Abstract
Insertional mutagenesis was applied to Cryptococcus neoformans to identify genes associated with virulence attributes. Using biolistic transformation, we generated 4,300 nourseothricin (NAT)-resistant strains, of which 590 exhibited stable resistance. We focused on mutants with defects in established virulence factors and identified two with reduced growth at 37°C, four with reduced production of the antioxidant pigment melanin, and two with an increased sensitivity to nitric oxide (NO). The NAT insertion and mutant phenotypes were genetically linked in five of eight mutants, and the DNA flanking the insertions was characterized. For the strains with altered growth at 37°C and altered melanin production, mutations were in previously uncharacterized genes, while the two NO-sensitive strains bore insertions in the flavohemoglobin gene FHB1, whose product counters NO stress. Because of the frequent instability of nourseothricin resistance associated with biolistic transformation, Agrobacterium-mediated transformation was tested. This transkingdom DNA delivery approach produced 100% stable nourseothricin-resistant transformants, and three melanin-defective strains were identified from 576 transformants, of which 2 were linked to NAT in segregation analysis. One of these mutants contained a T-DNA insertion in the promoter of the LAC1 (laccase) gene, which encodes a key enzyme required for melanin production, while the second contained an insertion in the promoter of the CLC1 gene, encoding a voltage-gated chloride channel. Clc1 and its homologs are required for ion homeostasis, and in their absence Cu+ transport into the secretory pathway is compromised, depriving laccase and other Cu+-dependent proteins of their essential cofactor. The NAT resistance cassette was optimized for cryptococcal codon usage and GC content and was then used to disrupt a mitogen-activated protein kinase gene, a predicted gene, and two putative chloride channel genes to analyze their contributions to fungal physiology. Our findings demonstrate that both insertional mutagenesis methods can be applied to gene identification, but Agrobacterium-mediated transformation is more efficient and generates exclusively stable insertion mutations.
Cryptococcus neoformans is a basidiomycetous fungus that infects animals and humans worldwide. Three varieties of the fungus have been identified, of which C. neoformans var. grubii (serotype A) is the one most frequently isolated from patients, accounting for 95% of clinically derived strains (7, 24). Nevertheless, genetic research on C. neoformans has focused on C. neoformans var. neoformans (serotype D) isolates because of their established mating system with congenic strains of the opposite mating type (23, 29). The recent discovery of serotype A MATa strains (30, 48), the establishment of their sexual cycle, and the isolation of congenic mating partners now enable comparable genetic studies with the predominant clinical form of this pathogen (26, 37).
C. neoformans is an excellent model system for the study of the virulence of human pathogenic fungi. This fungus grows as a haploid yeast and can be readily transformed, facilitating gene disruption by homologous recombination (7). Auxotrophic mutants, including ade2 and ura5, are available as transformation recipients, and dominant drug resistance markers conferring resistance to hygromycin, nourseothricin, or G418 are also available. The congenic serotype A mating partners (KN99a and KN99α) enable genetic analyses of mutations and studies of genetic linkage (33, 37). Virulence models have been established in mice, rats, rabbits, and in vitro cell cultures of alveolar macrophages, and more recently, in heterologous environmental hosts, including amoebae, nematodes, and slime mold (34, 43, 44). Finally, the genomes of two serotypes (A and D) are nearing completion and the genome of a serotype B strain has been sequenced to 6× coverage.
Several factors have been identified that are required for C. neoformans virulence (reviewed in references 7, 42). Further understanding of the genes necessary for virulence will provide a foundation for better strategies to manage cryptococcosis. The fungus must be able to (i) synthesize amino acids and nucleotides found in limiting amounts in the host, (ii) produce the antioxidant pigment melanin and an antiphagocytic polysaccharide capsule, (iii) grow within the host at 37°C, and (iv) avoid host defense systems such as the generation of reactive oxygen and nitrogen species. Genes involved in these abilities have been identified through the use of both forward and reverse genetic approaches. Forward genetic approaches with serotype A include two insertional mutagenesis screens. Erickson et al. (16) screened 1,000 mutants and identified the VPH1 gene, encoding a subunit of the vacuolar H+-ATPase, which is involved in melanin production and virulence. Nelson et al. (36) used insertional mutagenesis with plasmids containing signature tags to screen 672 mutants in mice, identifying 39 isolates with altered virulence. In about half of the strains that were characterized further, the plasmid had inserted into the actin gene, and gene identification in the other mutant strains is ongoing.
Insertional mutagenesis is a powerful tool for identifying new genes and their functions. Studies with other human pathogenic fungi have revealed the Epa1 adhesin of Candida glabrata (8), PABA synthetase as a virulence factor in Aspergillus fumigatus (3), and 146 genes regulating filamentation in Candida albicans (46). Insertional mutagenesis has also been applied to plant pathogenic fungi to identify more than 20 genes that are necessary for fungal virulence on susceptible host plant species (25).
One tool for insertional mutagenesis in fungi that so far has been underutilized is the transfer of T-DNA from Agrobacterium tumefaciens into the fungal target cell. A. tumefaciens is a soilborne bacterium that in nature causes crown gall on plants by introducing bacterial genes encoding proteins that produce metabolites mimicking plant hormones into the host plant genome. This ability to transfer DNA has been manipulated in the laboratory to allow the bacterium to transfer a variety of DNA molecules into plant cells, and more recently, into other organisms, including animal cells, oomycetes, and myriad fungal species (4, 9, 28, 47). This method has the potential to circumvent many time-consuming steps of fungal transformation and may also reduce the generation of extraneous mutations.
The aims of this study were to apply insertional mutagenesis to the clinically derived serotype A strain H99, use the recently developed congenic MATa strains for genetic analysis, and identify new genes involved in regulating known virulence attributes. We also developed Agrobacterium-mediated integration and tested its applicability as an insertional mutagen in C. neoformans compared to current biolistic methods of DNA delivery. Our findings resolved several technological complications for conducting insertional mutagenesis and now provide a foundation from which to subject the entire genome to saturating insertional mutagenesis screens to determine the molecular determinants of development and virulence.
MATERIALS AND METHODS
Strains.
C. neoformans serotype A strains H99, KN99-5, KN99a, and KN99α and serotype D strains JEC20 and JEC21 were used (23, 29, 37, 39). Strains generated by insertional mutagenesis with altered phenotypes are listed in Table 1. The fhb1 strain was generated with a targeted mutation in the flavohemoglobin gene (14). Escherichia coli strain DH5α was used to rescue ligated plasmids. A. tumefaciens strain LBA4404 (Invitrogen) was employed for the transformation of C. neoformans. The Saccharomyces cerevisiae BY4743 reference strain and gef1 mutant diploid strain were obtained from the S. cerevisiae gene deletion set, and the gef1 disruption was confirmed by PCR (20).
TABLE 1.
C. neoformans strains created by insertional mutagenesis
| Strain | Phenotypea | Phenotype linked to nourseothicin resistance | Gene(s) flanking insertion | Method of transformation | GenBank accession no. |
|---|---|---|---|---|---|
| 1F2 | Melbrown | Yes | MPK2, predicted gene | Biolistic | CG865111 |
| 4F2 | Melleaky | No | Biolistic | ||
| 5D12 | ts | Yes | WD40, MCM2 | Biolistic | CG865112, CG865113 |
| 5E2 | Melbrown | Yes | Predicted gene | Biolistic | CG865116 |
| 3C12 | NOhs | Yes | FHB1 | Biolistic | CG865114, CG865115 |
| 3E7 | NOhs | Yes | FHB1 | Biolistic | |
| 7E3 | Mel− | No | Biolistic | ||
| 7E12 | ts, sterile | NA | Biolistic | ||
| At-mel1 | Mel− | Yes | CLC1 | Agrobacterium mediated | CG865117, CG865118 |
| At-mel2 | Mel− | Yes | LAC1 | Agrobacterium mediated | CG865119, CG865120 |
| At-mel3 | Mel− | No | Agrobacterium mediated |
ts, reduction in growth at 37°C; Mel, altered melanin production; NOhs, hypersensitivity to nitric oxide.
Insertional mutagenesis.
For biolistic transformation, plasmid pCH233, which contains the nourseothricin acetyltransferase (NAT) gene controlled by the H99 actin (ACT1) promoter and the phosphoribosyl anthranilate isomerase (TRP1) terminator (32; C. M. Hull and J. Heitman, unpublished data), was transformed into strain H99. The transformed plasmid was either circular or was linearized with the BamHI or XhoI restriction enzyme. Biolistic transformations were performed as described by Toffaletti et al. (45), using a Bio-Rad model PDS-1000/He biolistic particle delivery system. Transformed cells were initially grown on YPD medium including 1 M sorbitol as an osmotic stabilizer and then were transferred to YPD medium containing nourseothricin (100 μg/ml) 4 to 6 h after particle delivery.
For Agrobacterium-mediated transformation, a plasmid was constructed by excising the NAT cassette from pCH233 with HindIII and XbaI and ligating it into the polylinker region of plasmid pPZP-201BK (9) cleaved with HindIII and XbaI. The plasmid was electroporated into A. tumefaciens LBA4404 electrocompetent cells and selected on Luria-Bertani medium containing kanamycin. Agrobacterium cells were grown for 48 h at 25°C in Luria-Bertani medium with kanamycin in shaking cultures, transferred to induction medium (4) with 100 μM acetosyringone at an optical density at 600 nm of 0.15, and incubated a further 6 h. C. neoformans H99 cells grown overnight in YPD were washed in induction medium and resuspended at 104, 105, 106, 107, or 108 cells per ml. Equal aliquots (200 μl) of C. neoformans and A. tumefaciens were mixed and plated onto induction medium agar. The organisms were cocultured for 48 h, scraped from the plates, and transferred to YPD medium with nourseothricin and cefotaxime (each at 100 μg/ml).
Phenotypic analysis of insertional mutants.
The stability of transformed strains was examined by growing strains in YPD medium without nourseothricin for 4 to 7 days and replica plating them by use of a 48-prong transfer device (Dan-Kar Corporation, Woburn, Mass.) to YPD with and without nourseothricin. Strains that did not exhibit equal growth were discarded. Strains generated by biolistic transformation were examined for alterations in several phenotypes associated with virulence. Strains were replica plated by use of a 48-prong transfer device to a rich medium (YPD), a minimal medium (YNB), and a melanin-inducing medium containing l-dihydroxyphenylalanine (l-DOPA; 100 mg/liter), and one set of YPD plates was incubated at 37°C. Capsule production was assessed under a microscope to examine the exclusion of India ink from yeast cells after 4 days of growth in a medium with a low iron concentration containing the iron chelation agent EDDHA (ethylene diamine di-o-phenylacetic acid; 20 mg/liter). Resistance to nitric oxide was examined by replica plating strains into 96-well plates and comparing their growth after overnight culturing in the present or absence of DETA-NONOate {(Z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate, 1 mM; Cayman Chemical Company} by measuring the optical densities at 600 nm with a plate reader. Strains generated by Agrobacterium-mediated transformation were replica plated with velvet squares to l-DOPA plates for the examination of melanin production.
For assessment of the linkage between nourseothricin resistance and the altered phenotype, strains were crossed to KN99-5, a serotype A MATa strain from the 5th backcross during the construction of the congenic serotype A strains, or KN99a, the product of the 10th backcross (37). Individual basidiospores were isolated with a micromanipulator, and progeny were scored for a mutant phenotype and nourseothricin resistance. The mating types of these progeny were determined by crossing to serotype D tester strains JEC20 (MATa) and JEC21 (MATα) as independent genetic markers.
Reconstruction of nourseothricin acetyltransferase and targeted disruption of genes.
For an improvement of the efficiency of PCR from the GC-rich template of the nourseothricin acetyltransferase gene, the gene was reconstructed by use of eight oligomers (Table 2) to reduce the GC content and then was optimized for cryptococcal codon usage (35; http://www.kazusa.or.jp/codon/). Primers were paired and filled in with ThermalAce DNA polymerase (Invitrogen) and were amplified with primers JOHE8758 and JOHE8759. The nourseothricin acetyltransferase coding region was placed between the ACT1 promoter and the TRP1 terminator derived from strain JEC21 or H99 by overlap PCR and subcloning of DNA fragments to produce plasmids pAI3 and pAI4.
TABLE 2.
Oligonucleotides used for this study
| Primer name or use | Sequence (5′-3′) |
|---|---|
| Nourseothricin acetyltransferase (NAT) reconstruction | |
| JOHE8758 | TTATGGACAAGGCATACTCATATAAAG |
| JOHE8759 | ATGGCGGCCGCCACTCTTGAC |
| JOHE8760 | ATGGCGGCCGCCACTCTTGACGATACGGCTTACCGTTACAGAACCAGTGTCCCAGGTGATGCTGAAGCCATCGAAGCACTTGATGGTAG |
| JOHE8761 | CATCGAAGCACTTGATGGTAGTTTCACAACCGATACTGTCTTCCGAGTCACAGCCACCGGTGATGGATTCACTTTGCGAGAAGTCCCTGTTG |
| JOHE8762 | TTTGCGAGAAGTCCCTGTTGATCCACCTCTTACTAAAGTATTTCCTGATGATGAATCAGATGATGAATCAGATGCAGGTGAAGACGGCG |
| JOHE8763 | GATGCAGGTGAAGACGGCGATCCAGACTCCAGAACATTCGTCGCTTACGGTGACGATGGAGACCTTGCAGGATTCGTTGTCGTCTCTTAG |
| JOHE8764 | GGATTCGTTGTCGTCTCTTACTCCGGCTGGAACAGACGTCTTACCGTCGAAGATATCGAAGTCGCCCCAGAACACCGTGGTCACGGTGTCG |
| JOHE8765 | CACCGTGGTCACGGTGTCGGTAGAGCTTTGATGGGTCTTGCTACAGAATTTGCACGTGAAAGAGGTGCAGGTCACCTCTGGTTGGAAGT |
| JOHE8766 | GGTCACCTCTGGTTGGAAGTCACTAACGTTAACGCACCTGCTATCCATGCGTACCGACGTATGGGTTTCACCCTTTGTGGACTGGATACC |
| JOHE8767 | CCCTTTGTGGACTGGATACCGCACTGTACGACGGAACCGCATCTGACGGAGAGCAAGCTCTTTATATGAGTAATGCCTTGTCCATAA |
| Voltage-gated chloride channel 1 (CLC1) disruption | |
| 1 JOHE8986 | ACTCTTACGCTTCAACTTCGG |
| 2 JOHE8988 | CAACGGTGACGCTGTGAGGCTTGGTGTTCGCCGTGG |
| 3 JOHE8987 | CCACGGCGAACACCAAGCCTCACAGCGTCACCGTTG |
| 4 JOHE8990 | CATTCTGTATCCCATCCAGAAGAGATGTAGAAACGAG |
| 5 JOHE8989 | CTCGTTTCTACATCTCTTCTGGATGGGATACAGAATG |
| 6 JOHE8992 | TTGTCAAGAAGGTCGAGCAC |
| Voltage-gated chloride channel 2 (CLC2) disruption | |
| 1 JOHE9227 | GACGATCGGGATCGGAAAC |
| 2 JOHE9229 | CAGCTCACATCCTCGCAGCCGTATAGTGGAAAGGGAG |
| 3 JOHE9228 | CTCCCTTTCCACTATACGGCTGCGAGGATGTGAGCTG |
| 4 JOHE9230 | CTTCAATGCCCTGTGCCGAAGAGATGTAGAAACTAG |
| 5 JOHE9231 | CTAGTTTCTACATCTCTTCGGCACAGGGCATTGAAG |
| 6 JOHE9232 | ATCCGAACCTCCAGAACC |
| MAP kinase 2 (MPK2) disruption | |
| 1 JOHE9584 | ACAACTCTGACCGAGAAC |
| 2 JOHE9586 | CAGCTCACATCCTCGCAGCTTCCGTAGAGTGGGACAAG |
| 3 JOHE9585 | CTTGTCCCACTCTACGGAAGCTGCGAGGATGTGAGCTG |
| 4 JOHE9588 | GCCAAATACCGCTTGATCAAGAGATGTAGAAACTAG |
| 5 JOHE9587 | CTAGTTTCTACATCTCTTGATCAAGCGGTATTTGGC |
| 6 JOHE9589 | GACTAGCGTATGAGCAAG |
| MAP kinase 2 (MPK2) plus upstream predicted gene disruption | |
| 1 JOHE9929 | GGCGAGATAAACTGGAAC |
| 2 JOHE9931 | CAGCTCACATCCTCGCAGGATTTCACAGCGTCTTCG |
| 3 JOHE9930 | CGAAGACGCTGTGAAATCCTGCGAGGATGTGAGCTG |
| 4 JOHE9588 | GCCAAATACCGCTTGATCAAGAGATGTAGAAACTAG |
| 5 JOHE9587 | CTAGTTTCTACATCTCTTGATCAAGCGGTATTTGGC |
| 6 JOHE9589 | GACTAGCGTATGAGCAAG |
| Predicted gene upstream of MPK2 disruption | |
| 1 JOHE9929 | GGCGAGATAAACTGGAAC |
| 2 JOHE10952 | CAGCTCACCTCCCGCAGATTCCTTCTCCTTTCAAC |
| 3 JOHE8672 | AGGCTGCGGGAGGTGAGC |
| 4 JOHE8677 | GAAGAGATGTAGAAACGAG |
| 5 JOHE10943 | CTCGTTTCTACATCTCTTCGCTTTGGATGAGAAGGAG |
| 6 JOHE9404 | CCTTGTCAAACACTCTGG |
| Inverse PCR from T-DNA | |
| JOHE8956 | AACAGTTGCGCAGCCTGAATG |
| JOHE8957 | AGAGGCGGTTTGCGTATTGG |
Overlap PCR was used as described previously (11, 17) to prepare gene disruption alleles that were transformed into H99 or KN99α cells by biolistic transformation as described above. PCR amplifications were performed on a Biometra T3 Thermocycler with Extaq polymerase (Takara). Primers were used in a combination of primers 1 and 2 or primers 5 and 6 with H99 DNA to provide approximately 1.5 kb of DNA for homologous targeting or in a combination of primers 3 and 4 with a plasmid with the modified NAT (Table 2) to provide the selectable marker. The three PCR products were mixed in equimolar amounts and amplified with primers 1 and 6 to produce a disruption allele with the NAT marker flanked by homologous DNA as a single contiguous DNA fragment.
Nucleic acid manipulations.
Plasmid or T-DNA insertions or gene disruptions were confirmed by Southern blot analysis. Genomic DNA was extracted as described previously (40) and was digested with restriction enzymes, separated in agarose gels, and blotted to nitrocellulose (Zeta-Probe; Bio-Rad) by standard methods. Total RNA was isolated by use of TRIzol reagent according to the manufacturer's instructions (Gibco BRL), separated on denaturing agarose gels, and blotted. Probes were generated with a Prime-It II kit (Amersham) incorporating [α-32P]dCTP. For the rescue of regions flanking the inserted DNA, genomic DNA (2.5 μg) was digested with a restriction enzyme and purified through a Qiagen column, and then 8.5 of the 30 μl eluted was ligated to itself with T4 DNA ligase (New England BioLabs). Ligations were desalted and used for inverse PCRs with various primers (Table 2; more details will be provided upon request) or were electroporated into E. coli DH5α cells.
The genomic regions obtained by inverse PCR or plasmid rescue were sequenced and compared to the H99 genome database (Duke University; http://cneo.genetics.duke.edu) to determine the junction of plasmid insertion into the fungal genomic DNA. FGENESH software (SoftBerry) was used to search for coding regions within the sequence, and BLAST searches were conducted against the GenBank database to infer gene functions.
Nucleotide sequence accession numbers.
The sequences of the flanking regions were deposited in GenBank under accession numbers CG865111 to CG865120. The DNA sequence of the new NAT gene was deposited in GenBank under accession no. AY483215.
RESULTS AND DISCUSSION
Insertional mutagenesis by biolistic transformation.
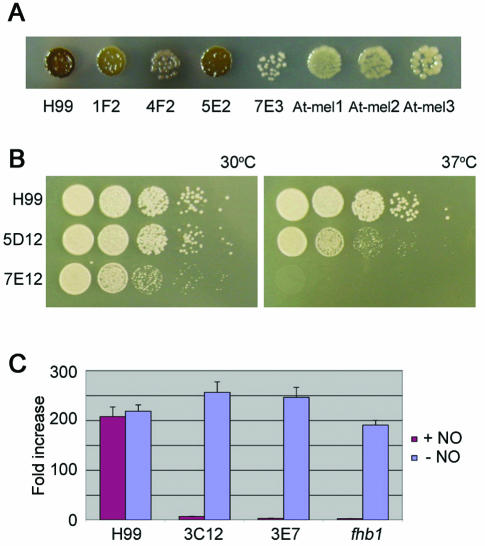
Approximately 4,300 nourseothricin-resistant transformants were obtained after biolistic transformation of a linear or circular plasmid into C. neoformans strain H99. Approximately 14% (590) were found to be stable transformants in which the NAT marker was integrated into the genome to confer stable nourseothricin resistance. Southern blot analysis of a subset of transformants confirmed that the plasmid had integrated into the genome (data not shown). An analysis of the uncut genomic DNA of an unstable transformant probed with the plasmid revealed a fast-migrating DNA fragment, indicating an episomal plasmid, consistent with the known spontaneous addition of telomeric sequences to linear transforming DNA (15). The stable transformants were examined for changes in phenotypes associated with virulence. Four strains with reduced melanin production, two with reduced growth at 37°C, and two with increased sensitivity to nitric oxide were identified (Fig. 1). No strains that lacked a capsule or were unable to grow on minimal medium were found. The four melanin mutants (mel) showed a range in defect, from white to dark brown, and one mutant appeared to leak laccase or melanin into the medium to give rise to pigmented halos surrounding the colony. The two mutants with reduced growth at 37°C differed, since isolate 5D12 showed reduced growth whereas isolate 7E12 failed to grow at 37°C. The two NO-hypersensitive mutants were virtually identical in their response to 1 mM DETA-NONOate.
FIG. 1.
Phenotypes of C. neoformans insertional mutants. (A) Four strains generated by biolistic transformation and three generated by Agrobacterium-mediated transformation with altered production of melanin compared to the wild type (H99) were grown on l-DOPA medium for 4 days at room temperature and were then photographed. (B) Strains generated by biolistic transformation with altered sensitivities to 37°C. Serial dilutions of strains were grown for 2 days at 30 or 37°C on YPD medium. (C) Two strains generated by biolistic transformation with altered sensitivities to nitric oxide compared to a mutant in the FHB1 gene and to strain H99. Strains were grown in 0 or 1 mM DETA-NONOate (NO) overnight in YPD liquid medium. Optical densities were measured at 600 nm with a plate reader, and fold increases in growth were plotted.
A disadvantage of working with serotype A in the past was the inability to use genetic analyses due to the absence of a MATa (30, 48) mating partner. The discovery of serotype A MATa strains and the development of congenic strains now allow classical Mendelian genetic approaches to be applied to serotype A strains (26, 37). Genetic linkage between the observed mutant phenotypes and the inserted NAT marker was established by genetic crosses. Mutant strains were crossed with congenic serotype A MATa strains (KN99-5 or KN99a), and 16 to 32 (average, 22.5) progeny from each cross were examined for the segregation of nourseothricin resistance and the mutant trait. Cosegregation was observed for two of the four melanin-deficient mutants, one of the two mutants with impaired growth at 37°C, and both strains that were hypersensitive to nitric oxide (five of eight total, or 67.5% linked). The mating-type locus in the progeny, as assessed by mating with the tester serotype D strains JEC20 (MATa) and JEC21 (MATα), segregated independently from the mutant phenotypes and nourseothricin resistance, indicating that micromanipulation of the crosses was successful and that bona fide basidiospores were analyzed rather than contaminating parental yeast cells. Moreover, these findings revealed that none of the insertions was linked to the mating-type locus. We noted that the temperature-sensitive isolate 7E12 was sterile, and thus, linkage could not be established by this approach. Isolate 4F2 had an unusual phenotype, which was unfortunately unlinked to NAT, as it appeared to leak laccase or melanin into the medium and may harbor a mutation affecting cell wall targeting or the attachment of melanin.
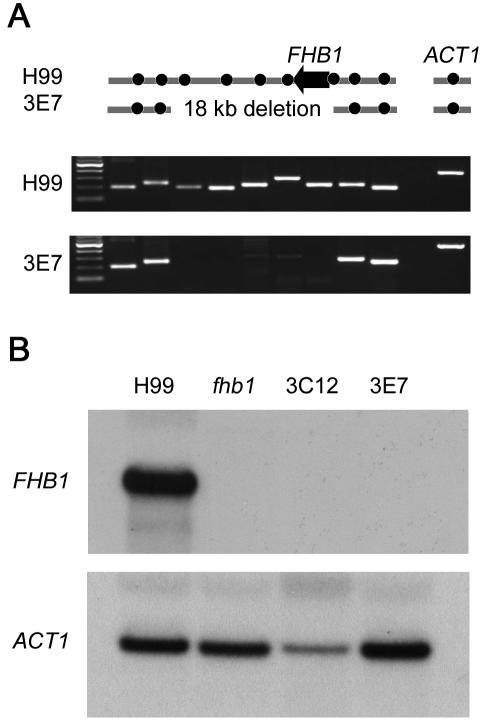
The DNAs flanking the insertion in mutant strains were identified by inverse PCR or plasmid rescue of the flanking regions in E. coli (Table 1). The temperature-sensitive isolate 5D12 had a full-length plasmid as well as a truncated copy (2,459 bp) inserted in the promoter region between two divergent genes encoding a predicted protein with WD40 repeats and a homolog of a DNA helicase involved in early steps of DNA replication (CDC19 of Schizosaccharomyces pombe or MCM2 of S. cerevisiae). Whether either or both of these genes are responsible for the temperature-sensitive growth defect remains to be established, but we note that MCM2 is essential in S. cerevisiae (20). The other temperature-sensitive isolate (7E12, which was sterile) had an estimated 50 to 60 copies of the plasmid in tandem, based on Southern blot analysis, and the junctions have not been analyzed for this mutant. The strains with reduced melanin production had insertions into a predicted gene (isolate 5E2) or into a novel mitogen-activated protein (MAP) kinase (MPK2) predicted gene (isolate 1F2), both of which are discussed further below. The other flank of the insertion in these two strains was not obtained and there is evidence for small deletions (<1 kb) based on PCR analyses. The two mutants that were sensitive to nitric oxide both had the NAT marker inserted into a homolog of the S. cerevisiae flavohemoglobin gene (FHB1) (31, 50). Flavohemoglobin detoxifies nitric oxide to nitrite and has been identified in several bacterial species as well as S. cerevisiae. In recent independent studies, the C. neoformans FHB1 gene was identified by sequence similarity to the S. cerevisiae homolog and was shown to play a role in resistance to nitric oxide, survival in macrophages, and full virulence in mice (14). Based on Southern blot analysis, the mutations in the FHB1 gene generated by this study were independent of one another. A deletion of approximately 18 kb of genomic DNA occurred in the NO-hypersensitive mutant 3E7 according to PCR analysis (Fig. 2), whereas the NAT marker inserted into the promoter region 411 bp upstream from the start codon in isolate 3C12. A Northern blot analysis (Fig. 2) showed that no FHB1 transcripts were produced in either NO-hypersensitive mutant strain.
FIG. 2.
Nitric oxide-hypersensitive strains fail to express flavohemoglobin (FHB1). (A) PCR analysis of wild-type (H99) and insertional mutant (3E7) strains showing a deletion of approximately 18 kb in strain 3E7. Dots indicate PCR amplicons. Actin (ACT1) was used as a control for DNA amplification of both strains. (B) Transcription of the FHB1 gene in wild-type (H99) and fhb1 mutants (targeted deletion of fhb1 and insertional mutants 3E7 and 3C12) was analyzed by Northern blotting with a radiolabeled probe for the FHB1 gene. A probe for the actin gene (ACT1) was used as a control for RNA loading and transfer.
Insertional mutagenesis with A. tumefaciens.
Agrobacterium-mediated transformation was found to yield stable and less complicated insertional mutations. For this DNA delivery approach, the nourseothricin resistance cassette was cloned into an Agrobacterium Ti plasmid between the right and left borders of the T-DNA. The plasmid was electroporated into A. tumefaciens cells. C. neoformans strain H99 was coincubated with A. tumefaciens cells containing the NAT delivery plasmid. Up to 120 transformants were obtained per transformation, with concentrations of 105 or 106 initial Cryptococcus cells/ml producing the highest yields. Serotype D strains were also successfully transformed. Of 148 nourseothricin-resistant strains examined, all showed stability of nourseothricin resistance in the absence of the drug, indicating that the frequency of stable transformants was >99.5%. The difference in the stability of NAT for the two transformation methods probably reflects the difference in the nature of DNA delivered into the cell. Biolistic transformation inserts naked DNA, whereas Agrobacterium inserts a complex of single-stranded DNA covered with proteins (53). The proteinaceous covering may protect the DNA from telomerase, thereby reducing the generation of episomal replicons.
To test whether T-DNA insertion provides a suitable tool for insertional mutagenesis, we replica plated 576 NAT isolates obtained by transconjugation onto l-DOPA medium to identify melanin-deficient mutants. Three mel− (white) strains were identified; these were crossed with a congenic serotype A MATa strain (KN99-5), and in two of the strains the pigmentation defect and nourseothricin resistance were linked and exhibited cosegregation in meiotic segregants after the genetic cross. The regions flanking the T-DNA insert were obtained by inverse PCR, sequenced (GenBank accession no. CG865117 to CG865120), and compared to the H99 genomic sequence (Fig. 3). One mutant had a replacement of 12 bp with the T-DNA, 575 bp upstream from the start of a laccase (LAC1) gene (41). The second mutant had 18 bp replaced 80 bp upstream from the start of the CLC1 gene, which encodes a putative voltage-gated chloride channel.
FIG. 3.
Arrangement of insertion of T-DNA by Agrobacterium into the genomes of two mel− mutants of C. neoformans. Sequences of the T-DNA left and right borders, the mutants, and wild-type H99 genomic DNA were aligned. One T-DNA insertion was in the promoter of the CLC1 gene and the other was in the promoter of the LAC1 gene. Small deletions of DNA-containing sequences similar to the T-DNA borders (e.g., TGGCAGGA in left border and region deleted in At-mel2) are associated with the insertion of T-DNA.
Insertional mutagenesis bias and generation of unlinked mutations.
An ideal insertional mutation process creates random mutations in the genome without causing any additional genetic rearrangements. Two mutants generated by Agrobacterium-mediated transformation that were characterized further had insertions of the T-DNA into the promoters of genes. In a study of nearly 100,000 T-DNA insertion points in transgenic Arabidopsis thaliana, bias was observed towards promoters and 5′ and 3′ untranslated regions of genes, as well as certain chromosomal positions (1). Both of the C. neoformans mutants analyzed here also had small deletions of DNA with sequence similarities to the T-DNA borders (Fig. 3), suggesting that T-DNA integration may occur in regions of modest sequence similarity and could cause a bias in insertion sites. For the transformation of S. cerevisiae with Agrobacterium T-DNA, small deletions of target DNA with a sequence bias occurred in 2 of 11 cases analyzed, and no bias towards insertions into the promoters of genes was observed (5, 6). In another insertional mutagenesis study of C. neoformans using biolistic transformation, Nelson et al. (36) observed a high frequency of targeting of the plasmid into the actin promoter, as this promoter was used to control antibiotic resistance. We did not observe this bias for the strains analyzed here. This may reflect the fact that we used predominantly linearized plasmid DNA whereas Nelson et al. employed circular plasmid DNA. Four of the insertion mutations generated by biolistic transformation were in the promoter regions of genes. Our screen may have been biased towards fitter strains, as we isolated transformants with robust growth in a rich medium (YPD), and this may have selected for events that reduce, rather than abolish, gene production. An alternative explanation for insertions into promoters is that these regions may represent chromatin structures into which exogenous DNA can more readily integrate.
The Agrobacterium-mediated and biolistic methods also yielded strains in which the mutant phenotype was unlinked to the insertion of nourseothricin resistance. The method of biolistic transformation relies on the delivery of DNA on gold beads into the cell and nucleus. Transformation using auxotrophic markers reveals a pattern of dead cells in the middle of the plate where the highest density of beads have struck, suggesting that this process is damaging and may cause DNA rearrangements. In an independent insertional mutagenesis study with serotype D, only 1 of 12 temperature-sensitive mutants showed cosegregation of temperature sensitivity with nourseothricin resistance (P. R. Kraus and J. Heitman, unpublished data). Undesired events following Agrobacterium-mediated transformation have yet to be determined, and our study represents one of the first insertional mutagenesis studies reported that used this method. Transposable elements have been identified in C. neoformans, such as the T1 and T2 elements that spontaneously inserted into the FRR1 gene during the isolation of FK506-resistant mutants, and they may be responsible for generating some unlinked mutations (10, 21). In addition, phenotypic switching may also be involved in the additional generation of unstable phenotypes (18). For example, we initially identified two strains that failed to grow at 37°C, but both subsequently reverted to a wild-type growth phenotype.
Reconstruction of nourseothricin acetyltransferase resistance cassette and targeted disruption of the CLC1, CLC2, and MPK2 genes.
The nourseothricin acetyltransferase (NAT) gene can be recalcitrant to PCR amplification, which is likely a consequence of its high GC content (71.2%). The NAT gene was reconstructed to reduce the GC content and also to incorporate C. neoformans preferred codons while maintaining the original amino acid sequence. Eight oligonucleotides were designed, and overlap PCR was used to create a single contiguous DNA molecule that was cloned into plasmid pCR2.1 TOPO (Invitrogen). Clones were sequenced to identify one without errors, and one clone was used to generate plasmids in which the ACT1 promoter and TRP1 terminator from strains JEC21 or H99 flanked the preferred codon- and GC content-adjusted nourseothricin acetyltransferase gene (GenBank accession no. AY483215).
Two strains with reduced melanization were obtained by biolistic and Agrobacterium-mediated transformation and were found to have mutations in the MPK2 and CLC1 genes, respectively (Fig. 1). In addition to the evidence of genetic analysis that the insertions cause reduced melanization, we created independent mutations of these genes to confirm that the phenotype of the original insertional mutant was attributable to these defined mutations.
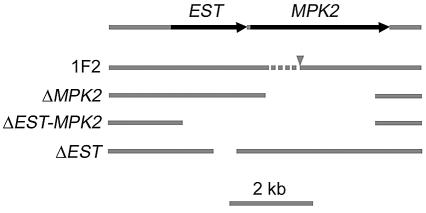
A plasmid bearing the optimized NAT gene was employed in overlap PCR to generate disruption alleles corresponding to insertional mutants 1F2 and At-mel1. In the case of isolate 1F2, three deletion strains were made, comprising the MAP kinase MPK2 gene (2,640 bp deleted), both MPK2 and an upstream gene (4,599 bp deleted), or only the upstream gene (525 bp deleted), with strain KN99α (Fig. 4). In similar experiments for At-mel1, the modified NAT marker was also employed to disrupt the chloride channel gene CLC1 and a second highly similar gene CLC2 in strain H99. Gene disruption in nourseothricin-resistant transformants was confirmed by PCR and Southern blot analyses (data not shown). A voltage-gated chloride channel double mutant (clc1 clc2) was generated by mating a MATa clc1 and a MATα clc2 mutant and then isolating meiotic segregants in which both mutations were present.
FIG. 4.
Diagram of deletion alleles used to examine gene functioning of insertional mutant 1F2. The arrangement of two genes, one represented by an EST and the other being a MAP kinase (MPK2), was derived from The Institute for Genomic Research C. neoformans database. The MPK2 gene sequence was predicted and may be shorter at the 5′ end than is represented. Strain 1F2 had the plasmid conferring resistance to nourseothricin (NAT) inserted at the arrowhead, and a deletion of <1 kb occurred (dashed line) that was not further defined. Three alleles were used to replace the MPK2, the EST, or both genes with the NAT marker.
Phenotypic analysis of mutants in MAP kinase MPK2 and adjacent gene.
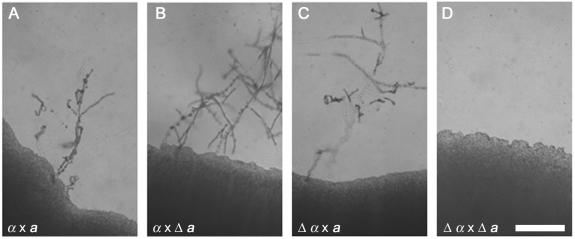
An initial exciting discovery was the MAP kinase mpk2 mutation in strain 1F2, which exhibited a melanin defect. Mpk2 is similar to the MAP kinase family members involved in cell wall integrity. There is a second MAP kinase, Mpk1, in C. neoformans that is closely related to this family and is involved in cell wall integrity, growth at 37°C, and resistance to caspofungin (27). Mutation of a third C. neoformans MAP kinase, Cpk1, results in a unilateral mating defect (12). Because MAP kinase mutants can have diverse cellular effects, we also tested whether the 1F2 mutation conferred any impairment in mating. In bilateral crosses (i.e., crosses in which both parents contain the mutation), no filaments were formed (Fig. 5). Nevertheless, when the MPK2 gene was mutated by replacing most of the predicted coding region with the nourseothricin resistance cassette described above, the resulting mpk2 strain did not exhibit any melanin or mating defect. Prediction of the gene structure within this region is ambiguous, and a second mutation of the same MPK2 coding region plus an additional 1,959 bp of flanking DNA upstream of the gene resulted in strains with the same melanin phenotype as strain 1F2. There is an expressed sequence within this region (transcribed in the same direction as MPK2), based on the presence of an expressed sequence tag (EST) according to the University of Oklahoma EST database. Finally, we created a third mutant in which 525 bp of DNA covering the EST were deleted (Fig. 4). This strain did not show a reduction in melanin production, but it was sterile in bilateral crosses, like the original insertion mutant. These data suggest either a combined effect of deletion of the predicted gene and MPK2 or the presence of a small unpredicted gene within the region. A Northern blot analysis of RNAs extracted from wild-type and four mutant strains did not resolve these hypotheses because transcript levels were low. The results of the analysis of sequences of regions flanking the insertional mutations and the investigation of the 1F2 mutant emphasize the importance of sequencing both sides flanking the inserted DNA and indicate that insertional mutagenesis will become much easier to interpret as the C. neoformans genome is annotated.
FIG. 5.
C. neoformans insertional mutant 1F2 exhibits a bilateral mating defect. Crosses between the wild type and the 1F2 mutant or a mutant strain derived by genetic crosses were cocultured on V8 medium (pH 5) in the dark and were examined 10 days later. (A) Wild-type H99 (MATα) × KN99-5 (MATa); (B) unilateral H99 (MATα) × mutant (Δ) 1F2 MATa progeny (NAT MATa); (C) unilateral 1F2 (NAT MATα) × KN99-5 (MATa); (D) bilateral 1F2 (NAT MATα) × 1F2 MATa (NAT MATa). Bar = 0.5 mm.
Phenotypic analysis of mutants in CLC1 and CLC2 chloride channel genes.
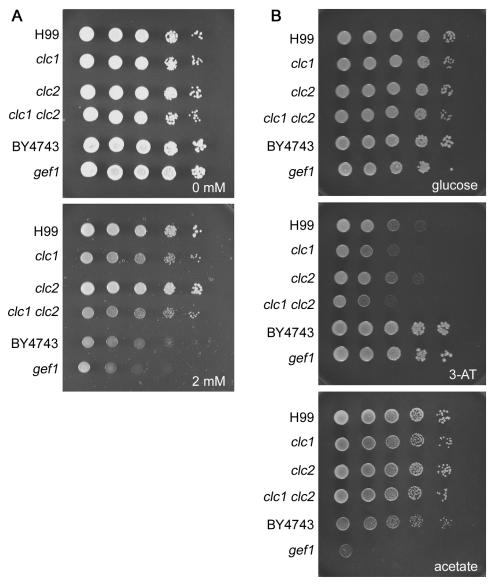
The mel− strain At-mel1 contains T-DNA inserted into the promoter of a gene encoding a voltage-gated chloride channel (CLC1). We identified a second chloride channel-like gene (CLC2) in the C. neoformans genome and mutated both genes to perform functional tests. The clc1 single and clc1 clc2 double mutants did not produce melanin on medium containing the diphenolic compound l-DOPA, whereas the clc2 single mutant produced melanin at a level comparable to that of the wild-type strain H99. The role of chloride channels in ion homeostasis has been investigated previously with S. cerevisiae (19, 22). Mutants in the single voltage-gated chloride channel gene, GEF1, of this fungus require copper for growth on minimal medium and are hypersensitive to iron-chelating agents (19, 22). We therefore analyzed the phenotype of the C. neoformans clc mutants based on previous observations from S. cerevisiae. Growth of the C. neoformans and S. cerevisiae clc/gef1 mutants in the presence or absence of the iron-chelating agent ferrozine was examined (Fig. 6A). C. neoformans clc1 mutant strains were hypersensitive to ferrozine (2 mM), whereas clc2 mutants were not. The S. cerevisiae reference (wild type) and gef1 mutant strains both grew more poorly than the C. neoformans strains on ferrozine, and the difference between the reference and gef1 strains was modest. The clc1 mutant strains were also hypersensitive to 3-amino-1,2,4-triazole (10 mM), which is a multifunctional molecule used as a herbicide that has multiple effects on eukaryotic cells, including the inhibition of catalase and imidazole glycerol-phosphate dehydratase (His3), and can also chelate metal ions such as aluminum, copper, and iron (2) (Fig. 6B). The growth of the clc1 mutants was comparable to that of the wild type on acetate or glycerol, whereas the S. cerevisiae gef1 mutant was unable to grow on acetate (Fig. 6B) but did grow on glycerol. The differences in growth on acetate as the sole carbon source between C. neoformans and S. cerevisiae mutants suggest that both conserved and divergent phenotypic consequences follow the loss of the CLC1/GEF1 encoded chloride channel in the two fungi.
FIG. 6.
C. neoformans voltage-gated chloride channel gene CLC1 is required for normal growth under low-iron conditions. The growth of wild-type (H99) and clc1 and clc2 mutant strains of C. neoformans was compared to that of reference wild-type (BY4743) and gef1 mutant strains of S. cerevisiae. Cells were serially diluted and grown on synthetic medium containing 0 or 2 mM iron chelation agent ferrozine (3 days at room temperature) (A) or glucose (2 days at 30°C), glucose plus 3-amino-1,2,4-triazole (3-AT) (10 mM; 2 days at 30°C), or acetate (2%; 4 days at 30°C) as the sole carbon source (B).
In S. cerevisiae, Gef1 functions to maintain ion balance in the Golgi complex to allow copper uptake by Ccc2 and H+ uptake by the vacuolar H+-ATPase, hence leading to iron uptake by Fet3 (19). There is also evidence that Cl− ions may directly regulate the loading of copper ions onto Fet3 (13). The C. neoformans Lac1 laccase, a member of the iron-copper oxidase family, like Fet3, must also bind copper to be active (49). The H+-ATPase (VPH1) is also involved in melanin production in C. neoformans (16, 51), and a CCC2 homolog is involved in melanin production (via the dihydroxynaphthalene pathway) and virulence in the bean pathogen Colletotrichum lindemuthianum (38). Our hypothesis is that the clc1 mutation perturbs Cl− counter-anion transport into the secretory pathway and thereby perturbs copper import, depriving laccase of its essential Cu+ ion cofactors and impairing the melanin biosynthesis potential. This hypothesis is further supported by the finding that the addition of 100 μM Cu2+ restores melanin production to clc1 mutant strains (data not shown).
Recently, Zhu and Williamson (52) discovered the CLC1 gene, providing independent evidence that this gene plays a role in melanin production and virulence. They also reported a reduction of capsule production in clc1 mutant strains grown on malt agar; however, we observed normal capsule production in liquid cultures under conditions of low glucose and low iron (data not shown).
Concluding remarks.
The genome sequences of C. neoformans serotypes A and D are nearly complete, providing a comprehensive view of the gene contents of related but divergent varieties of this ubiquitous human fungal pathogen. The next challenge in genome analysis is to determine the functions of these genes through mutational analysis. There is a consortium within the cryptococcal research community whose goal is to generate a systematic deletion set of genes in the fungus, starting with 10% of the genes. Nevertheless, the project to disrupt approximately 800 genes may require several years to accomplish. We embarked on generating a random mutant library to accelerate gene discovery in an approach complementary to the gene disruption project which will also allow more subtle phenotypes to be examined because random insertions that reduce gene functioning are proving invaluable for the analysis of essential genes. Our studies demonstrate that insertional mutagenesis can be used for the identification of genes in a pathogenic serotype A strain background and show the utility of the newly created congenic strains of serotype A for the genetic analysis of insertional, and other, mutations. Future work using insertional mutagenesis of C. neoformans serotype A will continue to apply Agrobacterium-mediated transformation with the goal of generating a collection of strains with the genome saturated with T-DNA insertions and using signature tags. Other laboratories have started to apply Agrobacterium as an insertional mutagen to C. neoformans (S. Chung et al., Abstr. 102nd Gen. Meet. Am. Soc. Microbiol., abstr. F-12, 2002). For this study, we have tested representative in vitro phenotypes associated with virulence attributes. These methods can now be extended and applied to the identification of additional novel virulence factors. These approaches can also be applied immediately to investigations of insertional mutants in animals or cultured cells, including mice, macrophages, amoebae, and Caenorhabditis elegans, to identify novel virulence attributes and their underlying molecular bases as novel targets for therapeutic intervention. The stage has therefore been set for a large-scale genetic analysis of the molecular determinants of virulence in a ubiquitous human fungal pathogen of significant clinical importance.
Acknowledgments
We thank Peter Kraus for advice on insertional mutagenesis in C. neoformans, Joseph Nairn and Barbara Howlett for plasmid pPZP-201BK and advice on Agrobacterium-mediated transformation, Rob Brazas for advice on reconstruction of nourseothricin acetyltransferase, and Arturo Casadevall and Javier Garcia-Rivera for examining laccase in the “leaky” melanin mutant. We thank Andy Alspaugh, John Perfect, and Dennis Thiele for comments on the manuscript. Gene identification was greatly facilitated by the following C. neoformans genome sequencing projects: Stanford Genome Technology Center (www-sequence.stanford.edu), The Institute for Genomic Research (www.tigr.org/tdb/e2k1/cna1), the Duke University project (cneo.genetics.duke.edu), the Whitehead Institute (www.broad.mit.edu/annotation/fungi/cryptococcus_neoformans/), and the University of Oklahoma project (www.genome.ou.edu/cneo.html).
This work was supported in part by NIAID R01 grant AI50113 to J. Heitman and by NIAID P01 program project grant AI44975 to the Duke University Mycology Research Unit. J. Heitman is a Burroughs Wellcome Scholar in Molecular Pathogenic Mycology and an investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Alonso, J. M., A. N. Stepanova, T. J. Leisse, C. J. Kim, H. Chen, P. Shinn, D. K. Stevenson, J. Zimmerman, P. Barajas, R. Cheuk, C. Gadrinab, C. Heller, A. Jeske, E. Koesema, C. C. Meyers, H. Parker, L. Prednis, Y. Ansari, N. Choy, H. Deen, M. Geralt, N. Hazari, E. Hom, M. Karnes, C. Mulholland, R. Ndubaku, I. Schmidt, P. Guzman, L. Aguilar-Henonin, M. Schmid, D. Weigel, D. E. Carter, T. Marchand, E. Risseeuw, D. Brogden, A. Zeko, W. L. Crosby, C. C. Berry, and J. R. Ecker. 2003. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:653-657. [DOI] [PubMed] [Google Scholar]
- 2.Brian, R. C. 1964. The classification of herbicides and types of toxicity, p. 1-37. In L. J. Audus (ed.), The physiology and biochemistry of herbicides. Academic Press, London, United Kingdom.
- 3.Brown, J. S., A. Aufauvre-Brown, J. Brown, J. M. Jennings, H. Arst, Jr., and D. W. Holden. 2000. Signature-tagged and directed mutagenesis identify PABA synthetase as essential for Aspergillus fumigatus pathogenicity. Mol. Microbiol. 36:1371-1380. [DOI] [PubMed] [Google Scholar]
- 4.Bundock, P., A. den Dulk-Ras, A. Beijersbergen, and P. J. J. Hooykaas. 1995. Trans-kingdom T-DNA transfer from Agrobacterium tumefaciens to Saccharomyces cerevisiae. EMBO J. 14:3206-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bundock, P., and P. J. J. Hooykaas. 1996. Integration of Agrobacterium tumefaciens T-DNA in the Saccharomyces cerevisiae genome by illegitimate recombination. Proc. Natl. Acad. Sci. USA 93:15272-15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bundock, P., H. van Attikum, A. den Dulk-Ras, and P. J. J. Hooykaas. 2002. Insertional mutagenesis in yeasts using T-DNA from Agrobacterium tumefaciens. Yeast 19:529-536. [DOI] [PubMed] [Google Scholar]
- 7.Casadevall, A., and J. Perfect. 1998. Cryptococcus neoformans. ASM Press, Washington, D.C.
- 8.Cormack, B. P., N. Ghori, and S. Falkow. 1999. An adhesin of the yeast pathogen Candida glabrata mediating adherence to human epithelial cells. Science 285:578-582. [DOI] [PubMed] [Google Scholar]
- 9.Covert, S. F., P. Kapoor, M.-H. Lee, A. Briley, and C. J. Nairn. 2001. Agrobacterium tumefaciens-mediated transformation of Fusarium circinatum. Mycol. Res. 105:259-264. [Google Scholar]
- 10.Cruz, M. C., L. M. Cavallo, J. M. Görlach, G. Cox, J. R. Perfect, M. E. Cardenas, and J. Heitman. 1999. Rapamycin antifungal action is mediated via conserved complexes with FKBP12 and TOR kinase homologs in Cryptococcus neoformans. Mol. Cell. Biol. 19:4101-4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson, R. C., J. R. Blankenship, P. R. Kraus, M. de Jesus Berrios, C. M. Hull, C. D'Souza, P. Wang, and J. Heitman. 2002. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology 148:2607-2615. [DOI] [PubMed] [Google Scholar]
- 12.Davidson, R. C., C. B. Nichols, G. M. Cox, J. R. Perfect, and J. Heitman. 2003. A MAP kinase cascade composed of cell type specific and non-specific elements controls mating and differentiation of the fungal pathogen Cryptococcus neoformans. Mol. Microbiol. 49:469-485. [DOI] [PubMed] [Google Scholar]
- 13.Davis-Kaplan, S. R., C. C. Askwith, A. C. Bengtzen, D. Radisky, and J. Kaplan. 1998. Chloride is an allosteric effector of copper assembly for the yeast multicopper oxidase Fet3p: an unexpected role for intracellular chloride channels. Proc. Natl. Acad. Sci. USA 95:13641-13645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jesús-Berríos, M., L. Liu, J. C. Nussbaum, G. M. Cox, J. S. Stamler, and J. Heitman. 2003. Enzymes that counteract nitrosative stress promote fungal virulence. Curr. Biol. 13:1963-1968. [DOI] [PubMed] [Google Scholar]
- 15.Edman, J. C. 1992. Isolation of telomere-like sequences from Cryptococcus neoformans and their use in high-efficiency transformation. Mol. Cell. Biol. 12:2777-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erickson, T., L. Liu, A. Gueyikian, X. Zhu, J. Gibbons, and P. R. Williamson. 2001. Multiple virulence factors of Cryptococcus neoformans are dependent on VPH1. Mol. Microbiol. 42:1121-1131. [DOI] [PubMed] [Google Scholar]
- 17.Fraser, J. A., R. L. Subaran, C. B. Nichols, and J. Heitman. 2003. Recapitulation of the sexual cycle of the primary fungal pathogen Cryptococcus neoformans var. gattii: implications for an outbreak on Vancouver Island, Canada. Eukaryot. Cell 2:1036-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fries, B. C., D. L. Goldman, and A. Casadevall. 2002. Phenotypic switching in Cryptococcus neoformans. Microbes Infect. 4:1345-1352. [DOI] [PubMed] [Google Scholar]
- 19.Gaxiola, R. A., D. S. Yuan, R. D. Klausner, and G. R. Fink. 1998. The yeast CLC chloride channel functions in cation homeostasis. Proc. Natl. Acad. Sci. USA 95:4046-4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giaever, G., A. M. Chu, L. Ni, C. Connelly, L. Riles, S. Véronneau, S. Dow, A. Lucau-Danila, K. Anderson, B. André, A. P. Arkin, A. Astromoff, M. El-Bakkoury, R. Bangham, R. Benito, S. Brachat, S. Campanaro, M. Curtiss, K. Davis, A. Deutschbauer, K.-D. Entian, P. Flaherty, F. Foury, D. J. Garfinkel, M. Gerstein, D. Gotte, U. Güldener, J. H. Hegemann, S. Hempel, Z. Herman, D. F. Jaramillo, D. E. Kelly, S. L. Kelly, P. Kötter, D. LaBonte, D. C. Lamb, N. Lan, H. Liang, H. Liao, L. Liu, C. Luo, M. Lussier, R. Mao, P. Menard, S. L. Ooi, J. L. Revuelta, C. J. Roberts, M. Rose, P. Ross-Macdonald, B. Scherens, G. Schimmack, B. Shafer, D. D. Shoemaker, S. Sookhai-Mahadeo, R. K. Storms, J. N. Strathern, G. Valle, M. Voet, G. Volckaert, C.-Y. Wang, T. R. Ward, J. Wilhelmy, E. A. Winzeler, Y. Yang, G. Yen, E. Youngman, K. Yu, H. Bussey, J. D. Boeke, M. Snyder, P. Philippsen, R. W. Davis, and M. Johnston. 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418:387-391. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin, T. J., and R. T. M. Poulter. 2001. The diversity of retrotransposons in the yeast Cryptococcus neoformans. Yeast 18:865-880. [DOI] [PubMed] [Google Scholar]
- 22.Greene, J. R., N. H. Brown, B. J. DiDomenico, J. Kaplan, and D. J. Eide. 1993. The GEF1 gene of Saccharomyces cerevisiae encodes an integral membrane protein, mutations in which have effects on respiration and iron-limited growth. Mol. Gen. Genet. 241:542-553. [DOI] [PubMed] [Google Scholar]
- 23.Heitman, J., B. Allen, J. A. Alspaugh, and K. J. Kwon-Chung. 1999. On the origins of congenic MATα and MATa strains of the pathogenic yeast Cryptococcus neoformans. Fungal Genet. Biol. 28:1-5. [DOI] [PubMed] [Google Scholar]
- 24.Hull, C. M., and J. Heitman. 2002. Genetics of Cryptococcus neoformans. Annu. Rev. Genet. 36:557-615. [DOI] [PubMed] [Google Scholar]
- 25.Idnurm, A., and B. J. Howlett. 2001. Pathogenicity genes of phytopathogenic fungi. Mol. Plant Pathol. 2:241-255. [DOI] [PubMed] [Google Scholar]
- 26.Keller, S. M., M. A. Viviani, M. C. Esposto, M. Cogliati, and B. L. Wickes. 2003. Molecular and genetic characterization of a serotype A MATa Cryptococcus neoformans isolate. Microbiology 149:131-142. [DOI] [PubMed] [Google Scholar]
- 27.Kraus, P. R., D. S. Fox, G. M. Cox, and J. Heitman. 2003. The Cryptococcus neoformans MAP kinase Mpk1 regulates cell integrity in response to antifungal drugs and loss of calcineurin function. Mol. Microbiol. 48:1377-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunik, T., T. Tzfira, Y. Kapulnik, Y. Gafni, C. Dingwall, and V. Citovsky. 2001. Genetic transformation of HeLa cells by Agrobacterium. Proc. Natl. Acad. Sci. USA 98:1871-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon-Chung, K. J., J. C. Edman, and B. L. Wickes. 1992. Genetic association of mating types and virulence in Cryptococcus neoformans. Infect. Immun. 60:602-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lengeler, K. B., P. Wang, G. M. Cox, J. R. Perfect, and J. Heitman. 2000. Identification of the MATa mating-type locus of Cryptococcus neoformans reveals a serotype A MATa strain thought to have been extinct. Proc. Natl. Acad. Sci. USA 97:14455-14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, L., M. Zeng, A. Hausladen, J. Heitman, and J. S. Stamler. 2000. Protection from nitrosative stress by yeast flavohemoglobin. Proc. Natl. Acad. Sci. USA 97:4672-4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDade, H. C., and G. M. Cox. 2001. A new dominant selectable marker for use in Cryptococcus neoformans. Med. Mycol. 39:151-154. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell, A. P. 2003. Updated view of Cryptococcus neoformans mating type and virulence. Infect. Immun. 71:4829-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mylonakis, E., F. M. Ausubel, J. R. Perfect, J. Heitman, and S. B. Calderwood. 2002. Killing of Caenorhabditis elegans by Cryptococcus neoformans as a model of yeast pathogenesis. Proc. Natl. Acad. Sci. USA 99:15675-15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura, Y., T. Gojobori, and T. Ikemura. 2000. Codon usage tabulated from international DNA sequence databases: status for the year 2000. Nucleic Acids Res. 28:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson, R. T., J. Hua, B. Pryor, and J. K. Lodge. 2001. Identification of virulence mutants of the fungal pathogen Cryptococcus neoformans using signature-tagged mutagenesis. Genetics 157:935-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen, K., G. M. Cox, P. Wang, D. L. Toffaletti, J. R. Perfect, and J. Heitman. 2003. Sexual cycle of Cryptococcus neoformans var. grubii and virulence of congenic a and α isolates. Infect. Immun. 71:4831-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parisot, D., M. Dufresne, C. Veneault, R. Laugé, and T. Langin. 2002. clap1, a gene encoding a copper-transporting ATPase involved in the process of infection by the phytopathogenic fungus Colletotrichum lindemuthianum. Mol. Genet. Genomics 268:139-151. [DOI] [PubMed] [Google Scholar]
- 39.Perfect, J. R., N. Ketabchi, G. M. Cox, C. W. Ingram, and C. L. Beiser. 1993. Karyotyping of Cryptococcus neoformans as an epidemiological tool. J. Clin. Microbiol. 31:3305-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pitkin, J. W., D. G. Panaccione, and J. D. Walton. 1996. A putative cyclic peptide efflux pump encoded by the TOXA gene of the plant-pathogenic fungus Cochliobolus carbonum. Microbiology 142:1557-1565. [DOI] [PubMed] [Google Scholar]
- 41.Salas, S. D., J. E. Bennett, K. J. Kwon-Chung, J. R. Perfect, and P. R. Williamson. 1996. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J. Exp. Med. 184:377-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steenbergen, J. N., and A. Casadevall. 2003. The origin and maintenance of virulence for the human pathogenic fungus Cryptococcus neoformans. Microbes Infect. 5:667-675. [DOI] [PubMed] [Google Scholar]
- 43.Steenbergen, J. N., J. D. Nosanchuk, S. D. Malliaris, and A. Casadevall. 2003. Cryptococcus neoformans virulence is enhanced after growth in the genetically malleable host Dictyostelium discoideum. Infect. Immun. 71:4862-4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steenbergen, J. N., H. A. Shuman, and A. Casadevall. 2001. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl. Acad. Sci. USA 98:15245-15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toffaletti, D. L., T. H. Rude, S. A. Johnston, D. T. Durack, and J. R. Perfect. 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 175:1405-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uhl, M. A., M. Biery, N. Craig, and A. D. Johnson. 2003. Haploinsufficiency-based large-scale forward genetic analysis of filamentous growth in the diploid human fungal pathogen C. albicans. EMBO J. 22:2668-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vijn, I., and F. Govers. 2003. Agrobacterium tumefaciens mediated transformation of the oomycete plant pathogen Phytophthora infestans. Mol. Plant Pathol. 4:459-468. [DOI] [PubMed] [Google Scholar]
- 48.Viviani, M. A., M. C. Esposto, M. Cogliati, M. T. Montagna, and B. L. Wickes. 2001. Isolation of a Cryptococcus neoformans serotype A MATa strain from the Italian environment. Med. Mycol. 39:383-386. [DOI] [PubMed] [Google Scholar]
- 49.Williamson, P. R. 1994. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. J. Bacteriol. 176:656-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu, H., and A. F. Riggs. 1992. Yeast flavohemoglobin is an ancient protein related to globins and a reductase family. Proc. Natl. Acad. Sci. USA 89:5015-5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu, X., J. Gibbons, S. Zhang, and P. R. Williamson. 2003. Copper-mediated reversal of defective laccase in a Δvph1 avirulent mutant of Cryptococcus neoformans. Mol. Microbiol. 47:1007-1014. [DOI] [PubMed] [Google Scholar]
- 52.Zhu, X., and P. R. Williamson. 2003. A CLC-type chloride channel gene is required for laccase activity and virulence in Cryptococcus neoformans. Mol. Microbiol. 50:1271-1282. [DOI] [PubMed] [Google Scholar]
- 53.Zupan, J., T. R. Muth, O. Draper, and P. Zambryski. 2000. The transfer of DNA from Agrobacterium tumefaciens into plants: a feast of fundamental insights. Plant J. 23:11-28. [DOI] [PubMed] [Google Scholar]