Abstract
Secondary metabolites, or biochemical indicators of fungal development, are of intense interest to humankind due to their pharmaceutical and/or toxic properties. We present here a novel Aspergillus nuclear protein, LaeA, as a global regulator of secondary metabolism in this genus. Deletion of laeA (ΔlaeA) blocks the expression of metabolic gene clusters, including the sterigmatocystin (carcinogen), penicillin (antibiotic), and lovastatin (antihypercholesterolemic agent) gene clusters. Conversely, overexpression of laeA triggers increased penicillin and lovastatin gene transcription and subsequent product formation. laeA expression is negatively regulated by AflR, a sterigmatocystin Zn2Cys6 transcription factor, in a unique feedback loop, as well as by two signal transduction elements, protein kinase A and RasA. Although these last two proteins also negatively regulate sporulation, ΔlaeA strains show little difference in spore production compared to the wild type, indicating that the primary role of LaeA is to regulate metabolic gene clusters.
A complex and fascinating aspect of fungal development is the production of secondary metabolites. These compounds, frequently associated with sporulation processes, are considered part of the chemical arsenal required for niche specialization and have garnered intense interest by virtue of their biotechnological and pharmaceutical applications (9, 11). Many of them display a broad range of useful antibiotic, antiviral, antitumor, antihypercholesterolemic, and immunosuppressant activities as well as less desirable phyto- and mycotoxic activities. Hawksworth's studies of fungal biodiversity led to the conclusion that nearly 1.5 million fungal species exist on Earth, with only 5% identified thus far (16). Thus, the potential for fungal secondary metabolite discovery is vast. Furthermore, the discovery of global regulators for fungal secondary metabolite production is critical, as it would allow for universal manipulation of secondary metabolite production.
A large number of known fungal secondary metabolites have been ascribed to the Ascomycete genus Aspergillus. Studies of Aspergillus nidulans have demonstrated the power of using a model system to elucidate the biochemistry and molecular genetics of fungal secondary metabolism, principally penicillin (PN, an antibiotic) and sterigmatocystin (ST, a carcinogen biochemically related to the agricultural contaminant aflatoxin) biosynthesis (6, 17). These studies have established several characteristics of fungal secondary metabolism, including the clustering of biosynthetic and regulatory genes and a genetic connection linking secondary metabolite biosynthesis with sporulation through a shared signal transduction pathway (9).
The discovery of the G protein-cyclic AMP (cAMP)-protein kinase A regulation of ST, aflatoxin, and other fungal secondary metabolites (4, 17, 29, 34) has been helpful for establishing a model of global regulation of secondary metabolism. However, all of the signal transduction mutants described in the literature have pleiotropic effects on fungi, the most notable effect being the gross impact on spore production and vegetative hyphal growth (1, 9, 18, 29). Similarly, mutations in other major regulators of ST biosynthesis, such as RcoA, a WD protein (19), and SpdA, or spermidine synthase (20), also have gross effects on fungal morphology. Thus, currently available Aspergillus mutants are so impaired in fungal development that further elucidation of genes that are specific for the regulation of secondary metabolism is difficult.
In a previous mutagenesis hunt, 23 A. nidulans mutants that displayed a phenotype of loss of ST production but had normal sporulation were isolated (8). Here we describe the identification of a gene called laeA that complements one of these mutants. laeA encodes a nuclear protein that is required for the expression of secondary metabolite genes. We propose that LaeA is a regulator of secondary metabolism in Aspergillus, as it is required not only for ST biosynthesis but also for PN biosynthesis and the biosynthesis of mycelial pigments in A. nidulans and gliotoxin and mycelial pigments in Aspergillus fumigatus. Furthermore, this protein is required for expression of the heterologous lovastatin (LOV) gene cluster in A. nidulans as well as for native LOV expression in Aspergillus terreus. Interestingly, the protein appears to be conserved in filamentous fungi, but it is not present in Saccharomyces cerevisiae, a fungus devoid of secondary metabolites. Unlike other genes that regulate secondary metabolism, the loss of laeA has a negligible impact on morphological developmental processes.
MATERIALS AND METHODS
Fungal strains and growth conditions.
Table 1 lists all of the fungal strains used for this study. Some strains are not discussed in the text but were used for sexual crosses to obtain the strains of interest. Sexual crosses of A. nidulans strains were conducted according to the method of Pontecorvo et al. (26). All strains were maintained as glycerol stocks and were grown at 37°C for A. nidulans and A. fumigatus or 32°C for A. terreus on glucose minimal medium (GMM) (29), threonine minimal medium (TMM) (29), or lactose minimal medium (LMM) (21) amended with 30 mM cyclopentanone. Threonine and cyclopentanone both induce alcAp, which was used to promote laeA expression. All media contained appropriate supplements to maintain auxotrophs (2).
TABLE 1.
Fungal strains used for this study
| Strains or category | Genotype | Source or reference |
|---|---|---|
| Wild type and controls | ||
| A. nidulans strains | ||
| FGSC 26 | biA1 veA1 | Fungal Genetics Stock Center |
| RDIT 2.1 | methG1 | D. Tsitsigiannis |
| RAMB38 | biA1 methG1 ΔaflR::argB trpC801 veA1 | A. M. Bergh |
| RDIT 2.3 | veA1 | D. Tsitsigiannis |
| RDIT 7.24 | methG1 veA1 | D. Tsitsigiannis |
| RDIT 30.34 | methG1 trpC801 pyrG89 veA1 | D. Tsitsigiannis |
| RJH26 | biA1 wA3 argB2 ΔstcE::argB veA1 trpC801 | This study |
| RJW3 | pyrG89 wA3 argB2 pyroA4 ΔstcE::argB veA1 trpC801 | This study |
| RJW51 | alcAp::lovB::pyr4 hygB::lov gene cluster | This study |
| RKIS 1 | pabaA1 yA2 veA1 | 29 |
| RMFV2 | pabaA1 yA2 veA1 argB2 ΔaflR::argB | 12 |
| TJH3.40 | biA1 wA3 argB2 methG1 ΔstcE::argB2 veA1 | 8 |
| TJH34.10 | pabaA1 yA2 veA1 alcA p::aflR::trpC | 29 |
| TPK1.1 | biA1 methG1 veA1 | N. Keller |
| WMH1739 | pabaA1 yA2 alcAp::lovB::pyr4 hygB::lov gene cluster | 21 |
| A. fumigatus strains | ||
| AF293 | G. May | |
| AF293.1 | pyrG− | G. May |
| TJW55.2 | pyrG−, A. parasiticus pyrG | This study |
| A. terreus strains | ||
| ATCC 20542 | American Type Culture Collection | |
| TJW58.9 | hygB | This study |
| laeA mutants | ||
| A. nidulans strains | ||
| MRB300 | biA1wA3 methG1 ΔstcE::argB2 veA1 laeA1 | 8 |
| RJW32 | biA1 wA3 argB2 methG1 ΔstcE::argB veA1 trpC801 ΔlaeA::methG | This study |
| RJW33.2 | wA3 argB2 methG1 pyroA4ΔstcE::argB veA1 trpC801 ΔlaeA::methG | This study |
| RJW 40.4 | biA1 methG1 veA1 ΔlaeA::methG | This study |
| RJW44.2 | biA1 methG1 alcAp::laeA::trpC veA1 ΔlaeA::methG | This study |
| RJW 46.4 | methG1 veA1ΔlaeA::methG | This study |
| RYJ 8 | biA1 wA3ΔstcE::argB veA1 trpC801 laeA1 | This study |
| RJW 52 | alcAp::laeA::trpC alcAp::lovB::pyr4 hygB::lov gene cluster | This study |
| RJW 53 | ΔlaeA::methG alcAp::lovB::pyr4 hygB::lov gene cluster | This study |
| TJW 46.16 | biA1 wA3 argB2 methG1 ΔstcE::argB veA1 alcAp::gfp::laeA::trpC ΔlaeA::methG | This study |
| TJW 57.9 | wA3 argB2 methG1 pyroA4 ΔstcE::argB veA1 aflR::trpCΔlaeA::methG | This study |
| A. fumigatus strain | ||
| TJW 54.2 | ΔlaeA::A. parasiticus pyrG pyrG− | This study |
| A. terreus strains | ||
| TJW 58.2 | hygB alcAp::laeA | This study |
| TJW 58.4 | hygB alcAp::laeA | This study |
| TJW 58.7 | hygB alcAp::laeA | This study |
| TJW 58.8 | hygB alcAp::laeA | This study |
| TJW 58.14 | hygB alcAp::laeA | This study |
| Signal transduction mutants | ||
| H1FAD4 | biA1 veA1 fadAG42R | 40 |
| RKIS 11.1 | pabaA1 yA2 veA1 argB2 ΔfadA::argB | 29 |
| RKIS 28.5 | pabaA1 yA2 veA1 alcAp::rasA17V::argB | 29 |
| TBN 39.5 | biA1 methG1 argB2 ΔflbA::argB veAI | 23 |
| TJH 34.10 | pabaA1 yA2 trpC801 trpC::alcA::aflR veA1 | J. Hicks |
| TKIS 18.11 | pabaA1 yA2 ΔargB::trpC trpC801 veA1 ΔpkaA::argB | 29 |
| TKIS 20.1 | pabaAI yA2 veA1 alcp::pkaA::trpC | 29 |
| TSRB 1.38 | biA1 methG1 argB2 ΔsfaD::argB veA1 | 27 |
Cloning and sequence of A. nidulans and A. fumigatus laeA genes.
The A. nidulans aflR expression mutant, RYJ8 (derived from strain MRB300), was transformed with an A. nidulans genomic cosmid library. Norsolorinic acid-producing transformants were purified, and a cosmid, pCOSJW3, that complemented the mutation was rescued from one transformant. Norsolorinic acid is an orange pigmented precursor in the ST biosynthetic pathway and is commonly used as an indicator of ST production (8). pJW15, a 4.5-kb KpnI-EcoRI subclone of pCOSJW3, also complemented the mutation and was sequenced by use of synthetic primers and an ABI PRISM DNA sequencing kit (Perkin-Elmer Life Science). The mutant allele laeA1 was sequenced after subcloning of a 3-kb PCR fragment from RYJ8 genomic DNA amplified with primers LAE1 and LAE2 (Table 2) into the Zero Blunt TOPO vector (Invitrogen) to produce pJW31. Rapid amplification of cDNA ends technology using a Gene Racer kit (Invitrogen) was employed to clone laeA cDNA according to the manufacturer's instructions. The cloned cDNA was then sequenced. The Institute for Genomic Research (TIGR) database contains a partial A. fumigatus genome sequence (http://www.tigr.org/tdb/e2k1/afu1/). A putative A. fumigatus laeA homolog was obtained by comparing the A. fumigatus genomic data with the A. nidulans laeA sequence.
TABLE 2.
Primers
| Primer | Sequencea | Restric- tion site |
|---|---|---|
| LAE1 | ATCTACCTTTCTGGGCTCCTGG | |
| LAE2 | CGTGAAGAACTTGGCGTTGTAG | |
| LA2 | GACGAGCTCGTGGAACAGTGGAAGGAAC | SacI |
| LA3 | GCGAAGCTTATGAACCGCATCAACCGA | HindIII |
| OEF | GCTGTGAAGCTTTGTACCCTGTTTCGCC | HindIII |
| OER | GATTTGAAGCTTTGCTGGCATGGAACGG | HindIII |
| MT1 | ATGCTGAAGCTTGGAAACTGGGAAAGGGGTC | HindIII |
| GFP2 | TGACGAATTCTCTTAATGGTTTCCTAGCCTG | EcoRI |
| GFP31 | TGCGGAATTCATGAGCAAGGGCGAGGAA | EcoRI |
| GFP4 | GGATGCCTCGAGTTTGTACAGCTCGTCCATGC | XhoI |
| GFP5 | AAGCAGCTCGAGTAAGAGCAAAAGGCGACCAC | XhoI |
| GF1 | CTAGCGAAGCTTGCCACCATGAGCAAGGGCG | HindIII |
| GF2 | CGGCGAATTCCTTGTACAGCTCGTCCATGC | EcoRI |
| GF3 | TTTGGAATTCGTTTCGCCGCTGATGTTTGAG | EcoRI |
| FUM1 | GCGCACTTCTTTGTTTTCCCCT | |
| FUM2 | CATCGGAATTCTTTCTTGAGCGGCC | EcoRI |
| FUM3 | TACCAGGATCCAAAACCTCTCGCCA | BamHI |
| FUM4 | CATGACGGTAACTAAGGATTTGG |
Underlined sequences show the placement of restriction sites.
Nucleic acid analysis.
The extraction of DNAs from fungi and bacteria, restriction enzyme digestion, gel electrophoresis, blotting, hybridization, and probe preparation were performed by standard methods (28, 29). Total RNAs were extracted from Aspergillus strains by use of Trizol reagent (Invitrogen) according to the manufacturer's instructions. RNA blots were hybridized with a 0.7-kb SacII-KpnI fragment from pRB7 containing the stcU coding region (18), a 1.3-kb EcoRV-XhoI fragment from pJW19 containing the aflR coding region, a 3-kb HindIII fragment from pJW45.4 containing the laeA coding region, a 1.1-kb EcoRI-HindIII fragment from pUCHH(458) containing the ipnA coding region (36), a 5-kb BamHI fragment from pWHM1401 containing the lovE coding region (21), and a 1.3-kb PCR product from pWHM1263 containing the lovC coding region (21). Also, A. nidulans cosmids pW07H03, pL11C09, and pL24B03 were used as probes. pL11C09 contains most of the ST gene cluster, whereas pW07H03 and pL24B03 primarily contain genes located upstream and downstream of the ST gene cluster, respectively (7).
Construction of transformation vectors and strains.
Plasmids were generated by standard techniques, and the primers used for this study are listed in Table 2. Pfu Turbo (Stratagene) was used for PCRs. The A. nidulans disruption plasmid pJW34 was constructed by ligating a 1.2-kb DNA fragment upstream of the laeA start codon (primers LAE1 and LA2) and a 1.2-kb DNA fragment downstream of the laeA stop codon (primers LA3 and LAE2) to either side of the methG gene in the pUG11-41 vector (31). The 5′-end PCR product and 3′-end PCR product were inserted into the SacI site and HindIII site of pUG11-41, respectively, by blunt end ligation. pJW34 was used to disrupt the laeA gene (ΔlaeA) in strain TJH3.40 to create TJW35.5. TJW35.5 was subsequently sexually crossed to RDIT2.1 to create RJW46.4. Plasmid pJW47.4 was constructed to overexpress laeA from the alcA promoter. The 2.5-kb coding sequence of laeA was amplified with primers OEF and OER and ligated into the HindIII site of pCN2, which contains the 5′ half of the trpC gene and the alcA promoter (19). This resulted in an alcAp::laeA fusion, referred to as OE::laeA hereafter. pJW47.4 was used to transform RJW32 to tryptophan auxotrophy to yield the strain TJW44.39. TJW44.39 was subsequently sexually crossed to RDIT2.1 to create RJW47.3. pJW47.4 and a hygromycin B (hygB) resistance gene in plasmid pUCH2-8 (3) were used for cotransformation to introduce the overexpression laeA construct into A. terreus ATCC 20542. Transformants were selected in hygromycin B (500 μg/ml)-containing medium and confirmed by PCR and Southern hybridization. Five transformants, namely TJW58.2, TJW58.4, TJW58.7, TJW58.8, and TJW58.14, containing hygB and OE::laeA, were examined for LOV production, and TJW58.9 containing hygB alone was used as a control (Table 1). pJW45.4, containing a wild-type copy of the laeA gene, was used to complement the ΔlaeA strain RJW33.2. pJW45.4 was created by ligating the 3-kb laeA gene (primers MT1 and OER) into the HindIII site of pSH96. pSH96 contains the 5′ half of the trpC gene (39). RJW33.2 is a sexual progeny of a cross between strains TJW35.5 and RJW3. pJW45.4 was used to transform RJW33.2 to produce TJW42.7. TJW42.7 was crossed with RDIT7.24 sexually to create RJW49.1. Plasmids pJW48 and pJW49 were created to visualize LaeA by fusing of the green fluorescent protein (GFP) gene (10, 13) to the N-terminal and C-terminal ends, respectively, of LaeA. pJW48 was made by ligating the 0.7-kb gfp gene (primers GF1 and GF2) to the 5′ end of the 2.5-kb encoding region of the laeA gene (primers GF3 and OER) and then inserting the ligated fragment into the pCN2 HindIII site to yield an alcAp::gfp::laeA chimera. pJW49 was constructed by consecutively ligating a 2-kb laeA coding region (primers OEF and GFP2), a 0.7-kb gfp gene (primers GFP31 and GFP4), and a 0.5-kb laeA termination cassette (primers GFP5 and OER) into the HindIII site of pCN2 to yield an alcAp::laeA::gfp::laeAterm chimera. pJW48 and pJW49 were used to transform RJW32 to yield transformants TJW46.16 (5′ GFP) and TJW47.9 (3′ GFP), respectively. The A. fumigatus laeA gene disruption vector, pJW58, was constructed by insertion of a 0.9-kb DNA fragment upstream of the laeA start codon (primers FUM1 and FUM2) and a 1.0-kb DNA fragment downstream of the laeA stop codon (primers FUM3 and FUM4) on either side of the Aspergillus parasiticus pyrG marker gene obtained from pBZ5 (32). pJW58 was used to disrupt the A. fumigatus laeA gene in strain AF293.1 to create strain TJW54.2.
Fungal transformation procedures.
Fungal transformation was done essentially as described by Miller et al. (24), with the modification of embedding the protoplasts in top agar (0.75%) rather than spreading them by a glass rod on solid medium.
Secondary metabolite analysis.
Published procedures were used to extract and analyze ST (12), gliotoxin (5), LOV (21), and monocolin J (MONJ) (21). All metabolites were extracted with chloroform from both mycelial and culture filtrates. MONJ and LOV concentrations in each strain were estimated by comparisons to standard spots on thin-layer chromatography (TLC) plates by dilution spotting. Pictures of TLC plates were taken at 254 nm. ST was extracted from either 50-ml shake cultures in GMM inoculated with 107 spores/ml and grown for 60 h or solid medium cultures spread with 106 spores/plate and grown for 5 days. Dried ST extracts were resuspended in 100 μl of chloroform, and 10 μl was separated in chloroform-acetone (8:2) on TLC plates. ST (Sigma) was spotted as a standard. MONJ was extracted from 50-ml GMM shake cultures inoculated with 107 spores/ml and grown for 72 h. MONJ from WT/lov+ and OE::laeA/lov+ strains was extracted from cultures grown in 50 ml of shaking liquid GMM for 14 h at 37°C and then transferred to shaking liquid TMM for 24 h. Dried MONJ extracts were resuspended in 100 μl of methanol, and 10 μl was separated in methanol-0.1% phosphoric acid (9:1) on C18 reversed-phase TLC plates. The MONJ standard was extracted from A. nidulans strain WMH1739 (Table 1). All experiments were performed in triplicate. Gliotoxin production in A. fumigatus was analyzed by modification of the TLC method of Belkacemi et al. (5). Gliotoxin was extracted from 50-ml GMM shake cultures inoculated with 107 spores/ml and grown for 3 days. Dried chloroform extracts were resuspended in 100 μl of methanol, and 10 μl was separated in chloroform-methanol (9:1). Gliotoxin (Sigma) was spotted as a standard. All experiments were performed in triplicate. For the assessment of PN production, Micrococcus luteus ATCC 9341 was grown in TBS (17 g of Bacto Tryptone, 3 g of Bacto Soytone, 5 g of NaCl, 2.5 g of K2HPO4, and 2.5 g of glucose in a 1-liter total volume) at 37°C at 180 rpm until it reached an optical density of 1. Three and one-third milliliters of M. luteus culture was mixed with 40 ml of TSA (15 g of Bacto Tryptone, 5 g of Bacto Soytone, 5 g of NaCl, and 10 g of agar in a 1-liter total volume) and poured into 150-cm-diameter plates to solidify. Fifty-milliliter cultures of the wild-type (WT), ΔlaeA, and OE::laeA strains (107 spores/ml) were grown in GMM with shaking for 14 h at 37°C and then were transferred to LMM shake cultures amended with 30 mM cyclopentanone for 24 h. For each strain, 6 ml was removed, lyophilized, and resuspended in 1 ml of distilled water. One-hundred-microliter samples, with or without 6 U of β-lactamase, were placed in 10-cm-diameter wells of the M. luteus plates. Plates were placed for 2 h at 4°C and then incubated overnight at 37°C to evaluate PN inhibition zones. All experiments were duplicated. LOV was extracted from A. terreus cultures grown in 50-ml GMM shake flasks for 18 h at 32°C and then transferred to LMM shake cultures with 30 mM cyclopentanone for 36 h at 32°C. Extraction and identification on TLC were followed by the previously described method of MONJ examination. LOV (Merck Co.) was spotted onto TLC plates as a control. All experiments were duplicated.
Nucleotide sequence accession numbers.
GenBank numbers for the A. nidulans and A. fumigatus laeA genes are AY394722 and AY422723, respectively.
RESULTS
Cloning and characterization of A. nidulans and A. fumigatus laeA.
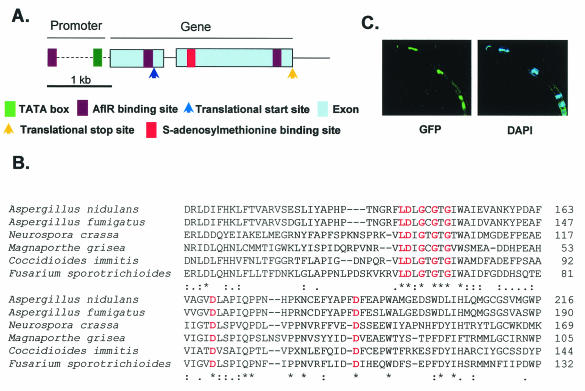
A mutagenesis screen previously led to the isolation of 23 mutants displaying a loss of ST production but normal sporulation in A. nidulans (8). Three of the mutants were unable to express aflR, which encodes an ST cluster Zn2Cys6 transcription factor regulating ST biosynthetic gene expression (12). We were able to complement one of these three mutants, RYJ8, with an A. nidulans trpC genomic cosmid library. Sequencing of a 4.5-kb subclone (pJW15) of the complementing cosmid pCOSJW3 revealed a 3-kb open reading frame designated laeA (for loss of aflR expression). Sequencing of the mutant allele, laeA1, from RYJ8 showed that it has a base pair transversion (at position 1455; C→G) and a 1-bp deletion (at position 1453) in the gene. The deletion resulted in a premature stop codon. An examination of genomic and cDNA sequences revealed that laeA has one intron and three putative AflR binding sites (12), one in the promoter (−607) and two in the encoding region (positions 607 and 1487) (Fig. 1A). cDNA analysis showed that laeA possesses a 5′ untranslated region (642 bp) (Fig. 1A). Analyses of available genomic databases indicated that only filamentous fungi, including A. fumigatus (human pathogen causing aspergillosis; TIGR [http://www.tigr.org/tdb/e2k1/afu1/]), Neurospora crassa (model fungus; GenBank), Magnaporthe grisea (plant pathogen causing rice blast fungus [http://www-genome.wi.mit.edu/annotation/fungi/magnaporthe]), Coccidioides immitis (human pathogen causing coccidioidomycosis; GenBank), and Fusarium sporotrichioides (plant pathogen producing trichothecene mycotoxin [http://www.genome.ou.edu/fsporo.html]), have possible LaeA homologs (Fig. 1B). An examination of the LaeA amino acid sequence (375 amino acids) revealed a conserved S-adenosylmethionine (SAM) binding site found in nuclear protein methyltransferases (Fig. 1B) (15). Although the amino acid sequence of LaeA did not show the presence of a nuclear localization motif, GFP tagging of either the 5′ or 3′ end of A. nidulans laeA showed LaeA to be primarily localized in the nucleus (Fig. 1C).
FIG. 1.
Overview of LaeA. (A) Schematic of laeA gene. Although LaeA contains the exact SAM motif found in histone methyltransferases and arginine methyltransferases, it lacks other conserved domains (e.g., a SET domain and a double E loop) typically found in these proteins. In addition, likely histone methyltransferase and arginine methyltransferase candidates are found in the Aspergillus database (1e−42 and 1e−94). Therefore, LaeA appears to be a unique protein methyltransferase. (B) Amino acid comparison of A. nidulans, A. fumigatus, N. crassa, Magnaporthe grisea, C. immitis, and F. sporotrichioides LaeA proteins showing conserved protein methyltransferase SAM binding sites in red. (C) A. nidulans LaeA protein localizes to the nucleus. GFP was fused to the N-terminal end of LaeA. Nuclei were stained with the DNA-specific dye 4,6-diamidino-2-phenylindole (DAPI).
LaeA is required for secondary metabolite production.
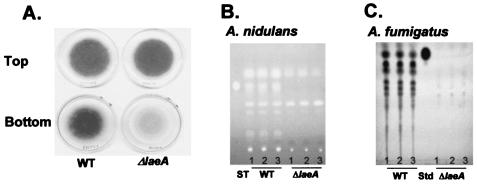
laeA null mutants (ΔlaeA) were created by replacing laeA with methG and pyrG in A. nidulans TJH3.40 (a methG1 auxotroph) and A. fumigatus AF293.1 (a pyrG auxotroph), respectively. Southern blot and PCR analyses were carried out to confirm single gene replacement events in several transformants, including A. nidulans TJW35.5 and A. fumigatus TJW54.2. Prototroph RJW46.4 was obtained from TJW35.5 by a sexual cross, as described in Materials and Methods. Strains RJW46.4 and TJW54.2 were used for our study. For both species, ΔlaeA strains were visually detectable due to the loss of mycelial pigment from the backside of the colonies (Fig. 2A and data not shown). A TLC examination of chloroform extracts of A. nidulans and A. fumigatus ΔlaeA strains showed that the production of several metabolites, including ST in A. nidulans (Fig. 2B) and the immunotoxin gliotoxin in A. fumigatus, was reduced (Fig. 2C). The identities of the other metabolites are not known. Interestingly, the levels of two of the A. nidulans metabolites were not reduced and even appeared to be somewhat increased in the ΔlaeA strain (Fig. 2B). Verification that these defects were caused by the loss of laeA was obtained by the transformation of A. nidulans ΔlaeA with wild-type laeA and the observed remediation of metabolite production (data not shown).
FIG. 2.
Phenotypes of laeA. (A) Asexual sporulation (top) and mycelial pigmentation (bottom) patterns of A. nidulans wild-type (RDIT2.3) (WT) and ΔlaeA (RJW46.4) strains after 5 days of cultivation on GMM. A. fumigatus ΔlaeA presented a similar loss of mycelial pigmentation (data not shown). (B) TLC analysis of chloroform extracts of RDIT2.3 and RJW46.4 after 5 days of cultivation on solid GMM. (C) TLC analysis of chloroform extracts of A. fumigatus AF293 (WT) and TJW54.2 (ΔlaeA) grown in liquid shaking GMM for 3 days. The experiment was performed in triplicate. Std, gliotoxin standard.
Transcriptional regulation of secondary metabolism gene clusters by LaeA. (i) Native cluster regulation.
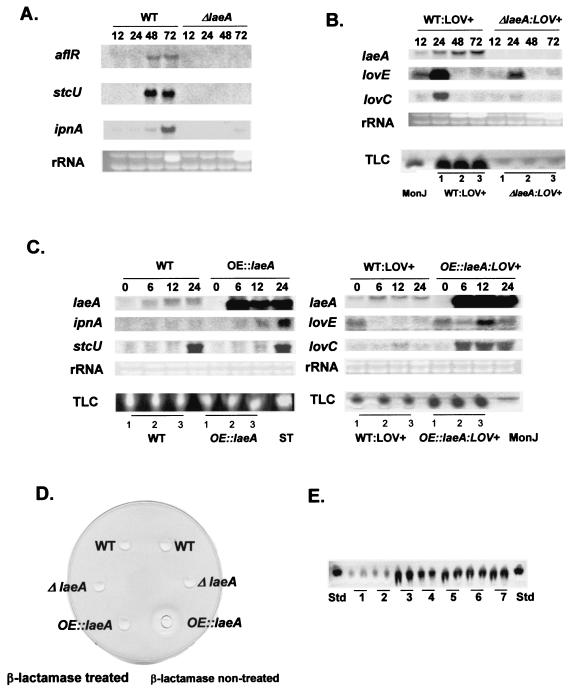
To confirm our initial observation that laeA is required for ST gene regulation, we assessed aflR and stcU (a gene encoding a biosynthetic enzyme required for ST production) (17) expression in the ΔlaeA background. Neither gene was expressed (Fig. 3A). A transcriptional profile of the entire ST gene cluster, which covers ca. 60 kb and contains ca. 26 genes (7), suggested that LaeA transcriptional control is ST cluster specific, as transcription of the upstream and downstream genes of the ST cluster was unaffected (data not shown). Because many uncharacterized metabolites were reduced in the ΔlaeA strains (Fig. 2B and C), we thought it possible that LaeA is a global regulator for secondary metabolite gene expression. To address this hypothesis, we examined PN gene expression in the A. nidulans ΔlaeA strain. Figure 3A shows that ipnA (encoding isopenicillin N synthetase, a biosynthetic enzyme required for PN biosynthesis) (6) expression was greatly reduced in the ΔlaeA strain.
FIG. 3.
laeA regulation of secondary metabolism. (A) aflR, stcU, and ipnA gene expression in A. nidulans wild-type (WT) (RDIT2.3) and ΔlaeA (RJW46.4) strains grown in liquid shaking GMM for 12, 24, 48, and 72 h at 37°C. Ethidium bromide-stained rRNA is indicated as a loading control. (B) laeA, lovE, and lovC gene expression in A. nidulans WT/lov+ (RJW51) and ΔlaeA/lov+ (RJW53) strains grown in liquid shaking GMM for 12, 24, 48, and 72 h at 37°C. Ethidium bromide-stained rRNA is indicated as a loading control. MONJ was extracted from WT/lov+ (RJW51) and ΔlaeA/lov+ (RJW53) A. nidulans strains grown in liquid shaking GMM for 3 days. The experiment was performed in triplicate. (C) laeA, ipnA, stcU, lovE, and lovC gene expression in A. nidulans wild-type (WT) (RDIT2.3), OE::laeA (RJW47.3), WT/lov+ (RJW51), and OE::laeA/lov+ (RJW52) strains grown in liquid shaking GMM for 14 h at 37°C and then transferred to liquid shaking TMM for the induction of laeA expression. Time points were 0, 6, 12, and 24 h after transfer. ST and MONJ were extracted from A. nidulans WT (RDIT2.3), OE::laeA (RJW47.3), WT/lov+ (RJW51), and OE::laeA/lov+ (RJW52) strains grown in liquid shaking GMM for 14 h at 37°C and then transferred to liquid shaking TMM for 24 h. ST, ST standard. The MONJ standard was extracted from A. nidulans strain WMH1739. The experiment was performed in triplicate. (D) PN bioassay. Wild-type (FGSC26), ΔlaeA (RJW40.4), and OE::laeA (RJW44.2) strains were grown in liquid shaking GMM for 14 h at 37°C and then transferred to LMM amended with 30 mM cyclopentanone for the induction of laeA for 24 h at 37°C. (E) TLC examination of LOV production in A. terreus laeA overexpression strains. The wild type (ATCC 20542; lane 1), TJW58.9 (hygB resistance gene-containing transformant used as a control; lane 2), and OE::laeA strains containing hygB (TJW58.2, TJW58.4, TJW58.7, TJW58.8, and TJW58.14, in lanes 3 to 7, respectively) were grown in liquid shaking GMM for 18 h at 32°C and then transferred to LMM with 30 mM cyclopentanone for the induction of laeA for 36 h at 32°C. Std, LOV standard. The experiment was performed in duplicate.
(ii) Heterologous cluster regulation.
Our results for ST and PN gene expression suggested a role for LaeA in secondary metabolite gene cluster regulation. To further address this potential role, we examined the expression of the heterologous LOV gene cluster in the A. nidulans ΔlaeA background. The partial LOV cluster, derived from A. terreus, was originally transformed into A. nidulans to study aspects of LOV biosynthesis (21). This strain was used to cross the LOV cluster (LOV+) into appropriate mutant laeA backgrounds. Figure 3B shows that the ΔlaeA/ LOV+ strain displayed very diminished levels of both lovE (encoding a LOV-specific Zn2Cys6 transcription factor) and lovC (a LOV biosynthetic gene) transcripts. Chloroform extracts of this strain also showed diminished production of MONJ (Fig. 3B), the LOV intermediate produced by the partial LOV cluster (21).
Overexpression of laeA upregulates PN and LOV gene expression but not ST gene expression.
We next constructed laeA overexpression strains (OE::laeA) of both A. nidulans and A. terreus to examine secondary metabolite gene expression and product formation. As shown in Fig. 3C, ipnA, lovE, and lovC, but not stcU, expression levels were remarkably elevated in the A. nidulans OE::laeA background. Secondary metabolite production was correlated with transcript levels. MONJ production was increased ∼400%, and high levels of PN were produced during times when the wild type showed no PN activity (Fig. 3D). ST levels remained the same as that of the wild type in the OE::laeA background (Fig. 3C). Overexpression of the A. nidulans laeA gene in the LOV-producing fungus A. terreus led to 400 to 700% increases in LOV (Fig. 3E).
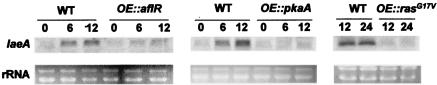
Feedback regulation of laeA by aflR.
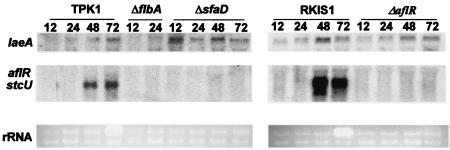
An examination of the laeA transcript in wild-type strains showed that it is an inducible, low-expression-level gene that is observed in Northern blots before and after aflR transcripts are observed (data not shown). Due to the presence of three AflR binding sites in the gene (Fig. 1A), we thought it possible that AflR regulates laeA expression. As shown in Fig. 4, overexpression of aflR (OE::aflR) downregulates laeA expression, although elimination of aflR (ΔaflR) does not affect the laeA transcript level (Fig. 5). This indicates that there are both negative (laeA) and positive (stc genes) regulatory effects of AflR on gene transcription.
FIG. 4.
Regulation of laeA. Effects of overexpression of aflR, pkaA, and rasG17V on laeA expression. Wild-type (RKIS1), OE::aflR (TJH34.10), OE::pkaA (TKIS20.1), and OE::rasG17V (RKIS28.5) strains were grown in liquid shaking GMM for 14 h at 37°C and then transferred to TMM. Time points were 0, 6, 12, and 24 h after transfer.
FIG. 5.
laeA expression is not affected in ΔflbA, ΔsfaD, and ΔaflR strains. laeA, aflR, and stcU gene expression was examined in A. nidulans wild-type (TPK1.1 and RKIS1), ΔflbA (TBN39.5), ΔsfaD (TSRB1.38), and ΔaflR (RMFV2) strains grown in liquid shaking GMM for 12, 24, 48, and 72 h at 37°C. Ethidium bromide-stained rRNA is indicated as a loading control.
Protein kinase A and RasA negatively regulate laeA expression.
ST biosynthesis is regulated in A. nidulans via a signal transduction pathway, and many of the genes involved in this signaling pathway are known (9). Therefore, we looked at the possible interactions with laeA of five signaling genes, encoding two members of a heterotrimeric G protein (fadA and sfaD) (27, 40), a regulator of G-protein signaling protein regulating FadA activity (flbA) (23), a cAMP-dependent kinase (pkaA) (29), and a Ras protein (rasA) (33). laeA expression was examined in the wild type and in strains carrying the following alleles: ΔflbA, fadAG42R, ΔfadA, ΔsfaD, ΔpkaA, OE::pkaA, and OE::rasAG17A (Table 1). mRNA analyses of these mutants showed that OE::pkaA and OE::rasAG17A completely inhibited laeA expression (Fig. 4), whereas laeA transcription was not repressed in any of the other strains (Fig. 5 and data not shown). Interestingly, the laeA transcript level was elevated in the ΔsfaD strain (Fig. 5). The presence of laeA transcripts in these mutants (Fig. 5 and data not shown) shows that laeA is not sufficient for aflR expression, as aflR was not expressed in these strains (18). Figure 6 depicts our current understanding of LaeA involvement in secondary metabolite regulation.
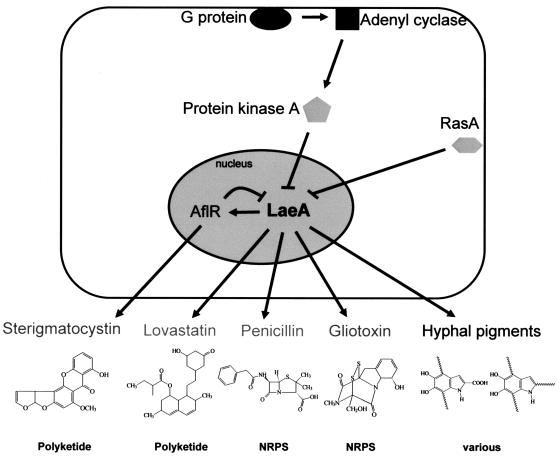
FIG. 6.
Proposed model of secondary metabolite regulation by LaeA. Gliotoxin is formed from serine and alanine and is proposed to be produced by a nonribosomal peptide synthase (NRPS). Fungal pigments belong to several chemical classes, including polyketides, terpenes, and DOPA melanins.
DISCUSSION
Secondary metabolite biosynthesis is often associated with the advent of sporulation and cellular development in filamentous fungi (9) and filamentous Streptomyces bacteria (38). These developmental processes reflect the need to access multiple nutrients and to optimize cellular morphology and metabolic differentiation for effective competition in complex environments. We are interested in the identification of secondary metabolism-specific global regulators that can uncouple sporulation and secondary metabolism. Such regulatory elements are extremely desirable because they would possess broad specificity for the activation and/or repression of entire families of secondary metabolite gene clusters while providing strains that are capable of otherwise normal or near-normal development and growth. The identification of such regulatory elements would enable the increased production of secondary metabolites by providing improved strains of engineered organisms and would also contribute to a broader understanding of molecular mechanisms by which secondary metabolites are produced. Through complementation of an ST developmental mutant, we have identified such a protein, called LaeA, which is an archetypal global regulator of secondary metabolism in fungi.
LaeA regulation of metabolite production is transcriptional, as assessed by the effects of ΔlaeA and OE::laeA alleles on ST, PN, and LOV gene expression in A. nidulans and A. terreus. In all cases, gene transcripts were reduced or eliminated in ΔlaeA strains. However, although overexpression of laeA increased PN and LOV gene transcript and concomitant production formation (Fig. 3C, D, and E), this was not the case for ST gene transcription or production (Fig. 3A). The steady-state level of ST transcripts and product formation in the OE::laeA background, in contrast to the increased PN and LOV transcripts and product formation, suggested a unique interaction between laeA and ST gene regulation.
Due to the presence of three potential AflR binding sites in the A. nidulans gene (Fig. 1A) and the lack of ST cluster gene upregulation in the OE::laeA background, we thought it possible that AflR negatively regulates laeA expression. As shown in Fig. 4, overexpression of aflR (OE::aflR) downregulates laeA expression, although elimination of aflR (ΔaflR) does not affect laeA transcript levels (Fig. 5). This indicates that there are both negative (laeA) and positive (stc genes) (12) regulatory effects of AflR on gene transcription. To our knowledge, this is the first description of a putative secondary metabolite feedback mechanism. As overproduction of ST negatively affects fungal growth (N. P. Keller, unpublished data), we speculate that this feedback loop may have evolved as a fitness trait. In contrast, neither the promoter nor the encoding region of A. fumigatus laeA contained AflR binding sites, and no aflR ortholog was found in the genome. Some A. fumigatus strains are reported to produce ST (14); it would be interesting to see if laeA genes from those isolates contained AflR binding sites. Initial examinations of the Aspergillus ΔlaeA strains showed them to be more susceptible to killing by neutrophils in vitro than the wild type (S. Balajee, L. Delbridge, J. Bok, N. Keller, and K. Marr, unpublished data). Presumably this is due to a loss of toxin secondary metabolites or melanins, known virulence factors in several fungal systems (5, 22).
laeA expression is also negatively regulated by two signal transduction molecules, PkaA and RasA. Both proteins have been shown to transcriptionally and posttranscriptionally regulate aflR (29, 30). It appears that LaeA mediates PkaA transcriptional regulation of aflR, since overexpression of laeA in a pkaA overexpression background, a condition that normally suppresses stc expression, partially restored stc gene expression (29; data not shown). With regard to PkaA regulation of laeA, the lack of conventional PkaA phosphorylation consensus sequences in LaeA indicates that PkaA regulation of LaeA is not direct. Alternatively, LaeA may contain unconventional PkaA phosphorylation sites. RasA regulation of laeA gene expression may occur through PkaA and/or another pathway(s).
The requirement of a kinase for laeA function is reminiscent—to a degree—of a Streptomyces global regulatory system involving the protein AfsR. AfsR is a transcription factor that regulates secondary metabolism in Streptomyces coelicolor but regulates morphogenesis in Streptomyces griseus (contrast this to the similar role LaeA has in the three Aspergillus spp. examined here). Phosphorylation of AfsR enhances its activity (37). Like the case for AfsR, LaeA regulation occurs at the transcriptional level, but it shows no homology to transcription factors. Its nuclear location, its role in transcriptional regulation, and the presence of a SAM motif in LaeA suggest that it may be a protein methyltransferase. Well-known protein methyltransferases include histone and arginine methyltransferases that play important roles in the regulation of gene expression in eukaryotes, in part through modification of the chromatin structure (15, 25, 35).
Regardless of the mechanism, these findings with LaeA present an advance toward understanding the complex regulation of secondary metabolite production and provide a means for the discovery of new metabolites. Indeed, initial comparative microarray studies between ΔlaeA and the wild type have identified putative secondary metabolism gene clusters in the Aspergillus genome (L. Maggio-Hall, J. Bok, and N. Keller, unpublished data). The manipulation of LaeA in filamentous fungi may enable the increased production of pharmaceuticals or the elimination of fungal toxins by providing improved strains of engineered organisms and may also contribute to the broader understanding of molecular mechanisms by which secondary metabolites are produced.
Acknowledgments
This research was funded by NSF grant MCB-9874646 to N.P.K.
REFERENCES
- 1.Adams, T. H., and J. H. Yu. 1998. Coordinate control of secondary metabolite production and asexual sporulation in Aspergillus nidulans. Curr. Opin. Microbiol. 1:674-677. [DOI] [PubMed] [Google Scholar]
- 2.Adams, T. H., and W. E. Timberlake. 1990. Developmental repression of growth and gene expression in Aspergillus. Proc. Natl. Acad. Sci. USA 87:5405-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander, N. J., T. H. Hohn, and S. P. McCormick. 1998. The TRI11 gene of Fusarium sporotrichioides encodes a cytochrome P-450 monooxygenase required for C-15 hydroxylation in trichothecene biosynthesis. Appl. Environ. Microbiol. 64:221-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alspaugh, J. A., J. R. Perfect, and J. Heitman. 1997. Cryptococcus neoformans mating and virulence are regulated by the G-protein alpha subunit GPA1 and cAMP. Genes Dev. 11:3206-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belkacemi, L., R. C. Barton, V. Hopwood, and E. G. V. Evans. 1999. Determination of optimum growth conditions for gliotoxin production by Aspergillus fumigatus and development of a novel method for gliotoxin detection. Med. Mycol. 37:227-233. [PubMed] [Google Scholar]
- 6.Brakhage, A. A. 1998. Molecular regulation of beta-lactam biosynthesis in filamentous fungi. Microbiol. Mol. Biol. Rev. 62:547-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, D. W., J. H. Yu, H. S. Kelkar, M. Fernandes, T. C. Nesbitt, N. P. Keller, T. H. Adams, and T. J. Leonard. 1996. Twenty-five coregulated transcripts define a sterigmatocystin gene cluster in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 93:1418-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butchko, R. A., T. H. Adams, and N. P. Keller. 1999. Aspergillus nidulans mutants defective in stc gene cluster regulation. Genetics 153:715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvo, A. M., R. A. Wilson, J. W. Bok, and N. P. Keller. 2002. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 66:447-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalfie, M., Y. Tu, G. Euskirchen, W. W. Ward, and D. C. Prasher. 1994. Green fluorescent protein as a marker for gene expression. Science 263:802-805. [DOI] [PubMed] [Google Scholar]
- 11.Demain, A., and A. Fang. 2000. The natural functions of secondary metabolites. Adv. Biochem. Eng. Biotechnol. 69:1-39. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes, M., N. P. Keller, and T. H. Adams. 1998. Sequence-specific binding by Aspergillus nidulans AflR, a C6 zinc cluster protein regulating mycotoxin biosynthesis. Mol. Microbiol. 28:1355-1365. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Abalos, J. M., H. Fox, C. Pitt, B. Wells, and J. H. Doonan. 1998. Plant-adapted green fluorescent protein is a versatile vital reporter for gene expression, protein localization and mitosis in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 27:121-130. [DOI] [PubMed] [Google Scholar]
- 14.Hah, H. 1993. Differential action of cercoran and topsin-M on sensitive and tolerant strains of toxic fungi. Cryptogamie Mycologie 14:61-68. [Google Scholar]
- 15.Hamahata, A., Y. Takata, T. Gomi, and M. Fujioka. 1996. Probing the S-adenosylmethionine-binding site of rat guanidinoacetate methyltransferase. Effect of site-directed mutagenesis of residues that are conserved across mammalian non-nucleic acid methyltransferases. Biochem. J. 317:141-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawksworth, D. L. 2001. The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol. Res. 105:1422-1432. [Google Scholar]
- 17.Hicks, J. K., K. Shimizu, and N. P. Keller. 2002. Genetics and biosynthesis of aflatoxins and sterigmatocystin, p. 55-69. In F. Kempken and J. W. Bennett (ed.), The mycota, vol. XI. Springer-Verlag, Berlin, Germany.
- 18.Hicks, J. K., J.-H. Yu, N. P. Keller, and T. H. Adams. 1997. Aspergillus sporulation and mycotoxin production both require inactivation of the FadA G alpha protein-dependent signaling pathway. EMBO J. 16:4916-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hicks, J., R. A. Lockington, J. Strauss, D. Dieringer, C. P. Kubicek, J. Kelly, and N. Keller. 2001. RcoA has pleiotropic effects on Aspergillus nidulans cellular development. Mol. Microbiol. 39:1482-1493. [DOI] [PubMed] [Google Scholar]
- 20.Jin, Y., J. W. Bok, D. Guzman-de-Peña, and N. P. Keller. 2002. Requirement of spermidine for developmental transitions in Aspergillus nidulans. Mol. Microbiol. 46:801-812. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy, J., K. Auclair, S. G. Kendrew, C. Park, J. C. Vederas, and C. R. Hutchinson. 1999. Modulation of polyketide synthase activity by accessory proteins during lovastatin biosynthesis. Science 284:1368-1372. [DOI] [PubMed] [Google Scholar]
- 22.Langfelder, K., M. Streibel, B. Jahn, G. Haase, and A. A. Brakhage. 2003. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 38:143-158. [DOI] [PubMed] [Google Scholar]
- 23.Lee, B. N., and T. H. Adams. 1994. Overexpression of flbA, an early regulator of Aspergillus asexual sporulation, leads to activation of brlA and premature initiation of development. Mol. Microbiol. 14:323-334. [DOI] [PubMed] [Google Scholar]
- 24.Miller, B. L., K. Y. Miller, and W. E. Timberlake. 1985. Direct and indirect gene replacements in Aspergillus nidulans. Mol. Cell. Biol. 5:1714-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mowen, K. A., J. Tang, W. Zhu, B. T. Schurter, K. Shuai, H. R. Herschman, and M. David. 2001. Arginine methylation of STAT1 modulates IFN alpha/beta-induced transcription. Cell 104:731-741. [DOI] [PubMed] [Google Scholar]
- 26.Pontecorvo, G., J. A. Roper, L. M. Hemmons, K. P. Macdonald, and A. W. J. Bufton. 1953. The genetics of Aspergillus nidulans. Adv. Genet. 5:141-238. [DOI] [PubMed] [Google Scholar]
- 27.Rosén, S., J.-H. Yu, and T. H. Adams. 1999. The Aspergillus nidulans sfaD gene encodes a G protein beta subunit that is required for normal growth and repression of sporulation. EMBO J. 18:5592-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Shimizu, K., and N. P. Keller. 2001. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics 157:591-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimizu, K., J.-K. Hicks, T.-P. Huang, and N. P. Keller. 2003. Pka, Ras and RGS protein interactions regulate activity of AflR, a Zn(II)2Cys6 transcription factor in Aspergillus nidulans. Genetics 165:1095-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sienko, M., and A. Paszewski. 1999. The metG gene of Aspergillus nidulans encoding cystathionine beta-lyase: cloning and analysis. Curr. Genet. 35:638-646. [DOI] [PubMed] [Google Scholar]
- 32.Skory, C. D., P. K. Chang, J. Cary, and J. E. Linz. 1992. Isolation and characterization of a gene from Aspergillus parasiticus associated with the conversion of versicolorin A to sterigmatocystin in aflatoxin biosynthesis. Appl. Environ. Microbiol. 58:3527-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Som, T., and V. S. Kolaparthi. 1994. Developmental decisions in Aspergillus nidulans are modulated by Ras activity. Mol. Cell. Biol. 14:5333-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tag, A., J. Hicks, G. Garifullina, M. Beremand, and N. Keller. 2000. G-protein signalling mediates differential production of toxic secondary metabolites. Mol. Microbiol. 38:658-665. [DOI] [PubMed] [Google Scholar]
- 35.Tamaru, H., and E. U. Selker. 2001. A histone H3 methyltransferase controls DNA methylation in Neurospora crassa. Nature 414:277-283. [DOI] [PubMed] [Google Scholar]
- 36.Tilburn, J., S. Sarkar, D. A. Widdick, E. A. Espeso, M. Orejas, J. Mungroo, M. A. Penalva, and H. N. Arst, Jr. 1995. The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. EMBO J. 14:779-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umeyama, T., P. C. Lee, and S. Horinouchi. 2002. Protein serine/threonine kinases in signal transduction for secondary metabolism and morphogenesis in Streptomyces. Appl. Microbiol. Biotechnol. 59:419-425. [DOI] [PubMed] [Google Scholar]
- 38.Wang, L., and L. C. Vining. 2003. Control of growth, secondary metabolism and sporulation in Streptomyces venezuelae ISP5230 by jadW (1), a member of the afsA family of gamma-butyrolactone regulatory genes. Microbiology 149:1991-2004. [DOI] [PubMed] [Google Scholar]
- 39.Wieser, J., and T. H. Adams. 1995. flbD encodes a Myb-like DNA-binding protein that coordinates initiation of Aspergillus nidulans conidiophore development. Genes Dev. 9:491-502. [DOI] [PubMed] [Google Scholar]
- 40.Yu, J.-H., J. Wieser, and T. H. Adams. 1996. The Aspergillus FlbA RGS domain protein antagonizes G protein signaling to block proliferation and allow development. EMBO J. 15:5184-5190. [PMC free article] [PubMed] [Google Scholar]