Abstract
Background
Physical injury, including surgery, can result in chronic pain; yet chronic pain following childbirth, including cesarean delivery in women, is rare. The mechanisms involved in this protection by pregnancy or delivery have not been explored.
Methods
We examined the effect of pregnancy and delivery on hypersensitivity to mechanical stimuli of the rat hindpaw induced by peripheral nerve injury (spinal nerve ligation) and after intrathecal oxytocin, atosiban and naloxone. Additionally, oxytocin concentration in lumbar spinal cerebrospinal fluid was determined.
Results
Spinal nerve ligation performed at mid-pregnancy resulted in similar hypersensitivity to nonpregnant controls, but hypersensitivity partially resolved beginning after delivery. Removal of pups after delivery prevented this partial resolution. Cerebrospinal fluid concentrations of oxytocin were greater in normal postpartum rats prior to weaning. To examine the effect of injury at the time of delivery rather than during pregnancy, spinal nerve ligation was performed within 24 h of delivery. This resulted in acute hypersensitivity that partially resolved over the next 2–3 weeks. Weaning of pups resulted only in a temporary return of hypersensitivity. Intrathecal oxytocin effectively reversed the hypersensitivity following separation of the pups. Postpartum resolution of hypersensitivity was transiently abolished by intrathecal injection of the oxytocin receptor antagonist, atosiban.
Conclusions
These results suggest that the postpartum period rather than pregnancy protects against chronic hypersensitivity from peripheral nerve injury and that this protection may reflect sustained oxytocin signaling in the central nervous system during this period.
Introduction
Physical injury can result in chronic pain, usually with neuropathic characteristics, and occurs in 5–50% of patients undergoing surgery, depending on the surgical procedure. 1 For example, chest wall pain is present in nearly half of women 2 yr after mastectomy for cancer, and half of these women rate their pain as moderate to severe with interference of activities of daily living and utilization of health care resources. 2 Although many patient and surgical factors are associated with increased risk for chronic pain after surgery, the mechanisms underlying the lack of resolution of pain in these individuals are unknown. Peripheral nerve injury in rodents results in long lasting hypersensitivity to mechanical stimuli 3, a surrogate for allodynia in patients that can accompany chronic pain after surgery. 4 This hypersensitivity is associated with multiple changes in neuronal function and neuronal-glial interactions in the spinal cord, and pharmacologic blockade of many of these changes results in temporary resolution of hypersensitivity. 5–6 Social interactions also modulate hypersensitivity in rodents with peripheral nerve injury, with hypersensitivity reduced in pair-housed mice compared to isolated animals by a mechanism that involve oxytocin. 7
In contrast to chronic pain after surgery, childbirth is not associated with such a high incidence of chronic pain. Thus, chronic pain related to surgery is nearly ten-fold greater in incidence following abdominal hysterectomy for non-cancer indications compared to cesarean delivery. 8–11 This could reflect a lesser degree of injury to peripheral nerves from cesarean delivery, which typically is of shorter duration than abdominal hysterectomy, although peripheral nerve injury from cesarean delivery has been documented to produce chronic pain. 12,13 Alternatively, psychological factors or active protection from pregnancy or delivery could be responsible for this low incidence of chronic pain. The primary purpose of the current study was to determine whether protection occurs also in rodents during the peripartum period from hypersensitivity induced by spinal nerve ligation, a surgical model of chronic neuropathic pain. We observed a protective effect which was related to the postpartum period, but not during pregnancy.
A protective effect in the post-, but not prepartum period, is not consistent with an etiology dependent on circulating progesterone and estrogen, which decline rapidly following delivery. We therefore focused our initial mechanistic studies on oxytocin. Oxytocin is released from the pituitary into the systemic circulation to accomplish multiple functions during labor and thereafter. 14–16 In addition, oxytocin is also released in the central nervous system, as evidenced by increased concentrations in cerebrospinal fluid (CSF) during labor. 17 Since oxytocin does not cross the blood brain barrier 18 this increase likely reflects sustained activation of oxytocin containing neurons in the central nervous system. In addition to its effects on maternal-neonatal bonding, oxytocin, released in the spinal cord from descending projections from the paraventricular nucleus, is analgesic. 19 A secondary purpose of this study was to probe the role of spinal oxytocin receptor signaling in protection from surgery-induced hypersensitivity.
Material and Methods
Animals
Female Sprague-Dawley rats (250–350 g) from Harlan Industries (Indianapolis, IN), housed under a 12-h light-dark cycle with food and water ad libitum, were used. All experiments were approved by Animal Care and Use Committee at Wake Forest University (Winston Salem, North Carolina).
Surgical preparations
One week before delivery or the day of delivery, animals were anesthetized with 2% isoflurane in oxygen and spinal nerve ligation (SNL) or sham surgery was performed. For SNL, the right L6 transverse process was removed and the right L5 and L6 spinal nerves were tightly ligated using 5.0 silk sutures as previously described. 3 Sham surgery consisted of exposure of the L5 and L6 spinal nerves, but no ligation. After surgery, animals were housed individually with or without their pups according to the study design in plastic cages in a climate-controlled room under a 12 h/12 h light-dark cycle, with free access to food and water.
Behavioral testing
Withdrawal threshold to punctate mechanical stimulation was determined before and after SNL or sham surgery by the application of calibrated von Frey filaments (Stoelting, Wood Dale, IL) to the hindpaw. Animals were separated from pups, if present, and placed on a plastic mesh floor in individual clear plastic boxes and allowed to accommodate to their environment for at least 30 min. Filaments were applied to the bending point for 5 s, and a brisk paw withdrawal was considered a positive response. Withdrawal threshold was determined by application of filaments of different bending strengths using an up-down statistical method. 20 Behavioral testing was performed prior to surgery and commencing 3 days thereafter. The person performing the behavioral test was blinded to surgery and treatment.
A total of 168 animals were tested in these protocols. All animals recovered from surgery without evidence of infection, and all deliveries occurred spontaneously with an average litter size of 11.
Timing of surgery and behavioral measures
To investigate the effect of pregnancy and postpartum over the SNL-induced hypersensitivity, SNL surgery was performed one week before delivery in a total of 37 pregnant rats. In a group of these (20/37), the pups were housed with the dam until weaning and their withdrawal thresholds was monitored and compared to nonpregnant animals with SNL (n = 17). The other group of the pregnant rats with SNL (n = 17/37) were separated from the pups within 24 h of delivery. Their withdrawal thresholds was monitored and compared as well, to controls (nonpregnant rats with SNL, n = 6).
To study the resolution of SNL-induced hypersensitivity during postpartum period and after weaning, surgery was performed in 30 animals (15 SNL and 15 sham) within 24 h after delivery and compared to virgin controls (SNL or sham, n = 13 each). Withdrawal threshold was determined prior to surgery and 3, 10, 14, and 17 days afterwards. In addition, in 18/30 animals with surgery within 24 h of delivery (9 Sham and 9 SNL), their withdrawal thresholds was monitored also at 22, 28, 31, and 34 days after surgery.
Oxytocin concentration in lumbar spinal cerebrospinal fluid
In 11 animals without previous surgery (four nonpregnant, four with pups 22 days after delivery, and three, also 22 days after delivery, but with pups removed 24 h previously), anesthesia was induced with 2% isoflurane and a T13-L1 laminectomy was performed. A sharp glass pipette with connection to a microinjection syringe was used to penetrate into the intrathecal space corresponding to L4-L5 segments. Approximately 100 µL of CSF was withdrawn for oxytocin assay, using a commercially available ELISA kit (Assay Designs Inc., Ann Arbor, MI) as previously reported. 21
Intrathecal drug administration
Rats were briefly anesthetized with isoflurane in oxygen on day 21 following delivery and 12 µL containing 12 µg of the oxytocin receptor antagonist atosiban (1-deamino-2D-Tyr(O-ethyl)-4-Thr-8-Orn-oxytocin (n = 7 for SNL, n = 8 for no surgery), the opioid receptor antagonist naloxone (10 µg, n = 7 for SNL), or 12 µL saline (n = 6 for SNL) were injected intrathecally by acute puncture between L3 and L4 vertebrae of the spine using a 30-gauge ½-inch needle as previously described. 22 Needle insertion was assumed by a brief tail flick. Prior training using this method and injection of local anesthetic in other rats resulted reliably (>95%) in temporary hindlimb paralysis, indicative of intrathecal injection. Behavioral testing was conducted before and at hourly intervals after injection. On day 22 following delivery, the day after weaning, another group of animals received 10 µL containing 120 ng of oxytocin (n = 7) or µL saline (n = 6) by the similar procedure.
Statistical analysis
To examine our hypotheses we used generalized estimating equations. Generalized estimating equations (are a method for analyzing multilevel longitudinal data. 23 Because both the nature of the outcome as well as the correlation structure of the repeated measures can be specified, generalized estimating equations provide accurate estimates of the standard errors, allowing for precise estimation of confidence intervals. In general, we utilized two-factor models with experimental groups (or sides) as a between subjects factor and time as a repeated measures factor. A normal distribution with identity link was specified for the outcomes and repeated measures modeled with a independent covariance structure. For these models time is expressed as days since surgery, injury, or delivery as appropriate. We report the main effects (group, time) and interactions (group × time) for all models. Only where indicated by either a statistically significant main effect or group × time interaction (for group differences across time) was post hoc testing conducted. To control the error rate for each effect, Bonferroni corrected post hoc tests were conducted on a priori designated pairs (e.g., groups at synchronous times). Because of the modest sample size and heterogeneous variance, we conducted a Kruskal-Wallis test on CSF oxytocin levels by experimental group with post hoc Mann-Whitney U tests and Bonferroni corrections. Data are presented as mean ± SD, except for CSF oxytocin concentrations that exhibited considerable heterogeneity of variances so these data are presented using scatter plots. All analyses were conducted using SPSS 18.0 (IBM, Chicago, IL). Where appropriate, all hypothesis testing is two-tailed with p < 0.05 considered statistically significant.
Results
The postpartum period, but not pregnancy, alleviates SNL hypersensitivity
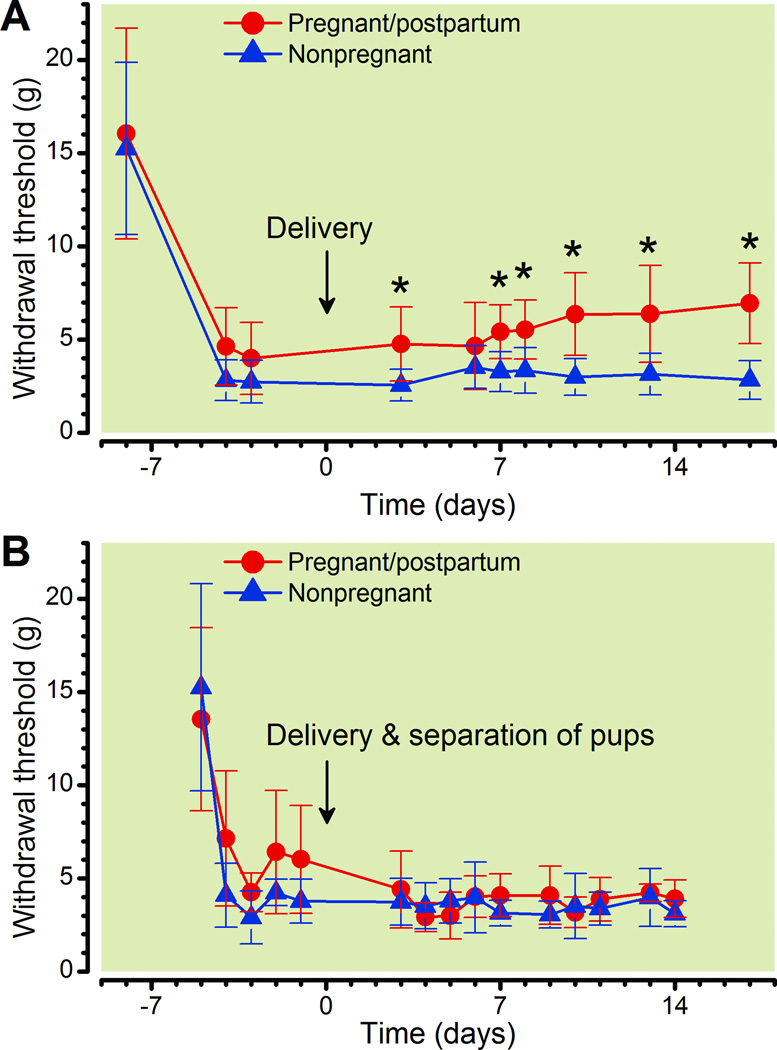
SNL 1 week before delivery resulted in slightly less hypersensitivity in pregnant animals compared to nonpregnant animals (fig. 1A), whereas sham surgery was without effect on paw withdrawal threshold in either group (data not shown). Overall pregnant rats were less sensitive as compared to nonpregnant rats (Group p < 0.0001) with both groups exhibiting a similar sharp drop in withdrawal thresholds after injury (Time: p < 0.001), but following delivery there were increasing thresholds among postpartum rats, whereas it was relatively unchanged over a similar time period in nonpregnant animals (fig. 1A: Group × Time: P < 0.001). Separation of the pups on the first day after delivery prevented the postpartum decrease in hypersensitivity (fig. 1B, Group: p = 0.131) with both groups exhibiting similar drop in withdrawal thresholds after injury (Time: p < 0.001, Group × Time: p < 0.001).
Figure 1.
Effect of spinal nerve ligation (SNL) surgery, when performed duringpregnancy, on withdrawal threshold.
A. Withdrawal threshold over time in pregnant and nonpregnant rats, with surgery performed one week prior to delivery.
B. Withdrawal threshold over time in pregnant an nonpregnant rats, with surgery performed one week prior to delivery and pups removed within 24 hr of delivery.
Data are presented as mean ± SD, Bonferroni's Multiple Comparison Test: *p <0.05 compared to nonpregnant.
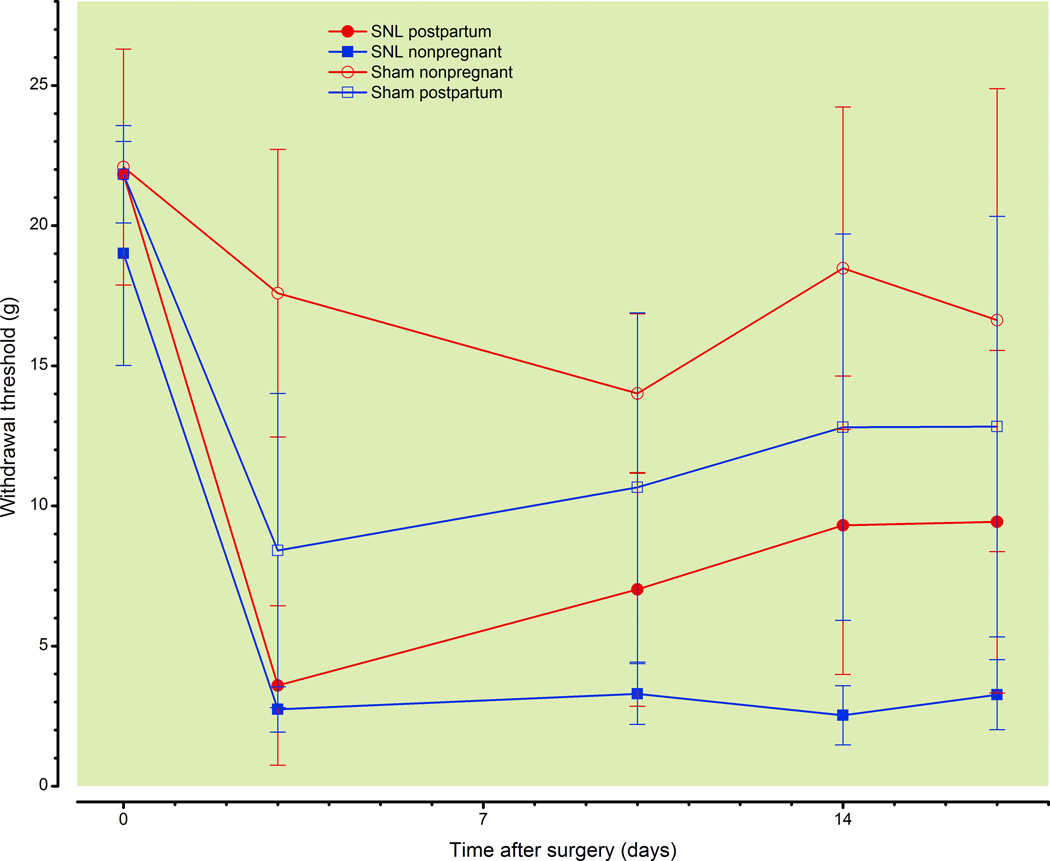
Hypersensitivity from SNL is also alleviated when the injury occurs at the time of delivery
When SNL injury was performed on the day of delivery, ipsilateral tactile hypersensitivity developed rapidly and to a similar degree compared to nonpregnant rats (fig. 2A). However, hypersensitivity diminished thereafter in postpartum, but not in nonpregnant rats (Group: p < 0.0001, Group × Time: p < 0.0001). A much lesser degree of hypersensitivity was observed after sham surgery in both groups (fig. 2A, Time: p < 0.0001), and as in SNL, with lesser hypersensitivity overall in postpartum than nonpregnant animals. There were small reductions in withdrawal threshold contralateral to surgery in both sham and SNL groups (fig. 2B; Time: p < 0.001, Group: p = 0.002; Group × Time: p = 0.001), with overall differences between the groups.
Figure 2.
Effect of spinal nerve ligation (SNL) or sham surgery, when performed within 24 hr of delivery, on withdrawal threshold.
Withdrawal threshold ipsilateral (A) or contralateral (B) to SNL or sham surgery in virgin, nonpregnant and in postpartum rats when surgery was performed within 24 hr of delivery.
Data are presented as mean ± SD, Bonferroni's Multiple Comparison Test: *p<0.05 comparing postpartum SNL to nonpregnant SNL; ^p<0.05 comparing postpartum SNL to postpartum Sham, &p<0.05 comparing postpartum SNL to nonpregnant Sham; ^p<0.05 comparing nonpregnat SNL to nonpregnant Sham, @p<0.05 comparing nonpregnant Sham to postpartum Sham, #p<0.05 comparing nonpregnant Sham to postpartum SNL.
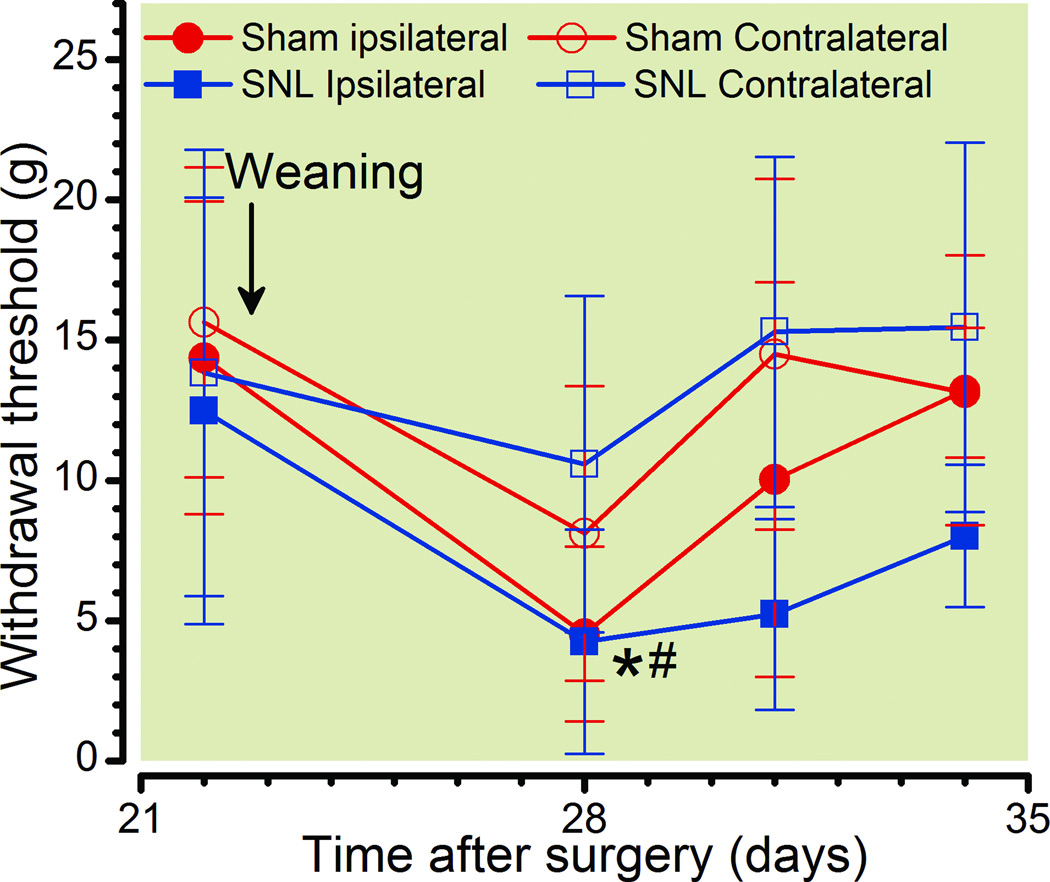
Weaning of pups produces temporary hypersensitivity
Weaning of pups 21 days after delivery resulted in a temporary reduction in withdrawal threshold regardless of whether SNL or sham surgery had been performed (fig. 3; Time: p < 0.0001, Side × Group p = 0.004) and this effect was greater ipsilateral to surgery in SNL than sham animals (fig. 3; Group: p < 0.001)
Figure 3.
Effect of pups separation at the time of weaning on withdrawal threshold. Withdrawal threshold over time before and after weaning and separation of pups 22 days after delivery and with spinal nerve ligation (SNL) or sham surgery performed within 24 hr of delivery.
Data are presented as mean ± SD, Bonferroni's Multiple Comparison Test: *p<0.05 comparing nonpregnant Sham to postpartum Sham; #p<0.05 comparing nonpregnant Sham to postpartum SNL.
Role of spinal oxytocin in postpartum action on SNL-induced hypersensitivity
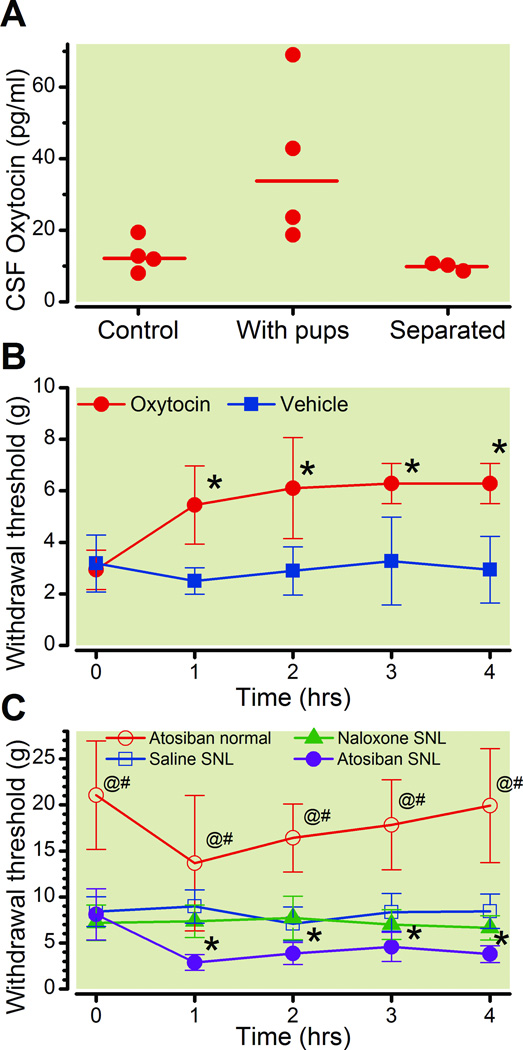
Three results suggested a prominent role of spinal oxytocin release on the resolving effect of the postpartum period on SNL-induced hypersensitivity. First, there were significant differences among postpartum rats that were kept with or separated from their pups 24 h earlier and the controls in the oxytocin concentration in lumbar CSF 22 days after delivery (fig. 4A, Group p = 0.036). Second, acute administration of intrathecal oxytocin, but not vehicle, one day after separation from pups produced an antihypersensitivity effect (fig. 4B, Group p < 0.001, Time: p < 0.001, Group × Time p < 0.001). Finally, in rats with SNL performed 1 week before delivery, the partial resolution of tactile hypersensitivity 3 weeks after delivery was reversed by the acute lumbar intrathecal injection of the oxytocin receptor antagonist, atosiban, but not by the opioid receptor antagonist, naloxone (fig. 4C; Group: p < 0.001; Time: p < 0.001; Group × Time: p < 0.001).
Figure 4.
Role of spinal oxytocin in postpartum antihypersensitivity.
A. Cerebrospinal fluid (CSF) concentrations of oxytocin in nonpregnant animals (control), postpartum rats 22 days after delivery (With pups) or those 22 days after delivery with pups separated 24 hr earlier (Separated). The Bar represents the Mean.
B. Withdrawal threshold over time in postpartum rats with spinal nerve ligation(SNL)following separation of pups and after intrathecal injection, at time 0, of oxytocin or vehicle.*p<0.05 compared to vehicle.
C. Withdrawal threshold over time in postpartum rats 3 weeks after SNL at the time ofdelivery and receiving intrathecal injection of saline, the oxytocin receptor antagonist atosiban, or the opioid receptor antagonist, naloxone. For comparison, the effect of intrathecal atosiban in nonpregnant, non-postpartum rats is also shown.
Data are presented as mean ± SD. Bonferroni's Multiple Comparison Test: *p<0.05 comparing atosiban SNL to saline SNL; @p < 0.05 comparing atosiban normal to atosiban SNL; #p<0.05 comparing atosiban normal to saline SNL.
Discussion
Chronic pain after major physical injury, including surgery, affects as many as 50 million individuals globally each year, yet little is known regarding the processes which result in resolution or lack of resolution of acute pain following injury. Although backache and headache are common pain complaints following childbirth, these usually predate the delivery itself 24 and pain in the perineum, pelvis, or site of cesarean delivery scar 1 yr after delivery is remarkably low. 11 This observation suggests that the puerperium may alter processes of pain resolution and thereby influence the trajectory of recovery from pain following physical injury. The current study provides an animal correlate to these clinical observations and an initial examination of the mechanisms for this protection. We employed a surgical injury of peripheral nerves resulting in hypersensitivity to mechanical stimuli rather than an abdominal incision such as would occur with cesarean delivery in order to more rigorously examine our hypothesis that factors during pregnancy or the puerperium protect against chronic pain after neuropathic surgical injury.
Hypersensitivity to tactile stimuli after peripheral nerve injury in rodents is used as a surrogate for allodynia 3, 24 which occurs in many patients with chronic neuropathic pain. Although patients suffer and seek medical treatment for chronic neuropathic pain because of spontaneous pain rather than allodynia per se, allodynia nonetheless may exacerbate pain during activities of daily living, resulting in fear of pain and curtailment of normal activities. In addition, there is a strong correlation between the area of tactile allodynia surrounding the surgical wound following surgery and the risk for chronic pain, 25 suggesting that this measure is meaningful in the study of mechanisms of resolution of pain following injury.
Using this model of neuropathic hypersensitivity, we observed a very minor protective effect of pregnancy on resolution, at least over the last 7 days of pregnancy. Although it is conceivable that resolution took more than 7 days to become evident, the reduction in hypersensitivity which occurred within a few days of delivery, whether neuropathic injury occurred at the time of delivery of 7 days previously, argues strongly against a primary effect of factors present during pregnancy on this resolution. Gestational hormones and neurosteroids can produce antinociceptive and antihypersensitivity effects when acutely administered, 26–28 and we did not probe the role of these hormones using selective antagonists, so we cannot completely exclude their role in the resolution of hypersensitivity observed.
Minimal resolution of hypersensitivity was observed over a 3-week period following SNL in virgin female rats, consistent with the very shallow recovery observed after this surgical nerve injury, typically requiring 3–6 months for resolution, with many animals showing no resolution at all. 3, 29 In contrast, hypersensitivity began to recover starting 3 days after delivery with continued improvement over the subsequent month, although complete recovery was not observed in all animals at this time period. The presence of pups was important for this recovery, since it did not occur in animals whose pups had been removed shortly after delivery in agreement with previous studies that suggests that social isolation increases mechanical allodynia.30
Although many factors could affect central nervous system plasticity during the puerperium, we focused our initial studies on oxytocin. This neuropeptide, classically examined for its effect on the labor process and lactation after release from the pituitary, has more recently received attention as a neurotransmitter within the central nervous system important to social interactions. Oxytocin containing neurons in the paraventricular and supraoptic regions of the hypothalamus send diffuse projections to the forebrain, limbic structures, brainstem, and spinal cord. Of special importance to pain are oxytocin’s effects in the amygdala, a key center regarding negative emotional experiences and reaction to pain, and the spinal cord, the initial site of pain neurotransmission. Others have shown that this paraventricular nucleus – spinal cord oxytocin pathway, when stimulated, produces acute antinociception and acutely reduces hypersensitivity after peripheral nerve injury. 19, 31–32
Several experiments in the current study support a role for spinally released oxytocin in the resolution of injury-induced tactile hypersensitivity during the puerperium. Lumbar CSF concentrations of oxytocin were increased during this time period, rapidly decreasing after removal of pups in normal animals and coincident with a transient return of hypersensitivity in both normal and injured animals. We did not measure CSF concentrations of oxytocin in injured animals and our sample size was small, both factors limiting the interpretation of our data, yet it is consistent with the known effects of lactation to stimulate release of oxytocin 33 not only in the blood, but also in the central nervous system, and is consistent with the reliance on the presence of pups, and hence lactation for the resolution of hypersensitivity in postpartum animals. The observation that pregnancy did not alleviates SNL-induced hypersensitivity is consistent with the fact that oxytocin released in the paraventricular hypothalamic nucleus of pregnant rats did not differ from that of nonpregnant animals. 34 35 Following labor, there is a significant but transient increment of oxytocin in the paraventricular hypothalamic nucleus (3 fold) and during lactation central oxytocin remains high during the postpartum period. 34 It is also consistent with the very transient reduction in withdrawal threshold, even in normal animals, when the pups were weaned at the normal time after delivery. Lumbar intrathecal injection of oxytocin shortly after weaning reinstated the antihypersensitivity effect. Finally, intrathecal injection of the oxytocin receptor preferring antagonist, atosiban, prior to weaning transiently reversed the postpartum antihypersensitivity effect. Atosiban also reduced withdrawal threshold in normal animals, and although this decrease was not statistically significant it was not specific to the nerve injury state and is consistent with tonic oxytocinergic tone in the spinal cord which affects response to peripheral stimuli. Additionally, we recognize that a weakness of these pharmacologic studies is that oxytocin and atosiban act on both oxytocin and vasopressin receptors and, antinociception from systemically administered oxytocin is dependent on vasopressin-1A receptor signaling. 36 We cannot conclude from the current experiments which of these receptors are primarily responsible for these effects.
Endogenous opioids modulate the antinociceptive effect of oxytocin in normal rats. 37 However, intrathecal naloxone failed in the current study to reverse the postpartum alleviation of SNL-induced hypersensitivity suggesting that oxytocin may act by a mechanism different than the opioids during the postpartum period and in chronic pain condition. The mechanisms by which oxytocin might prevent the development of SNL-induced hypersensitivity in rats after delivery were not otherwise examined in these studies. Others have shown that oxytocin reduces behavioral hypersensitivity in male rats by stimulating γ-amino-butyric acid release from spinal interneurons 38 and by preventing long-term potentiation in wide dynamic range neurons in the spinal cord 32. It is conceivable that oxytocin released onto the spinal cord during the postpartum period acts by similar mechanisms to prevent the sensitization of superficial spinal cord neurons.
The current work has limitations in its interpretation. For one, it focuses on spinal mechanisms of oxytocin action, although other sites may also be relevant to analgesia. For instance, injection of oxytocin into the periaqueductal gray and nucleus raphe magnus produces antinociception in normal rats 39–40 and oxytocin signaling is also important in modulating nociception in the central nucleus of the amygdala 41 and the nucleus accumbens, 42 the latter dependent on opioid receptor activation. It is conceivable that part of the enhanced resolution of hypersensitivity observed in the current study could be due to increased oxytocin signaling at these and other supraspinal sites. Another limitation is that, like most mechanistic studies in rodents, it relies on evoked response to mechanical stimuli and hypersensitivity following nerve injury as an important reflection of chronic pain in humans. Allodynia is far from universally present in patients with chronic pain after surgery, and its importance to the subjective experience of pain may often be small.
In conclusion, clinical experience suggests the likelihood of chronic pain after childbirth is small, and we show here a gradual, partial resolution of tactile hypersensitivity from peripheral surgical trauma during the puerperium in rats. This protective effect requires the presence of pups but outlasts weaning if the injury occurs near the time of delivery. Reversal of this protective effect by lumbar intrathecal delivery of an oxytocin, but not opioid-receptor antagonist and its association with increased CSF concentrations of oxytocin are consistent with a hypothesis that supraspinal-spinal oxytocin drive increases after delivery and is at least partially responsible. A better elucidation of the causes of puerperal protection from hypersensitivity after peripheral nerve injury could provide important targets for prevention and treatment of chronic neuropathic pain.
FINAL BOX SUMMARY STATEMENT.
What we already know about this topic
Chronic pain develops after many surgeries
The prevalence of chronic pain depends upon the particular type of surgery
What this article tells us that is new
There is a low incidence of chronic pain after cesarean delivery; this could be related to hormones regulated during pregnancy or after delivery
Increased postpartum oxytocin reduces mechanosensitivity after nerve injury and may be part of a protective mechanism against the development of chronic pain after cesarean delivery
Acknowledgements
We would like to thank Renee Parker, Technician III of Anesthesiology, Wake Forest School of Medicine, Winston-Salem, North Carolina, for her excellent technical assistance.
Supported from grant GM48085 from the National Institute of Health, Bethesda, Maryland
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 2.Gartner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302:1985–1992. doi: 10.1001/jama.2009.1568. [DOI] [PubMed] [Google Scholar]
- 3.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 4.Romundstad L, Breivik H, Roald H, Skolleborg K, Romundstad PR, Stubhaug A. Chronic pain and sensory changes after augmentation mammoplasty: Long term effects of preincisional administration of methylprednisolone. Pain. 2006;124:92–99. doi: 10.1016/j.pain.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics. 2010;7:482–493. doi: 10.1016/j.nurt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benarroch EE. Central neuron-glia interactions and neuropathic pain: Overview of recent concepts and clinical implications. Neurology. 2010;75:273–278. doi: 10.1212/WNL.0b013e3181e8e984. [DOI] [PubMed] [Google Scholar]
- 7.Norman GJ, Karelina K, Morris JS, Zhang N, Cochran M, Courtney DeVries A. Social interaction prevents the development of depressive-like behavior post nerve injury in mice: A potential role for oxytocin. Psychosom Med. 2010;72:519–526. doi: 10.1097/PSY.0b013e3181de8678. [DOI] [PubMed] [Google Scholar]
- 8.Brandsborg B, Nikolajsen L, Hansen CT, Kehlet H, Jensen TS. Risk factors for chronic pain after hysterectomy: A nationwide questionnaire and database study. Anesthesiology. 2007;106:1003–1012. doi: 10.1097/01.anes.0000265161.39932.e8. [DOI] [PubMed] [Google Scholar]
- 9.Brandsborg B. Pain following hysterectomy: Epidemiological and clinical aspects. Dan Med J. 2012;59:B4374. [PubMed] [Google Scholar]
- 10.Khelemsky Y, Noto CJ. Preventing post-thoracotomy pain syndrome. Mt Sinai J Med. 2012;79:133–139. doi: 10.1002/msj.21286. [DOI] [PubMed] [Google Scholar]
- 11.Eisenach C, Chen ZH, Grefen C, Blatt MR. The trafficking protein SYP121 of Arabidopsis connects programmed stomatal closure and K channel activity with vegetative growth. Plant J. 2012;69:241–251. doi: 10.1111/j.1365-313X.2011.04786.x. [DOI] [PubMed] [Google Scholar]
- 12.Loos MJ, Scheltinga MR, Roumen RM. Surgical management of inguinal neuralgia after a low transverse Pfannenstiel incision. Ann Surg. 2008;248:880–885. doi: 10.1097/SLA.0b013e318185da2e. [DOI] [PubMed] [Google Scholar]
- 13.Ducic I, Moxley M, Al-Attar A. Algorithm for treatment of postoperative incisional groin pain after cesarean delivery or hysterectomy. Obstet Gynecol. 2006;108:27–31. doi: 10.1097/01.AOG.0000223864.63747.ce. [DOI] [PubMed] [Google Scholar]
- 14.Viero C, Shibuya I, Kitamura N, Verkhratsky A, Fujihara H, Katoh A, Ueta Y, Zingg HH, Chvatal A, Sykova E, Dayanithi G. Oxytocin: Crossing the bridge between basic science and pharmacotherapy. CNS Neurosci Ther. 2010;16:e138–e56. doi: 10.1111/j.1755-5949.2010.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biol Psychiatry. 2010;68:377–382. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morales T. Recent findings on neuroprotection against excitotoxicity in the hippocampus of female rats. J Neuroendocrinol. 2011;23:994–1001. doi: 10.1111/j.1365-2826.2011.02141.x. [DOI] [PubMed] [Google Scholar]
- 17.Takeda S, Kuwabara Y, Mizuno M. Effects of pregnancy and labor on oxytocin levels in human plasma and cerebrospinal fluid. Endocrinol Jpn. 1985;32:875–880. doi: 10.1507/endocrj1954.32.875. [DOI] [PubMed] [Google Scholar]
- 18.Kang YS, Park JH. Brain uptake and the analgesic effect of oxytocin--its usefulness as an analgesic agent. Arch Pharm Res. 2000;23:391–395. doi: 10.1007/BF02975453. [DOI] [PubMed] [Google Scholar]
- 19.Condes-Lara M, Maie IA, Dickenson AH. Oxytocin actions on afferent evoked spinal cord neuronal activities in neuropathic but not in normal rats. Brain Research. 2005;1045:124–133. doi: 10.1016/j.brainres.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 20.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 21.Devarajan K, Rusak B. Oxytocin levels in the plasma and cerebrospinal fluid of male rats: Effects of circadian phase, light and stress. Neurosci Lett. 2004;367:144–147. doi: 10.1016/j.neulet.2004.05.112. [DOI] [PubMed] [Google Scholar]
- 22.Peters CM, Eisenach JC. Contribution of the chemokine (C-C motif) ligand 2 (CCL2) to mechanical hypersensitivity after surgical incision in rats. Anesthesiology. 2010;112:1250–1258. doi: 10.1097/ALN.0b013e3181d3d978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: A generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- 24.Decosterd I, Woolf CJ. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 25.Eisenach JC. Preventing chronic pain after surgery: Who how, and when? Reg Anesth Pain Med. 2006;31:1–3. doi: 10.1016/j.rapm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Caba M, Gonzalez-Mariscal G, Beyer C. Perispinal progestins enhance the antinociceptive effects of muscimol in the rat. Pharmacol Biochem Behav. 1994;47:177–182. doi: 10.1016/0091-3057(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 27.Lacroix-Fralish ML, Tawfik VL, Nutile-McMenemy N, DeLeo JA. Progesterone mediates gonadal hormone differences in tactile and thermal hypersensitivity following L5 nerve root ligation in female rats. Neuroscience. 2006;138:601–608. doi: 10.1016/j.neuroscience.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 28.Lacroix-Fralish ML, Tawfik VL, Nutile-McMenemy N, Deleo JA. Neuregulin 1 is a pronociceptive cytokine that is regulated by progesterone in the spinal cord: Implications for sex specific pain modulation. Eur J Pain. 2008;12:94–103. doi: 10.1016/j.ejpain.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Ririe DG, Eisenach JC. Age-dependent responses to nerve injury-induced mechanical allodynia. Anesthesiology. 2006;104:344–350. doi: 10.1097/00000542-200602000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Norman GJ, Zhang N, Morris JS, Karelina K, Berntson GG, DeVries AC. Social interaction modulates autonomic, inflammatory, and depressive-like responses to cardiac arrest and cardiopulmonary resuscitation. Proc Natl Acad Sci U S A. 2010;107:16342–16347. doi: 10.1073/pnas.1007583107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miranda-Cardenas Y, Rojas-Piloni G, Martinez-Lorenzana G, Rodriguez-Jimenez J, Lopez-Hidalgo M, Freund-Mercier MJ, Condes-Lara M. Oxytocin and electrical stimulation of the paraventricular hypothalamic nucleus produce antinociceptive effects that are reversed by an oxytocin antagonist. Pain. 2006;122:182–189. doi: 10.1016/j.pain.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 32.DeLaTorre S, Rojas-Piloni G, Martinez-Lorenzana G, Rodriguez-Jimenez J, Villanueva L, Condes-Lara M. Paraventricular oxytocinergic hypothalamic prevention or interruption of long-term potentiation in dorsal horn nociceptive neurons: Electrophysiological and behavioral evidence. Pain. 2009;144:320–328. doi: 10.1016/j.pain.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Lipschitz DL, Crowley WR, Bealer SL. Central blockade of oxytocin receptors during late gestation disrupts systemic release of oxytocin during suckling in rats. J Neuroendocrinol. 2003;15:743–748. doi: 10.1046/j.1365-2826.2003.01052.x. [DOI] [PubMed] [Google Scholar]
- 34.Lipschitz DL, Crowley WR, Bealer SL. Differential sensitivity of intranuclear and systemic oxytocin release to central noradrenergic receptor stimulation during mid- and late gestation in rats. Am J Physiol Endocrinol Metab. 2004;287:E523–E528. doi: 10.1152/ajpendo.00572.2003. [DOI] [PubMed] [Google Scholar]
- 35.Neumann I, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: A microdialysis study. Neuroscience. 1993;53:65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- 36.Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, Crawley JN, Hu SB, Nishimori K, Young LJ, Tribollet E, Quirion R, Mogil JS. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci. 2010;30:8274–8284. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersson M, Alster P, Lundeberg T, Uvnas-Moberg K. Oxytocin increases nociceptive thresholds in a long-term perspective in female and male rats. Neuroscience Letters. 1996;212:87–90. doi: 10.1016/0304-3940(96)12773-7. [DOI] [PubMed] [Google Scholar]
- 38.Rojas-Piloni G, Lopez-Hidalgo M, Martinez-Lorenzana G, Rodriguez-Jimenez J, Condes-Lara M. GABA-mediated oxytocinergic inhibition in dorsal horn neurons by hypothalamic paraventricular nucleus stimulation. Brain Res. 2007;1137:69–77. doi: 10.1016/j.brainres.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 39.Ge Y, Lundeberg T, Yu LC. Blockade effect of mu and kappa opioid antagonists on the anti-nociception induced by intra-periaqueductal grey injection of oxytocin in rats. Brain Res. 2002;927:204–207. doi: 10.1016/s0006-8993(01)03346-7. [DOI] [PubMed] [Google Scholar]
- 40.Wang JW, Lundeberg T, Yu LC. Antinociceptive role of oxytocin in the nucleus raphe magnus of rats, an involvement of mu-opioid receptor. Regul Pept. 2003;115:153–159. doi: 10.1016/s0167-0115(03)00152-6. [DOI] [PubMed] [Google Scholar]
- 41.Han Y, Yu LC. Involvement of oxytocin and its receptor in nociceptive modulation in the central nucleus of amygdala of rats. Neurosci Lett. 2009;454:101–104. doi: 10.1016/j.neulet.2009.02.062. [DOI] [PubMed] [Google Scholar]
- 42.Gu XL, Yu LC. Involvement of opioid receptors in oxytocin-induced antinociception in the nucleus accumbens of rats. J Pain. 2007;8:85–90. doi: 10.1016/j.jpain.2006.07.001. [DOI] [PubMed] [Google Scholar]