Abstract
After changing empiric treatment of febrile neutropenia from meropenem to cefepime, the effect on C. difficile infection (CDI) was investigated. The change was assessed using an autoregressive model. A significant increase in CDI rates occurred following the introduction of cefepime. There may be an association between increased cefepime usage and CDI.
Background
Clostridium difficile is a major cause of healthcare-associated infections. Infection prevention measures and antimicrobial stewardship programs have been associated with decreased C. difficile infection (CDI) rates.[1, 2]
Current Infectious Diseases Society of America (IDSA) guidelines for febrile neutropenia recommend an anti-pseudomonal cephalosporin, a carbapenem or piperacillin-tazobactam, as first line therapy.[3] Individual institutions may favor certain antibiotics based on availability, costs, ease of administration and local antibiogram.
We sought to evaluate the intervention of changing the institutional first-line antibiotic for febrile neutropenia on the rates of CDI in the hematology and oncology ward using a quasi-experimental design.
Methods
A retrospective investigation of antimicrobial usage and incidence of CDI on the oncology/hematology inpatient floor at Tufts Medical Center (TMC) was performed. TMC is an urban tertiary care, university affiliated hospital with 417 beds in Boston, MA.
Prior to 2010, meropenem was the institutional choice as empiric initial therapy for neutropenic fever. After literature review, cost analysis and antibiograms, a change to cefepime was recommended by the antimicrobial subcommittee of the pharmacy and therapeutics committee; this occurred in July 2010.
Monthly antimicrobial usage data from January 2009 through December 2011 were obtained from the pharmacy information system and converted into defined daily dose (DDD; ATC/DDD version 2010), and expressed as DDD per 1000 bed-days. Case mix index data was collected for the same time period.
The number of hospital-acquired CDI cases was based on infection preventionist reports which are maintained using standard National Health and Safety Network definitions, which includes hospital onset cases only. The CDI rate was collected on a monthly basis and expressed per 1000 patient-days. The microbiology laboratory used two different testing methods during the specified time period. From January 2009 until January 2011, a toxin based qualitative enzyme immunoassay was used (Premier toxins A & B – Meridian biosciences, Cincinnati OH, USA). From January 2011 a DNA amplification assay (Illumigene C. difficile – Meridian Biosciences) was used. In the only study which compares the two tests directly, the tests have a reported sensitivity of 83.3% and 100% respectively compared to toxigenic culture.[4] A CDI case was defined as a positive test (by either method) in the presence of clinical diarrhea.
The effect of the intervention was assessed using an autoregressive model to estimate changes in the CDI rates before and after the antibiotic change, serial correlations between the data were evaluated. Individual effects of case mix index and other antibiotic use were examined and those that had statistically significant effect were included in the final model.
Results
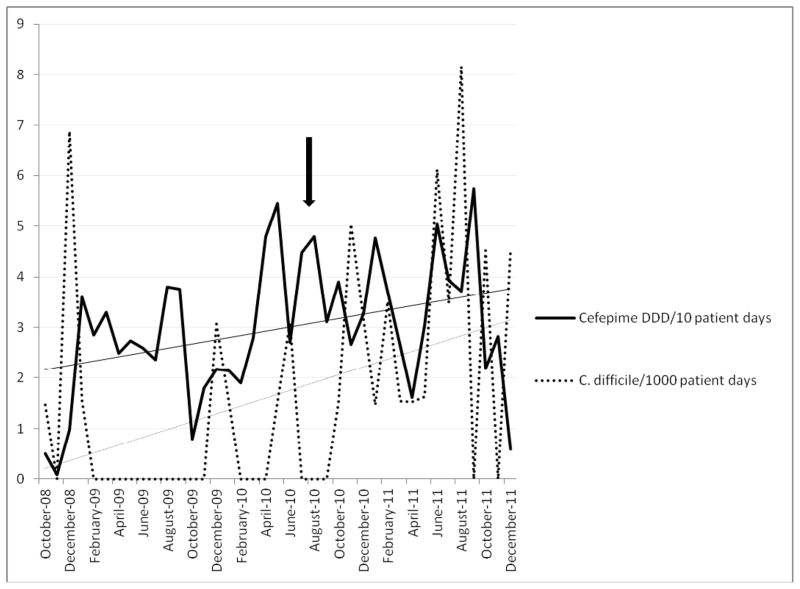
Prior to the change in empiric therapy for neutropenic fever, the CDI rate on the hematology/oncology ward was 0.45/1000 patient days and DDD of cefepime was 290/1000 patient days, while the mean meropenem DDD was 180/1000 patient days. After the change, the mean CDI rate was 2.59/1000 patient days, the mean DDD of cefepime was 340/1000 patient days, and the mean DDD of meropenem was 109/1000 patient days.
Using an autoregressive linear model, we identified a significant upward trend in the CDI rate following change to cefepime from meropenem as the preferred agent for empiric neutropenic fever therapy. The rate increased by 0.3 units for every additional month post intervention (p=0.008). However, tests for serial correlations were non-significant, indicating independent residuals. Therefore, the data were analyzed using a linear regression model, and the results were similar to those obtained from the autoregressive model. There was a significant increase in the trend of the CDI rate (p<0.001) after the switch from meropenem to cefepime.
All other antibiotics used on the ward and case mix index were included in the models. However, there were no significant associations between other individual antibiotics or case mix index and C. difficile rate. There was no change in the rate of MRSA or VRE infection during the same time period, and no major changes in infection control practices were made until after the increase in CDI rates was recognized.
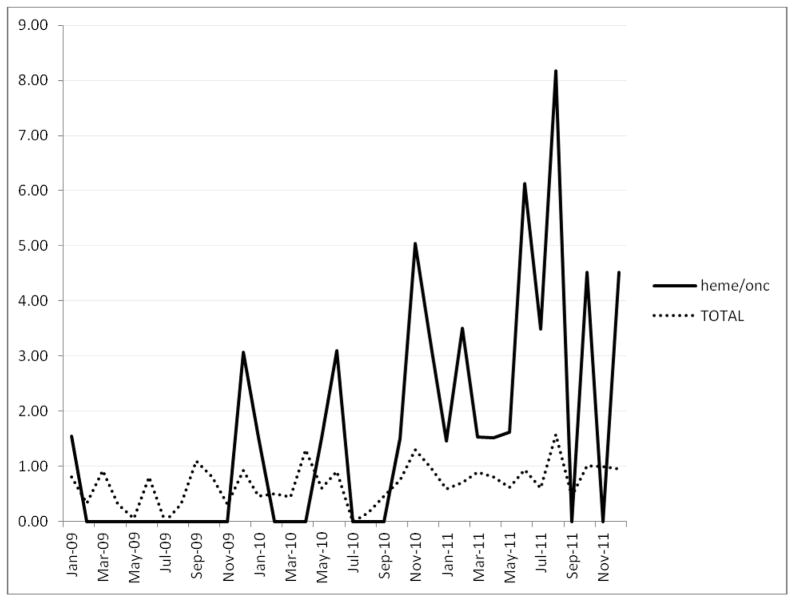
The hospital-wide CDI rate increased as expected from 0.61/1000 patient days while the EIA toxin test was utilized and 0.84 after the introduction of the DNA amplification assay (figure 2) p=0.06.
Figure 2.
C. difficile rates per 1000 patient days in heme/onc ward and the hospital as a whole.
Discussion
Cephalosporins have long been implicated as a risk factor for the acquisition of CDI. Broad spectrum antibiotics are thought to disturb the microflora of the gastrointestinal tract, allowing pathogenic bacteria such as C. difficile to overgrow. This may occur to a lesser extent with antimicrobials like meropenem, which have activity against C. difficile and therefore may inhibit its growth.[5, 6]
Studies done on the risk factors for CDI in an endemic setting have shown that the administration of a fourth generation cephalosporin is an independent risk factor for the acquisition of CDI[7]. Similarly, in outbreaks of CDI, cefepime has been associated with an increased odds ratio (OR 2.1) of developing CDI[8] and restriction of cephalosporin administration has been associated with the control of epidemic CDI.[9] In a comparative study of cefepime and piperacillin/tazobactam in neutropenic patients, CDI was more often observed among the patients receiving cefepime (2.3% vs. 6.8%, p=0.012)[10].
While this study suggests that there may be an association between increased cefepime use and C. difficile rates on a hematology-oncology ward, it has a number of limitations. Being a quasi-experimental study, there may have been other unrecognized factors which affected the CDI rates, such as changes in infection control practices. The stable rates of hospital-acquired MRSA and VRE suggest that no significant changes in practice occurred. The change in diagnostic methodology most certainly impacted CDI rates, however comparison with the hospital as a whole shows that the CDI rate on the hematology/oncology floor was disproportionately higher than rates in the rest of the hospital (figure 2). Furthermore the increase in CDI rate in the hematology/oncology ward was substantially higher than would be expected had the difference been due to the change in testing methodology alone.
This study shows that there may be an association between increased cefepime use for empiric neutropenic fever and CDI rates. This finding may have significant clinical implications and warrants further investigation.
Figure 1.
C. difficile rate/1000 patient days pre- and post- intervention and DDD of cefepime/10 patient days pre- and post- intervention. Arrow indicates date of change to cefepime as empiric therapy.
The empiric therapy for neutropenic fever was changed from meropenem to cefepime.
Time series analysis used to assess any change in C. difficile rates.
There was an association between the switch to cefepime and C. difficile rates.
Acknowledgments
Funding: Eavan G. Muldoon is the inaugural recipient of the Francis P. Tally endowed fellowship in infectious diseases, Tufts Medical Center. Lauren Epstein is supported by NIH training grant number 5T32AI055412-07. The project described was supported by the National Center for Research Resources Grant Number UL1 RR025752 and the National Center for Advancing Translational Sciences, National Institutes of Health, Grant Number UL1 TR000073. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Data from this manuscript was presented as a poster presentation at IDWeek; 2012 October 17–21; San Diego, CA.
All authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aldeyab MA, Kearney MP, Scott MG, et al. An evaluation of the impact of antibiotic stewardship on reducing the use of high-risk antibiotics and its effect on the incidence of Clostridium difficile infection in hospital settings. J Antimicrob Chemother. Aug 16; doi: 10.1093/jac/dks330. [DOI] [PubMed] [Google Scholar]
- 2.Talpaert MJ, Gopal Rao G, Cooper BS, Wade P. Impact of guidelines and enhanced antibiotic stewardship on reducing broad-spectrum antibiotic usage and its effect on incidence of Clostridium difficile infection. J Antimicrob Chemother. Sep;66(9):2168–74. doi: 10.1093/jac/dkr253. [DOI] [PubMed] [Google Scholar]
- 3.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis. Feb 15;52(4):427–31. doi: 10.1093/cid/ciq147. [DOI] [PubMed] [Google Scholar]
- 4.Barkin JAGA, Miller N, Nandi N, Sussman D, Poppiti R, Barkin JS. Evaluation of a loop-mediated isothermal amplification (LAMP) assay for Clostriduium difficile as a stand-alone test for diagnosis of C difficile infection. 111th General Meeting; American society for Microbiology; 2011. [Google Scholar]
- 5.Owens RC, Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008 Jan 15;46(Suppl 1):S19–31. doi: 10.1086/521859. [DOI] [PubMed] [Google Scholar]
- 6.Sheikh W, Pitkin DH, Nadler H. Antibacterial activity of meropenem and selected comparative agents against anaerobic bacteria at seven North American centers. Clin Infect Dis. 1993 Jun;16(Suppl 4):S361–6. doi: 10.1093/clinids/16.supplement_4.s361. [DOI] [PubMed] [Google Scholar]
- 7.Dubberke ER, Reske KA, Yan Y, Olsen MA, McDonald LC, Fraser VJ. Clostridium difficile--associated disease in a setting of endemicity: identification of novel risk factors. Clin Infect Dis. 2007 Dec 15;45(12):1543–9. doi: 10.1086/523582. [DOI] [PubMed] [Google Scholar]
- 8.Muto CA, Pokrywka M, Shutt K, et al. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005 Mar;26(3):273–80. doi: 10.1086/502539. [DOI] [PubMed] [Google Scholar]
- 9.Debast SB, Vaessen N, Choudry A, Wiegers-Ligtvoet EA, van den Berg RJ, Kuijper EJ. Successful combat of an outbreak due to Clostridium difficile PCR ribotype 027 and recognition of specific risk factors. Clin Microbiol Infect. 2009 May;15(5):427–34. doi: 10.1111/j.1469-0691.2009.02713.x. [DOI] [PubMed] [Google Scholar]
- 10.Bow EJ, Rotstein C, Noskin GA, et al. A randomized, open-label, multicenter comparative study of the efficacy and safety of piperacillin-tazobactam and cefepime for the empirical treatment of febrile neutropenic episodes in patients with hematologic malignancies. Clin Infect Dis. 2006 Aug 15;43(4):447–59. doi: 10.1086/505393. [DOI] [PubMed] [Google Scholar]