Fig. 9.
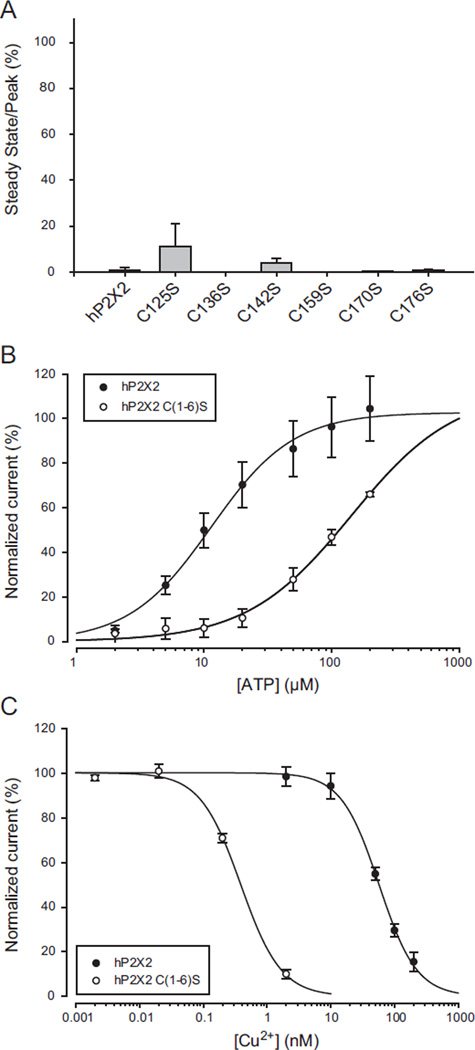
Effects of mutation of extracellular cysteines on copper inhibition. A) Responses to copper of single mutations of each of the first six extracellular cysteines to serines. The data plotted are the responses to the EC10 concentration of ATP plus 20 µM copper divided by the responses to ATP alone. None were significantly different from wild type. Mutation of each of the last four extracellular cysteines to serine produced nonfunctional proteins. B) ATP concentration response relation of the hP2X2 C(1–6)S mutant in which the first six cysteines were simultaneously mutated to serine. C) Copper concentration response relation for hP2X2 C(1–6)S. The data for the wild type controls illustrated were collected on the same day as the data for the mutant receptors.