Abstract
Context:
Altered fractionated radiotherapy may have better result than conventional radiotherapy and concomitant chemoradiotherapy to treat locally advanced head and neck cancers.
Aims:
Evaluation of the response and toxicities in different fractionated radiotherapy schedules in locally advanced head and neck cancer.
Materials and Methods:
Sixty four histologically proved patients of locally advanced head and neck cancer were included in the study according to protocol and were randomized into three arms. Arm A (n = 21) received 66 Gy in 33 fractions (5 fractions/week from Monday to Friday) single fraction daily in 6½ weeks along with concomitant chemotherapy (injection Cisplatin 30 mg/m2 intravenous once weekly) for 6 weeks. Arm B (n = 21) received 66 Gy in 33 fractions (6 fractions per week) single fraction daily in 5½ weeks, and arm C (n = 22) received late hyperfractionation after 3 weeks; 30 Gy in 15 fractions in 3 weeks followed by 1.4 Gy twice daily (time gap between 2 fractions were 6 hours) for 15 days with a total of 72 Gy in 6 weeks. Response to treatment, compliance, and toxicities were compared in all the three arms.
Statistical Analysis Used:
Frequency table and chi square tests done.
Results:
Baseline data were comparable in all the three arms. Complete response in arm A, arm B, and arm C were 15%, 26.315%, and 23.81%, respectively (P = 0.339). Grade 1 Neutropenia in arm A was 15%, arm B was 26.32%, and arm C was nil (P = 0.0486).
Conclusion:
Altered fractionation and concurrent chemoradiation showed similar response with comparable acute toxicities except nutropenia, which was significantly higher in arm B.
Keywords: Accelerated fractionation, concomitant chemoradiotherapy, hyperfractionation, locally advanced head and neck cancer
Introduction
Worldwide, nearly 650000 people have head and neck cancer each year with approximately 350000 deaths.[1,2] One of the major oncological problems in Indian population is head and neck malignancies.[3,4] The age adjusted incidence for head and neck cancers in Indian male population range from 10.8 to 38.8 per 100000 population and in 6.4-14.9 in 100000 female population.[3,4,5] There were 25.1% registered head and neck cancer cases in a hospital-based cancer registry in 1998. Its frequency was no different from those seen over the years 1984-1998, that is, 27%.[6]
Head and neck carcinoma (HNC) includes the common squamous-cell carcinomas of the oral cavity, pharynx, and larynx, and the less frequent tumors of the nasal cavity, paranasal sinuses, and the salivary glands. Radiation therapy the mainstay of treatment offered nearly 75% of all head and neck cancers with either curative or palliative intent, alone or as a part of multimodality approach.[7] The prognosis of patients with locally advanced head and neck cancer (LAHNC) is still poor, 5 year survival rate with conventional radiotherapy is 40-50%.[8]
One of the important causes of failure is accelerated repopulation of tumor clonogen, which usually starts around the 4th week of radiotherapy.[9] To combat this, 60 cGy of extra dose per day is needed.[9] Hence to increase local control and survival, several strategy of altered fractionation is used.
Another reason to deliver radiation therapy with altered fractionation schedules was designed as a means of maximizing the therapeutic ratio. The use of small multiple daily fractions allows to increase the therapeutic differential between late-responding normal tissues and acutely responding tumors with the advantage that the overall treatment time is shortened, thereby limiting the opportunities for proliferation.[9]
To improve the outcome of head and neck cancer concomitant chemoradiotherapy is another novel approach. The goal of using concomitant chemotherapy and radiation therapy is to eradicate systemic microscopic disease while simultaneously enhancing the cytotoxicity of radiation against macroscopic neoplastic disease. Several recent studies and meta-analyses have indicated superior loco-regional control and/or survival rates after concomitant chemoradiotherapy when compared with radiotherapy alone particularly when chemotherapy includes cisplatin or analogues.[10]
Based on these considerations, we began a prospective, single institutional study to compare different schedules of radiation therapy in patient LAHNC. The present report compares the outcome and toxicity profile observed in these three consecutive patient cohorts.
Materials and Methods
To conduct the study we included (n = 64) patients with head and neck cancer with histological or cytological proof, attending the outpatient service of radiotherapy of our iInstitution. The initial assessment included in all patients a complete medical history and physical examination, endoscopy and biopsy, complete blood count and biochemical profile, chest X-ray, and computerized tomography (CT) of the head and neck. Bone scan and abdominal ultrasound were performed at the discretion of the treating physician. Pretreatment evaluation also included complete dental evaluation and nutritional assessment. Patients were simulated before the start of the treatment with an appropriate immobilization device. In most of the patients, lateral opposed fields were used to treat the primary tumor and involved lymph nodes. Uninvolved level II to V nodes were included in the initial treatment volume in all patients and uninvolved level I nodes were also included in oral cavity cancers to ensure microscopic coverage. A third anterior field was used to treat the uninvolved supraclavicular nodes. CT-based three-dimensional treatment planning was used in all cases. The radiation dose was prescribed to the International Commission on Radiation Units (ICRU) point. Radiation treatments were delivered with Co-60 photon beams. The patients were randomized into three groups. The randomization has been done by computer generated numbers.
Arm A (n = 21) received 66 Gy in 33 fractions (5 fractions/week from Monday to Friday) single fraction daily in 6 ½ weeks and will receive concomitant chemotherapy with injection Cisplatin 30 mg/m2 body surface area intravenous on every Saturday for 6 weeks.
Arm B (n = 21) received 66 Gy in 33 fractions (6 fractions/week) single fraction daily in 5½ weeks,
Arm C (n = 22) received late hyperfractionation after 3 weeks; 30 Gy in 15 fractions in 3 weeks followed by 1.4 Gy twice daily (time gap between 2 fractions were 6 hours) for 15 days with a total of 72 Gy in 6 weeks.
Response will be assessed using the response evaluation criteria in solid tumors (RECIST) version 1.1.[11] Acute and late toxicities will be assessed using the Radiation Therapy Oncology Group (RTOG) criteria for adverse events.[12]
Statistical analysis was done by MedCalc Software Version 11.6.1 – © 1993-2011 (Last modified: June 6, 2011). Before proceeding to a larger trial, we conducted this pilot study. We have consulted our statistics department before the study started. They have guided us in selecting the sample size.
Chi-square and independent samples t-tests were used for comparison between the patient groups. Mann–Whitney test was done for independent samples to compare toxicities and response.
Results
The characteristics of the patients included in three series were comparable. Median age for arm A, arm B, and arm C were 63 years (44-72), 63 years (45-70), and 64 years (42-71), respectively. All patients had performance status eastern cooperative oncology group (ECOG) score less than or equal to 2. Stage III patients were predominant in all the arms (61.9% in arm A, 57.142% in arm B, and 59.09% in arm C). The median age of all the patients were similar in the three series (63, 63, and 64 years, respectively). All the 64 patients with locally advanced squamous cell carcinoma of the head and neck were randomly assigned into three treatment arms,
Table 1 shows the pretreatment patients characteristics. They were well balanced among the both treatment groups. Our patient pool had male preponderance. All the three arms had male patients more than female (arm A 81%, arm B 76.2%, and arm C 77.3%). Based on histopathology reports most patients had mainly well differentiated (WD) and moderately differentiated (MD) squamous cell cancer of head and neck rather than poorly differentiated (PD) cancer. (WD cancer in arm A, arm B, and arm C are 38.09%, 42.85%, and 36.363% whereas PD cancer in these three arms were respectively 14.286%, 9.5%, and 9.1%).
Table 1.
Patients characteristics
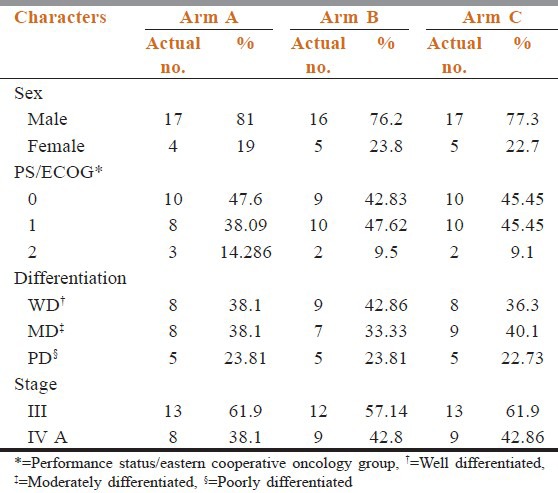
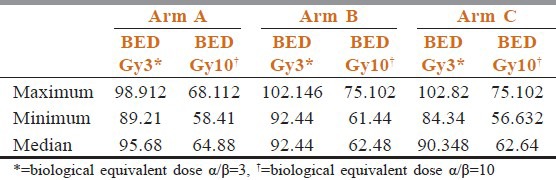
Table 2 shows that compliance of treatment in conventional chemoradiotherapy arm was better than the other two arms. Estimated time of completion of treatment were 6.5 weeks in arm A, 5.5 weeks in arm B, and 6 weeks in arm C. In between 6 and 8 weeks 55% patients of arm A had completed their treatment but only 31.58% and 38.1% patients of arm B and arm C completed treatments. Overall responses were assessed after 6 months of completion of treatment. Table 3 shows there were no incidence of progressive disease in arm B and arm C patients. Number of stable disease patients in arm A was 7 (35%), whereas in arm B and arm C patients, it was 2 (10.526%) and 3 (14.29%), respectively. Patients having complete response were 26.35% in arm B, 23.81% in arm C, whereas it was only 15% in arm A (P = 0.3385). Patients show the site and grade of acute and late adverse effects by treatment groups. The most common sites of grade 3 or worse acute side effects were the mucous membranes. However, the most common sites of grade 3 or worse late effects were the mucous membranes, the pharynx, and the salivary gland. Table 4 compared toxicities of conventional fractionated radiotherapy with concomitant chemotherapy and other two arms. Grade 3 skin toxicities more in arm C (52.38%) and arm B (47.36%) than arm A (30%). Grade 3 mucositis in arm B (63.16%) and arm C (61.9%) was more than in arm A (35%). Grade 2 anemia in arm A was 10% and in arm B and arm C, it was 10.32% and 5.26%, respectively (P = 0.9247). No Grade 2 neutropenia in arm C (P = 0.048). Median value of biological effective dose (BED) for α/β = 10 were 64.88, 61.44, and 62.64 in arm A, arm B, and arm C, respectively, [vide Table 5] carrying no statistical significance.
Table 2.
Patients compliance
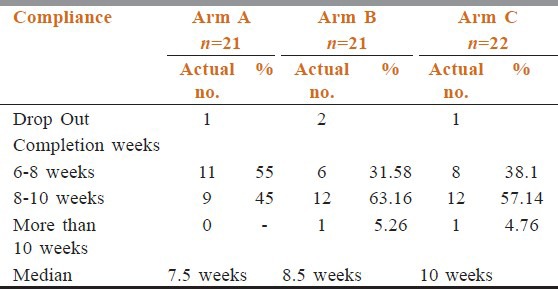
Table 3.
Response
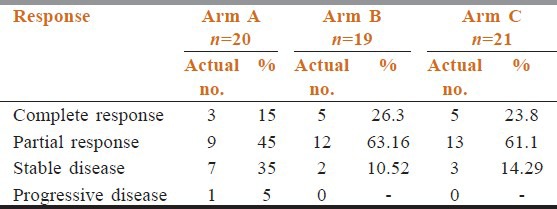
Table 4.
Toxicities
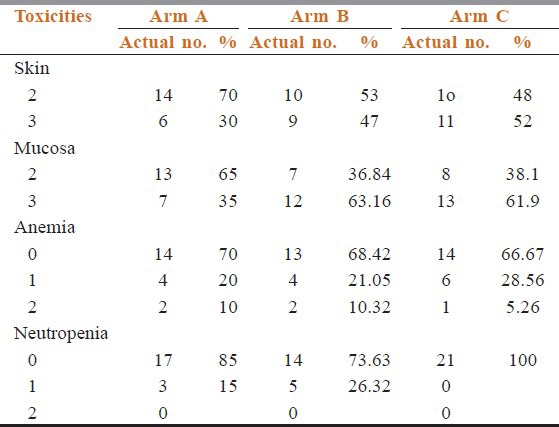
Table 5.
Biological equivalent dose
Discussion
Treatment of advanced squamous cell cancer of head and neck has been the subject of intensive investigations in the past few decades. Radiotherapy alone was the standard nonsurgical treatment for advanced disease for a long time. It was observed that radiotherapy alone resulted in local control of 50-70% and disease-free survival of 30-40%. The phenomenon of accelerated repopulation is one of reason of treatment failure in cancers of head and neck. This refers to the triggering of the surviving tumor cells [clonogens] to divide more rapidly as a tumor shrinks after irradiation or chemotherapy. It starts after about the 4th weeks of radiation in head and neck cancers. This suggests that treatment should be completed as soon as possible once it is started.
Accelerated treatment strategy aims to deliver the same total dose over a shorter time. In purely fractionated regime, total dose is delivered in half the overall time without changing the size of the fraction. But in practice it is difficult to follow it because of acute toxicities.[2,9] In this study we have followed a modest acceleration regime by giving 6 fractions of radiotherapy per week.
For concomitant chemo radiation: Meta analysis of chemotherapy on head and neck cancers [MACH-NC] studied > 10000 patients in 63 trials conducted prior to 1993. It demonstrated that adding chemotherapy to radiation therapy (RT) resulted in 12% reduction in the risk of death and 4% increase in 5 year survival.[10] Sharma et al. performed a phase III trial on concomitant chemoradiation versus radiotherapy in advanced cancers of oropharynx and nasopharynx with weekly Cisplatin. This showed the concomitant chemoradiotherapy regime to be safe and superior in advanced cases.[13] In our study we found better compliance in concomitant chemoradiotherapy arm is good (median compliance arm A = 7.5 weeks, arm B = 8.5 weeks, and arm C = 10 weeks).
For radiotherapy with accelerated fractionation: Overgaard et al. conducted Danish Head and Neck Cancer Study Group (DAHANCA)-6 and DAHANCA-7 trials, which are one of the largest trials of altered fractionation. The study compared five versus six fractions per week with same fraction size. Local control, disease free survival (DFS), and probability of voice preservation were improved with 6 fraction/week arm.[14] RTOG-90-03 used an accelerated fractionation scheme to deliver 72 Gy in 6 weeks. The 5 years locoregional control was increased from 45% to 54% but with increased acute and late toxicity.[15] In another clinical trial 101 patients with the squamous cell carcinoma of head and neck underwent either hyperfractionated radiotherapy, with 74.4-79.2 Gy delivered in 6.2-7 weeks (1.2 Gy per fraction twice a day), or accelerated fractionation with concomitant boost, which delivered 68.7-72 Gy in 6 weeks (1.8 Gy per fraction a day and 1.5 Gy per fraction a day to a boost filed as a second daily treatment for the last 11-12 treatment days). No significant differences were observed among the patients treated with conventional, hyperfractionated, or accelerated radiotherapy modalities either in locoregional control rate (41% vs 35% vs 49%, respectively; P = 0.690) or overall survival rate (50% vs 40% vs 51%, respectively; P = 0.760).[16]
A randomized clinical trial was conducted at Poland to evaluate tumour and normal tissues 3-year response to 7-day-a-week continuous accelerated irradiation (CAIR) compared to a conventional treatment (5 days per week). Actuarial 3-year local tumour control was 82% in the CAIR and 37% in the control group (P < 0.0001) with reduction in local recurrence rate of 83%. Actuarial 3-year overall survival was 78 and 32% (P < 0.0001), respectively. Confluent mucositis was significantly more severe and lasted longer in the CAIR than in control arm.[17] A study was conducted by Sanguineti et al.,[18] in Genoa, Italy, who randomized patients from four institutions with one or more high-risk features after surgery to conventional 60 Gy in 6 weeks versus 64 Gy in 5 weeks with twice daily treatment in the first and last weeks of treatment there was no difference in outcome between the two arms; however, there was a trend for improved locoregional control for patients who had a delay in starting radiotherapy and who were treated with altered fractionation (AF) compared with those with a delay who were treated with conventional fractionation (CF) (hazard ratio = 0.5; 95% confidence interval 0.2-1.1).
Altered fractionation regimens such as hyperfractionation or accelerated fractionation should be considered for patients being treated with radiation alone, as this approach has been demonstrated to improve the likelihood of locoregional tumor control.[15] The RTOG 90-03 altered fractionation randomized trial comparing conventional fractionation to hyperfractionation, split-course, and concomitant boost technique demonstrated a significant improvement in disease-free survival for the hyperfractionation and concomitant boost arms.[19] These altered fractionation regimens were associated with higher incidence of grade 3 or worse acute mucosal toxicity, but no significant difference in overall toxicity at 2 years following completion of treatment.
In our study, Grade 1 neutropenia found 26.32% in arm B, 15% in arm A, and no neutropenia in arm C (P = 0.048), which has got statistical significance. Grade 3 skin toxicities found in arm B and arm C were 47.36% and 52.38%, respectively, but in arm A, it was 30%. Mucositis Grade 3 was higher in arm B (63.16%) and arm C (61.9%) compared with arm A (35%) but no statistical significance can be drawn. Complete response in arm B and arm C were 26.315% and 23.809%, respectively. Stable disease in arm A at 6 months follow up was 35% whereas in arm B and arm C, it was 10.526% and 14.29%, respectively, again carrying no statistical significance (P = 0.3385).
Conclusions
There is a trend toward improved overall survival with hyperfractionation, but no difference in cause-specific survival in literatures. In our study no significant difference in response to all the three arms found. Grade 1 neutropenia found to be higher in arm B, which is statistically significant. So there is need for more studies to validate the concepts further.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Perez CA, Brady LW, Halperin CE. Lippincott Williams and Wilkins. 4th ed. A Wolters Kluwer business; 2008. Principles and practice of radiation oncology. Oral cavity cancer, oropharynx, hypopharynx cancer; pp. 891–958. [Google Scholar]
- 2.Mendenhall WM, Werning JW, Pfister DG. Treatment of Head and Neck Cancers. In: Devita VT, Lawrence TS, Rosenberg SA, editors. CANCER Principles and Practice of Oncology. Lippincott Williams and Wilkins. 8th ed. A Wolters Kluwer business; 2008. pp. 809–13. [Google Scholar]
- 3.Development of an atlas of cancer in India. National cancer registry programme (Indian Council of Medical Research). April. 2004. [Last accessed date 27th June, 2012]. Available from: http://www.canceratlasindia.org .
- 4.Rath GK, Mohanty BK, Bahadur S, Lal P, Gairola M. 1st ed. Chapter 7. Elsevier: Reed Elsevier India private limited. 2000 reprinted 2007; Cancer of head and neck, Text book of radiation oncology-principles and practice; pp. 131–99. [Google Scholar]
- 5.Park K. 19th ed. M/S Banarsidas Bhanot; 2000. Park's textbook of preventive and social medicine; pp. 319–20. [Google Scholar]
- 6.Magnitude and leading sites of cancer. [Last accessed on 2010 Oct]. Available from: http://www.ncrpindia.org/Old_Reports/Five_Years_consolidated_HBCR_report_1994_98.pdf .
- 7.Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80:827–41. doi: 10.1002/(sici)1097-0215(19990315)80:6<827::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 8.Cummings B, Keane T, Pintilie M, Warde P, Waldron J, Payne D, et al. Five year results of a randomized trial comparing hyperfractionated to conventional radiotherapy over four weeks in locally advanced head and neck cancer. Radiother Oncol. 2007;85:7–16. doi: 10.1016/j.radonc.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Hall Eric J, Giaccia Amato J. 6th edition. E. J Hall, Lippincott Williams and Wilkins; 2006. Time, Dose and Fractionation in Radiotherapy, Radiology For The Radiologists; pp. 378–397. [Google Scholar]
- 10.Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head neck squamous cell cancer: Three meta analyses of updated individual data. MACH-NC Collaborative Group. Meta Analyses of Chemotherapy on Head and Neck cancer. Lancet. 2000;355:949–55. [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–6. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 13.Sharma A, Mohanti BK, Thakar A, Bahadur S, Bhasker S, Bahl A. Concomitant chemoradiation versus radiotherapy in advanced squamous cell carcinoma of oropharynx and nasopharynx using weekly cisplatin: Final result of a phase III trial. J Clin Oncol. 2007;25:6030. doi: 10.1093/annonc/mdq219. [DOI] [PubMed] [Google Scholar]
- 14.Overgaard J, Hansen HS, Specht L, Overgaard M, Grau C, Andersen E, et al. Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head neck: DAHANCA 6 and 7 randomized controlled trial. Lancet. 2003;362:933–40. doi: 10.1016/s0140-6736(03)14361-9. [DOI] [PubMed] [Google Scholar]
- 15.Fu KK, Pajak TF, Trotti A, Jones CU, Spencer SA, Phillips TL, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: First report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 16.Krstevska V, Crvenkova S. Altered and Conventional Fractionated Radiotherapy in Locoregional Control and Survival of Patients with Squamous Cell Carcinoma of the Larynx, Oropharynx, and Hypopharynx. Croat Med J. 2006;47:42–52. [PMC free article] [PubMed] [Google Scholar]
- 17.Skladowski K, Maciejewski B, Golen M, Pilecki B, Przeorek W, Tarnawski R. Randomized clinical trial on 7-day-continuous accelerated irradiation (CAIR) of head and neck cancer-report on 3-year tumour control and normal tissue toxicity. Radiother Oncol. 2000;55:101–10. doi: 10.1016/s0167-8140(00)00139-0. [DOI] [PubMed] [Google Scholar]
- 18.Sanguineti G, Richetti A, Bignardi M, Corvo R, Gabriele P, Sormani MP, et al. Accelerated versus conventional fractionated postoperative radiotherapy for advanced head and neck cancer: Results of a multicenter Phase III study. Int J Radiat Oncol Biol Phys. 2006;61:762–71. doi: 10.1016/j.ijrobp.2004.07.682. [DOI] [PubMed] [Google Scholar]
- 19.Trotti A, Fu KK, Pajak TF. Long term outcomes of RTOG 90-03: A comparison of hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2005;63:S70–1. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]


