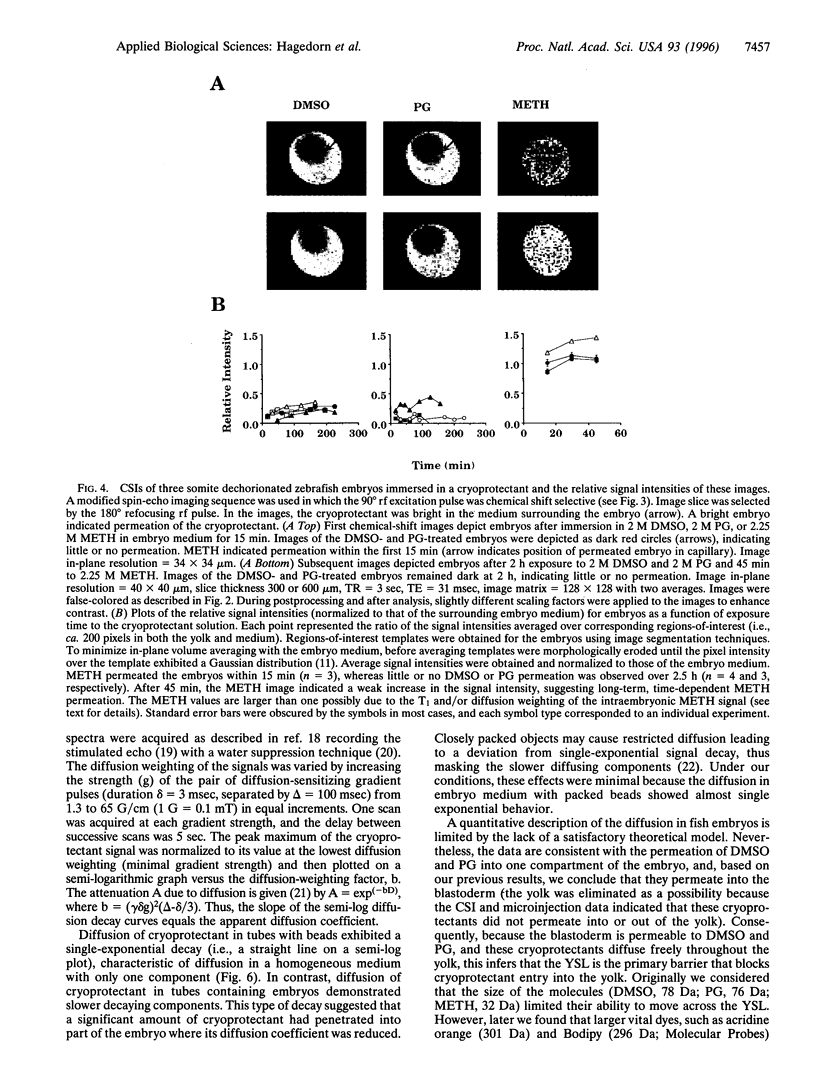
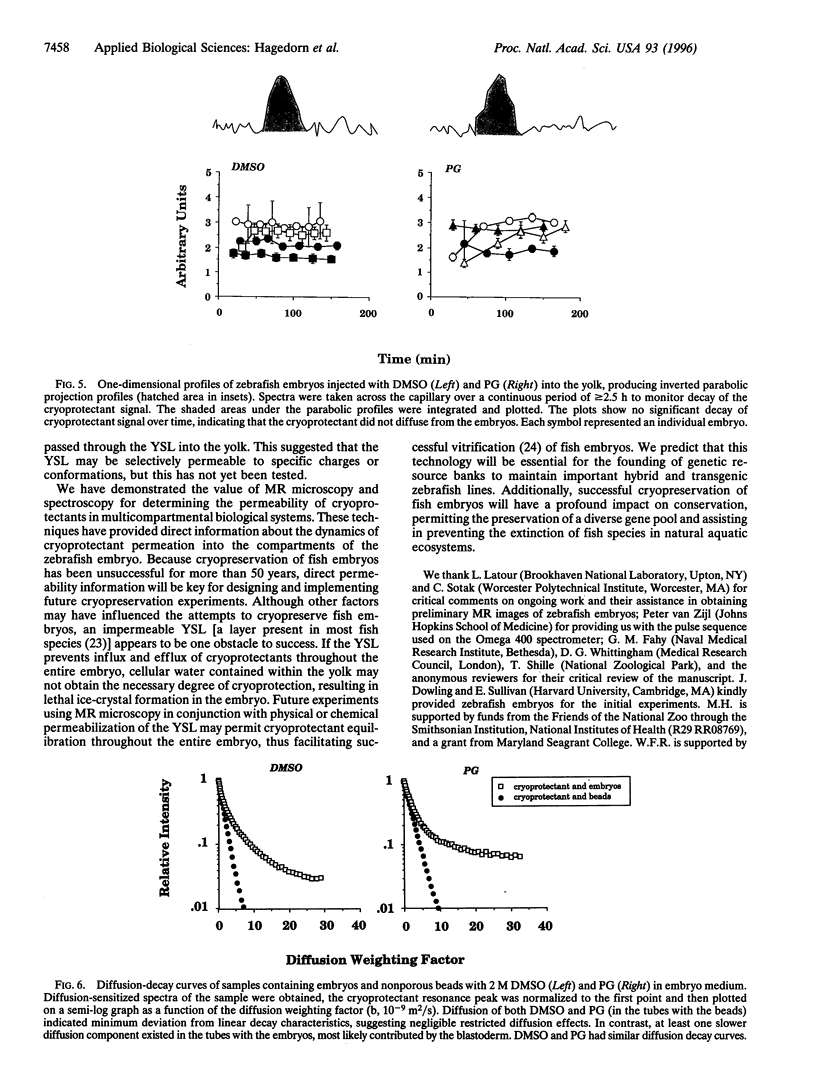
Abstract
Successful cryopreservation of most multicompartmental biological systems has not been achieved. One prerequisite for success is quantitative information on cryoprotectant permeation into and amongst the compartments. This report describes direct measurements of cryoprotectant permeation into a multicompartmental system using chemical shift selective magnetic resonance (MR) microscopy and MR spectroscopy. We used the developing zebrafish embryo as a model for studying these complex systems because these embryos are composed of two membrane-limited compartments: (i) a large yolk (surrounded by the yolk syncytial layer) and (ii) differentiating blastoderm cells (each surrounded by a plasma membrane). MR images of the spatial distribution of three cryoprotectants (dimethyl sulfoxide, propylene glycol, and methanol) demonstrated that methanol permeated the entire embryo within 15 min. In contrast, the other cryoprotectants exhibited little or no permeation over 2.5 h. MR spectroscopy and microinjections of cryoprotectants into the yolk inferred that the yolk syncytial layer plays a critical role in limiting the permeation of some cryoprotectants throughout the embryo. This study demonstrates the power of MR technology combined with micromanipulation for elucidating key physiological factors in cryobiology.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betchaku T., Trinkaus J. P. Contact relations, surface activity, and cortical microfilaments of marginal cells of the enveloping layer and of the yolk syncytial and yolk cytoplasmic layers of fundulus before and during epiboly. J Exp Zool. 1978 Dec;206(3):381–426. doi: 10.1002/jez.1402060310. [DOI] [PubMed] [Google Scholar]
- Gottesfeld Z., Neeman M. Ferritin effect on the transverse relaxation of water: NMR microscopy at 9.4 T. Magn Reson Med. 1996 Apr;35(4):514–520. doi: 10.1002/mrm.1910350410. [DOI] [PubMed] [Google Scholar]
- Haase A., Frahm J., Hänicke W., Matthaei D. 1H NMR chemical shift selective (CHESS) imaging. Phys Med Biol. 1985 Apr;30(4):341–344. doi: 10.1088/0031-9155/30/4/008. [DOI] [PubMed] [Google Scholar]
- Harvey B., Kelley R. N., Ashwood-Smith M. J. Permeability of intact and dechorionated zebra fish embryos to glycerol and dimethyl sulfoxide. Cryobiology. 1983 Aug;20(4):432–439. doi: 10.1016/0011-2240(83)90033-0. [DOI] [PubMed] [Google Scholar]
- Jacobs R. E., Fraser S. E. Magnetic resonance microscopy of embryonic cell lineages and movements. Science. 1994 Feb 4;263(5147):681–684. doi: 10.1126/science.7508143. [DOI] [PubMed] [Google Scholar]
- Joseph P. M. A spin echo chemical shift MR imaging technique. J Comput Assist Tomogr. 1985 Jul-Aug;9(4):651–658. doi: 10.1097/00004728-198507010-00001. [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Law R. D. Cell lineage of zebrafish blastomeres. II. Formation of the yolk syncytial layer. Dev Biol. 1985 Mar;108(1):86–93. doi: 10.1016/0012-1606(85)90011-9. [DOI] [PubMed] [Google Scholar]
- Päuser S., Keller K., Zschunke A., Mügge C. Study of the membrane permeability of a paramagnetic metal complex on single cells by NMR microscopy. Magn Reson Imaging. 1993;11(3):419–424. doi: 10.1016/0730-725x(93)90075-o. [DOI] [PubMed] [Google Scholar]
- Schoeniger J. S., Aiken N., Hsu E., Blackband S. J. Relaxation-time and diffusion NMR microscopy of single neurons. J Magn Reson B. 1994 Mar;103(3):261–273. doi: 10.1006/jmrb.1994.1039. [DOI] [PubMed] [Google Scholar]
- Schoeniger J. S., Blackband S. J. The design and construction of a NMR microscopy probe. J Magn Reson B. 1994 Jun;104(2):127–134. doi: 10.1006/jmrb.1994.1065. [DOI] [PubMed] [Google Scholar]
- Trinkaus J. P. The yolk syncytial layer of Fundulus: its origin and history and its significance for early embryogenesis. J Exp Zool. 1993 Mar 1;265(3):258–284. doi: 10.1002/jez.1402650308. [DOI] [PubMed] [Google Scholar]
- Van Zijl P. C., Moonen C. T., Faustino P., Pekar J., Kaplan O., Cohen J. S. Complete separation of intracellular and extracellular information in NMR spectra of perfused cells by diffusion-weighted spectroscopy. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3228–3232. doi: 10.1073/pnas.88.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]