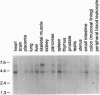
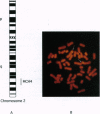
Abstract
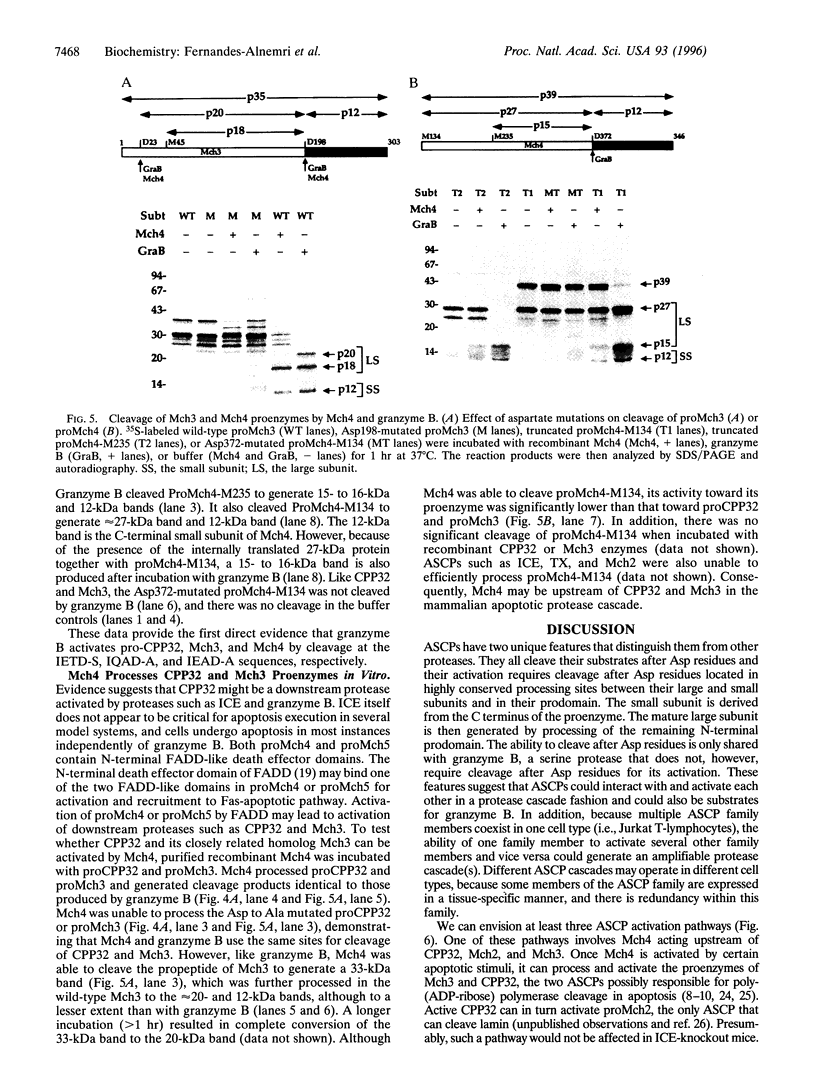
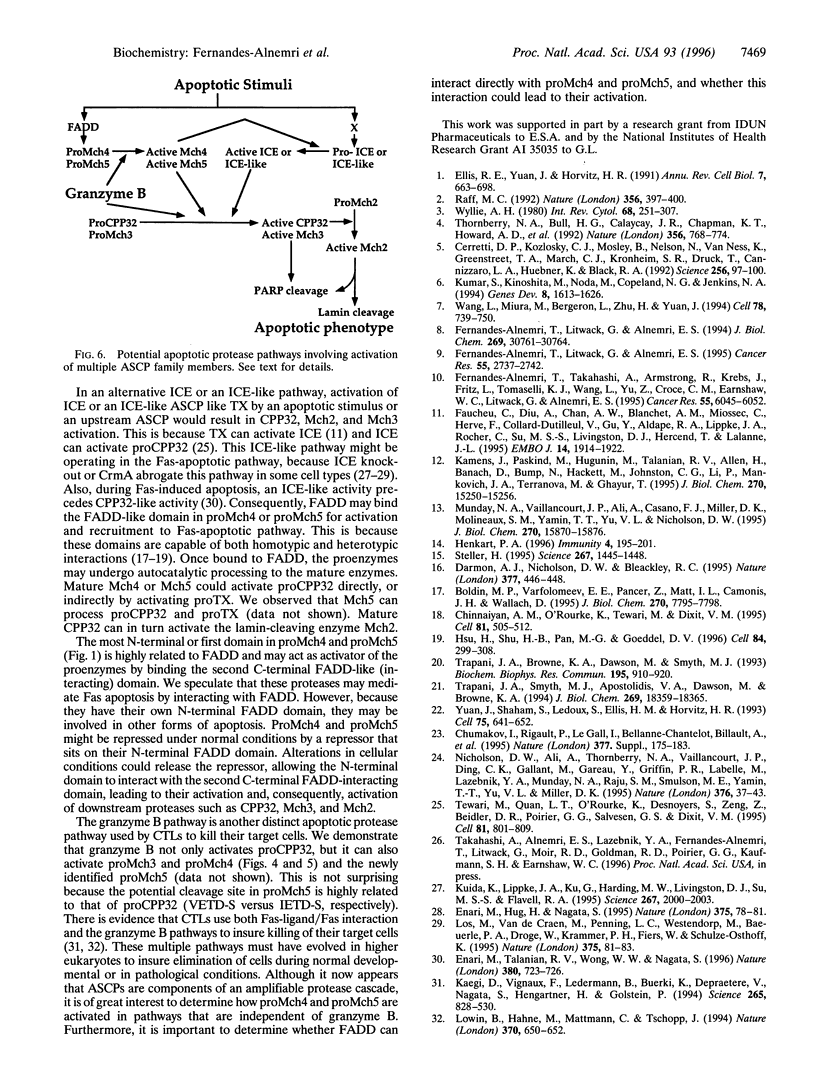
Emerging evidence suggests that an amplifiable protease cascade consisting of multiple aspartate specific cysteine proteases (ASCPs) is responsible for the apoptotic changes observed in mammalian cells undergoing programmed cell death. Here we describe the cloning of two novel ASCPs from human Jurkat T-lymphocytes. Like other ASCPs, the new proteases, named Mch4 and Mch5, are derived from single chain proenzymes. However, their putative active sites contain a QACQG pentapeptide instead of the QACRG present in ail known ASCPs. Also, their N termini contain FADD-like death effector domains, suggesting possible interaction with FADD. Expression of Mch4 in Escherichia coli produced an active protease that, like other ASCPs, was potently inhibited (Kj = 14 nM) by the tetrapeptide aldehyde DEVD-CHO. Interestingly, both Mch4 and the serine protease granzyme B cleave recombinant proCPP32 and proMch3 at a conserved IXXD-S sequence to produce the large and small subunits of the active proteases. Granzyme B also cleaves proMch4 at a homologous IXXD-A processing sequence to produce mature Mch4. These observations suggest that CPP32 and Mch3 are targets of mature Mch4 protease in apoptotic cells. The presence of the FADD-like domains in Mch4 and Mch5 suggests a role for these proteases in the Fas-apoptotic pathway. In addition, these proteases could participate in the granzyme B apoptotic pathways.
Full text
PDF

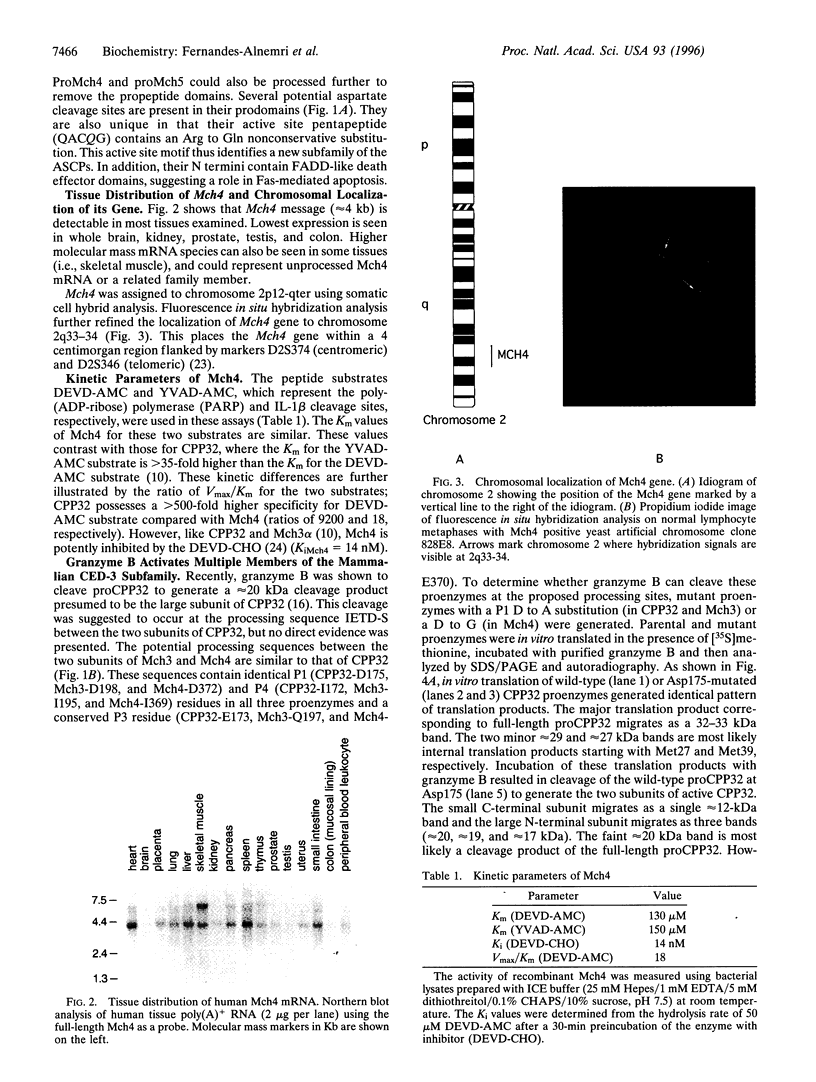
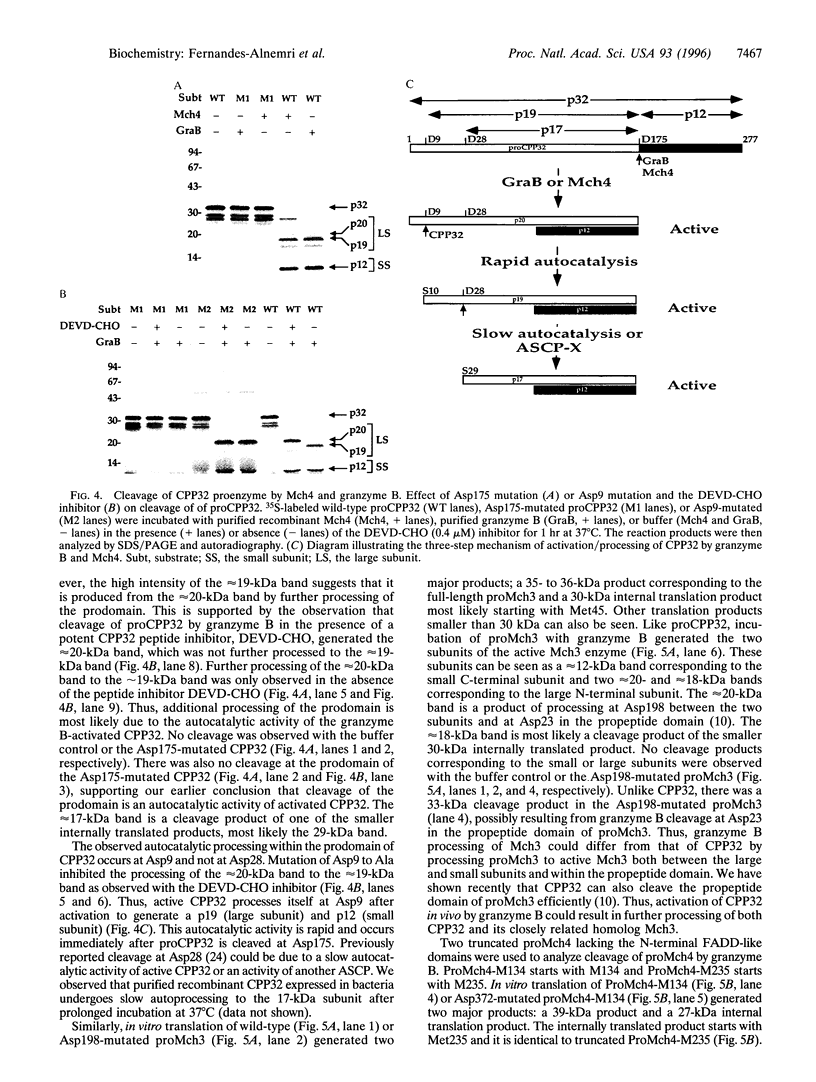
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boldin M. P., Varfolomeev E. E., Pancer Z., Mett I. L., Camonis J. H., Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem. 1995 Apr 7;270(14):7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- Cerretti D. P., Kozlosky C. J., Mosley B., Nelson N., Van Ness K., Greenstreet T. A., March C. J., Kronheim S. R., Druck T., Cannizzaro L. A. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992 Apr 3;256(5053):97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan A. M., O'Rourke K., Tewari M., Dixit V. M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995 May 19;81(4):505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Chumakov I. M., Rigault P., Le Gall I., Bellanné-Chantelot C., Billault A., Guillou S., Soularue P., Guasconi G., Poullier E., Gros I. A YAC contig map of the human genome. Nature. 1995 Sep 28;377(6547 Suppl):175–297. doi: 10.1038/377175a0. [DOI] [PubMed] [Google Scholar]
- Darmon A. J., Nicholson D. W., Bleackley R. C. Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature. 1995 Oct 5;377(6548):446–448. doi: 10.1038/377446a0. [DOI] [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Enari M., Hug H., Nagata S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature. 1995 May 4;375(6526):78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- Enari M., Talanian R. V., Wong W. W., Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996 Apr 25;380(6576):723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- Faucheu C., Diu A., Chan A. W., Blanchet A. M., Miossec C., Hervé F., Collard-Dutilleul V., Gu Y., Aldape R. A., Lippke J. A. A novel human protease similar to the interleukin-1 beta converting enzyme induces apoptosis in transfected cells. EMBO J. 1995 May 1;14(9):1914–1922. doi: 10.1002/j.1460-2075.1995.tb07183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T., Litwack G., Alnemri E. S. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994 Dec 9;269(49):30761–30764. [PubMed] [Google Scholar]
- Fernandes-Alnemri T., Litwack G., Alnemri E. S. Mch2, a new member of the apoptotic Ced-3/Ice cysteine protease gene family. Cancer Res. 1995 Jul 1;55(13):2737–2742. [PubMed] [Google Scholar]
- Fernandes-Alnemri T., Takahashi A., Armstrong R., Krebs J., Fritz L., Tomaselli K. J., Wang L., Yu Z., Croce C. M., Salveson G. Mch3, a novel human apoptotic cysteine protease highly related to CPP32. Cancer Res. 1995 Dec 15;55(24):6045–6052. [PubMed] [Google Scholar]
- Henkart P. A. ICE family proteases: mediators of all apoptotic cell death? Immunity. 1996 Mar;4(3):195–201. doi: 10.1016/s1074-7613(00)80428-8. [DOI] [PubMed] [Google Scholar]
- Hsu H., Shu H. B., Pan M. G., Goeddel D. V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996 Jan 26;84(2):299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- Kamens J., Paskind M., Hugunin M., Talanian R. V., Allen H., Banach D., Bump N., Hackett M., Johnston C. G., Li P. Identification and characterization of ICH-2, a novel member of the interleukin-1 beta-converting enzyme family of cysteine proteases. J Biol Chem. 1995 Jun 23;270(25):15250–15256. doi: 10.1074/jbc.270.25.15250. [DOI] [PubMed] [Google Scholar]
- Kuida K., Lippke J. A., Ku G., Harding M. W., Livingston D. J., Su M. S., Flavell R. A. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995 Mar 31;267(5206):2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- Kumar S., Kinoshita M., Noda M., Copeland N. G., Jenkins N. A. Induction of apoptosis by the mouse Nedd2 gene, which encodes a protein similar to the product of the Caenorhabditis elegans cell death gene ced-3 and the mammalian IL-1 beta-converting enzyme. Genes Dev. 1994 Jul 15;8(14):1613–1626. doi: 10.1101/gad.8.14.1613. [DOI] [PubMed] [Google Scholar]
- Kägi D., Vignaux F., Ledermann B., Bürki K., Depraetere V., Nagata S., Hengartner H., Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994 Jul 22;265(5171):528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- Los M., Van de Craen M., Penning L. C., Schenk H., Westendorp M., Baeuerle P. A., Dröge W., Krammer P. H., Fiers W., Schulze-Osthoff K. Requirement of an ICE/CED-3 protease for Fas/APO-1-mediated apoptosis. Nature. 1995 May 4;375(6526):81–83. doi: 10.1038/375081a0. [DOI] [PubMed] [Google Scholar]
- Lowin B., Hahne M., Mattmann C., Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994 Aug 25;370(6491):650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- Munday N. A., Vaillancourt J. P., Ali A., Casano F. J., Miller D. K., Molineaux S. M., Yamin T. T., Yu V. L., Nicholson D. W. Molecular cloning and pro-apoptotic activity of ICErelII and ICErelIII, members of the ICE/CED-3 family of cysteine proteases. J Biol Chem. 1995 Jun 30;270(26):15870–15876. doi: 10.1074/jbc.270.26.15870. [DOI] [PubMed] [Google Scholar]
- Nicholson D. W., Ali A., Thornberry N. A., Vaillancourt J. P., Ding C. K., Gallant M., Gareau Y., Griffin P. R., Labelle M., Lazebnik Y. A. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995 Jul 6;376(6535):37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Steller H. Mechanisms and genes of cellular suicide. Science. 1995 Mar 10;267(5203):1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- Tewari M., Quan L. T., O'Rourke K., Desnoyers S., Zeng Z., Beidler D. R., Poirier G. G., Salvesen G. S., Dixit V. M. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995 Jun 2;81(5):801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- Thornberry N. A., Bull H. G., Calaycay J. R., Chapman K. T., Howard A. D., Kostura M. J., Miller D. K., Molineaux S. M., Weidner J. R., Aunins J. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992 Apr 30;356(6372):768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- Trapani J. A., Browne K. A., Dawson M., Smyth M. J. Immunopurification of functional Asp-ase (natural killer cell granzyme B) using a monoclonal antibody. Biochem Biophys Res Commun. 1993 Sep 15;195(2):910–920. doi: 10.1006/bbrc.1993.2131. [DOI] [PubMed] [Google Scholar]
- Trapani J. A., Smyth M. J., Apostolidis V. A., Dawson M., Browne K. A. Granule serine proteases are normal nuclear constituents of natural killer cells. J Biol Chem. 1994 Jul 15;269(28):18359–18365. [PubMed] [Google Scholar]
- Wang L., Miura M., Bergeron L., Zhu H., Yuan J. Ich-1, an Ice/ced-3-related gene, encodes both positive and negative regulators of programmed cell death. Cell. 1994 Sep 9;78(5):739–750. doi: 10.1016/s0092-8674(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yuan J., Shaham S., Ledoux S., Ellis H. M., Horvitz H. R. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993 Nov 19;75(4):641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]