Abstract
Background:
Mediastinal tumors are an uncommon abnormalities found in clinical practice. Anterior mediastinum is the common site and tissue diagnoses of anterior mediastinal masses (AMMs) are very important for correct therapeutic decision.
Objective:
We evaluate the different malignant AMMs in various age groups and the sensitivity of fine needle aspiration cytology (FNAC) and core needle biopsy (CNB). Cytology smears are reviewed with particular emphasis on pitfalls in the cytological diagnosis.
Materials and Methods:
This was a prospective study of 50 patients who were consulted for AMMs and underwent FNAC and CNB under guidance of ultrasound or computed tomography (CT) scan from 2006 to 2011. Cytology smears and histological sections were evaluated in all patients.
Results:
Among 50 cases, 36 were male and 14 were female. Most AMMs (52%) were identified in the fifth and sixth decades of life. Metastatic carcinoma and nonHodgkin's lymphoma are the common AMMs. Adequate tissue material was obtained in 49 of 50 cases by CNB. Of these 49 patients, 35 (71.42%) cases were diagnosed correctly by FNAC, whereas 14 (28.57%) cases were not diagnosed definitely by FNAC. The sensitivity of CNB for AMMs was 97.95%, significantly higher than FNAC (71.42%) (P < 0.05). CNB had statistically significant higher diagnostic rate than FNAC in the noncarcinoma group (100% versus 62.96%) (P < 0.05). There is no significant difference of CNB and FNAC in carcinoma group (P > 0.05). Diagnostic rate of FNAC was higher for carcinomatous lesions (81.81%) than for noncarcinomatous lesions (62.96%).
Conclusion:
Ultrasound or CT scan-guided CNB in combination with FNAC are safe, minimally invasive, and cost-effective procedure, which can provide a precise diagnosis in the AMMs, and may obviate the need for invasive surgical approach. FNAC usually suffice for carcinomatous lesions but CNB should be performed whenever the diagnosis of carcinoma is equivocal or noncarcinoma lesions are suspected.
Keywords: Anterior mediastinal masses, core needle biopsy, fine needle aspiration cytology
Introduction
Mediastinal mass is an uncommon abnormality found in clinical practices.[1,2] Malignant mediastinal tumors are increasing since past four decades.[3] Anterior mediastinal masses (AMMs) are common and include a wide variety of tumors and remain an interesting diagnostic challenge.[1,2,3,4,5] Management strategies are diverse and depend strongly on the pathological diagnosis and the extent of the disease.[1,4,6,7,8] Procedures for the tissue diagnosis of AMMs have been diverse, including minimally invasive transthoracic or transbronchial fine needle aspiration cytology (FNAC), core needle biopsy (CNB), mediastinoscopy, video-assisted thoracoscopy, and more traumatic open biopsy.[1,4,6,7,8,9] Most groups have used FNAC techniques.[4] However, adequacy of cytology samples recovered from FNAC is the critical point of question among pathologists and physicians.[1,6] Repeated FNAC procedure due to inadequate cytological material delays the diagnosis and specific therapy, which increases the risk of invasive surgical approach and prolonged hospital stay.[1,6] A larger tissue sample obtained by CNB allows more architectural, cytological, and immunohistochemical studies, which will increase the diagnostic accuracy. CNB may obviate the need for more invasive diagnostic procedures.[1,6,9,10,11] There are very few reports available in the literature regarding the diagnostic methods, cyto-histological correlation and changing patterns of anterior mediastinal tumors.[1,4,12] We report a prospective study comparing the usefulness of CNB and FNAC under local anesthesia and guidance of ultrasound or CT scan for AMMs. In our observation, CNB in combination with FNAC has a major role in diagnosis of AMMs. Most malignant lesion can be accurately diagnosed on CNB. CNB have higher diagnostic rate than FNAC in noncarcinomatous patients. We examined cytological features of different AMMs with its histological correlation and immunohistochemistry confirmation in some cases. The reason for discordant cytological diagnosis and pitfalls of entities are discussed.
Materials and Methods
This was a prospective study performed on 50 patients who underwent CNB and FNAC for their AMMs over a 3-year period from 2008 to 2011. Clinical history was obtained from patients and case record file. Out of 50 patients, 47 patients were symptomatic with presenting clinical features of dyspnea, cough, chest pain, hoarseness of voice, weight loss, and features suggestive of myasthenia gravis. In other three patients, AMMs were detected on routine physical examination. In evaluating AMMs we obtained both cytologic slides and tissue for histology. The patients were informed about the procedure. Coagulation tests were obtained. After skin cleaning, local anesthesia (10 cc of 2% xylocaine) was given, taking particular care to infiltrate the periosteum of the sternal margin. All procedures were performed under guidance of ultrasound or computed tomography (CT) scan. Tumor size ranged from large infiltrative tumor (7 cm) to small nodules (1.5 cm). All lesions were considered potentially malignant. The area from skin to the mass was evaluated for vessels and the distance from the skin to mass was determined. The second, third, or fourth intercostal space on both sides can be approached, depending on the location and the extension of the lesion. We used a 22-gauge spinal needle (15 cm in length) attached to a 10 ml disposable syringe to obtain cytological material. CNB were performed using a 14- or 16-gauge semi-automated cut needle (15 cm in length) to obtain histological tissue. The number of needles passed depended on adequacy of the sample retrieved. Usually one to three passes was made. A repeat ultrasound examination was performed to evaluate any complication such as localized hematoma or pneumothorax. In all cases the chest radiograph was done 4 hours after biopsy and it was remained unchanged from the prebiopsy chest radiograph except in one case showing moderate pneumothorax. If the vital parameters remained normal, the patient was discharged after about 4-6 hours.
Air-dried and wet-fixed (95% alcohol) cytology smears were prepared and stained by May–Grunwald–Giemsa (MGG) and Papanicolaou stains or hematoxylin–eosin stain, respectively. The biopsy tissues were fixed in 10% formalin, processed, embedded in paraffin, 4 micron thin sections were cut and stained by hematoxylin–eosin stain. If the result of histopathologic study was not definite, immunohistochemical studies were performed on biopsies by direct avidin–biotin–peroxidase method using various antibodies. The antibodies like cytokeratins, epithelial membrane antigen, leukocyte common antigen, alpha-fetoprotein, beta human gonadotropins, neuron-specific enolase, and chromogranins were used. In addition, since prediction of malignancy in thymoma is difficult to make on purely cellular features, all thymomas diagnosed in this series were considered potentially malignant. The AMMs were also divided into a carcinomatous group and a noncarcinomatous group. Diagnostic accuracy of these groups by FNAC and CNB were compared using, Chi-square test. The P < 0.05 was considered statistically significant difference. Cyto-histological correlation was done and incorrect cytological diagnoses were reviewed with special attention. The reasons for discordant cytology diagnosis and pitfalls of entities were evaluated. The patients were followed up for their definite diagnosis either by noting their response to therapy or by surgical resection of the masses.
Results
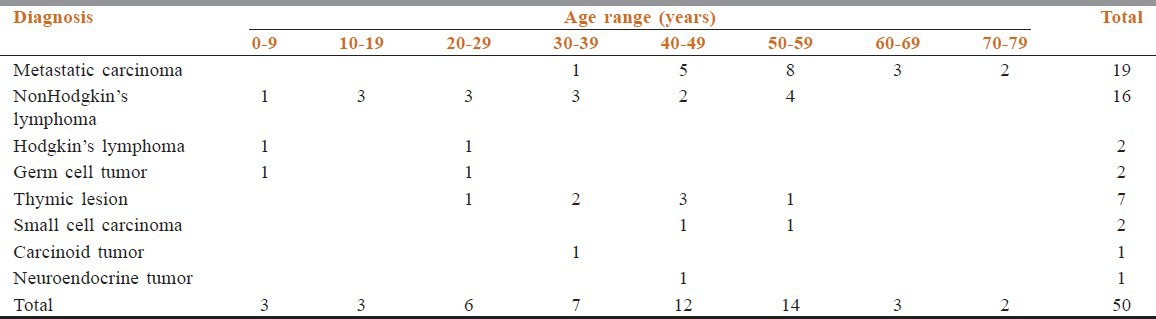
Among 50 patients who underwent FNAC and CNB for their AMMs, there were 36 male and 14 female with the mean age of 38.6 years (range 1-76 years). The male to female ratio was 2.5:1. Age wise distribution of the patients along with different AMMs is shown in Table 1. Most anterior mediastinal tumors (52%) were identified in the fifth and sixth decades of life. Metastatic carcinoma remains the most common entity followed by nonHodgkin's lymphoma (NHL). Metastatic carcinomas were more common in fifth to eighth decade of life. The most common malignancy during the first four decades of life was NHL [Table 1].
Table 1.
Distribution of anterior mediastinal masses in relation to age groups
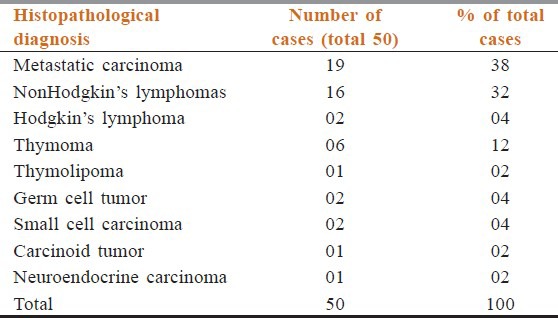
In 49 out of 50 cases, the CNB material was adequate for evaluation. However, histological diagnosis was achieved in 48 out of 49 cases. One case had unsatisfactory material to evaluate both cyto-histological features, mini-mediastinotomy suggest nodular sclerosing Hodgkin's lymphoma. One case had inadequate material on CNB in form of only necrotic tissue but on FNAC definitive diagnosis of squamous cell carcinoma was evident. Histopathologically, 19 (38%) patients had metastatic carcinoma, 16 (32%) had NHL, 2 (04%) had Hodgkin's lymphoma, 6 (12%) had thymic pathology, 1 (02%) had thymolipoma, 2 (04%) had germ cell tumor, 2 (04%) had small cell carcinoma, 1 (02%) had carcinoid tumor, and 1 (02%) had neuroendocrine carcinoma [Table 2].
Table 2.
Histopathological diagnosis of anterior mediastinal masses
In 49 patients, 35 (71.42%) patients were diagnosed correctly by FNAC and cyto-histologic correlation was found; whereas 14 (28.57%) patients were not diagnosed definitely by FNAC. In three patients cytological material was inadequate for examination due to hemorrhagic smear and very scanty cellularity. Hence, overall sensitivity was 71.42% for FNAC. The sensitivity of CNB for AMMs was 97.95%, significantly higher than FNAC (71.42%) and it was statistically significant (P < 0.05) [Tables 3 and 4]. A comparison of the results of FNAC and CNB is presented in Table 3. To further evaluate our results a biopsy was regarded as being accurate as further clinical follow up and subsequent investigations failed to establish an alternative diagnosis.
Table 3.
Comparison of histological and cytological diagnosis in carcinoma and noncarcinoma group
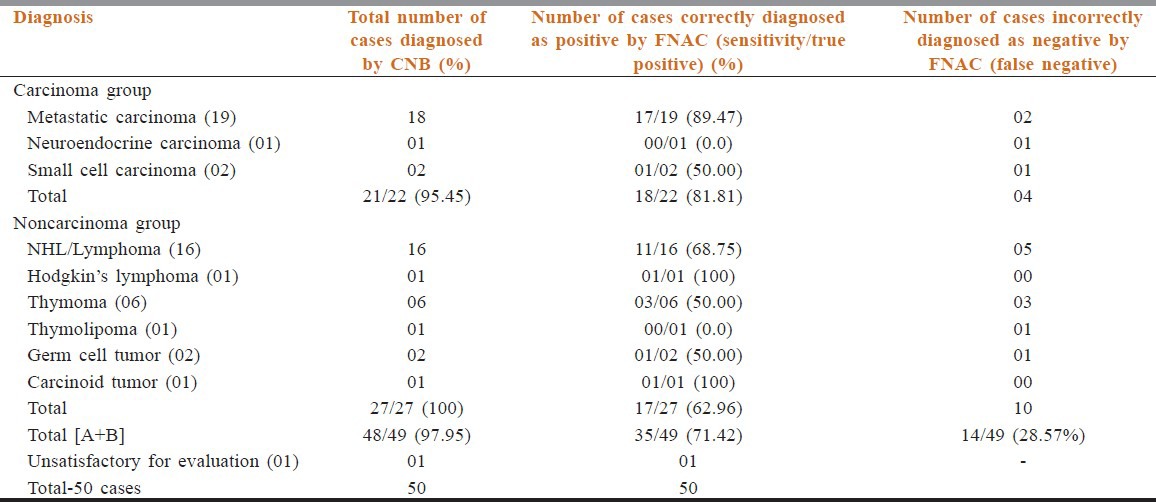
Table 4.
Diagnostic rate of anterior mediastinal masses by cytology and histology in carcinoma group compared with noncarcinoma group
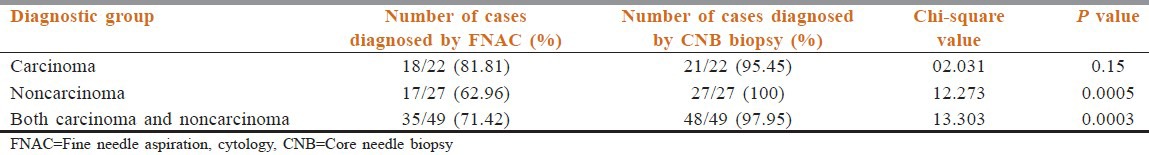
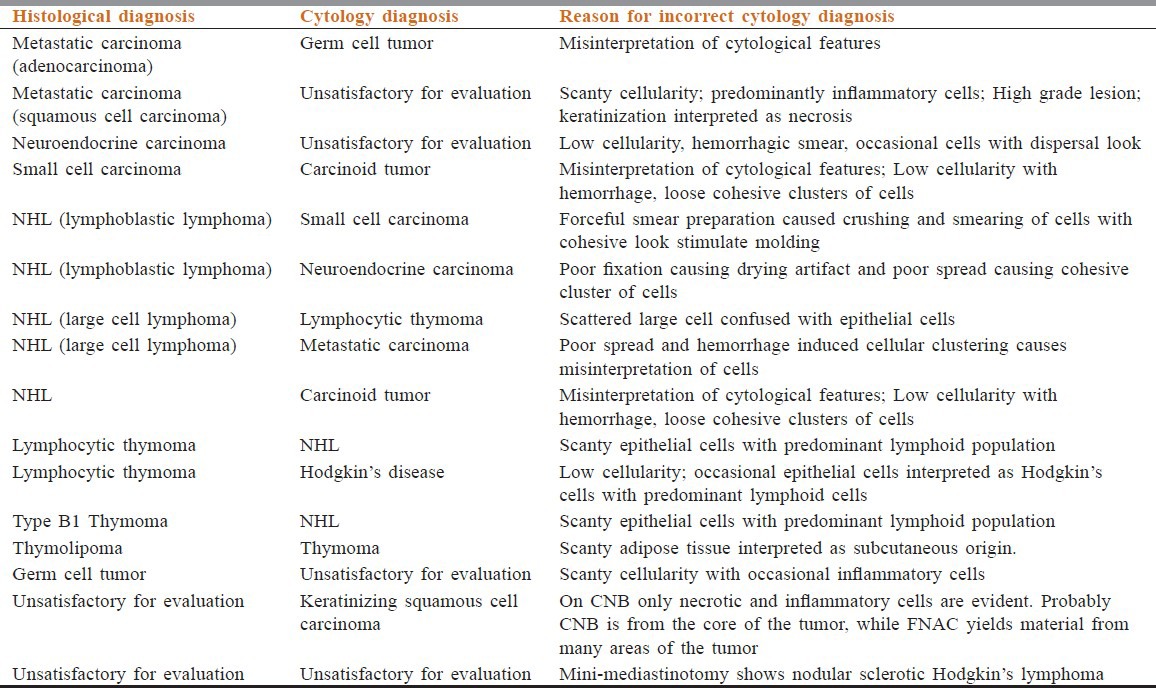
These 49 cases were divided into carcinoma and noncarcinoma group. The proportion of noncarcinoma group is more (27 cases; 55.10%) as compared with carcinoma group (22 cases; 44.89%). Carcinoma group composed of metastatic adenopathy of the mediastinal nodes (18), small cell lung cancer (2), and neuroendocrine carcinoma (1). In noncarcinomatous group, tumors include NHL (16), Hodgkin's lymphoma (01), thymoma (6), thymolipoma (1), germ cell tumor (2), and carcinoid tumor (1). Cytology of NHL showed dispersed monotonous population of cells with coarse granular chromatin and lymphoid globules in background. Hodgkin lymphoma showed Reed–Sternberg cells, Hodgkin cells in the background of lymphocytes, plasma cells, eosinophils, and histiocytes [Figure 1]. Cytology of thymic neoplasm revealed cohesive tissue fragments of oval to spindle epithelial cells admixed with lymphoid cells. Carcinoid tumor was suggested by dispersed monotonous small neoplastic ells with rounded to oval nuclei with salt-paper chromatin, small nucleoli with small intact cytoplasm. Features of teratoma were keratinous debris, squamous cells, glandular epithelium with hair shaft material. Germinoma showed dispersed as well as loose clusters of large cells having rounded nuclei, prominent single or multiple nucleoli and abundant fragile cytoplasm in tigroid and lymphocytic background. FNAC of carcinoma (81.81%) showed higher diagnostic rate than noncarcinoma group (62.96%). There was statistically significant higher diagnostic rate of CNB than FNAC in noncarcinoma group (100% versus 62.96%) (P < 0.05). However, CNB in combination with FNAC neutralized this difference in diagnostic rate in noncarcinoma and carcinoma group [Table 4]. The cases with discordant cytological diagnosis along with possible reasons were evaluated [Table 5]. Only one of the 50 patients (2% of patient) developed moderate pneumothorax following CNB requiring intercostal drainage. No mortality was evident.
Figure 1.
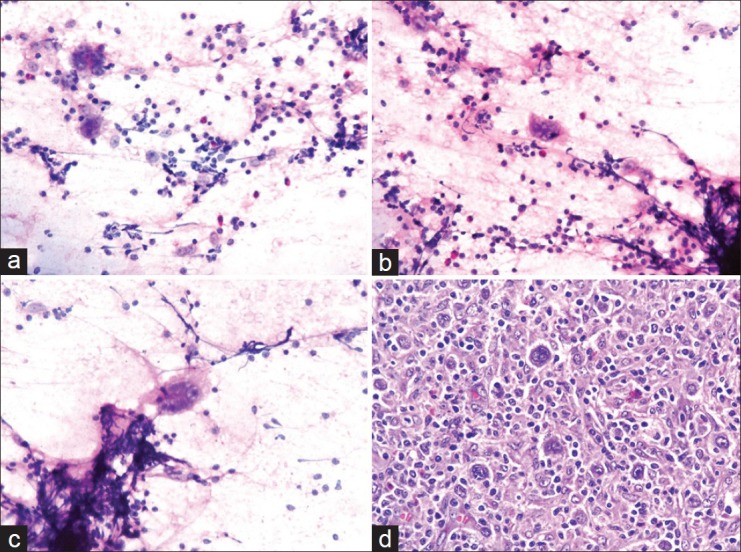
(a-c) Cytology smear shows binucleated Reed–Sternberg cells and mononuclear Hodgkin cells in the background of lymphocytes, eosinophils, plasma cells, and histiocytes (Hematoxylin–eosin stain, ×40); (d) Histological section shows mixed cellularity Hodgkin's lymphoma. Several Reed–Sternberg cells are seen with a polymorphic population of lymphocytes, eosinophils, plasma cells, and histiocytes (Hematoxylin–eosin stain, ×40)
Table 5.
Evaluation of anterior mediastinal masses with discordant cytology diagnosis
Discussion
Mediastinum is the site of variety of lesions, ranging from inflammatory to neoplastic, benign to malignant, primary to metastatic lesions.[5,7] Primary mediastinal tumors are uncommon representing about 3% of tumors within the chest wall.[3,5] As many as 25-40% of these lesions are malignant.[5] Adler et al. and Jereb et al. reported a higher incidence of about 72% prevalence of malignancy in their study.[13,14] Vaziri et al. showed 60% of malignancy in their study.[3] Majority of tumors are seen in the anterior mediastinum.[1,2,3,5] The knowledge of the nature of AMMs is very important for making correct diagnosis and therapeutic decisions.[2,3,4,5,6,10] Usually mediastinal masses are picked-up by clinical examination and radio-imaging appearance.[1,5,6] Radio-imaging are widely used for detection of mediastinal masses and their extension. Tissue characterization by these techniques is not sufficient and even for distinguishing malignant from benign tumors.[6] Anterior mediastinum includes a variety of lesions, which are medically treatable such as lymphomas; surgically treatable such as thymoma or that are clearly nonresectable such as metastatic carcinoma, it is clear that a precise histopathological diagnosis is essential.[1,3,4,5,6,10,12]
Tissue diagnosis of AMMs can be performed by a variety of techniques ranging from FNAC and CNB to surgical procedures allowing biopsy as well as resection.[1,4,6,7,8,9] The first priority is to provide positive histological diagnosis with the lowest possible risk.[8] Procedures like mediastinoscopy, thoracoscopy, mediastinotomy, or thoracotomy are traditionally used for determining the nature of these tumors, which require intubation and general anesthesia.[6,7,9,10] Open biopsy can certainly assure a definite histological diagnosis. Although the diagnostic rate might be as high as 100%, they are associated with significant morbidity, increased chance of pleural dissemination, and poor long-term results.[7,8,10] For this reason, the surgically oriented strategies are no longer considered suitable for malignancies of the anterior mediastinum.[3,6,7,11] An ideal diagnostic procedure should have a high yield and as minimally invasive as possible.[7,8] The present study is the fourth report as far as literature surveyed are concerned in comparing FNAC and CNB results in the diagnosis of AMMs.[1,4,11] The purpose of this study was to evaluate the clinical utility of CNB and FNAC in the diagnosis of AMMs and to determine whether CNB in combination with FNAC should be the initial diagnostic procedure.
Percutaneous FNAC and CNB under ultrasound or CT scan guidance have a major role in the diagnosis of AMMs and several advantages over open biopsy.[1,4,6,10,11,12,15] Both FNAC and CNB biopsies were undertaken to evaluate AMMs in this series. FNAC and CNB performed safely and easily without any discomfort to the patient. Moreover, hospitalization was short and the total costs were minimal. Ultrasound and CT scan permitted ready identification of the major vessels, the needle tip can be clearly identified within the lesion, and it was relatively easy to maintain a sterile field during the procedure. Ultrasound had the advantage over CT of allowing real time imaging leading to quicker procedure.
In our study, cyto-histologic correlation was found in 71.42% cases, similar to study by Desai et al.[4] Sensitivity of CNB in the diagnosis of AMMs was 97.95%, which was similar to study by Annessi et al.[9] and higher than study by Hsu et al.[16] and Safavi et al.[6] The sensitivity of FNAC in our series was 71.42%, significantly lower than CNB (97.95%). A total of 14 cases were misdiagnosed by FNAC. FNAC seems to be the least invasive diagnostic measure than CNB. However, they were usually associated with unsatisfactory results like in other studies.[6,7,17,18,19] The failure was due to the minimal amount of tissue harvested through fine needle aspiration. In many studies, making a specific diagnosis is not possible by FNAC, and only a classification into “malignant cells” or “non malignant cells” will be achieved. FNAC may suggest a diagnosis of lymphoma, but differentiation between Hodgkin's disease, NHL, and thymoma can be difficult.[6,17,18,19] In such instances larger core specimens by CNB are usually required for more precise diagnosis, allowing histological rather than cytological evaluation and special staining methods including immunohistochemical techniques.[6,7,8,11,20,21,22] It is possible to establish the histological subtypes on CNB.[9,10] We found that most malignant lesions can be diagnosed on CNB more accurately than FNAC. Hence, CNB may be alternative to surgical procedures with high diagnostic accuracy and less morbidity. A major advantage of FNAC was that immediate cytological examination of the specimen was possible and then pathologist can direct the clinician appropriately.
In our study most patients with AMMs were symptomatic with chief complaint of dyspnea, cough, chest pain, and weight loss. Most AMMs in our series were malignant. It is to be noted that the incidence of benign versus malignant lesions varies with the lesion under consideration, the location of the mass, and the hospital referral patterns.[3] In our cases, there was a male preponderance. Most AMMs in this series were identified in the fifth and sixth decade of life (52%), while Vaziri et al. study showed that third and fifth decades were common.[3] The most common tumor in our series was metastatic carcinoma (38%) followed by NHL (32%), while NHL were common in the study by Shrivastava et al.[2] In the study by Shabb et al., lymphoma followed by metastatic tumors was the most common lesions.[5] From the first to fourth decades, NHL was the most common malignant tumor, similar to study by Vaziri et al.[3]
In our study, malignant AMMs were divided into carcinoma and noncarcinoma groups. The proportion of noncarcinoma group was more (27 cases; 55.10%) as compared with carcinoma group (22 cases; 44.89%). Our best results of FNAC were achieved in the diagnosis of carcinoma lesions (81.81%), with lower figures for the diagnosis of noncarcinoma lesions (62.96%). FNAC is diagnostic in majority of carcinoma including metastatic carcinoma. CNB had statistically significant higher diagnostic rate than FNAC in the noncarcinomatous group (P < 0.05). Our findings are similar to previous studies by Hsu et al.,[23] Tscheikuna et al.,[1] Yang et al.,[24] and Morrissey et al.[11] There was no significant difference between CNB and FNAC in carcinoma group. When comparing CNB in combination with FNAC, this difference was not significant and this combination yields the diagnosis of 98%. CNB is more valuable and helpful than FNAC in the diagnosis of malignant AMMs. On the basis of our experience we suggest that, when carcinoma is suspected, FNAC should be performed as the initial procedure. If this provides the diagnosis of carcinoma, further biopsy is usually unnecessary. If insufficient material is obtained or chances of noncarcinomatous lesion is more, then CNB should be performed whenever practical for greater diagnostic accuracy.
FNAC material was unsatisfactory in 4 out of 50 (8%) cases in our study, compared with 18.51% cases in the study by Desai et al.[4] CNB was inadequate in 1 out of 50 (2%) cases in our study, compared with 11.76% cases in study by Safavi et al.[6] The reason for failing to achieve a definite diagnosis via CNB lies not in the amount of tissue harvested, as all specimens obtained in Fang Wen-Tao's series and in Watanabe's series were sufficient for histological examination like in our case.[7] One reason is the location of tissue harvested during biopsy; since all locally advanced tumors have necrotic center due to rapid tumor growth. When biopsy was carried out via a cutting core needle, the target area would be either at or close to the center of the tumor. It is difficult, if not entirely impossible, to tell if the target area is necrotic or not under CT or ultrasonography.[7] This problem is probably avoided by FNAC as cytology material is taken from multiple areas of the tumor, from center to edge of the tumor. This explains the failure to achieve the diagnosis on CNB in single case, while FNAC gives definite diagnosis as viable tumor cells are evident on cytology.
In the 14 patients the cytology diagnosis was changed by CNB. The common technical factors associated with misdiagnosis were poor spread, poor fixation low cellularity, and hemorrhage. When cellularity was found to be adequate without any technical fault, the cell size and patterns of different lesion were confused with each other. In most cases, more than one factor was responsible. Our findings are similar to those found by other studies.[4,25,26] Our findings suggest that to avoid diagnostic errors on optimally prepared smears, one should have adequate knowledge and experience of the site specific common lesion. The ultrasound or CT scan guided use of large-bore needles helps to overcome diagnostic difficulties encountered with the FNAC. CNB provides more confident diagnosis and important additional information. The diagnostic rate of FNAC and CNB in AMMs is dependent on the amount of the specimens, which depend on the size of the needles used and number of passes.
The incidence of complications in this series were very small and compares favorably with previously published series.[1,3,6,9,10,11] Pneumothorax is the most frequent complication resulting from CNB in thoracic lesion.[1,6,9,17] In our series only a single patient required an intercostals drain for a moderate pneumothorax. In most cases the mediastinal lesion would have been in direct contact with the chest wall and accessible without traversing the lung or pleura. This explanation supports the low incidence of pneumothorax in our study. This low episode of complication might be due to real time observing of the needle by ultrasound during biopsy procedure.
Conclusion
The rates of nonsurgical tumors such as lymphoma are higher and the rates of traditionally surgical diseases such as thymomas are lower. Prompt and correct diagnosis of AMMs is the key process in therapeutic decision. The precise nature of AMMs cannot be determined without histology examination of the tissue. In variety of AMMs, an extensive resection without a definitive preoperative diagnosis is not indicated. In such instances, FNAC and CNB under ultrasound or CT scan guidance allows adequate sampling of tissue with lowest possible risk and discomfort to patients. This procedure is safe, easy, and may obviate the need for more extensive diagnostic surgical procedures while yielding comparable results. When many etiologic cells of origin cannot be diagnosed accurately by cytology alone, the CNB for small histology section is recommended as an initial investigation method with FNAC. The advantage of CNB over FNAC is high diagnostic yield and sensitivity. We recommend the use of FNAC as the initial procedure when the probability of carcinoma is high. The use of CNB to obtain larger tissue specimens is recommended when cytology diagnosis of carcinoma is uncertain, or when a chance of noncarcinoma lesion is more likely.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Tscheikuna J, Suttinont P. Is cytology necessary in diagnosis of mediastinal mass? J Med Assoc Thai. 2009;92:S24–9. [PubMed] [Google Scholar]
- 2.Shrivastava CP, Devgarha S, Ahlawat V. Mediastinal tumors: A clinicopathological analysis. Asian Cardiovasc Thorac Ann. 2006;14:102–4. doi: 10.1177/021849230601400204. [DOI] [PubMed] [Google Scholar]
- 3.Vaziri M, Pazooki M, Zahedi-Shoolami L. Mediastinal Masses: Review of 105 Cases. Acta Med Iran. 2009;47:297–300. [Google Scholar]
- 4.Desai F, Shah M, Patel S, Shukla SN. Fine needle aspiration cytology of anterior mediastinal masses. Indian J Pathol Microbiol. 2008;51:88–90. doi: 10.4103/0377-4929.40413. [DOI] [PubMed] [Google Scholar]
- 5.Karki S, Chalise S. Analysis of mediastinal lesions: A study of 27 cases. Journal of Pathology of Nepal. 2011;1:114–7. [Google Scholar]
- 6.Safavi E, Hosseinian SM, Firoozbakhsh S. The value of percutaneous core needle biopsy in the diagnosis of anterior mediastinal tumors. Tanaffos. 2004;3:7–11. [Google Scholar]
- 7.Fang WT, Xu MY, Chen G, Chen Y, Chen WH. Minimally invasive approaches for histological diagnosis of anterior mediastinal masses. Chin Med J (Engl) 2007;120:675–9. [PubMed] [Google Scholar]
- 8.Rendina EA, Venuta F, De Giacomo T, Ciccone AM, Moretti MS, Ibrahim M, et al. Biopsy of anterior mediastinal masses under local anesthesia. Ann Thorac Surg. 2002;74:1720–2. doi: 10.1016/s0003-4975(02)03821-3. [DOI] [PubMed] [Google Scholar]
- 9.Annessi V, Paci M, Ferrari G, Sgarbi G. Ultrasonically guided biopsy of anterior mediastinal masses. Interact Cardiovasc Thorac Surg. 2003;2:319–21. doi: 10.1016/S1569-9293(03)00068-9. [DOI] [PubMed] [Google Scholar]
- 10.Zinzani PL, Corneli G, Cancellieri A, Magagnoli M, Lacava N, Gherlinzoni F, et al. Core needle biopsy is effective in the initial diagnosis of mediastinal lymphoma. Haematologica. 1999;84:600–3. [PubMed] [Google Scholar]
- 11.Morrissey B, Adams H, Gibbs AR, Crane MD. Percutaneous needle biopsy of the mediastinum: Review of 94 procedures. Thorax. 1993;48:632–7. doi: 10.1136/thx.48.6.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samad SA, Sharifah NA, Zulfiqar MA, Maimunah A, Yahya A, Zainudin W. Ultrasound guided percutaneous biopsies of suspected mediastinal lesions. Med J Malaysia. 1993;48:421–6. [PubMed] [Google Scholar]
- 13.Adler OB, Rosenberger A, Peleg H. Fine-needle aspiration biopsy of mediastinal masses: Evaluation of 136 experiences. AJR Am J Roentgenol. 1983;140:893–6. doi: 10.2214/ajr.140.5.893. [DOI] [PubMed] [Google Scholar]
- 14.Jereb M, Us-Krasovec M. Transthoracic needle biopsy of mediastinal and hilar lesions. Cancer. 1977;40:1354–7. doi: 10.1002/1097-0142(197709)40:3<1354::aid-cncr2820400353>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 15.Weisbrod GL. Percutaneous fine-needle aspiration biopsy of the mediastinum. Clin Chest Med. 1987;8:27–41. [PubMed] [Google Scholar]
- 16.Hsu WH, Chiang CD, Hsu JY, Kwan PC, Chen CL, Chen CY. Ultrasonically guided needle biopsy of anterior mediastinal masses: Comparison of carcinomatous and non-carcinomatous masses. J Clin Ultrasound. 1995;23:349–56. doi: 10.1002/jcu.1870230604. [DOI] [PubMed] [Google Scholar]
- 17.Zafar N, Moinuddin S. Mediastinal needle biopsy. A 15-year experience with 139 cases. Cancer. 1995;76:1065–8. doi: 10.1002/1097-0142(19950915)76:6<1065::aid-cncr2820760622>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 18.Wernecke K, Vassallo P, Peters PE, von Bassewitz DB. Mediastinal tumors: Biopsy under US guidance. Radiology. 1989;172:473–6. doi: 10.1148/radiology.172.2.2664870. [DOI] [PubMed] [Google Scholar]
- 19.Weisbrod GL, Lyons DJ, Tao LC, Chamberlain DW. Percutaneous fine-needle aspiration biopsy of mediastinal lesions. AJR Am J Roentgenol. 1984;143:525–9. doi: 10.2214/ajr.143.3.525. [DOI] [PubMed] [Google Scholar]
- 20.Böcking A, Klose KC, Kyll HJ, Hauptmann S. Cytologic versus histologic evaluation of needle biopsy of the lung, hilum and mediastinum. Sensitivity, specificity and typing accuracy. Acta Cytol. 1995;39:463–71. [PubMed] [Google Scholar]
- 21.Sawhney S, Jain R, Berry M. Tru-Cut biopsy of mediastinal masses guided by real-time sonography. Clin Radiol. 1991;44:16–9. doi: 10.1016/s0009-9260(05)80219-3. [DOI] [PubMed] [Google Scholar]
- 22.Yu CJ, Yang PC, Chang DB, Wu HD, Lee LN, Lee YC, et al. Evaluation of ultrasonically guided biopsies of mediastinal masses. Chest. 1999;100:399–405. doi: 10.1378/chest.100.2.399. [DOI] [PubMed] [Google Scholar]
- 23.Hsu WH, Chiang CD, Hsu JY, Chen CY, Chiang CS, Lee T. Value of ultrasonically guided needle biopsy of pleural masses: An under-utilized technique. J Clin Ultrasound. 1997;25:119–25. doi: 10.1002/(sici)1097-0096(199703)25:3<119::aid-jcu4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 24.Yang PC, Lee YC, Yu CJ, Chang DB, Wu HD, Lee LN, et al. Ultrasonographically guided biopsy of thoracic tumors. A comparison of large-bore cutting biopsy with fine-needle aspiration. Cancer. 1992;69:2553–60. doi: 10.1002/1097-0142(19920515)69:10<2553::aid-cncr2820691027>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Singh HK, Silverman JF, Powers CN, Geisinger KR, Frable WJ. Diagnostic pitfalls in fine-needle aspiration biopsy of the mediastinum. Diagn Cytopathol. 1997;17:121–6. doi: 10.1002/(sici)1097-0339(199708)17:2<121::aid-dc7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 26.Geisinger KR. Differential diagnostic considerations and potential pitfalls in fine-needle aspiration biopsies of the mediastinum. Diagn Cytopathol. 1995;13:436–42. doi: 10.1002/dc.2840130512. [DOI] [PubMed] [Google Scholar]


