1 (0–2) | 3 (1–5) | 3 (1–6) | 8 (4–14) | |
| >10.0 | OC (n = 191) | 69 (64–74) | 42 (36–48) | 34 (28–40) | 33 (26–40) | 23 (15–32) |
| EPE (n = 121) | 27 (22–31) | 42 (36–47) | 28 (32–45) | 39 (31–47) | 33 (24–44) | |
| SV+ (n = 28) | 3 (2–5) | 13 (9–18) | 20 (14–27) | 20 (12–28) | 25 (15–36) | |
| LN+ (n = 14) | 0 (0–1) | 3 (1–5) | 8 (4–14) | 8 (3–14) | 18 (9–30) | |
| Clinical stage T2a (n = 897) 0–2.5 |
OC (n = 140) | 90 (87–92) | 76 (70–81) | 72 (65–79) | 71 (62–79) | 65 (51–76) |
| EPE (n = 23) | 10 (7–13) | 22 (17–28) | 24 (17–30) | 24 (18–33) | 27 (18–39) | |
| SV+ (n = 1) | 0 (0–1) | 2 (0–4) | 3 (1–7) | 3 (1–7) | 6 (1–13) | |
| LN+ (n = 1) | 0 (0–0) | 0 (0–1) | 1 (0–4) | 1 (0–3) | 2 (0–9) | |
| 2.6–4.0 | OC (n = 139) | 82 (78–84) | 61 (56–66) | 56 (48–62) | 54 (46–63) | 45 (33–56) |
| EPE (n = 52) | 18 (15–21) | 34 (29–39) | 35 (29–42) | 36 (29–44) | 36 (26–49) | |
| SV+ (n = 5) | 1 (0–1) | 3 (1–5) | 5 (2–8) | 5 (2–9) | 7(3–14) | |
| LN+ (n = 5) | 0 (0–0) | 1 (0–3) | 4(1–8) | 4 (1–10) | 11 (4–23) | |
| 4.1–6.0 | OC (n = 183) | 78 (74–81) | 56 (51–60) | 49 (43–56) | 48 (40–56) | 39 (28–50) |
| EPE (n = 91) | 21 (18–24) | 38 (34–43) | 39 (33–46) | 40 (32–48) | 39 (28–50) | |
| SV+ (n = 8) | 1(1–1) | 4 (3–6) | 7 (4–10) | 7 (4–11) | 10 (5–16) | |
| LN+ (n = 3) | 0 (0–0) | 2 (1–3) | 4 (2–7) | 4 (2–8) | 11 (4–21) | |
| 6.1–10.0 | OC (n = 104) | 73 (68–77) | 48 (43–54) | 42 (36–49) | 41 (33–50) | 32 (23–43) |
| EPE (n = 72) | 26 (22–30) | 44 (39–49) | 44 (37–50) | 45 (36–52) | 43 (31–54) | |
| SV+ (n = 10) | 1 (1–2) | 6 (4–9) | 10 (6–15) | 10 (5–16) | 14 (7–22) | |
| LN+ (n = 4) | 0 (0–0) | 1 (1–3) | 4 (2–7) | 4(1–8) | 10 (4–20) | |
| >10.0 | OC (n = 22) | 60 (53–66) | 32 (26–39) | 25 (20–31) | 24 (18–32) | 16 (10–24) |
| EPE (n = 22) | 36 (30–42) | 50 (43–56) | 44 (36–53) | 45 (35–55) | 37 (25–49) | |
| SV+ (n = 10) | 4 (2–6) | 14 (8–20) | 20 (12–29) | 20 (11–30) | 24 (13–38) | |
| LN+ (n = 2) | 1 (0–2) | 4 (2–7) | 10 (4–18) | 10 (4–20) | 22 (10–37) | |
| Clinical stage T2b or T2c (n = 352) 0–2.5 |
OC (n = 26) | 82 (76–87) | 61 (52–70) | 55 (45–66) | 54 (44–66) | 45 (32–60) |
| EPE (n = 13) | 17 (12–23) | 33 (25–42) | 34 (25–44) | 35 (24–46) | 35 (23–48) | |
| SV+ (n = 0) | 1 (0–2) | 5 (1–10) | 8 (2–16) | 8 (2–16) | 13 (3–24) | |
| LN+ (n = 0) | 0 (0–0) | 1 (0–3) | 2 (0–9) | 3 (0–9) | 7 (0–21) | |
| 2.6–4.0 | OC (n = 27) | 70 (63–75) | 44 (37–51) | 36 (29–44) | 35 (27–44) | 24 (16–35) |
| EPE (n = 30) | 28 (22–35) | 46 (39–53) | 43 (35–51) | 44 (34–53) | 37 (26–51) | |
| SV+ (n = 3) | 2 (1–3) | 6 (3–10) | 10 (5–16) | 10 (5–17) | 13 (6–23) | |
| LN+ (n = 2) | 1 (0–2) | 4(2–8) | 11 (5–20) | 11 (4–21) | 25 (12–42) | |
| 4.1–6.0 | OC (n = 52) | 64 (58–70) | 38 (32–44) | 30 (24–37) | 30 (22–37) | 20 (13–29) |
| EPE (n = 45) | 32 (27–39) | 49 (42–56) | 45 (38–52) | 46 (37–55) | 38 (26–51) | |
| SV+ (n = 14) | 2 (1–4) | 9 (6–13) | 14 (9–20) | 13 (8–21) | 17 (9–28) | |
| LN+ (n = 12) | 1 (0–2) | 4(2–8) | 11 (5–17) | 11 (5–19) | 24 (12–40) | |
| 6.1–10.0 | OC (n = 25) | 58 (50–65) | 31(25–37) | 24 (19–31) | 24 (18–31) | 16 (10–23) |
| EPE (n = 36) | 38 (32–45) | 52 (46–59) | 47 (40–55) | 48 (39–57) | 40 (28–52) | |
| SV+ (n = 7) | 4 (2–6) | 12 (8–18) | 19 (12–25) | 18 (10–26) | 23 (12–34) | |
| LN+ (n = 5) | 1 (0–2) | 4 (2–7) | 10 (5–16) | 10 (5–18) | 22 (10–35) | |
| >10.0 | OC (n = 8) | 42 (34–50) | 17 (13–23) | 12 (8–16) | 11 (8–16) | 6 (4–11) |
| EPE (n = 21) | 47 (39–55) | 50 (41–59) | 39 (30–49) | 40 (28–51) | 27 (18–40) | |
| SV+ (n = 18) | 9 (5–14) | 23 (15–33) | 30 (20–41) | 29 (18–42) | 30 (17–45) | |
| LN+ (n = 8) | 2 (0–4) | 9 (4–16) | 20 (10–31) | 20 (9–32) | 36 (20–53) | |
PSA, prostate-specific antigen; RP, radical prostatectomy; OC, organ confined; EPE, extraprostatic extension; SV+, seminal vesicle involvement; LN+, lymph node involvement.
We also combined PSA categories 6.1 to 8.0 and 8.1 to 10.0 ng/mL in a single predictor category because clinical decisions are usually similar within that range, and using separate categories in the model did not improve predictive performance (c-indexes for EPE vs OC, SV+ vs OC, LN+ vs OC were 0.702, 0.854, 0.916 for the model with separate PSA categories).
Several interesting trends are apparent in these tables. Within the range of PSA levels from 2.6 to 10.0 ng/mL, the predicted probability of SV+ or LN+ is relatively insensitive to PSA, but probabilities increase twofold to threefold when comparing PSA ≤ 10 with >10.0 ng/mL. In our previous version of these tables a similar trend was observed for LN+ but not for SV+, which tended to increase more linearly. In a similar vein, the biggest increases in probability of EPE occur for biopsy Gleason score 6 vs 3+4, with much smaller increases above 3+4. Again, in our previous version the risk of EPE tended to increase more linearly with biopsy Gleason. Finally, regardless of clinical stage or PSA level, men with biopsy Gleason score 4+3 and 8 had similar predicted probabilities for all pathological stages.
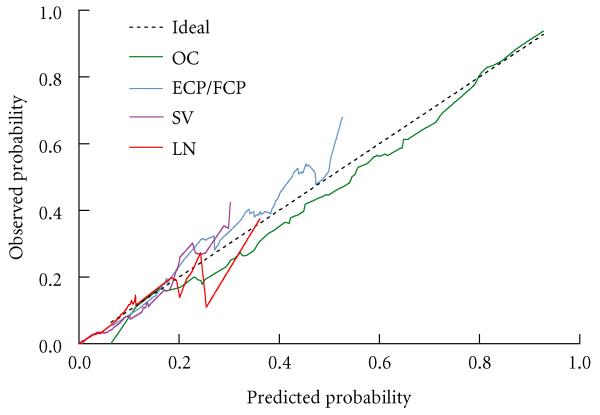
Figure 1 shows the calibration plots comparing predicted and actual probabilities for each pathological stage. The model tended to overestimate the probability of OC and underestimate the probability of EPE, though it was very accurate for predicted probability of OC > 80% and EPE < 20%. Predicted probabilities of SV+ and LN+ were close to the observed values for probability ≤20%. Only 2.2% of participants had predicted probability of SV+ > 20% and only 0.6% had predicted probability of LN+ > 20%, so model predictions for advanced stage are likely to be accurate.
Fig. 1.
Calibration plots showing predicted vs observed probability of each pathological stage, and comparison to ideal calibration (predicted = observed).
Discussion
The use of nomograms to predict clinical outcomes assumes that contemporary patients will behave similarly to patients with comparable clinical characteristics in the nomogram population [21]. As PSA screening became widespread, a continuously increasing proportion of men diagnosed with prostate cancer have presented with localized disease [22] amenable to surgical therapy [23], making older nomograms progressively obsolete.
In the current report, the distribution of men in each pathological stage is identical to that in our previous 2007 iteration of these tables [4]. Hence, stage migration appears to have stabilized at our institution. The predicted probabilities from the current nomogram are similar to those of our 2007 report, which used a patient cohort treated from 2000 to 2005 [4]. The most important differences are that risk of advanced stage is higher for Gleason 9-10 than Gleason 8 tumours, Gleason 4+3 and 8 tumours exhibit similar risks for each category of clinical stage and PSA, and the prognostic impact of PSA level >10 ng/mL for predicting pT3 and N1 disease is particularly strong.
In the contemporary population, fewer men presented with biopsy Gleason 6 disease (63% vs 77%, respectively) as well as pathological Gleason sum 6 disease (50% vs 65%, respectively) than in the previous Partin table population, and more men presented with ‘high-risk’ disease of 4+3 or higher on the biopsy (15% vs 9%, respectively) and the prostatectomy (20% vs 13%, respectively) than in the previous Partin table population. Despite the shift to higher grade, the prevalence of high-risk PSA values (>10 ng/mL) was somewhat lower (8% vs 12%, respectively) than in the previous Partin tables. This, and the lack of change in advanced stage cases, supports the notion that the grade changes are a result of the update to the Gleason scoring system, rather than an increase in more biologically aggressive disease. However, increasing employment of active surveillance among men with low-risk disease [24,25] and increased use of RP among those with high-risk disease [26-29] probably play a role as well [24,30]. Previous studies have found the new Gleason scoring system to provide more accurate predictions of biochemical recurrence as well as greater correlation between biopsy and pathological Gleason score [17,18,31,32]. The current nomogram may also have increased accuracy resulting from the new Gleason scoring system, though this remains to be verified in an independent patient population.
In recent years the most common use of the Partin nomograms has been in deciding to perform a lymphadenectomy at the time of RP. In a recent head-to-head comparison of the 2007 Partin tables with the National Comprehensive Cancer Network lymph node invasion nomogram [33] and the D’Amico risk classification [34] for the purpose of predicting LN+ disease in a lymphadenectomy, the Partin tables showed the greatest net benefit for a decision threshold probability of LN+ ≤ 4%. That is, when the decision to perform lymphadenectomy is based on predicted probability of LN+ that exceeds a threshold set at ≤4%, predictions from the Partin tables gave the best ratio of appropriate to inappropriate lymphadenectomies [35,36]. It should be noted that most men in the study population underwent limited pelvic lymph node dissection rather than extended pelvic lymph node dissection.
Though lymphadenectomy is generally well tolerated, an increased risk of lymphocoele [37], thromboembolism [38] and, rarely, injury to the ureter, pelvic nerves or vessels [39] has been reported. Overall complication rates range from 2 to 20% for limited lymphadenectomy, with higher complication rates reported in extended lymphadenectomy [40-42]. To limit complications in patients unlikely to benefit from lymphadenectomy, the National Comprehensive Cancer Network suggests that lymphadenectomy may be excluded if the risk of lymph node involvement is <2% [43], a policy which has been adopted for patients at one high-volume centre [44]. Abdollah et al. [35] showed that the 2007 Partin tables are particularly accurate within this range, and given the close calibration of the current model for predicted probabilities of N+ < 20% (see Fig. 1) it is likely that the current model will continue to be an excellent tool for the decision to perform lymphadenectomy. Using the current tables and a cut-off of <2%, lymphadenectomy may be omitted in all men with Gleason 6 disease unless they have both PSA level >10 ng/mL and clinical stage T2b or greater. Men with Gleason 3+4 disease and clinical stage T1c may avoid lymphadenectomy unless PSA levels are >10 ng/mL. When patients have Gleason 3+4 and palpable disease, lymphadenectomy should be performed in most cases, and in our opinion, all men with Gleason 4+3 or greater disease should undergo lymphadenectomy regardless of clinical stage or serum PSA.
Since the publication of our previous version of the Partin tables there is an increasing trend toward the use of active surveillance to delay or forgo definitive treatment. With the recent US Preventive Services Task Force report on prostate cancer screening and over-treatment [45], and the National Institutes of Health State-of-the-Science Conference: Role of Active Surveillance in the Management of Men with Localized Prostate Cancer [25], there is increased deliberation about the benefits vs harms of treatment for low-risk disease. A major use of the tables in the past was to predict the likelihood of low-risk disease that was curable by surgery. If additional evidence accrues that many men with low-risk disease are treated without benefit, then the use of the tables may shift toward identifying men with intermediate- to high-risk disease for whom the ratio of treatment benefit to harm may be greatest, or to identify men with low- to intermediate-risk disease who may benefit from active surveillance.
A limitation of the current analysis is that the data represent the demographics of the RP population at a single tertiary centre rather than that of the general population of men with prostate cancer. Whether the pathological stage distribution seen at our centre reflects a new equilibrium for RP candidates remains unclear, as other institutions have actually found decreasing OC disease over time [24]. Furthermore, whether these data reflect that pathological stage has stabilized only in those patients undergoing RP or all prostate cancer patients is unclear.
In conclusion, the current analysis represents an update of the ‘Partin tables’ based on the population of men treated from 2006 to 2011 at our institution. Pathological stage did not change between RP specimens from 2006 to 2010 and those from 2000 to 2005, possibly indicating a new equilibrium of pathological stage. Urologists may use these nomograms to predict pathological stage as a treatment decision aid for men newly diagnosed with prostate cancer.
What’s known on the subject? and What does the study add?
Pathological stage after radical prostatectomy can be accurately predicted by serum prostate-specific antigen level, clinical stage and biopsy Gleason sum, the ‘Partin tables’.
Since the previous publication of the Partin tables, an updated Gleason scoring system has been established and incremental changes have occurred in the clinical characteristics of patients diagnosed with prostate cancer. The current analysis updates the Partin nomogram in a contemporary cohort of patients.
Abbreviations
- RP
radical prostatectomy
- OC
organ confined
- EPE
extraprostatic extension
- SV+
seminal vesicle involvement
- LN+
lymph node involvement
Footnotes
Conflict of Interest
None declared.
References
- 1.Partin AW, Yoo J, Carter HB, et al. The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancer. J Urol. 1993;150:110–14. doi: 10.1016/s0022-5347(17)35410-1. [DOI] [PubMed] [Google Scholar]
- 2.Partin AW, Kattan MW, Subong EN, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA. 1997;277:1445–51. [PubMed] [Google Scholar]
- 3.Partin AW, Mangold LA, Lamm DM, Walsh PC, Epstein JI, Pearson JD. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001;58:843–8. doi: 10.1016/s0090-4295(01)01441-8. [DOI] [PubMed] [Google Scholar]
- 4.Makarov DV, Trock BJ, Humphreys EB, et al. Updated nomogram to predict pathologic stage of prostate cancer given prostate-specific antigen level, clinical stage, and biopsy Gleason score (Partin tables) based on cases from 2000 to 2005. Urology. 2007;69:1095–101. doi: 10.1016/j.urology.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu JB, Makarov DV, Sharma R, Peschel RE, Partin AW, Gross CP. Validation of the partin nomogram for prostate cancer in a national sample. J Urol. 2010;183:105–11. doi: 10.1016/j.juro.2009.08.143. [DOI] [PubMed] [Google Scholar]
- 6.Blute ML, Bergstralh EJ, Partin AW, et al. Validation of Partin tables for predicting pathological stage of clinically localized prostate cancer. J Urol. 2000;164:1591–5. [PubMed] [Google Scholar]
- 7.Karakiewicz PI, Lattouf JB, Perrotte P, et al. Validation of 1997 Partin Tables’ lymph node invasion predictions in men treated with radical prostatectomy in Montreal Quebec. Can J Urol. 2005;12:2588–92. [PubMed] [Google Scholar]
- 8.Eskicorapci SY, Karabulut E, Turkeri L, et al. Validation of 2001 Partin tables in Turkey: a multicenter study. Eur Urol. 2005;47:185–9. doi: 10.1016/j.eururo.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Isharwal S, Haese A, et al. Prediction of patient-specific risk and percentile cohort risk of pathological stage outcome using continuous prostate-specific antigen measurement, clinical stage and biopsy Gleason score. BJU Int. 2011;107:1562–9. doi: 10.1111/j.1464-410X.2010.09692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augustin H, Isbarn H, Auprich M, et al. Head to head comparison of three generations of Partin tables to predict final pathological stage in clinically localised prostate cancer. Eur J Cancer. 2010;46:2235–41. doi: 10.1016/j.ejca.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Fanning DM, Fan Y, Fitzpatrick JM, Watson RW. External validation of the 2007 and 2001 Partin tables in Irish prostate cancer patients. Urol Int. 2010;84:174–9. doi: 10.1159/000277594. [DOI] [PubMed] [Google Scholar]
- 12.Naito S, Kuroiwa K, Kinukawa N, et al. Validation of Partin tables and development of a preoperative nomogram for Japanese patients with clinically localized prostate cancer using 2005 International Society of Urological Pathology consensus on Gleason grading: data from the Clinicopathological Research Group for Localized Prostate Cancer. J Urol. 2008;180:904–9. doi: 10.1016/j.juro.2008.05.047. discussion 9-10. [DOI] [PubMed] [Google Scholar]
- 13.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 14.Shao YH, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101:1280–3. doi: 10.1093/jnci/djp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178:S14–19. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL. The 2005 international society of urological pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29:1228–42. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 17.Lotan TL, Epstein JI. Clinical implications of changing definitions within the Gleason grading system. Nat Rev Urol. 2010;7:136–42. doi: 10.1038/nrurol.2010.9. [DOI] [PubMed] [Google Scholar]
- 18.Epstein JI. An update of the Gleason grading system. J Urol. 2010;183:433–40. doi: 10.1016/j.juro.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 19.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Chuang AY, Nielsen ME, Hernandez DJ, Walsh PC, Epstein JI. The significance of positive surgical margin in areas of capsular incision in otherwise organ confined disease at radical prostatectomy. J Urol. 2007;178:1306–10. doi: 10.1016/j.juro.2007.05.159. [DOI] [PubMed] [Google Scholar]
- 21.Guillonneau B. Ceteris paribus and nomograms in medicine. Eur Urol. 2007;52:1287–9. doi: 10.1016/j.eururo.2007.04.085. [DOI] [PubMed] [Google Scholar]
- 22.Han M, Partin AW, Piantadosi S, Epstein JI, Walsh PC. Era specific biochemical recurrence-free survival following radical prostatectomy for clinically localized prostate cancer. J Urol. 2001;166:416–19. [PubMed] [Google Scholar]
- 23.Epstein JI, Pizov G, Walsh PC. Correlation of pathologic findings with progression after radical retropubic prostatectomy. Cancer. 1993;71:3582–93. doi: 10.1002/1097-0142(19930601)71:11<3582::aid-cncr2820711120>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 24.Silberstein JL, Vickers AJ, Power NE, et al. Reverse stage shift at a tertiary care center: escalating risk in men undergoing radical prostatectomy. Cancer. 2011;117:4855–60. doi: 10.1002/cncr.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganz PA, Barry JM, Burke W, et al. National Institutes of Health State-of-the-Science Conference: role of active surveillance in the management of men with localized prostate cancer. Ann Intern Med. 2012;156:591–5. doi: 10.7326/0003-4819-156-8-201204170-00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zelefsky MJ, Eastham JA, Cronin AM, et al. Metastasis after radical prostatectomy or external beam radiotherapy for patients with clinically localized prostate cancer: a comparison of clinical cohorts adjusted for case mix. J Clin Oncol. 2010;28:1508–13. doi: 10.1200/JCO.2009.22.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierorazio PM, Ross AE, Han M, et al. Evolution of the clinical presentation of men undergoing radical prostatectomy for high-risk prostate cancer. BJU Int. 2012;109:988–93. doi: 10.1111/j.1464-410X.2011.10514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierorazio PM, Ross AE, Schaeffer EM, et al. A contemporary analysis of outcomes of adenocarcinoma of the prostate with seminal vesicle invasion (pT3b) after radical prostatectomy. J Urol. 2011;185:1691–7. doi: 10.1016/j.juro.2010.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loeb S, Schaeffer EM, Trock BJ, Epstein JI, Humphreys EB, Walsh PC. What are the outcomes of radical prostatectomy for high-risk prostate cancer? Urology. 2010;76:710–14. doi: 10.1016/j.urology.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Budaus L, Spethmann J, Isbarn H, et al. Inverse stage migration in patients undergoing radical prostatectomy: results of 8916 European patients treated within the last decade. BJU Int. 2011;108:1256–61. doi: 10.1111/j.1464-410X.2010.09982.x. [DOI] [PubMed] [Google Scholar]
- 31.Billis A, Guimaraes MS, Freitas LL, Meirelles L, Magna LA, Ferreira U. The impact of the 2005 international society of urological pathology consensus conference on standard Gleason grading of prostatic carcinoma in needle biopsies. J Urol. 2008;180:548–52. doi: 10.1016/j.juro.2008.04.018. discussion 52-3. [DOI] [PubMed] [Google Scholar]
- 32.Helpap B, Egevad L. The significance of modified Gleason grading of prostatic carcinoma in biopsy and radical prostatectomy specimens. Virchows Arch. 2006;449:622–7. doi: 10.1007/s00428-006-0310-6. [DOI] [PubMed] [Google Scholar]
- 33.Cagiannos I, Karakiewicz P, Eastham JA, et al. A preoperative nomogram identifying decreased risk of positive pelvic lymph nodes in patients with prostate cancer. J Urol. 2003;170:1798–803. doi: 10.1097/01.ju.0000091805.98960.13. [DOI] [PubMed] [Google Scholar]
- 34.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 35.Abdollah F, Schmitges J, Sun M, et al. Head-to-head comparison of three commonly used preoperative tools for prediction of lymph node invasion at radical prostatectomy. Urology. 2011;78:1363–7. doi: 10.1016/j.urology.2011.07.1423. [DOI] [PubMed] [Google Scholar]
- 36.Vickers AJ. Editorial comment. Urology. 2011;78:1368. doi: 10.1016/j.urology.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Orvieto MA, Coelho RF, Chauhan S, Palmer KJ, Rocco B, Patel VR. Incidence of lymphoceles after robot-assisted pelvic lymph node dissection. BJU Int. 2011;108:1185–90. doi: 10.1111/j.1464-410X.2011.10094.x. [DOI] [PubMed] [Google Scholar]
- 38.Eifler JB, Levinson AW, Hyndman ME, Trock BJ, Pavlovich CP. Pelvic lymph node dissection is associated with symptomatic venous thromboembolism risk during laparoscopic radical prostatectomy. J Urol. 2011;185:1661–5. doi: 10.1016/j.juro.2010.12.044. [DOI] [PubMed] [Google Scholar]
- 39.Musch M, Klevecka V, Roggenbuck U, Kroepfl D. Complications of pelvic lymphadenectomy in 1,380 patients undergoing radical retropubic prostatectomy between 1993 and 2006. J Urol. 2008;179:923–8. doi: 10.1016/j.juro.2007.10.072. discussion 8-9. [DOI] [PubMed] [Google Scholar]
- 40.Briganti A, Blute ML, Eastham JH, et al. Pelvic lymph node dissection in prostate cancer. Eur Urol. 2009;55:1251–65. doi: 10.1016/j.eururo.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Stone NN, Stock RG, Unger P. Laparoscopic pelvic lymph node dissection for prostate cancer: comparison of the extended and modified techniques. J Urol. 1997;158:1891–4. doi: 10.1016/s0022-5347(01)64161-2. [DOI] [PubMed] [Google Scholar]
- 42.Herrell SD, Trachtenberg J, Theodorescu D. Staging pelvic lymphadenectomy for localized carcinoma of the prostate: a comparison of 3 surgical techniques. J Urol. 1997;157:1337–9. [PubMed] [Google Scholar]
- 43.Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw. 2010;8:145. doi: 10.6004/jnccn.2010.0010. [DOI] [PubMed] [Google Scholar]
- 44.Silberstein JL, Vickers AJ, Power NE, et al. Pelvic lymph node dissection for patients with elevated risk of lymph node invasion during radical prostatectomy: comparison of open, laparoscopic and robot-assisted procedures. J Endourol. 2011;26:748–53. doi: 10.1089/end.2011.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin K, Croswell JM, Koenig H, Lam C, Maltz A. Prostate-Specific Antigen-Based Screening for Prostate Cancer: An Evidence Update for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality (US); Rockville, MD: 2011. October Update for the U.S. Preventive Services Task Force. [PubMed] [Google Scholar]