Abstract
P2X receptors are ion channels gated by ATP. In rodents these channels are modulated by zinc and copper. Zinc is co-released with neurotransmitter at some synapses and can modulate neuronal activity, but the role of copper in the brain is unclear. Rat P2X2 receptors show potentiation by 2–100 µM zinc or copper in the presence of a submaximal concentration of ATP but are inhibited by zinc or copper at concentrations above 100 µM. In contrast, human P2X2 (hP2X2) receptors show no potentiation and are strongly inhibited by zinc over the range of 2–100 µM. The effect of copper on hP2X2 is of interest because there are human brain disorders in which copper concentration is altered.
We found that hP2X2 receptors are potently inhibited by copper (IC50 = 40 nM). ATP responsiveness recovered extremely slowly after copper washout, with full recovery requiring over 1 h. ATP binding facilitated copper binding but not unbinding from this inhibitory site. A mutant receptor in which the first six extracellular cysteines were deleted, C(1–6)S, showed normal copper inhibition, however reducing agents dramatically accelerated recovery from copper inhibition in wild type hP2X2 and the C(1–6)S mutant, indicating that the final two disulfide bonds are required to maintain the high affinity copper binding site. Three histidine residues required for normal zinc inhibition were also required for normal copper inhibition. Humans with untreated Wilson’s disease have excess amounts of copper in the brain. The high copper sensitivity of hP2X2 receptors suggests that they are non-functional in these patients.
Keywords: P2X2 receptor, Copper inhibition, Potent, Wilson disease
1. Introduction
Copper has been reported to be present in many regions of the brain including the cerebral cortex, hippocampus and the cerebellum (Kozma et al., 1981; Sato et al., 1994) and a recent study producing a 3D atlas of copper distribution in the brain further supports this observation (Hare et al., 2012). It is clear that much of the copper in the brain is tightly bound to enzymes, where it functions as an essential cofactor for catalysis. However, there is reason to suspect that copper may also be involved in normal or pathological signaling. When copper levels in the brain are too low, as occurs with Menkes disease, a disorder caused by mutations in the X-linked gene encoding the copper transporter ATP7A, serious problems arise during central nervous system development (Keen et al., 1998). Menkes patients who have only null alleles typically are born with severe mental retardation and die perinatally. Copper levels that are too high are also problematic for brain function. In Wilson disease, mutations to the autosomal gene encoding another copper transporter, ATP7B, lead to dramatic, but highly variable effects on brain function (Huster, 2010). The major site of expression of the ATP7B protein is the liver and little or none is expressed in the brain, so the effect of the mutations on brain function must be indirect, with excess copper building up in the liver, leading to secondary increases in brain copper (Lutsenko et al., 2007). Typically people homozygous for mutations that cause Wilson disease are symptom less until late in their teens. In some individuals, only hepatic symptoms are present, but when brain effects appear they vary widely from patient to patient, and can sometimes be neurological (movement disorders, seizures, migraine, dystonia) and sometimes psychiatric (depression, personality changes, psychosis) (Lorincz, 2010). Interestingly, mice with the ATP7B gene knocked out show only hepatic symptoms (Huster et al., 2006) but the reason mice do not also show brain symptoms is unknown. A likely explanation is that some key protein or set of proteins respond differently to copper in the mouse and human brain.
We have demonstrated that human and rodent P2X2 receptors respond very differently to binding the divalent metal zinc (Tittle and Hume, 2008) and recently worked out some of the molecular reasons for this difference (Punthambaker et al., 2012). As many proteins that bind zinc can also bind Cu2+, we decided to test the effect of this metal on human P2X2. We report here that Cu2+ also has opposite effects on the function of human and rodent P2X2 receptors. The inhibitory effects of copper on human P2X2 begin in the low nanomolar range, making copper much more potent than zinc, and raise the possibility that these receptors may be modulated by copper during normal brain function or in copper overload disorders like Wilson disease. For this reason, we explored the mechanisms that allow such high potency in detail.
2. Materials and methods
2.1. Site directed mutagenesis
The source of the human P2X2b and rat P2X2a cDNAs has been previously described (Tittle and Hume, 2008). Mutations were made using the QuickChange mutagenesis kit (Agilent Technologies, Santa Clara, CA) and are referred to by the original single letter amino acid codon followed by the residue number and the substituted single letter codon. All mutations were confirmed by DNA sequencing from the University of Michigan DNA sequencing core.
2.2. Expression
RNAs encoding wild-type and mutant P2X2 receptors were synthesized using the mMessage Machine T7 kit (Life Technologies Corporation, Grand Island, NY) and expressed in stage V–VI Xenopus laevis oocytes. Oocytes were surgically extracted and harvested following methods approved by the University of Michigan Committee on the Use and Care of Vertebrate Animals. All efforts were made to minimize animal suffering, reduce the number of animals used and utilize alternatives to in vivo techniques, if available. Each oocyte was injected with 50 nl of RNA (50–100 ng/µl).
2.3. Solutions
The external recording solution consisted of (in mM): 90 NaCl, 1 KCl, 1.3 MgCl2 and 10 HEPES, pH 7.5. Disodium ATP (Sigma-Aldrich, St. Louis MO) was prepared as a 100mM stock in external recording solution and stored at −20 °C. The ATP solutions were made by diluting the stock in external recording solution and adjusting the pH to 7.5 to obtain the desired concentrations. Recording electrodes were filled with an internal solution of 3 M KCl. Zinc Chloride was prepared as a 10mM stock in external recording solution that was acidified with 0.01 M HCl to prevent precipitation. Copper was made up as a 100 mM stock of CuCl2 to generate Cu2+. Under the conditions of these experiments, little or no Cu1+ is expected to be present.
Cycloheximide (Sigma) was prepared as a 100 mg/ml stock in DMSO and diluted 1/100 in Barth’s solution just before being added to oocytes.
To treat oocytes with dithiothreitol (DTT) (Sigma) during recordings, it was prepared as a 100 mM stock in water, and stored frozen in small aliquots. Just before use it was diluted to its final concentration with recording solution. Because over time DTT can degrade, in every experiment that used DTT, a positive control was included to verify that the DTT was present at sufficient concentration to quickly break accessible disulfide bonds.
H2O2 was obtained as a 30% solution in water plus stabilizers that are described as keeping it at full strength for 5 years. It was diluted to its final working concentration (usually 0.3%) with recording solution just before use.
Apyrase (~ 200 units/mg) (Sigma) was prepared in recording solution (which in appropriate conditions also contained ATP and or copper) and used on the day of the experiment. The final concentration used was 1 mg/ml.
Suramin (Sigma) was prepared as a 1 mM stock on the day of each experiment, and then diluted to its final concentration with recording solution.
2.4. Electrophysiological recordings
Two-electrode voltage clamp studies were performed on oocytes expressing wild-type or mutant receptors 1–5 days after injection. For all recordings, the holding potential was −50 mV. Recording electrodes consisted of thin-walled borosilicate glass pipettes pulled on a P-97 Flaming Brown puller (Sutter Instrument Company, Novato, CA) that had resistances of 0.5–1 MΩ. Currents were recorded using a Turbo-Tec3 or Turbo-Tec10 amplifier (npi electronic GmBH, Tamm, Germany). Data acquisition was done using a Digidata 1322A interface controlled by Clampex 9 or 10 (Molecular Devices, Sunnyvale, CA).
The standard drug delivery system used in our electrophysiological recordings applies solutions to cells through a computer controlled, 8-way mixing valve (ALA Scientific Instruments, Farmingdale, NY). However, special procedures were used to apply copper containing solutions during recording because when flow is temporarily stopped (for instance when changing oocytes) a very small amount of mixing can occur between the lines. This clears within a second or two of resuming flow, and so was of no consequence when studying rapidly recovering zinc inhibition. However, because of the very slow recovery from copper inhibition, even a very small amount of copper exposure would alter the initial conditions of the experiment. Therefore, in most experiments copper was applied from a single completely isolated tube that had its tip kept just above the surface of the liquid in the recording chamber. When the valve controlling the control solution was shut off and the valve containing the copper solution opened, the solution “dripped” into the recording chamber. The only concern with this method of application was that the turbulence of the drops caused more frequent premature death of the oocyte than our standard drug application method. However, it was easy to monitor the health of the oocytes using the holding current at −50 mV.
2.5. Data analysis
Preliminary data analysis was performed using Clampfit 10 (Molecular Devices, Sunnyvale, CA), with additional analysis done with Microsoft Excel. Concentration-response relations were fit using the non-linear curve fitting program of Sigmaplot 9.0 or 10.0 (Systat Software, San Jose, CA). For ATP, the 3 parameter Hill equation was used.
where [X] = the concentration, Max = the maximum response (100%), EC50 = Concentration at the half-maximum response amplitude and n = the Hill coefficient.
For copper inhibition the 3 parameter Hill equation was used when the inhibition was complete, and the 4 parameter Hill equation was used when the maximal effective concentration of copper did not completely eliminate the ATP activated current.
For analysis of the magnitude of copper inhibition, the data from each cell were normalized to the maximum value of the ATP response, which was assigned a value of 100%. When parameters of the fit are given in text or figure legends, they were obtained by finding the best IC50 for each cell, and then reporting the average IC50± the standard error of the mean and the sample size. For display in figures, the normalized points at each copper concentration from all cells were averaged and plotted with error bars indicating the standard error of the mean. The fits shown on the graphs are to these averaged data, and therefore the IC50 of these fits sometimes differed slightly from the mean value of the IC50 reported in text.
3. Results
3.1. Copper inhibited human P2X2 receptors
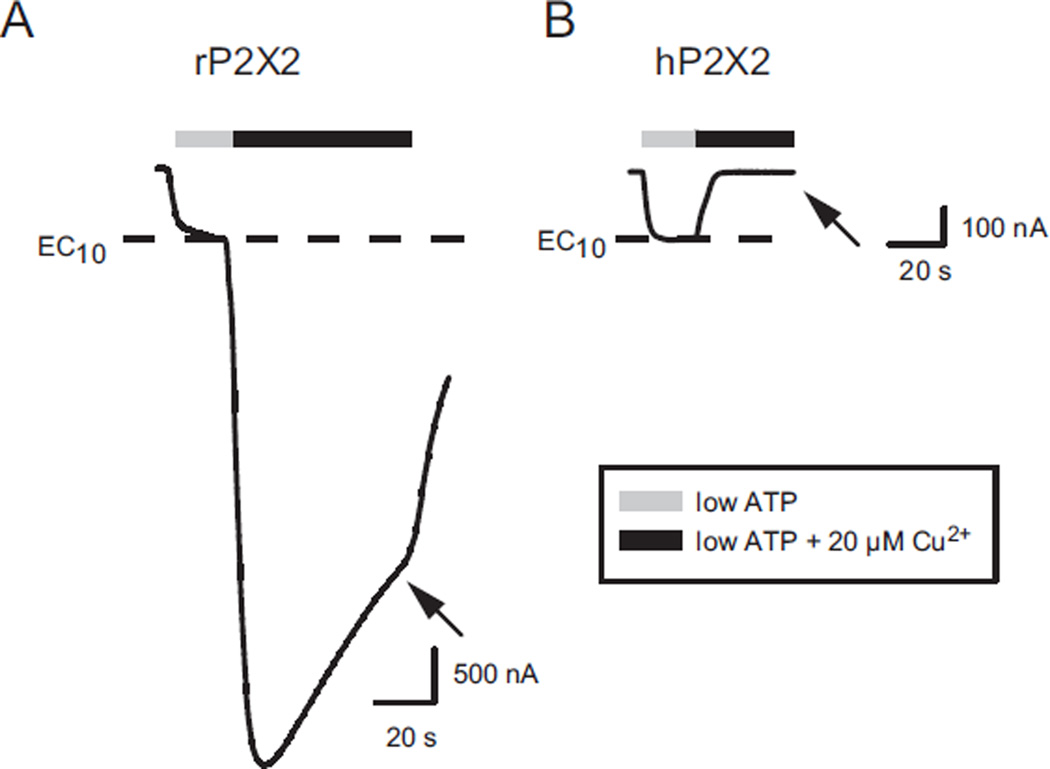
Rat P2X2 receptors are known to be greatly potentiated by micromolar levels of Cu2+ (Xiong et al., 1999). When ATP was set to its EC10, and 20 µM copper applied, the potentiation was approximately 10-fold (Fig. 1A). In contrast, when we studied human P2X2 receptors (hP2X2) at their EC10 for ATP, 20 µM copper caused complete inhibition of the ATP response (Fig. 1B). We had previously demonstrated that hP2X2 is inhibited by micromolar zinc, and that the IC50 was about 8 µM when EC10 ATP was used (Tittle and Hume, 2008; Punthambaker et al., 2012).
Fig. 1.
Effect of Copper on rat (rP2X2) and human (hP2X2) receptors. In this and all subsequent experiments the holding potential was −50 mV. A) Two-electrode voltage clamp recording from an oocyte expressing rP2X2. The low (EC10) concentration of ATP was 2 µM. The arrow indicates the current at the end of the copper application. B) Similar recording from an oocyte expressing hP2X2. The low (EC10) concentration of ATP was 5 µM.
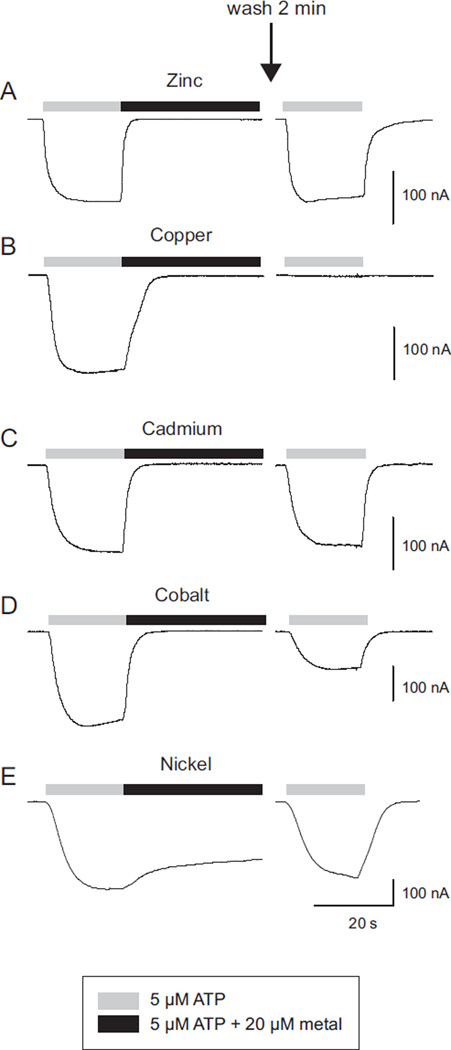
When oocytes expressing hP2X2 were treated with 5 µM ATP plus 20 µM zinc, washed for 2 min with recording solution and then retested with ATP only, their response amplitude was similar to the initial application of ATP alone (Fig. 2A). In contrast, when the identical paradigm was given, except that 20 µM copper was used as the inhibitor, there was little or no recovery of the ATP response detectable after 2 min of washout (Fig. 2B). On average the response at 1–2 min after washing out copper was 0.8 ± 0.02% (N = 10) of the initial response. We also tested a number of other divalent metals. At 20 µM, cobalt, cadmium and nickel all produced inhibition of hP2X2, but as with zinc, recovery was rapid (Fig. 2C–E).
Fig. 2.
Recovery of hP2X2 from inhibition by various divalent metals. Representative traces for five different metals tested with the identical paradigm are shown. After the initial test (ATP followed by ATP plus the metal), the cell was washed for 2 min with recording solution, and then retested with ATP alone. Only copper produced long-lasting inhibition.
3.2. Copper inhibition was long-lasting
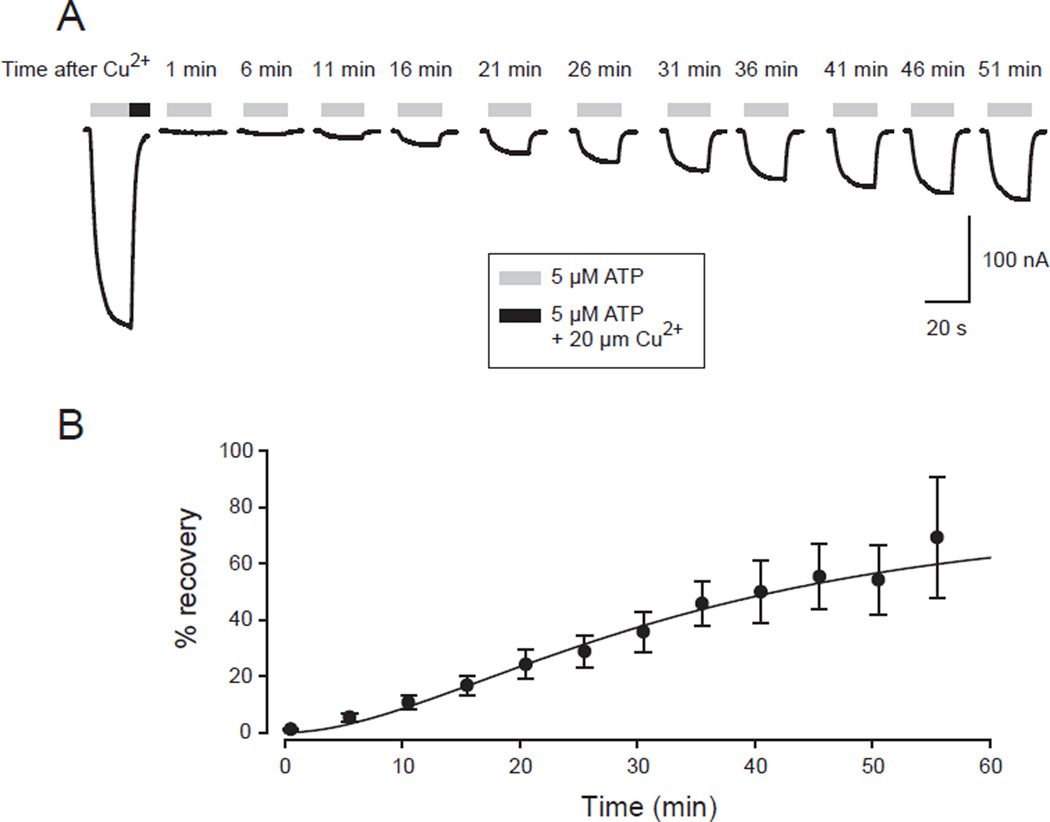
To characterize the time-course of recovery from copper inhibition, short ATP pulses were applied once every 5 min beginning 1 min after a complete inhibition of the ATP-evoked current by copper (Fig. 3A). By half an hour of washout, recovery was substantial, and responses had typically nearly reached a final steady state value by 1 h. In the case illustrated, which was typical, the final value reached was less than 100% of the initial response, as was also true of the average of a series of cells (Fig. 3B), but some cells stabilized at a final value of 100% or even greater. It was also notable that recovery always followed a sigmoid time course, so that after a lag period with very little recovery, the recovery rate accelerated for a while and then decelerated as the final steady state was approached. The data could usually be well fit by an exponential function raised to a power (the Chapman equation):
where Imin was the current immediately after copper was removed, Imax was the final value, τ (tau) was the time constant and Nwas the power. When ATP was at the EC10 and 2 or 20 µM copper was used, Imin was very close to 0. For a series of cells tested in this way the time constant of recovery (τ) averaged 19.5 ± 5.1 min, the power averaged 2.0 ± 0.3 and Imax averaged 83% ± 6% (N = 11).
Fig. 3.
Time course of recovery of hP2X2 from copper inhibition. A) An initial 20 s application of ATP was immediately followed by a 60 s application of ATP plus copper (only the first 10 s are shown) followed by a 60 s wash with recording solution alone. Additional 20 s pulses of 5 µM ATP were then given at the times shown. B) Results of a series of experiments as in A. All time points represent the average of data from at least 7 cells, except for the 55 min point, for which N = 4. The parameters of the fit to the Chapman equation are: Imin = 0, Imax = 73.9%, time constant (τ) = 24.2 min, N (power) = 2.0.
3.3. Copper inhibition was potent
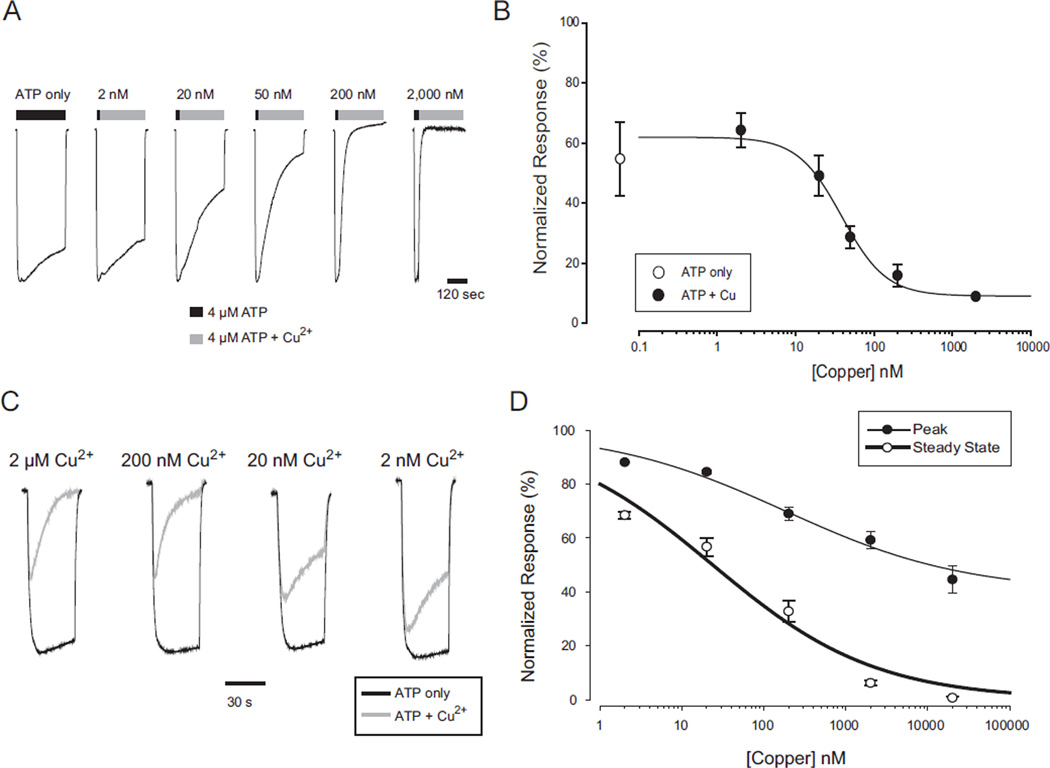
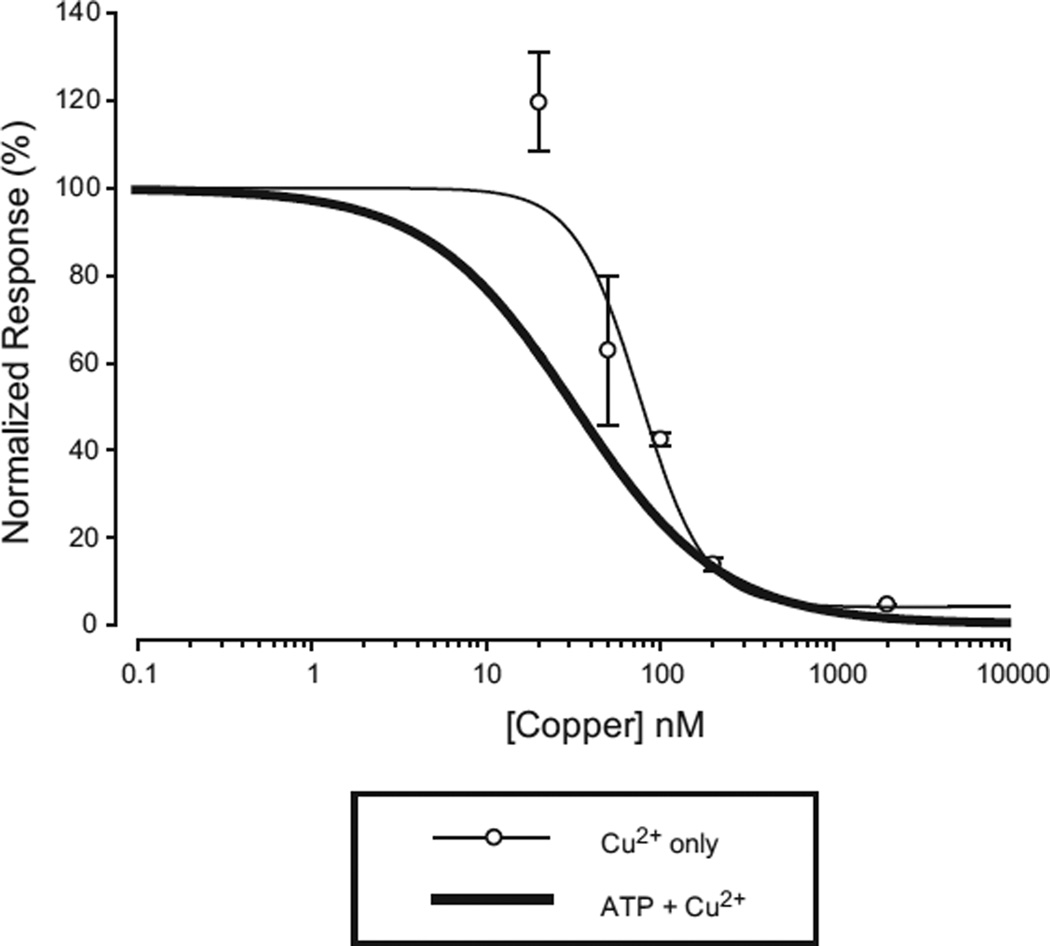
Because recovery from copper inhibition was extremely slow, to make a concentration response relation for the effect of copper we used protocols where each cell was exposed to copper only once, and then averaged data from many cells tested with the EC10 concentration of ATP, and a variety of copper concentrations. The usual protocol was to first apply a 2 step screening protocol of low ATP followed by saturating ATP (200 µM) to verify that the low concentration was sufficiently close to the EC10 (cells in the range EC5 to EC15 were used). All cells that were in range were then given a two-step test protocol of low ATP followed by low ATP + copper (Fig. 4A). Copper applications were maintained for 5 min, which was sufficient time to estimate steady-state inhibition. Copper inhibition measured in this way was extremely potent (Fig. 4B) with an IC50 of 39 nM. Furthermore, the rate at which the current declined depended on the concentration of copper used. We also used an alternative protocol in which cells that successfully passed the screening protocol were tested with low ATP alone, given 2 min to recover in recording solution alone and then tested by co-application of low ATP and copper (Fig. 4C). This protocol also indicated that inhibition was of very high potency and revealed an additional feature of interest. At all concentrations of copper used, there was an initial response to ATP, which then fell back to some level of steady state inhibition. Both the amplitude of the peak and the steady state response were copper dependent and indicated high potency (Fig. 4D). The observation that even extremely high copper concentrations were not able to completely suppress the initial responses to ATP indicates that the rate of channel opening is faster than the rate of inhibition and suggests a model in which the channels must be opened by ATP before they can be inhibited by copper.
Fig. 4.
Concentration response relation for copper inhibition of hP2X2 when ATP was present. A) Time dependent decrease in current in the presence of ATP alone or ATP plus the indicated concentration of copper. The initial 30 s application of ATP alone in each trace (black bar) was sufficient to produce a peak ATP response. The subsequent 5 min application of ATP (black bar) or ATP plus copper (gray bar) allowed an accurate assessment of the steady state level. Each trace is from a different oocyte, and all were studied at their EC10 for ATP. The concentration of copper is shown above each trace. To facilitate comparison of the magnitude and rate of inhibition at different copper concentrations, all traces were normalized to their peak response with ATP alone. B) Concentration–response relationship for a range of copper concentrations tested on a series of oocytes as shown in A. Each point is the average from 5 to 10 oocytes measured at the end of the 5 min application of ATP alone or ATP plus copper. Fitting the three parameter Hill equation to the averaged data gave an IC50 of 39 nM. C) Responses of hP2X2 expressing oocytes to several different concentrations of copper tested with a paradigm in which ATP only was applied (black traces), followed by a 2 min wash, and followed by co-application of ATP and copper (gray traces). The responses were normalized to the peak amplitude of the ATP only traces. Each recording is from a separate oocyte. D) Concentration response relations for the peak response and the steady state response for a series of experiments as in C. The data were normalized to the responses to application of ATP alone.
3.4. Effect of copper alone or in the presence of P2X2 receptor inhibitors
If inhibition by copper requires opening of the channel by ATP, one apparent prediction is that pre-applying copper without exogenous ATP, washing, and then testing with ATP alone should give no inhibition. This was not the result that was obtained. Applying 20 µM copper for 1 min or longer produced nearly complete and long-lasting inhibition. The results of one experiment to assess the concentration response relation in which a large number of cells at each copper concentration were tested are shown in Fig. 5. In this experiment, batches of 7–10 oocytes were first tested with ATP, removed from the recording chamber, washed with ATP free solution, and then incubated in copper containing solutions in 35 mm dishes. Beginning 10 min after incubation (long enough for steady state inhibition to become complete when ATP and copper were co-applied during recording, even for low concentrations of copper), we began returning oocytes to the recording chamber. Once impaled and voltage clamped, each cell was washed with copper free solution for 1 min and retested for ATP responses. The last cell of each batch was retested about 20 min after the first (but after only a 1–2 minwash). The % inhibition for the first and the last oocyte tested in each batch was similar. In this experiment, the IC50 for copper alone was 76 nM, only slightly higher than observed when ATP and copper were co-applied as in Fig. 4. However, the results of other experiments testing “copper alone” gave IC50s that were as much as 10-fold higher or lower than this. Furthermore, when individual oocytes were kept in the recording chamber for the entire sequence of solution changes, the identical concentration of “copper alone” could cause nearly complete inhibition of some cells, and virtually no inhibition of others.
Fig. 5.
Concentration response relation for copper inhibition of hP2X2 when no exogenous ATP was added. The thick line is the fit to the data from Fig. 4B. See text for details of how this experiment was carried out.
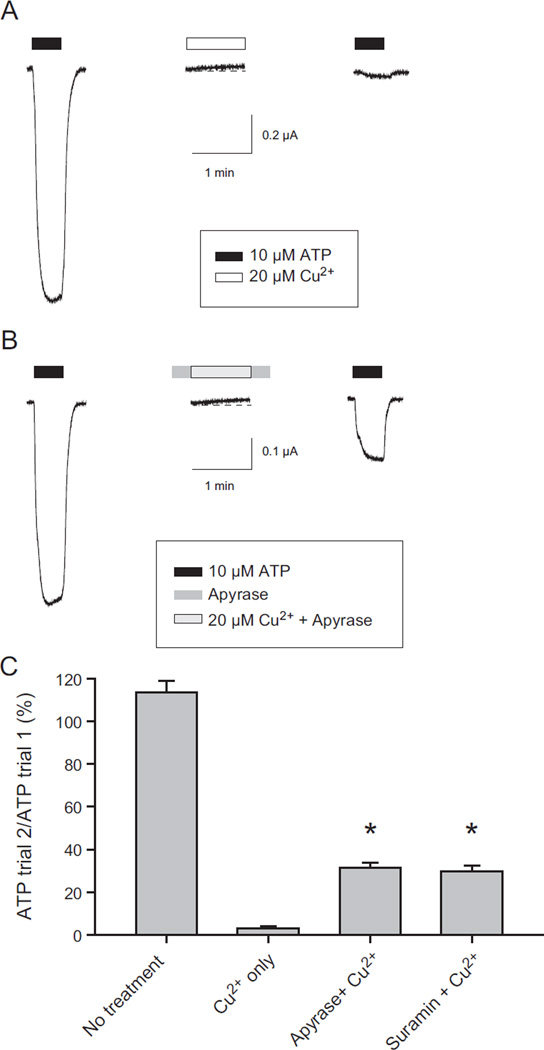
A plausible explanation for these variable results was that ATP is required for copper inhibition and that inhibition by copper occurred under “ATP free” conditions because oocytes release enough ATP to reach a concentration at the oocyte surface sufficient to activate some hP2X2 channels in the absence of exogenous ATP. The presence of a basal ATP dependent current in oocytes expressing hP2X2 in the absence of exogenous ATP was confirmed by the observation that in many cells the inward holding current at −50 mV became slightly less negative in the presence of either the ATP degrading enzyme apyrase, or the hP2X2 antagonist suramin. In a typical experiment, the shift in holding current with 25 µM suramin was +10.7 ± 2.9 nA (N = 7), while when recording solution was added through the same barrel of the drug application system the shift was negligible (+1.3 ± 0.8 nA, N = 8). Similarly, application of 20 µM copper alone also caused a decrease in inward holding current in most cells (for example Fig. 6A, middle trace).
Fig. 6.
Effect of apyrase and suramin on copper inhibition of hP2X2 when no exogenous ATP was present. A) Effect of copper alone. During the time periods with no drug bar, the cell was superfused with recording solution only. The time scale bar indicates the duration of the washes as well as the duration of the recordings. B) Effect when apyrase was present before, during and after the application of copper. C) Histogram of the extent of recovery of ATP-evoked currents under different conditions. The protocol used to test suramin was identical to the protocol used to test apyrase in B. Asterisks indicate a significant increase in current compared to copper alone.
We therefore carried out experiments to knock down the effect of any endogenously released ATP. In these experiments, apyrase or suramin alone was applied for 30 s before and after the application of the test compound plus copper and after a 2 min wash, copper inhibition was assayed by applying ATP alone. The inhibition by copper was dramatically decreased by pretreatment with either apyrase or suramin (Fig. 6B, C), but was not abolished. One interpretation of these results is that inhibition can occur without ATP, but at a much slower rate than in its presence. We favor an alternative interpretation that the highest inhibitor concentrations that were practical to use (due to cost and solubility issues) still allowed some hP2X2 receptors to be activated by endogenously released ATP and then inhibited by the exogenous copper. Evidence that supports this idea is that when copper was applied in the presence of apyrase (Fig. 6B, middle trace) it still often elicited a small outward current (which we interpret to be due to channels that were activated by the basal ATP that managed to elude the apyrase and then became inhibited when copper was added).
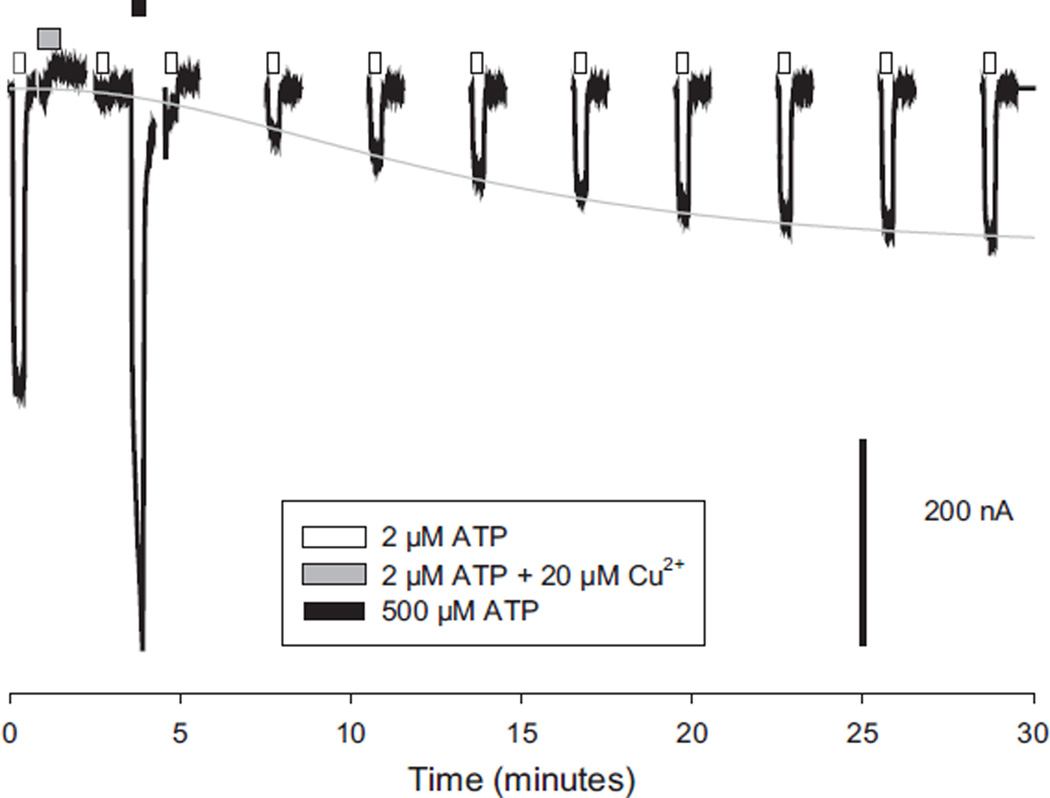
As ATP seemed to be necessary to allow copper to bind to the receptor, we also tested whether ATP could promote copper leaving the receptor. After inhibiting nearly 100% of the hP2X2 channels with 20 µM copper, we gave a series of test pulses of 2 µM ATP (approximately the EC10), to determine the time course of recovery from copper as in Fig. 3, or gave a single 1 min pulse of very high ATP (500 µM) soon after the removal of copper and then resumed the test pulses. Although 500 µM ATP produced a large response even shortly after the application of copper (Fig. 7), the next test pulse fell back down to exactly the amplitude expected if the high ATP had not been given, indicating that no enhancement of recovery had occurred. Analysis of the time constant of the recovery function confirmed the lack of an effect. In the absence of a high ATP pulse, the average time constant of recovery in this batch of oocytes was 7.0 ± 1.1 min and in its presence the time constant was 12.9 ± 1.7 min (N = 5 each).
Fig. 7.
Recovery from copper inhibition of hP2X2 is not enhanced by channel opening. Recovery from copper was monitored by applying test pulses of ATP every few minutes. After 9 min only every other pulse is illustrated. One minute after treatment with ATP plus copper (grey box), a pulse of very high ATP (black box) was interposed between the first and second recovery test pulses (open boxes).
3.5. Test of mechanisms for recovery from copper inhibition
The recovery from copper inhibition might arise by several mechanisms. One possibility is that copper is binding to a very high affinity site with a slow dissociation constant (off rate). Another possibility is that copper permanently inactivates the receptors and the rate of recovery represents the rate of insertion of intracellular receptors onto the surface. If recovery is by insertion, then under conditions in which the intracellular pool is depleted, the rate of recovery should be much slower and the final amplitude of the currents should be much smaller than the initial amplitude because fewer receptors are available to replace the inactivated ones. To deplete the intracellular pool, we treated oocytes with the protein synthesis inhibitor cycloheximide (CHX) beginning 24 h after RNA was injected. We then compared the rate of recovery from copper in CHX treated and untreated oocytes 24 or 48 h later.
An essential control was to demonstrate that we were using a dose of CHX that eliminated hP2X2 synthesis, and this indeed was the case, although it required a dose 10 times higher (1 mg/ml = 3.5 mM) than is typically used on mammalian cells in culture. When oocytes were bathed in CHX beginning immediately after RNA injection (t = 0), and then tested for responses to saturating ATP (200 µM) 24 h later, there were no detectable ATP evoked responses, while addition of the vehicle (DMSO) without CHX gave large responses of an average amplitude similar to oocytes that received no treatment (CHX treated = −2 ± 3 nA, N = 20; DMSO treated = −1230 ± 80 nA, N = 5; Untreated = −1160 ± 140 nA, N = 4). Consistent with potent inhibition of protein synthesis, the average amplitude of responses to saturating ATP (200 µM) were much smaller in oocytes treated with CHX beginning at 24 h after injection and assayed 24–48 h later (−10,500 ± 1400 nA) than in untreated oocyte (−68,300 ± 8400 nA).
In contrast to the predictions of the replacement hypothesis, the extent of recovery from copper inhibition was similar for oocytes that received CHX treatment beginning at 24 h after injection and were assayed 24–48 h later (78 ± 10%, N = 20) and for untreated oocytes, (which as reported above was 83% ± 6%). The rate of recovery was similar as well (untreated 19.5 ± 5.1 min; CHX treated 25.2 ± 3.7 min). As a final control, we conducted a set of experiments to assess the effect of CHX on a process that was known to be dependent on insertion of new receptors. Modification of I67C of rat P2X2 by large molecules blocks access of ATP to its binding site (Jiang et al., 2000). Alexa 488 maleimide (AM488) is a highly effective blocker at this site of rat P2X2 (Shlomo Dellal, personal communication), and since this compound does not cross the membrane and the bond is very stable, the rate of recovery of ATP sensitivity is a measure of the replacement rate from the intracellular pool. When we made the homologous mutation of hP2X2 (I79C) we found that recovery from AM488 treatment was different from recovery from copper treatment in several important features. First, it followed an exponential time course, while the recovery from copper had a lag phase and so required an exponential function raised to a power greater than 1. Second, in experiments in which CHX was added 24 h after RNA injection, and recovery tested at 48 h, the amplitude of the ATP responses after recovery from copper nearly reached the pretreatment value (93 ± 7%, N = 7) when sufficient time had elapsed, while the final value for the AM488 treated oocytes reached only 11 ± 2%, (N = 4). Third, after the same CHX treatment the time constant of the recovery function of AM488 was 3-fold slower than for copper. As these experiments demonstrate that ATP responsiveness after copper treatment is unchanged when delivery of internal receptors is severely impaired, we conclude that recovery from copper inhibition is likely due to recovery of the original surface receptors from inhibition by slowly unbinding the copper.
3.6. Inhibition by copper is allosteric
The ability of 500 µM ATP to transiently overcome copper inhibition of hP2X2 that was demonstrated in Fig. 7 suggested that the mechanism of inhibition is allosteric, rather than by blocking the channel, as channel blocking would not be overcome with additional agonist. We further explored this issue by assessing the ATP concentration response relation of wild type hP2X2 before and after copper treatment. The EC50 for ATP immediately after treatment with 20 µM copper was over 200 µM, a right shift of 9.8 ± 2.4-fold (N = 7). Furthermore, the maximal current at saturating ATP immediately after copper treatment was only 20 ± 4% of the pretreatment control. Thus, bound copper makes it harder for ATP to open the channel.
3.7. Effects of modifying cysteines on copper inhibition
The most common residues involved in copper binding sites are cysteines and histidines (Rubino and Franz, 2012). We first explored the possible role of the extracellular cysteines, because if any Cu1+ was present, it would represent a redox active metal that potentially might promote changes in the disulfide bonding state of the receptor. The only extracellular cysteines of hP2X2 are the ten that are highly conserved across all chordate P2X receptors. Studies of P2X1 and P2X2 strongly suggested that these form five disulfide bonds (Clyne et al., 2002; Ennion and Evans, 2002). This disulfide bond pattern was confirmed by the crystallization of the zebrafish P2X4.1 receptor (Kawate et al., 2009). The first six cysteines form three disulfide bonds (SS1, SS2 and SS3) located in the “head” region of this dolphin resembling structure, while the last four cysteines forming two disulfide bonds (SS4 and SS5) near the “dorsal fin” region (Kawate et al., 2009). Although it is clear that these residues can form disulfide bonds, there is a suggestion in the literature that under appropriate conditions some bonds become reduced and so their cysteines are available to participate in copper binding (Coddou et al., 2007).
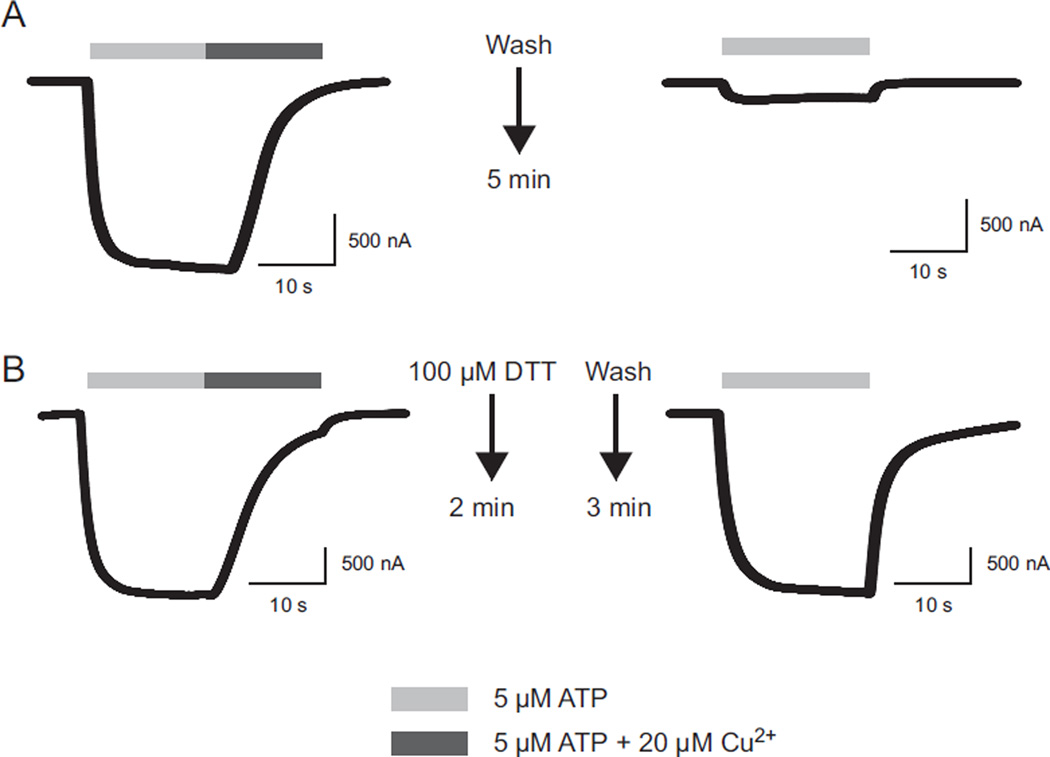
As an initial test of a potential role for non-disulfide bonded extracellular cysteines, we examined the properties of receptors that had their disulfide bonds chemically reduced by DTT or TCEP. When wild type hP2X2 was treated with DTT, the EC50 of the post-treatment ATP concentration response relation was 13.6 ± 2.8 µM (N = 7) which was indistinguishable from untreated oocytes. Similar results had previously been reported for rat P2X2 (Li et al., 1997; Rassendren et al., 1997; Clyne et al., 2002). However, DTT had a huge effect on responses to copper (Fig. 8). As noted previously, after a 5 min wash following treatment with 20 µM copper plus ATP, recovery was minimal. In the batch of control oocytes studied on the same day as the DTT experiments, the average amplitude was 2 ± 1% (N = 7) of the pretreatment value (Fig. 8A). However, when cells were treated with DTT or TCEP for 2 min immediately after treatment with copper and then washed with recording solution, by 3 min later (Fig. 8B) the responses had returned to 100% of the pre-copper amplitude (DTT 107 ± 5%, N = 7; TCEP 103 ± 5%, N = 3). When the sequence of drug application was reversed (DTT, then copper) the effect was time dependent. When cells were tested with copper within the first 5 min after DTT treatment, the inhibition by copper was less effective (the post copper current was to 17 ± 5% of the pretreatment level rather than the 2% shown without DTT pretreatment) and recovery was complete within 2 min. However, if the copper presentation was delayed until 30 min after DTT, the extent of inhibition was as great as in untreated oocytes and recovery was just as slow. A likely explanation for these results is that the cysteines gradually reform their disulfide bonds. Evidence for this is that once recovery had occurred, a second treatment with DTT could return the receptors to the state where copper had low potency and recovery was rapid.
Fig. 8.
Effect of the disulfide bond reducing agent dithiothreitol (DTT) on recovery from copper inhibition. A) Wash with recording solution only. B) Treatment with DTT.
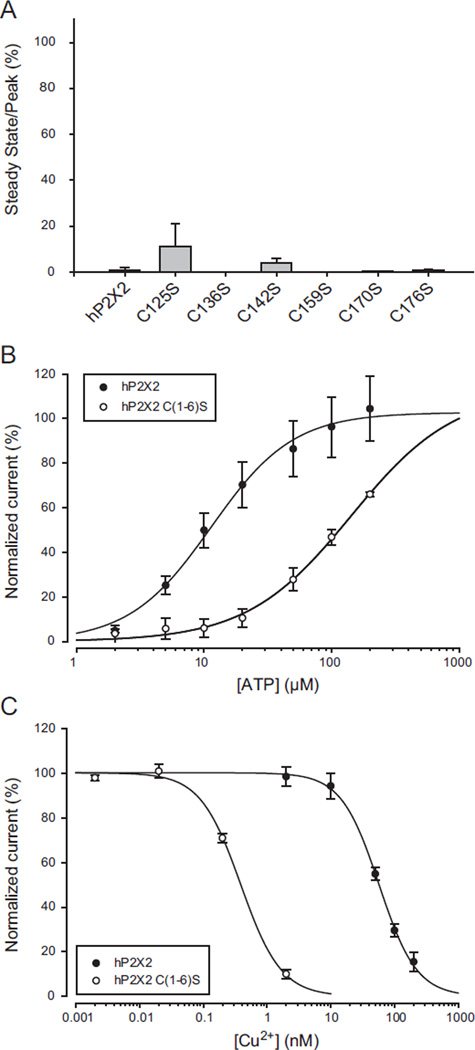
To test whether breaking of a specific disulfide bond was responsible for the effect of reducing agents on copper inhibition, single cysteine to serine mutations were made. No ATP evoked currents could be detected when the final four cysteines were mutated, but mutation of any one of the first six resulted in receptors that responded to ATP, although mutations at the first four positions had much lower potency than wild type (the EC50s for ATP were C125S = 363.4 ±76.9, N = 5; C136S = 392.2 ± 66.5, N = 5; C142S = 531.6 ± 117.7, N = 4; C159S = 255.5 ± 9.4, N = 6; C170S = 16.6 ± 2.3, N = 4 and C176S = 13.3 ± 2.6, N = 5). To assess the effect of these mutations on copper inhibition, we measured the amplitude of the currents present at steady state when the bathing solution was changed from EC10 ATP only to EC10 ATP plus 20 µM copper. All showed inhibition similar to wild type (Fig. 9A) and all recovered from application of 20 µM copper very slowly along a sigmoid time course well described by the Chapman equation with a time constant greater than 5 min.
Fig. 9.
Effects of mutation of extracellular cysteines on copper inhibition. A) Responses to copper of single mutations of each of the first six extracellular cysteines to serines. The data plotted are the responses to the EC10 concentration of ATP plus 20 µM copper divided by the responses to ATP alone. None were significantly different from wild type. Mutation of each of the last four extracellular cysteines to serine produced nonfunctional proteins. B) ATP concentration response relation of the hP2X2 C(1–6)S mutant in which the first six cysteines were simultaneously mutated to serine. C) Copper concentration response relation for hP2X2 C(1–6)S. The data for the wild type controls illustrated were collected on the same day as the data for the mutant receptors.
These single mutations would be expected to break one of three disulfide bonds that are found very close to each other, and so they might not cause much of a structural change. We therefore also studied the hP2X2 C(1–6)S sextuple mutant where the first six cysteines of the extracellular domain of the hP2X2 receptor were all replaced by serines. This mutant had low ATP potency, with its ATP concentration relationship shifted about 10-fold to the right (Fig. 9B). Surprisingly, when studied at its EC10 for ATP, this mutant showed higher potency for copper than wild type hP2X2 (Fig. 9C). Like wild type receptors, the time course of recovery from copper inhibition was very slow and followed a sigmoid time course, with a time constant averaging 11 ± 2.1 min (N = 5). Thus none of the first six cysteines are essential participants in copper binding.
We therefore tested the effect of reducing agents on the C(1–6)S mutant. Without treatment by a reducing agent, the response of C(1–6)S to ATP alone after treatment with 20 µM copper plus 20 µM ATP averaged 1.7 ± 0.2% of the pre-copper control at 1 min (N = 13) and 3.4 ± 0.5% at 4 min (N = 5). However, when a reducing agent was added 2 min after copper plus ATP and washed away 3 min after copper, the response to ATP alone at 4 min had nearly returned to the full control amplitude (10 mM TCEP 77.3 ± 1.8%, N = 4; 10 mM DTT 94.1 ± 3.3%, N = 4). As only the SS4 and SS5 disulfide bonds were present in this mutant, one or both of these is required to maintain the structure that allows high potency copper inhibition.
3.8. Effects of modifying histidines on copper inhibition
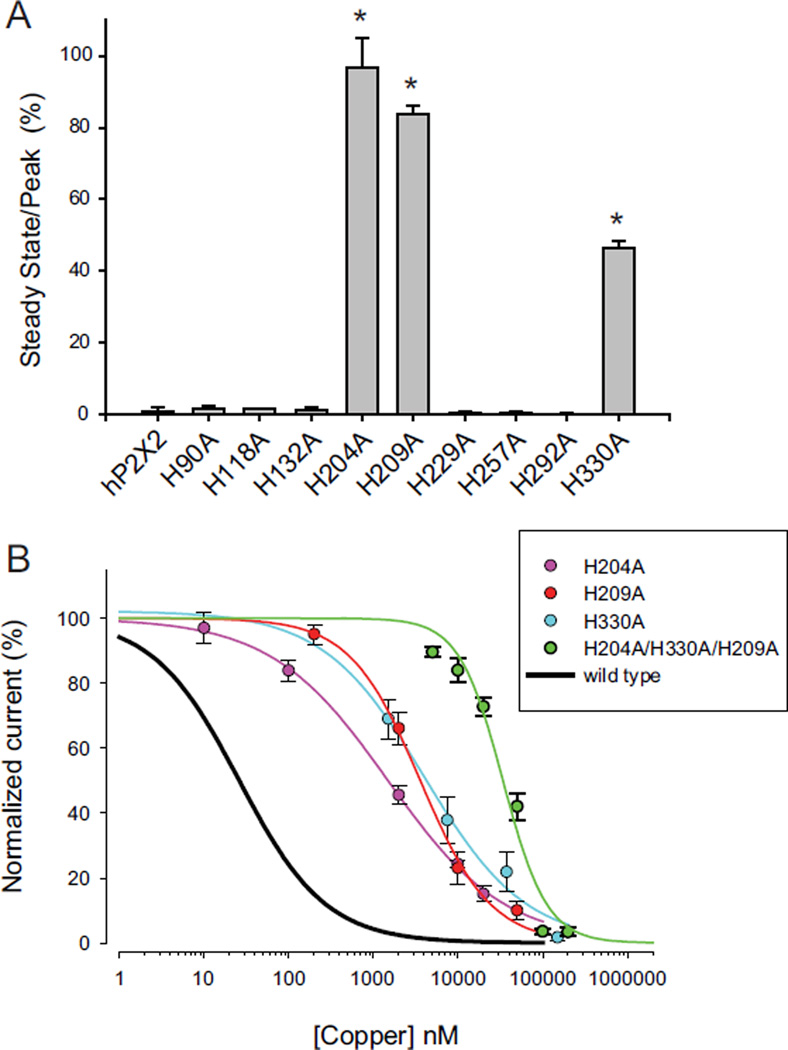
In contrast to the lack of effect with single cysteine mutagenesis, there were three H to A mutations that showed significantly reduced copper inhibition. When tested with 20 µM copper, the steady state inhibition of H204A, H209A and H330A were much less than for wild type or the other 6 histidine to alanine mutants (Fig. 10A). We therefore estimated the copper concentration response relations for these mutants. At the EC10 for ATP, the respective values for their IC50s were 1.5 µM, 3.6 µM and 4.0 µM (Fig. 10B). Thus copper inhibition is present, but is at least 40-fold less potent. We also tested the triple histidine to alanine mutant for its effect on copper inhibition and found that similar to its effect on the potency of zinc inhibition (Punthambaker et al., 2012), this mutant was shifted about a 1000-fold to the right on the concentration–response curve for copper and had an IC50 value of 34.0 µM. Even though copper is much more potent, the observation that this triple histidine mutation produced similar shifts in the potency of inhibition suggests that zinc and copper act by similar mechanisms. The relationship between the position of these three histidine residues in the structure of hP2X2 and the inhibition of ATP responses by zinc has recently been discussed in detail (Punthambaker et al., 2012).
Fig. 10.
Effects of mutation of extracellular histidines on copper inhibition. A) Responses to copper of single mutations of each of the nine extracellular histidines to alanines. The data plotted are the responses to the EC10 concentration of ATP plus 20 µM copper divided by the responses to ATP alone. Responses that were significantly different from hP2X2 are indicated with an asterisk. B) Copper concentration response relations for H204A, H209A H330A and the triple mutant of all three compared to wild type hP2X2.
4. Discussion
In rat and mouse P2X2, copper and zinc both strongly potentiate responses to low concentrations of ATP (Wildman et al., 1999; Xiong et al., 1999; Tittle and Hume, 2008). We had previously shown that in human P2X2, zinc strongly inhibits responses to low concentrations of ATP (Tittle and Hume, 2008) and our initial goal for this study was to see if copper does so too. We quickly discovered that copper inhibits hP2X2 with a potency approximately 200-fold greater than zinc, and that recovery from copper inhibition is extremely slow. The three other divalent metals we tested (cadmium, cobalt and nickel) had effects similar to zinc, with recovery from inhibition complete in just a few minutes. The time course of recovery from copper inhibition could usually be fit well by an exponential function raised to a power greater than 1, with a time constant around 15–20 min and a power near 2. These parameters indicated that full recovery will typically require nearly an hour. We therefore tested the possibility that recovery might be due to delivery of new receptors to the surface, but our studies with cycloheximide suggested that very slow unbinding of copper from a high affinity site is the more likely explanation.
The ability of apyrase and suramin to suppress copper inhibition suggested that binding ATP promotes entry into a state that enhances copper inhibition (and may even be required). Presumably ATP binding causes a conformational change that makes the site more easily accessible. Therefore, a likely explanation for the high affinity and slow off rate is that copper becomes conformationally trapped by the receptor once it inhibits it. This model is strikingly similar to results obtained in studies of the ASIC channel, which shares great structural homology with P2X receptors in the pore regions and vestibule cavities (Gonzales et al., 2009). ASIC channels are closed at pH 8 and undergo desensitization at low pH (pH 7 and below) which is accompanied by a substantial conformational change in the receptor. It has been demonstrated that when D79, a residue in the middle vestibule is mutated to a cysteine, it is accessible to cysteine-modifying agents when the channel is closed but not when the channel is desensitized (Cushman et al., 2007). As the middle vestibule of P2X receptors has been implicated as a potential binding site for divalent and trivalent cations (Kawate et al., 2009; Punthambaker et al., 2012), if the mechanism of copper inhibition is promoting entry into the desensitized state, it would explain why the copper is unable to leave its binding site and thus recovery is so slow.
Surprisingly, once copper is in its binding site, re-opening the channel with high ATP did not accelerate recovery, indicating that some other step is rate limiting for unbinding. However, mutations to residues that sit at the entrance to the middle vestibule (H204A, H209A, H330A) promoted rapid recovery. So did treatment with the reducing agents DTT or TCEP. The crystallization of the zebrafish P2X4.1 structure unequivocally confirmed the presence of 5 disulfide bonds between the 10 highly conserved cysteine residues (Kawate et al., 2009), which had been called into question by the claim that one of the cysteines in the cys-rich domain of rat P2X4 receptor played a role in zinc binding (Coddou et al., 2007). One possible explanation for the effect of reducing agents was that breaking of disulfide bonds and reforming them later is part of the normal mechanism for allowing copper to unbind. Indeed, we showed that within half an hour of chemically breaking the endogenous disulfide bonds they appear to spontaneously re-form, as high potency copper inhibition returns. However, the residue homologous to the specific cysteine proposed by Coddou et al. (2007) cannot be responsible for promoting either binding or unbinding copper in hP2X2, as it is absent in the C(1–6)S mutant which we showed binds copper with even higher potency than wild type hP2X2.
Considerable evidence suggests that in the mammalian central nervous system P2X2 receptors are expressed both on neurons and glia (Burnstock and Knight, 2004; Illes and Ribeiro, 2004), but the CNS phenotype of P2X2 knock-out mice is quite modest (Cockayne et al., 2005). Based on extrapolations of data measuring release of copper from synaptosomes, the free Cu2+ present beneath active synapses has been suggested to rise to as high as 2–3 µM (Hopt et al., 2003). However, there are reasons for suspecting that the copper concentration in many brain regions does not get this high. First, when rat hippocampal slices are bathed in 1 µM Cu2+, long term potentiation in CA1 is abolished (Doreulee et al., 1997). As these rats require LTP to learn, it seems unlikely that copper ever gets this high at these excitatory synapses. Similarly, if brain copper levels in humans routinely reached 200 nM Cu2+ or higher, the hP2X2 receptors would always be inactive. As nonfunctional genes typically rapidly evolve into pseudogenes, the fact that hP2X2 retains an open reading frame that encodes a functional protein implies that in the regions of the human brain where these receptors are expressed, copper rarely get this high in healthy individuals. However, the high potency of copper inhibition of hP2X2 makes one wonder if these receptors might be affected in humans with Wilson Disease, which is characterized by an excess of copper in the liver that secondarily raises copper levels in other tissues including the brain. If lack of activity via P2X2 signaling plays a role in the pathophysiology of Wilson Disease, it would provide a nice explanation for why mice with mutations in ATP7B show no brain symptoms similar to human Wilson patients. As noted in the Introduction, copper potentiates, rather than inhibits rat and mouse P2X2 receptors, so P2X2 signaling would be enhanced, rather than inhibited in these model organisms.
Acknowledgments
We thank Drs. Haoxing Xu, Gabrielle Rudenko and Matthew Chapman for helpful discussion of this work. A portion of this work was supported by NIH grant NS039196.
Abbreviations
- DMSO
dimethyl sulfoxide
- DTT
dithiotreitol
Footnotes
Conflict of interest
The authors declare no competing financial interests.
References
- Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int. Rev. Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- Clyne JD, Wang LF, Hume RI. Mutational analysis of the conserved cysteines of the rat P2X2 purinoceptor. J. Neurosci. 2002;22:3873–3880. doi: 10.1523/JNEUROSCI.22-10-03873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, Ruan HZ, Ma B, Yip P, Nunn P, McMahon SB, Burnstock G, Ford AP. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J. Physiol. 2005;567:621–639. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddou C, Acuna-Castillo C, Bull P, Huidobro-Toro JP. Dissecting the facilitator and inhibitor allosteric metal sites of the P2X4 receptor channel: critical roles of CYS132 for zinc potentiation and ASP138 for copper inhibition. J. Biol. Chem. 2007;282:36879–36886. doi: 10.1074/jbc.M706925200. [DOI] [PubMed] [Google Scholar]
- Cushman KA, Marsh-Haffner J, Adelman JP, McCleskey EW. A conformation change in the extracellular domain that accompanies desensitization of acid-sensing ion channel (ASIC) 3. J. Gen. Physiol. 2007;129:345–350. doi: 10.1085/jgp.200709757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doreulee N, Yanovsky Y, Haas HL. Suppression of long-term potentiation in hippocampal slices by copper. Hippocampus. 1997;7:666–669. doi: 10.1002/(SICI)1098-1063(1997)7:6<666::AID-HIPO8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Ennion SJ, Evans RJ. Conserved cysteine residues in the extracellular loop of the human P2X(1) receptor form disulfide bonds and are involved in receptor trafficking to the cell surface. Mol. Pharmacol. 2002;61:303–311. doi: 10.1124/mol.61.2.303. [DOI] [PubMed] [Google Scholar]
- Gonzales EB, Kawate T, Gouaux E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature. 2009;460:599–604. doi: 10.1038/nature08218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare DJ, Lee JK, Beavis AD, van Gramberg A, George J, Adlard PA, Finkelstein DI, Doble PA. Three-dimensional atlas of iron, copper, and zinc in the mouse cerebrum and brainstem. Anal Chem. 2012;84:3990–3997. doi: 10.1021/ac300374x. [DOI] [PubMed] [Google Scholar]
- Hopt A, Korte S, Fink H, Panne U, Niessner R, Jahn R, Kretzschmar H, Herms J. Methods for studying synaptosomal copper release. J. Neurosci. Methods. 2003;128:159–172. doi: 10.1016/s0165-0270(03)00173-0. [DOI] [PubMed] [Google Scholar]
- Huster D. Wilson disease. Best Pract. Res. Clin. Gastroenterol. 2010;24:531–539. doi: 10.1016/j.bpg.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Huster D, Finegold MJ, Morgan CT, Burkhead JL, Nixon R, Vanderwerf SM, Gilliam CT, Lutsenko S. Consequences of copper accumulation in the livers of the Atp7b−/− (Wilson disease gene) knockout mice. Am. J. Pathol. 2006;168:423–434. doi: 10.2353/ajpath.2006.050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes P, Ribeiro JA. Neuronal P2 receptors of the central nervous system. Curr. Top Med. Chem. 2004;4:831–838. doi: 10.2174/1568026043451032. [DOI] [PubMed] [Google Scholar]
- Jiang LH, Rassendren F, Surprenant A, North RA. Identification of amino acid residues contributing to the ATP-binding site of a purinergic P2X receptor. J. Biol. Chem. 2000;275:34190–34196. doi: 10.1074/jbc.M005481200. [DOI] [PubMed] [Google Scholar]
- Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen CL, Uriu-Hare JY, Hawk SN, Jankowski MA, Daston GP, Kwik-Uribe CL, Rucker RB. Effect of copper deficiency on prenatal development and pregnancy outcome. Am. J. Clin. Nutr. 1998;67:1003S–1011S. doi: 10.1093/ajcn/67.5.1003S. [DOI] [PubMed] [Google Scholar]
- Kozma M, Szerdahelyi P, Kasa P. Histochemical detection of zinc and copper in various neurons of the central nervous system. Acta Histochem. 1981;69:12–17. doi: 10.1016/S0065-1281(81)80003-7. [DOI] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. Inhibition of ATP-activated current by zinc in dorsal root ganglion neurones of bullfrog. J. Physiol. 1997;505(Pt 3):641–653. doi: 10.1111/j.1469-7793.1997.641ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz MT. Neurologic Wilson’s disease. Ann. N. Y Acad. Sci. 2010;1184:173–187. doi: 10.1111/j.1749-6632.2009.05109.x. [DOI] [PubMed] [Google Scholar]
- Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol. Rev. 2007;87:1011–1046. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- Punthambaker S, Blum JA, Hume RI. High potency zinc modulation of human P2X2 receptors and low potency zinc modulation of rat P2X2 receptors share a common molecular mechanism. J. Biol. Chem. 2012;287:22099–22111. doi: 10.1074/jbc.M112.369157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassendren F, Buell G, Newbolt A, North RA, Surprenant A. Identification of amino acid residues contributing to the pore of a P2X receptor. EMBO J. 1997;16:3446–3454. doi: 10.1093/emboj/16.12.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino JT, Franz KJ. Coordination chemistry of copper proteins: how nature handles a toxic cargo for essential function. J. Inorg. Biochem. 2012;107:129–143. doi: 10.1016/j.jinorgbio.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Sato M, Ohtomo K, Daimon T, Sugiyama T, Iijima K. Localization of copper to afferent terminals in rat locus ceruleus, in contrast to mitochondrial copper in cerebellum. J. Histochem. Cytochem. 1994;42:1585–1591. doi: 10.1177/42.12.7983358. [DOI] [PubMed] [Google Scholar]
- Tittle RK, Hume RI. Opposite effects of zinc on human and rat P2X2 receptors. J. Neurosci. 2008;28:11131–11140. doi: 10.1523/JNEUROSCI.2763-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildman SS, King BF, Burnstock G. Modulatory activity of extracellular H+ and Zn2+ on ATP-responses at rP2X1 and rP2X3 receptors. Br. J. Pharmacol. 1999;128:486–492. doi: 10.1038/sj.bjp.0702802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Peoples RW, Montgomery JP, Chiang Y, Stewart RR, Weight FF, Li C. Differential modulation by copper and zinc of P2X2 and P2X4 receptor function. J. Neurophysiol. 1999;81:2088–2094. doi: 10.1152/jn.1999.81.5.2088. [DOI] [PubMed] [Google Scholar]