Abstract
Background:
Patients undergone mechanical ventilation need rapid and reliable evaluation of their respiratory status. Monitoring of End-tidal carbon dioxide (ETCO2) as a surrogate, noninvasive measurement of arterial carbon dioxide (PaCO2) is one of the methods used for this purpose in intubated patients.
Objectives:
The aim of the present trial was to study the relationship between end-tidal CO2 tensions with PaCO2 measurements in mechanically ventilated patients.
Materials and Methods:
End-tidal carbon dioxide levels were recorded at the time of arterial blood gas sampling. Patients who were undergoing one of the mechanical ventilation methods such as: synchronized mandatory mechanical ventilation (SIMV), continuous positive airway pressure (CPAP) and T-Tube were enrolled in this study. The difference between ETCO2 and PaCO2 was tested with a paired t-test. The correlation of end-tidal carbon dioxide to (ETCO2) CO2 was obtained in all patients.
Results:
A total of 219 arterial blood gases were obtained from 87 patients (mean age, 71.7 ± 15.1 years). Statistical analysis demonstrated a good correlation between the mean of ETCO2 and PaCO2 in each of the modes of SIMV, CPAP and T-Tube; SIMV (42.5 ± 17.3 and 45.8 ± 17.1; r = 0.893, P < 0.0001), CPAP (37 ± 9.7 and 39.4 ± 10.1; r = 0.841, P < 0.0001) and T-Tube (36.1 ± 9.9 and 39.4 ± 11; r = 0.923, P < 0.0001), respectively.
Conclusions:
End-tidal CO2 measurement provides an accurate estimation of PaCO2 in mechanically ventilated patients. Its use may reduce the need for invasive monitoring and/or repeated arterial blood gas analyses.
Keywords: Blood Gas Analysis, Carbon Dioxide, Artificial Respiration
1. Background
End-tidal CO2 monitors are used to estimate arterial CO2 pressure (PaCO2), but appropriate use of this noninvasive method of assessing blood gases in ventilated patients remains unclear. It has been used extensively in operating rooms, intensive care units, emergency departments and in pre-hospital setting (1-4). In a study that was conducted by Flanagan et al., end-tidal CO2 measurement provided an accurate estimation of PaCO2, even during episodes of severe hypocarbia (5). One study indicated that measurements of end-tidal carbon dioxide concentrations correlated well with PaCO2 values in non-intubated patients presenting with a variety of conditions to emergency departments (6). End-tidal carbon dioxide measurements may be sufficient measures of PaCO2 in selected patients and obviate the need for repeat arterial blood gas determination. In a study that was carried out in ventilated head trauma patients, end-tidal PaCO2 monitoring correlated well with PaCO2 in patients without respiratory complications or without spontaneous breathing (7). However, its clinical validity is questionable in patients who have the greatest need for end-tidal PaCO2 monitoring (i.e., patients who have respiratory distress or who are breathing spontaneously and overriding the ventilator). Noninvasive end-tidal carbon dioxide pressure (ETCO2) monitoring may adequately predict PaCO2 in non-intubated emergency department patients with respiratory distress, who are able to produce a forced expiration (8). ETCO2 is a less accurate measure of PaCO2 with tidal volume breathing and in patients with pulmonary disease. PaCO2 cannot be estimated by the ETCO2 method in a pre-hospital setting (9). There is wide variation in the gradient between PaCO2 and ETCO2 depending on the patient’s condition, and this relationship does not remain constant over time, thus it is not useful in pre-hospital ventilation management. These data do not support routine monitoring of end-tidal CO2 during short transportation times in adult patients requiring mechanical ventilation. However, the monitor may prevent morbidity in patients requiring tight control of PaCO2 (10). One study reported that, PaCO2 gives a poor estimate of PaCO2 in patients with respiratory failure (11). In another study, that was carried out in mechanically ventilated patients with multisystem trauma, trends in the arterial to end-tidal carbon dioxide gradient magnitude were not reliable, and concordant direction changes in ETCO2 and PaCO2 are not assured (12).
2. Objectives
The aim of the present trial was to study the relationship between end-tidal CO2 tensions with PaCO2 measurements in mechanically ventilated patients.
3. Materials and Methods
This was a cross-sectional study conducted on 219 arterial blood gases in 87 adult patients with respiratory failure admitted to the Intensive Care Unit (ICU) of Shahid Beheshti Hospital, Kashan University of Medical Sciences, Iran, between March 2008 and February 2010. The mean age of the patients was 71.7 ± 15.1 years, 46 of the patients were male, and 43 were female. The study was approved by the local hospital Ethics Committee and informed consent was obtained. Blood samples were drawn by radial arterial puncture. Samples were immediately analyzed for PaCO2 using a blood gas analyzer (AVL-995, AVL Medical Instruments, Graz, Austria). The arterial to end-tidal CO2 gradient was determined. The ETCO2 was measured using an end-tidal CO2 analyzer (CAPNOGARD, Respironics, California, Inc. Carisbad, CA, USA), on the expiratory side of the circuit’s endotracheal tube connector. After proper calibration and an equilibration time of 20 minutes with stable hemodynamic and respiratory variables, ETCO2 were determined and the highest reading was recorded. Patients who were undergoing one of the mechanical ventilation methods such as; synchronized intermittent mandatory ventilation (SIMV), continuous positive airway pressure (CPAP) and T-Tube were enrolled in this study. The Mean ± SD of PaCO2, ETCO2 values and PaCO2 - ETCO2 gradients in all of the three groups were determined. The correlation between PaCO2 and ETCO2 in one of three modes of ventilation, SIMV, CPAP or T-tube, was done using linear regression. Paired t-test and a Kruskal-Wallis test were used to compare the gradients between PaCO2, and ETCO2 in each of the SIMV, CPAP and T-tube conditions. Data are presented as Mean ± SD. P < 0.05 was considered to indicate a significant difference.
4. Results
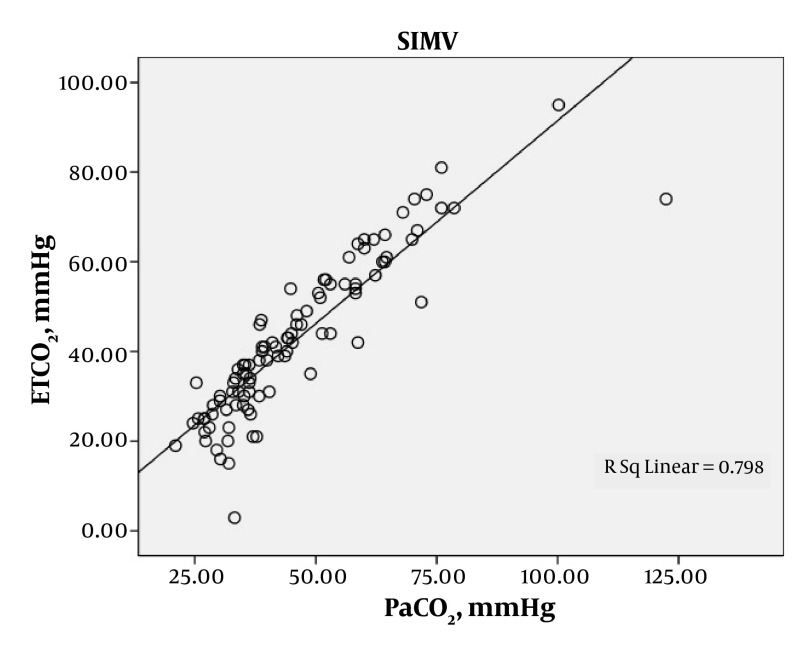
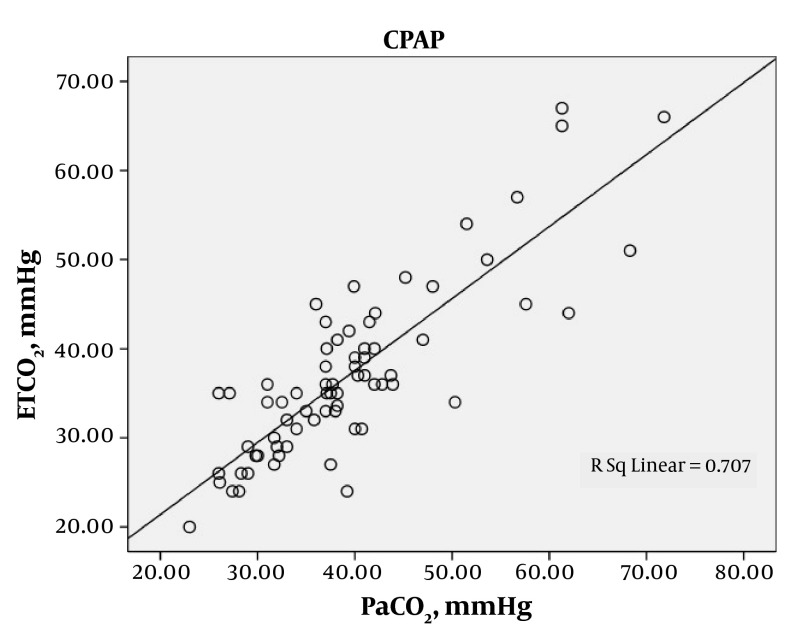
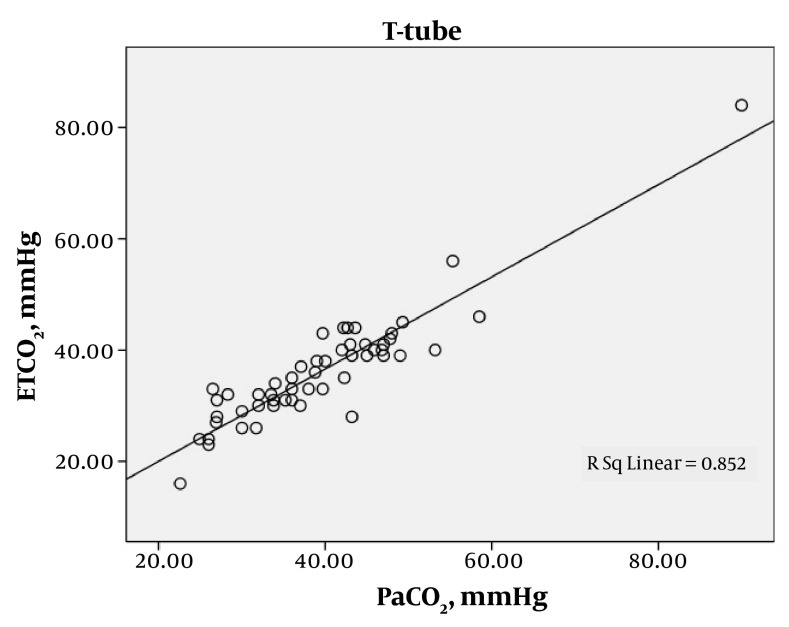
A total of 219 arterial blood gases were obtained from the 87 patients. The patients were ventilated with SIMV for 97 (44.3%) gas measurements, CPAP with support pressure for 70 (32%), and T-tube for 52 (23.7%). Statistical analysis demonstrated a good correlation between the mean of ETCO2 and PaCO2 in each of the modes of SIMV, CPAP and T-Tube; SIMV (42.5 ± 17.3 and 45.8 ± 17.1; r = 0.893, P < 0.0001), CPAP (37 ± 9.7 and 39.4 ± 10.1; r = 0.841, P < 0.0001) and T-Tube (36.1 ± 9.9 and 39.4 ± 11; r = 0.923, P < 0.0001), respectively (Table 1). In each of these modes the ETCO2 was generally lower than the PaCO2. The mean difference between the arterial to end-tidal carbon dioxide tension gradients were measured in each of the modes, SIMV, CPAP and T-Tube (Table 2). A positive correlation between PaCO2 and ETCO2 was found with each of the SIMV, CPAP and T-tube modes, indicating statistical significance (Figure 1, Figure 2 and Figure 3).
Table 1. Comparison of PaCO2 with ETCO2 in Patients with Various Modes of Ventilation a.
| PaCO2 a, Mean ± SD | ETCO2 a, Mean ± SD | R | R2 | P value | |
|---|---|---|---|---|---|
| Ventilation Setting | < 0.0001 | ||||
| SIMV a (n = 97) | 45.8 ± 17.1 | 42.5 ± 17.3 | 0.893 | 0.798 | |
| CPAP a (n = 70) | 39.4 ± 10.1 | 37 ± 9.7 | 0.841 | 0.707 | |
| T-tube a (n = 52) | 39.4 ± 11 | 36.1 ± 9.9 | 0.923 | 0.852 |
aAbbreviations: PaCO2, partial arterial carbon dioxide tension; ETCO2, end-tidal carbon dioxide; SIMV, synchronized mandatory mechanical ventilation; CPAP, continuous positive airway pressure
Table 2. Comparison of Mean Difference between PaCO2 and ETCO2 in Three Different Modes of Ventilation a.
| PaCO2 a - ETCO2 a, Mean ± SD | 95% CI | |
|---|---|---|
| SIMV a (n = 97) | 3.37 ± 7.93 | 1.77 - 4.97 |
| CPAP a (n = 70) | 2.32 ± 5.62 | 0.98 - 3.67 |
| T-tube a (n = 52) | 3.31 ± 4.26 | 2.13 - 4.50 |
aAbbreviations: PaCO2, partial arterial carbon dioxide tension; ETCO2, end-tidal carbon dioxide; SIMV, synchronized mandatory mechanical ventilation; CPAP, continuous positive airway pressure
Figure 1. Relationship between PaCO2 (mmHg) and ETCO2 (mmHg) in SIMV Mechanically Ventilated Patients, Showing a Significant Correlation (r = 0.893, P < 0.0001).
Figure 2. Relationship between PaCO2 (mmHg) and ETCO2 (mmHg) in CPAP Mechanically Ventilated Patients, Showing a Significant Correlation (r = 0.841, P < 0.0001).
Figure 3. Relationship between PaCO2 (mmHg) and ETCO2 (mmHg) in TTube Ventilated Patients, Showing a Significant Correlation (r = 0.923, P < 0.0001).
The relationship between PaCO2 (mmHg) and ETCO2 (mmHg) in CPAP mechanically ventilated patients is shown in Figure 2. There is a significant correlation (r = 0.841, P < 0.0001). The relationship between PaCO2 (mmHg) and ETCO2 (mmHg) in T-Tube ventilated patients is shown in Figure 3. There is a significant correlation (r = 0.923, P < 0.0001).
5. Discussion
Our results clearly show a strong correlation between arterial PaCO2 and ETCO2 in critically ill patients undergoing mechanical ventilation, and it provides a clinically reliable estimate of ventilation levels. Measurements of ETCO2 is an accepted standard of care for the monitoring of mechanically ventilated patients and this is often used during the ventilation of critically ill patients with respiratory failure (13). In healthy subjects there are close correlation between PaCO2 and ETCO2, and it is commonly accepted that PaCO2 measurements vary approximately 2-5 mmHg above ETCO2 values (14). Generally, PaCO2 is expected to exceed ETCO2 levels. Some studies have reported the correlation between ETCO2 and PaCO2 among ventilated patients and critical states (7, 13). Generally, ETCO2 measurements are affected by PaCO2 levels, dead space fraction, and pulmonary perfusion. ETCO2 is dependent on alveolar CO2 (PACO2) and the site of sampling. Non-uniform alveoli CO2 emptying patterns, in patients with large ventilation perfusion result in mismatching PACO2, and underestimation of PaCO2 levels (13). A high ventilation/perfusion ratio and dead space tends to cause low ETCO2 levels relative to PaCO2, whereas a low ventilation/perfusion ratio and shunt has little effect on causing a smaller ETCO2 measure relative to PaCO2. Among critically ill patients, increased intrapulmonary shunting from pulmonary parenchymal disease is relatively common. Yamanaka et al., reported that an admixture of this blood into the arterial circulation contributes to increased ETCO2 – PaCO2 gradients (11). This increase may be up to 20 mmHg in patients with severe pulmonary or major systemic disease. In other words, ETCO2 monitoring tends to underestimate PaCO2 levels. In a study that was conducted by Sivan et al., these discrepancies started to occur below a PaO2/PAO2 ratio of 0.3 (15). Although ETCO2 measurements have been identified as an invaluable tool to monitor airway patency and confirm endotracheal intubation, recent studies have suggested that it may also be used to guide ventilation as a surrogate measure of PaCO2 (16). Previous studies have shown conflicting results concerning the correlation between PaCO2 and ETCO2 in different clinical settings. McDonald et al., concluded that ETCO2 correlates with PaCO2 in critically ill patients undergoing conventional ventilation via an endotracheal tube and provides a clinically reliable estimate of ventilation (r2 = 0.716 and P < 0.001) (17). In their study ETCO2 (39.9 ± 12.7 mmHg) was lower than the PaCO2 (45.5 ± 14.1 mmHg). In the current study the values of ETCO2 and PaCO2 overall in the patients were 39.2 ± 13.9 mmHg versus 42.2 ± 14.1 mmHg. Kerr et al., reported that ETCO2 and PaCO2 correlated well (r2 = 0.77) in adults with traumatic brain injury, who did not have lung disease (defined as positive end-expiratory pressure of < 5 cm/H2O), however, they did not correlate when a lung injury was present (7). Good correlation was also observed in a study that included adults with and without lung disease (18). Barton et al., also reported that measurements of end-tidal carbon dioxide concentrations correlate well with PaCO2 values in non-intubated patients presenting with a variety of conditions to the emergency room (6). End-tidal carbon dioxide measurements may be sufficient measures of PaCO2 in selected patients and obviate the need for repeated arterial blood gas determination (6). Tobias and Meyer, concluded that measurement of transcutaneous CO2 was a more accurate predictor of PaCO2 than ETCO2 (19). The difference between transcutaneous CO2 and PaCO2 was less than the difference between ETCO2 and PaCO2, 2.3 ± 1.3 mmHg versus 6.8 ± 5.1 mmHg (19). Continuous monitoring of SpO2 (saturation of peripheral oxygen) and ETCO2 can be used to wean patients safely and effectively after coronary artery bypass grafting. ETCO2 was a good indicator of PaCO2 (r = 0.76), its sensitivity to detect hypercarbia (PaCO2 less than 45 mmHg) was 95% (20). In a study that was conducted on anesthetized, mechanically ventilated patients, transcutaneous monitoring of CO2 partial pressure gave a more accurate estimation of PaCO2 than ETCO2 monitoring (r2 = 0.73, and 0.50 respectively) (21). Weinger and Brimm measured arterial to end-tidal carbon dioxide gradient values of 4.24 ± 4.42 mmHg during intermittent mandatory ventilation (22). They reported good correlation between ETCO2 and PaCO2 in 25 adults with and without pulmonary disease (22). In patients without lung disease, ventilated either mechanically or spontaneously via a tracheal tube, the arterial to end-tidal carbon dioxide gradient values were 0.8-3.5 mmHg (23, 24). In the present study the difference between PaCO2 and ETCO2 in each of the SIMV, CPAP, and T-tube modes of the ventilator were; 3.37 ± 7.93 mmHg, 2.32 ± 5.62 mmHg, and 3.31 ± 4.26 mmHg, respectively. The objective of our study was to show that monitoring of ETCO2 provides a clinically useful and effective method for assessing ventilation. Several studies have indicated a poor correlation of ETCO2 with PaCO2 in an emergency setting (9, 25, 26). Russell and Graybeal, reported that in mechanically ventilated neurointensive care patients, there is significant variability in the relationship between PaCO2 and ETCO2 (27). The ETCO2 – PaCO2 gradients were reported as 6.9 ± 4.4 mmHg. In another study that was conducted by Russsell et al., during intraoperative craniotomies, this value was reported to be 7.2 ± 3.3 mmHg (28). They concluded that ETCO2 may not provide a statistically stable estimation of PaCO2 in mechanically ventilated neurosurgical patients undergoing craniotomies (28). Patients with respiratory failure and multisystem trauma have a much wider difference of 14 ± 11 mmHg (12). In a recent report, Warner et al., evaluated 180 intubated patients with isolated traumatic brain injury and demonstrated a poor correlation between ETCO2 and PaCO2 (r2 = 0.277) (29). In another study, in spite of a good correlation between ETCO2 and PaCO2 (r = 0.78, P < 0.001) in 20 intubated patients with respiratory failure, the changes in delta ETCO2 did not correlate as well with delta PaCO2 (r = 0.58, P ≤ 0.001) (30). The results of another study indicated that hypercapnia may be underestimated when ETCO2 is substituted for PaCO2 in patients breathing spontaneously via a cuffed oropharyngeal airway (31). Belpomme et al., concluded that in a pre-hospital setting, PaCO2 cannot be estimated by ETCO2 levels (9). Moreover, there is a wide variation in the gradient between PaCO2 and ETCO2 depending on the patient’s condition, and over time, this relationship does not remain constant and thus it is not useful in pre-hospital ventilation management (9). In a study that was conducted on hyperventilated neurosurgical patients, the values of ETCO2 showed a moderately acceptable correlation with PaCO2 measurements. However, changes in end-tidal carbon dioxide values failed to correlate with simultaneous changes in arterial carbon dioxide tension measures (32). Palmon et al., compared two groups of patients as no-monitor and monitor-blind groups that were under controlled with a capnograph during transport (10). The results of their study do not support routine monitoring of end-tidal CO2 during short transport times in adult patients requiring mechanical ventilation. However, the monitor may prevent morbidity in patients requiring tight control of PaCO2 (10). Kavanagh et al., found poor correlation between end-tidal and arterial PaCO2, as well as poor correlation between transcutaneous and arterial PaCO2 in extubated, spontaneously breathing patients recovering from general anesthesia (33). In conclusion, end-tidal CO2 measurement provides an accurate estimation of PaCO2 in mechanically ventilated patients. ETCO2 monitoring in adult ventilated patients may be a useful tool in their management. Its use may limit the need for invasive monitoring and/or repeated arterial blood gas analyses. The results of this study shows that ETCO2 monitoring accurately reflects PaCO2 during mechanical ventilation. A comparison of mean differences between PaCO2 and ETCO2 in three different modes of ventilation did not show any statistical significance. Additional studies in relation to the efficiency of CO2 monitoring during various phases of mechanical ventilation are recommended.
Acknowledgments
The authors wish to acknowledge Deputy of Research of Kashan University of Medical Sciences for its financial support in this study as a part of a MD thesis (grant No:8751).
Footnotes
Implication for health policy/practice/research/medical education: Substitution of ETCO2 monitoring as a noninvasive assessment of mechanical ventilation instead of PaCO2 measurement which is an invasive assessment of mechanical ventilation.
Please cite this paper as: Razi E, Moosavi GA, Omidi K, Khakpour Saebi A, Razi A. Correlation of End-Tidal Carbon Dioxide with Arterial Carbon Dioxide in Mechanically Ventilated Patients. Arch Trauma Res. 2012; 1(2):58-62. DOI: 10.5812/atr.6444
Authors’ Contribution: Ebrahim Razi (Study design and method), Gholam Abbass Moosavi (statistical analysis), Keivan Omidi (Data collection), Ashkan Khakpour Saebi (Data collection) and Armin Razi (Draft writing).
Financial Disclosure: None declared.
Funding/Support: None declared.
References
- 1.Bhende MS, LaCovey DC. End-tidal carbon dioxide monitoring in the prehospital setting. Prehosp Emerg Care. 2001;5(2):208–13. doi: 10.1080/10903120190940146. [DOI] [PubMed] [Google Scholar]
- 2.Helm M, Fischer S. The role of capnography in prehospital ventilation for trauma patients. Int J Int Care. 2005;12:124–30. [Google Scholar]
- 3.Sanders AB. Capnometry in emergency medicine. Ann Emerg Med. 1989;18(12):1287–90. doi: 10.1016/S0196-0644(89)80260-4. [DOI] [PubMed] [Google Scholar]
- 4.Santos LJ, Varon J, Pic-Aluas L, Combs AH. Practical uses of end-tidal carbon dioxide monitoring in the emergency department. J Emerg Med. 1994;12(5):633–44. doi: 10.1016/0736-4679(94)90416-2. [DOI] [PubMed] [Google Scholar]
- 5.Flanagan JF, Garrett JS, McDuffee A, Tobias JD. Noninvasive monitoring of end-tidal carbon dioxide tension via nasal cannulas in spontaneously breathing children with profound hypocarbia. Crit Care Med. 1995;23(6):1140–2. doi: 10.1097/00003246-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Barton CW, Wang ES. Correlation of end-tidal CO2 measurements to arterial PaCO2 in nonintubated patients. Ann Emerg Med. 1994;23(3):560–3. doi: 10.1016/S0196-0644(94)70078-8. [DOI] [PubMed] [Google Scholar]
- 7.Kerr ME, Zempsky J, Sereika S, Orndoff P, Rudy EB. Relationship between arterial carbon dioxide and end-tidal carbon dioxide in mechanically ventilated adults with severe head trauma. Crit Care Med. 1996;24(5):785–90. doi: 10.1097/00003246-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Plewa MC, Sikora S, Engoren M, Tome D, Thomas J, Deuster A. Evaluation of capnography in nonintubated emergency department patients with respiratory distress. Acad Emerg Med. 1995;2(10):901–8. doi: 10.1111/j.1553-2712.1995.tb03106.x. [DOI] [PubMed] [Google Scholar]
- 9.Belpomme V, Ricard-Hibon A, Devoir C, Dileseigres S, Devaud ML, Chollet C, et al. Correlation of arterial PaCO2 and ETCO2 in prehospital controlled ventilation. Am J Emerg Med. 2005;23(7):852–9. doi: 10.1016/j.ajem.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Palmon SC, Liu M, Moore LE, Kirsch JR. Capnography facilitates tight control of ventilation during transport. Crit Care Med. 1996;24(4):608–11. doi: 10.1097/00003246-199604000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Yamanaka MK, Sue DY. Comparison of arterial-end-tidal PaCO2 difference and dead space/tidal volume ratio in respiratory failure. Chest. 1987;92(5):832–5. doi: 10.1378/chest.92.5.832. [DOI] [PubMed] [Google Scholar]
- 12.Russell GB, Graybeal JM. Reliability of the arterial to end-tidal carbon dioxide gradient in mechanically ventilated patients with multisystem trauma. J Trauma. 1994;36(3):317–22. doi: 10.1097/00005373-199403000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Bhavani-Shankar K, Moseley H, Kumar AY, Delph Y. Capnometry and anaesthesia. Can J Anaesth. 1992;39(6):617–32. doi: 10.1007/BF03008330. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher R, Jonson B. Deadspace and the single breath test for carbon dioxide during anaesthesia and artificial ventilation. Effects of tidal volume and frequency of respiration. Br J Anaesth. 1984;56(2):109–19. doi: 10.1093/bja/56.2.109. [DOI] [PubMed] [Google Scholar]
- 15.Sivan Y, Eldadah MK, Cheah TE, Newth CJ. Estimation of arterial carbon dioxide by end-tidal and transcutaneous PaCO2 measurements in ventilated children. Pediatr Pulmonol. 1992;12(3):153–7. doi: 10.1002/ppul.1950120305. [DOI] [PubMed] [Google Scholar]
- 16.Brain Trauma Foundation. Guidelines for Prehospital Management of Traumatic Brain Injury. Brain Trauma Foundation; 2000. [Google Scholar]
- 17.McDonald MJ, Montgomery VL, Cerrito PB, Parrish CJ, Boland KA, Sullivan JE. Comparison of end-tidal CO2 and Paco2 in children receiving mechanical ventilation. Pediatr Crit Care Med. 2002;3(3):244–9. doi: 10.1097/00130478-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Morley TF, Giaimo J, Maroszan E, Bermingham J, Gordon R, Griesback R, et al. Use of capnography for assessment of the adequacy of alveolar ventilation during weaning from mechanical ventilation. Am Rev Respir Dis. 1993;148(2):339–44. doi: 10.1164/ajrccm/148.2.339. [DOI] [PubMed] [Google Scholar]
- 19.Tobias JD, Meyer DJ. Noninvasive monitoring of carbon dioxide during respiratory failure in toddlers and infants: end-tidal versus transcutaneous carbon dioxide. Anesth Analg. 1997;85(1):55–8. doi: 10.1097/00000539-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Thrush DN, Mentis SW, Downs JB. Weaning with end-tidal CO2 and pulse oximetry. J Clin Anesth. 1991;3(6):456–60. doi: 10.1016/0952-8180(91)90093-3. [DOI] [PubMed] [Google Scholar]
- 21.Casati A, Squicciarini G, Malagutti G, Baciarello M, Putzu M, Fanelli A. Transcutaneous monitoring of partial pressure of carbon dioxide in the elderly patient: a prospective, clinical comparison with end-tidal monitoring. J Clin Anesth. 2006;18(6):436–40. doi: 10.1016/j.jclinane.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Weinger MB, Brimm JE. End-tidal carbon dioxide as a measure of arterial carbon dioxide during intermittent mandatory ventilation. J Clin Monit. 1987;3(2):73–9. doi: 10.1007/BF00858353. [DOI] [PubMed] [Google Scholar]
- 23.Takki S, Aromaa U, Kauste A. The validity and usefulness of the end-tidal PaCO 2 during anaesthesia. Ann Clin Res. 1972;4(5):278–84. [PubMed] [Google Scholar]
- 24.Whitesell R, Asiddao C, Gollman D, Jablonski J. Relationship between arterial and peak expired carbon dioxide pressure during anesthesia and factors influencing the difference. Anesth Analg. 1981;60(7):508–12. doi: 10.1213/00000539-198107000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Prause G, Hetz H, Lauda P, Pojer H, Smolle-Juettner F, Smolle J. A comparison of the end-tidal-CO2 documented by capnometry and the arterial PaCO2 in emergency patients. Resuscitation. 1997;35(2):145–8. doi: 10.1016/S0300-9572(97)00043-9. [DOI] [PubMed] [Google Scholar]
- 26.Yosefy C, Hay E, Nasri Y, Magen E, Reisin L. End tidal carbon dioxide as a predictor of the arterial PaCO2 in the emergency department setting. Emerg Med J. 2004;21(5):557–9. doi: 10.1136/emj.2003.005819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell GB, Graybeal JM. End-tidal carbon dioxide as an indicator of arterial carbon dioxide in neurointensive care patients. J Neurosurg Anesthesiol. 1992;4(4):245–9. doi: 10.1097/00008506-199210000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Russell GB, Graybeal JM. The arterial to end-tidal carbon dioxide difference in neurosurgical patients during craniotomy. Anesth Analg. 1995;81(4):806–10. doi: 10.1213/00000539-199510000-00025. [DOI] [PubMed] [Google Scholar]
- 29.Warner KJ, Cuschieri J, Garland B, Carlbom D, Baker D, Copass MK, et al. The utility of early end-tidal capnography in monitoring ventilation status after severe injury. J Trauma. 2009;66(1):26–31. doi: 10.1097/TA.0b013e3181957a25. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman RA, Krieger BP, Kramer MR, Segel S, Bizousky F, Gazeroglu H, et al. End-tidal carbon dioxide in critically ill patients during changes in mechanical ventilation. Am Rev Respir Dis. 1989;140(5):1265–8. doi: 10.1164/ajrccm/140.5.1265. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi Y, Seki S, Ichimiya T, Iwasaki H, Namiki A. Cuffed oropharyngeal airway and capnometry: comparison of end-tidal and arterial carbon dioxide pressures. J Anesth. 1999;13(3):136–9. doi: 10.1007/s005400050044. [DOI] [PubMed] [Google Scholar]
- 32.Christensen MA, Bloom J, Sutton KR. Comparing arterial and end-tidal carbon dioxide values in hyperventilated neurosurgical patients. Am J Crit Care. 1995;4(2):116–21. [PubMed] [Google Scholar]
- 33.Kavanagh BP, Sandler AN, Turner KE, Wick V, Lawson S. Use of end-tidal PaCO2 and transcutaneous PaCO2 as noninvasive measurement of arterial PaCO2 in extubated patients recovering from general anesthesia. J Clin Monit. 1992;8(3):226–30. doi: 10.1007/BF01616780. [DOI] [PubMed] [Google Scholar]