Abstract
Resistance to enfuvirtide (ENF; T-20), a fusion inhibitor of human immunodeficiency virus type 1 (HIV-1), is conferred by mutations in the first heptad repeat of the gp41 ectodomain. The replicative fitness of recombinant viruses carrying ENF resistance mutations was studied in growth competition assays. ENF resistance mutations, selected in vitro or in vivo, were introduced into the env gene of HIV-1NL4-3 by site-directed mutagenesis and expressed in HIV-1 recombinants carrying sequence tags in nef. The doubling time of ENF-resistant viruses was highly correlated with decreasing ENF susceptibility (R2 = 0.859; P < 0.001). Initial fitness experiments focused on mutants identified by in vitro selection in the presence of ENF (L. T. Rimsky, D. C. Shugars, and T. J. Matthews, J. Virol. 72:986-993, 1998). In the absence of drug, these mutants displayed reduced fitness compared to wild-type virus with a relative order of fitness of wild type > I37T > V38 M > D36S/V38 M; this order was reversed in the presence of ENF. Likewise, recombinant viruses carrying ENF resistance mutations selected in vivo displayed reduced fitness in the absence of ENF with a relative order of wild type > N42T > V38A > N42T/N43K ≈ N42T/N43S > V38A/N42D ≈ V38A/N42T. Fitness and ENF susceptibility were inversely correlated (r = −0.988; P < 0.001). Similar results were obtained with recombinants expressing molecularly cloned full-length env genes obtained from patient-derived HIV-1 isolates before and after ENF treatment. Further studies are needed to determine whether the reduced fitness of ENF-resistant viruses alters their pathogenicity in vivo.
Emergence of drug-resistant variants of human immunodeficiency virus type 1 (HIV-1) limits the long-term success of antiretroviral therapy. Resistance to each of the agents currently available for treatment of HIV-1 infection has been described, and long-term exposure to partially suppressive regimens results in the eventual selection of multidrug-resistant HIV-1. Considerable effort therefore has been devoted to developing new classes of anti-HIV drugs directed against novel therapeutic targets.
Enfuvirtide (ENF; T-20; Trimeris, Inc., Durham, N.C.) is a synthetic 36-amino-acid oligopeptide fusion inhibitor belonging to the broader group of antiretroviral agents known as entry inhibitors. This drug, now approved for clinical use, exhibits potent and selective inhibition of HIV-1 in vitro and in vivo (7, 11, 22). After binding of virus particles to the CD4+ receptor on T lymphocytes and monocytes, molecular rearrangements in the transmembrane subunit of the HIV-1 envelope glycoprotein (gp41) result in fusion of the virus and cell membranes (2). These rearrangements involve the antiparallel association of two helically coiled heptad repeats (HR-1 and HR-2) located in the ectodomain of the trimeric gp41 complex (also referred to as a six-helix bundle) in the gp41 ectodomain. ENF is derived from HR-2 and corresponds to amino acids 127 to 162 of gp41 (21). Binding of ENF to the trimeric HR-1 complex prevents the association of HR-1 with HR-2, thereby inhibiting fusion and blocking virus entry.
Serial in vitro passage of HIV-1 in the presence of ENF selects for resistant viruses that carry mutations in HR-1 (17). These mutations map to a contiguous 3-amino-acid sequence at residues 36 to 38 of the gp41 ectodomain (GIV in Hxb2 and DIV in NL4-3). The I37T, V38 M, and D (or G) 36S/V38 M mutations reduce the affinity of HR-1 for ENF and confer resistance to ENF at concentrations as high as 10 μg/ml (17). Additional HR-1 mutations at positions 36 to 45 of gp41 have been identified in resistant isolates recovered from patients receiving ENF in phase II clinical trials. A V38A mutation has been found most often and confers approximately 20-fold resistance to ENF. The N42T and N42D mutations confer two- and fourfold resistance, respectively, whereas N43S and N43K mutations confer five- and sixfold resistance, respectively. Combinations of these mutations confer substantially higher levels of resistance, ranging from 32-fold for the N42T/N43K mutant to 149-fold for the V38A/N42T mutant (P. Sista, T. Melby, M. L. Greenburg, D. Davison, L. Jin, S. Mosier, M. Mink, E. Nelson, L. Fang, N. Cammack, M. Salgo, and T. J. Matthews, abstr. 21, Antivir. Ther. 7:S23, 2002). The significance of mutations at codons 36 and 38 in conferring resistance to ENF was confirmed by a detailed analysis of molecularly cloned env sequences from plasma samples of ENF-treated patients (20).
Accumulation of drug resistance mutations in HIV-1 protease and reverse transcriptase significantly impairs viral fitness (12, 13). Reduced viral fitness contributes to the continued benefit of antiretroviral therapy despite the presence of high-level drug resistance (3). However, not all resistance mutations necessarily reduce viral fitness in the absence of drug. For example, certain mutations selected during protease inhibitor therapy improve viral replication, leading to a mutant that is fitter than the initial wild-type (WT) isolate (15). We used a recombinant marker virus system developed in our laboratory (12) to perform growth competition assays in order to study the effects of ENF resistance mutations on viral fitness.
(These data were presented, in part, at the following meetings: [i] 5th International Workshop on HIV Drug Resistance and Treatment Strategies, Scottsdale, Ariz., 4 to 8 June 2001 [abstr. 23]; [ii] 1st International AIDS Society Conference on HIV Pathogenesis and Treatment, 8 to 11 July 2001, Buenos Aires, Argentina [abstr. LB 8]; and [iii] XIth International Workshop on HIV Drug Resistance, 2 to 5 July 2002, Seville, Spain [abstr. 67].)
MATERIALS AND METHODS
Cells and viruses.
The human T-lymphoblastoid cell lines CEM and MT-2 were maintained in R-10 medium (RPMI 1640 [Cellgro, Herndon, Va.] supplemented with fetal calf serum [10%], l-glutamine [2 mM], penicillin [100 U/ml], and streptomycin [50 μg/ml]). Peripheral blood mononuclear cells (PBMC) from HIV-seronegative donors were obtained by Ficoll-Hypaque density gradient centrifugation. Prior to HIV infection, PBMC were stimulated with phytohemagglutinin (5 μg/ml) for 3 days and maintained in R-20 medium (RPMI 1640 supplemented with fetal calf serum [20%], l-glutamine [2 mM], HEPES buffer [10 mM], recombinant human interleukin-2 [100 U/ml; kindly provided by Hoffman-LaRoche through the National Institutes of Health AIDS Research and Reference Reagent Program], penicillin [50 U/ml], and streptomycin [50 μg/ml]). Baseline and post-ENF treatment isolates of HIV-1 were obtained from subjects enrolled in a phase II study of ENF (T20-205) (10). Human subject aspects of this study were conducted in accordance with relevant federal guidelines. The study was approved by the appropriate institutional review board at the sites where subjects were enrolled, and all subjects provided signed informed consent. Infectious stocks of HIV-1 were prepared by transfection of MT-2 or CEM cells with the infectious molecular clone pNL4-3 or its derivatives. Recombinant HIV-1 isolates carrying ENF resistance mutations in HR-1 were constructed by site-directed mutagenesis as described below. Table 1 shows the HR-1 substitutions and associated 50% inhibitory concentrations (IC50) for ENF for these recombinants.
TABLE 1.
Characteristics of HIV-1NL4-3 site-directed mutants
| Subject | gp41 sequence (aa 36 to 45) | IC50 (μg/ml)a | Fold change in IC50 | Doubling time (days)a |
|---|---|---|---|---|
| In vitro-selected mutants | ||||
| NL4-3 WT (36D) | DIVQQQNNLL | 0.055 | 1.0 | 1.7 |
| 37T mutant | DTVQQQNNLL | 0.98 | 18 | 1.8 |
| 38M mutant | DIMQQQNNLL | 1.83 | 33 | 1.8 |
| 36S/38M mutant | SIMQQQNNLL | 4.38 | 81 | 2.0 |
| In vivo-selected mutants | ||||
| NL4-3 WT (36G) | GIVQQQNNLL | 0.020 | 1.0 | 1.6 |
| 1034 wk 48 | GIVQQQTNLL | 0.045 | 2.3 | 1.7 |
| 1071 wk 16 | GIAQQQNNLL | 0.16 | 8.0 | 1.7 |
| 1061 wk 62 | GIVQQQTKLL | 0.388 | 19 | 1.8 |
| 1065 wk 24 | GIVQQQTSLL | 0.727 | 36 | 1.8 |
| 1062 wk 16 | GIAQQQDNLL | 1.69 | 85 | 1.8 |
| 1036 wk 48 | GIAQQQTNLL | 1.78 | 89 | 1.8 |
| 1073 wk 16 | GIEQQQSNLL | 7.89 | 395 | 2.00 |
Results shown are means of replicate assays.
Site-directed mutagenesis and ENF susceptibility.
To introduce ENF resistance mutations selected in vitro (17) into the env gene of HIV-1NL4-3, a 3.2-kb fragment encompassing the entire coding sequence of HIV-1 env was amplified from the proviral clone pNL4-3 (provided by Malcolm Martin through the AIDS Research and Reference Reagent Program) and cloned into vector pCR2.1 (Stratagene, La Jolla, Calif.) to generate plasmid pTA-env. Mutations at gp41 codons 37 (I→T) and 38 (V→M) and the double mutation D36S/V38 M were introduced into the pTA-env using the QuikChange site-directed mutagenesis kit (Stratagene). The presence of mutant sequences was confirmed by DNA sequencing of the final plasmid clone on an ABI 377 automated sequencer (Perkin-Elmer, Foster City, Calif.). ENF susceptibility of the resultant recombinants was tested by a standardized assay modified for use with MT-2 cells (6).
To generate recombinant viruses carrying ENF resistance mutations selected in vivo, mutations were introduced into a plasmid carrying the 3′ half of the HIV-1NL4-3 sequence using synthetic oligonucleotide primers and the QuikChange site-directed mutagenesis kit (Stratagene). Sequences of the primers used for each reaction are available from the authors upon request. Replication-competent viruses were generated using a pNL4-3-derived system as described previously (17). The presence of the desired mutations was confirmed by sequencing with a Beckman CEQ L DNA analysis system. High-titer stocks of WT and mutant HIV-1NL4-3 were generated by transfection of pNL4-3 and its derivatives into CEM-x174 cells followed by passage of cell-free viral supernatants in CEM4 cells. Susceptibilities of the resulting recombinant viruses to ENF shown in Table 1 were determined by a MAGI cell assay as described previously (8).
Construction of recombinant marker vectors.
A 2,565-nucleotide segment of the env coding sequence (corresponding to nucleotides 6221 to 8786 in the HIV-1 NL4-3 sequence [http://hiv-web.lanl.gov]) was deleted from plasmid pTA-env, and a unique BstEII restriction enzyme site was introduced at the deletion junction by PCR-based site-directed mutagenesis using the ExSite kit (Stratagene). The env gene carrying this deletion was then cloned into pNL4-3 to yield pHIVΔenvBstEII. A segment of the Salmonella enterica serovar Typhimurium histidinol dehydrogenase (hisD) gene or the human placental heat-stable alkaline phosphatase (PLAP) gene was then introduced into nef to serve as a sequence tag. The hisD gene fragment was amplified with primers 5′-GGA CCT CGA GCG ATA TCT GGA-3′ and 5′-TCA GCC TGC TCG AGC AGG TCA GAA-3′, and the PLAP fragment was amplified with primers 5′-ATC GCT CTC GAG CTC ATC TCC AA-3′ and 5′-AGG CCT CGA GAG ACC TGT GGG ACA A-3′. The amplicons were subsequently cloned into an XhoI site in nef of pHIVΔenvBstEII to yield pHIVΔenvBstEIInef-hisD and pHIVΔenvBstEIInef-PLAP, respectively. Plasmid clones carrying the desired inserts were identified by restriction enzyme analysis, and the nucleotide sequences were confirmed by automated DNA sequencing on an ABI 377 automated sequencer (Perkin-Elmer).
Generation of recombinant marker viruses for growth competition assay.
Infectious recombinant viruses were generated by electroporation of 2.5 × 106 MT-2 cells with 10 μg of marker vector DNA (pHIVΔenvBstEIIΔnef-hisD or pHIVΔenvBstEIInef-PLAP) together with 1 to 10 μg of the env coding fragment of interest. Following electroporation, cells were suspended in 10 ml of R-10 medium in 25-cm2 tissue culture flasks and incubated at 37°C. Cell-free supernatants were harvested when peak cytopathic effect was observed and stored in 1-ml aliquots at −70°C. The env coding sequence was amplified from proviral DNA of HIV-1-infected cells at the end of virus culture and analyzed by automated DNA sequencing to verify presence of the correct HR-1 sequence and to ensure the absence of adventitious mutations.
Determination of viral replication kinetics.
To determine the growth kinetics of recombinant viruses, 103 tissue culture infectious doses were used to inoculate 10 × 106 MT-2 cells suspended in 1 ml of R-10 medium (multiplicity of infection, 0.0001). After incubation at 37°C for 2 h, cells were washed, resuspended in 10 ml of R-10 medium in 25-cm2 tissue culture flasks, and reincubated. Cultures were fed by medium exchange every 3 to 4 days, fresh cells were added every 7 days, and cell-free supernatant was tested for p24 antigen (Coulter Diagnostics, Hialeah, Fla.). Replication rates were determined by plotting the increase in p24 antigen over time.
Growth competition assay.
Recombinant marker viruses being tested were mixed together at a 50:50 ratio unless otherwise noted, and the mixed viral sample used to infect 2 × 106 MT-2 cells suspended in 200 μl of R-10 medium at a multiplicity of infection of 0.001 infectious units/cell in the absence or presence of ENF (Trimeris, Inc., Research Triangle Park, N.C.) at 5 μg/ml. After incubation at 37°C for 2 h, cells were washed twice with phosphate-buffered saline, resuspended in 5 ml of R-10 medium at a concentration of 0.4 × 106 cells/ml in 25-cm2 tissue culture flasks, and reincubated (day 0). Supernatants were harvested on days 1, 4, 7, 10, and 14. Starting from day 4, cultures were passaged by inoculating 200 μl of culture supernatant onto 10 × 106 fresh MT-2 cells. After incubation at 37°C for 2 h, cells were washed twice with phosphate-buffered saline, resuspended in 10 ml of R-10 medium, and reincubated at 37°C. Growth competition assays on PMBC were performed in a similar manner, except that 5 × 106 PBMC were used in place of MT-2 cells and R-20 medium was used in place of R-10 medium.
Viral RNA was extracted from culture supernatants on days 1, 4, 7, 10, and 14 using the Qiagen RNA kit and treated with RNase-free DNase (Qiagen, Valencia, Calif.). The proportion of hisD- or PLAP-tagged virus in the population was determined by real-time PCR. Briefly, amplification reactions were performed in MicroAmp optical tubes (Perkin-Elmer) in a 25-μl reaction mixture containing 8% glycerol, 1× TaqMan buffer A (500 mM KCl, 100 mM Tris-HCl, 0.1 M EDTA, 600 nM passive reference dye ROX; pH 8.3 at room temperature), 300 μM (each) dATP, dGTP, and dCTP and 600 μM dUTP, 5.5 mM MgCl2, 900 nM forward and reverse primers, 200 nM probe, 0.625 U of AmpliTaq Gold DNA polymerase (Perkin-Elmer), 6.25 U of Moloney murine leukemia virus reverse transcriptase (Life Technologies, Inc., Gaithersburg, Md.), 10 U of RNasin RNase inhibitor (Promega Corp., Madison, Wis.), and the template RNA in a final volume of 25 μl. Reverse transcription was performed at 48°C for 30 min followed by activation of TaqGold at 95°C for 10 min. Subsequently, 40 cycles of amplification were performed at 95°C for 15 s and 60°C for 1 min. All growth competition assays were performed at lease twice to verify results.
Statistical analysis.
Linear regression and Spearman rank correlation analyses were performed using SigmaStat 3.0 (SPSS, Inc., Chicago, Ill.). The IC50 data were log transformed prior to analysis.
RESULTS
Replication kinetics of recombinant viruses carrying ENF resistance mutations.
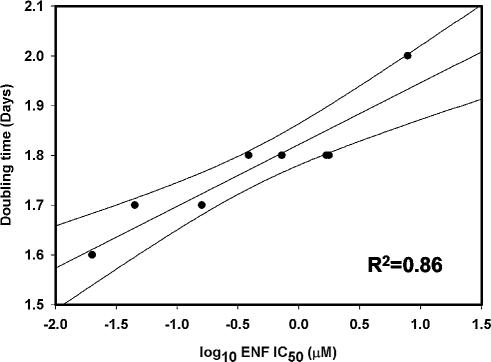
The first report of ENF resistance described the emergence of V38M, I37T, and D36S/V38M mutations in the HR-1 domain of HIV-1NL4-3 passaged in the presence of ENF in vitro (17). Therefore, initial experiments focused on determining the growth kinetics of these mutants. Table 1 shows the ENF susceptibilities and doubling times of WT and mutant HIV-1NL4-3. The data suggested a trend towards increasing doubling time with increasing ENF resistance. Similar experiments were performed with HIV-1 recombinants carrying ENF resistance mutations identified in clinical isolates from seven ENF-treated patients (Table 1). (For these experiments we used as WT a variant of HIV-1NL4-3 in which the aspartate at gp41 position 36 was replaced by glycine, which is more common in subtype B viruses [Los Alamos HIV sequence database; www.hiv.lanl.gov].) Linear regression analysis showed that doubling time and the log-transformed ENF IC50 were highly correlated (R2 = 0.859; P < 0.001) (Fig. 1).
FIG. 1.
Correlation of viral growth rate (expressed as doubling time) with log10 IC50 for ENF. The slope and 95% confidence intervals of the linear regression are shown.
Replication kinetics of recombinant viruses carrying hisD or PLAP sequence tags.
Recombinant viruses carrying the WT HIV-1NL4-3 env gene linked to PLAP or hisD (WT-PLAP and WT-hisD) were generated by cotransfecting the PCR-amplified env coding region of pNL4-3 into MT-2 cells together with marker vectors pHIVΔenvBstEIInef-PLAP and pHIVΔenvBstEIInef-hisD, respectively. Recombinant viruses carrying hisD or PLAP exhibited similar growth kinetics, with doubling times of 1.78 and 1.81 days, respectively. This minor difference in doubling time translated into a slight growth advantage of recombinants carrying the hisD sequence tag compared to those carrying the PLAP tag. In order to control for this minor fitness difference conferred by the sequence tag, reciprocal experiments were performed for all growth competition assays so that each env gene was tested at least twice—once linked to hisD and once linked to PLAP.
Relative replicative fitness of HIV-1 recombinants carrying ENF resistance mutations selected in vitro.
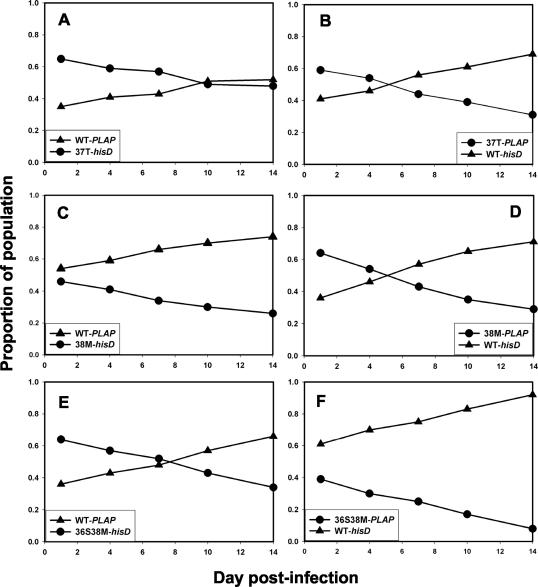
The replicative fitness of recombinant viruses carrying ENF resistance mutations selected in vitro was tested in a series of pairwise growth competition assays. The mutant viruses were each less fit than WT in the absence of ENF, whether carrying the PLAP or hisD sequence tag (Fig. 2). Growth competition assays also showed that the V38M mutant virus replicated less efficiently than the I37T mutant virus, and the double mutant D36S/V38M was less fit than the V38M single mutant (Fig. 3). From these results the order of relative fitness was deduced to be WT > I37T > V38M > D36S/V38M.
FIG. 2.
Reciprocal growth competition assays between WT (triangles) and ENF-resistant HIV-1NL4-3 (circles), inoculated at a ratio of 1:1. Recombinant viruses carrying ENF resistance mutations (selected during in vitro passage experiments) at gp41 codons 37 (A and B), 38 (C and D), or 36 and 38 (E and F) linked to a hisD (A, C, and E) or PLAP (B, D, and F) sequence tag in nef were competed against recombinants carrying WT gp41 linked to PLAP (A, C, and E) or hisD (B, D, and F).
FIG. 3.
Reciprocal growth competition assays between WT and ENF-resistant HIV-1NL4-3 carrying mutations in gp41, inoculated at a ratio of 1:1. Recombinants carrying the V38M mutation (triangles) linked to PLAP (A and C) or hisD (B and D), respectively, were compared with recombinants carrying the I37T mutation (squares) linked to hisD (A) or PLAP (B), respectively, or with the D36S/V38 M double mutant (circles) linked to hisD (C) or PLAP (D), respectively.
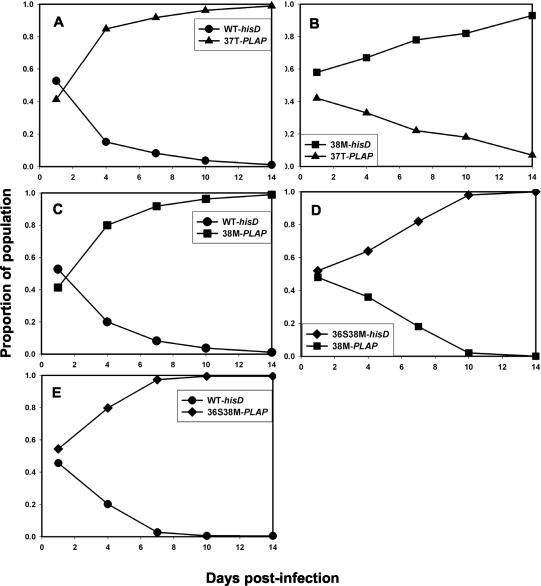
Growth competition assays performed in the presence of ENF (5 μg/ml) favored growth of the drug-resistant variants. For all three mutants (I37T, V38M, and D36S/V38M) the proportion of mutant virus relative to WT increased over time (Fig. 4). Likewise, in the presence of ENF the V38M variant was selected rapidly in mixed culture with I37T but was overgrown by the double mutant D36S/V38M. These results showed that the order of relative fitness was reversed in the presence of ENF and correlated with the relative degree of ENF resistance.
FIG. 4.
Growth competition experiments between WT and ENF-resistant HIV-1NL4-3 recombinants inoculated at a ratio of 1:1, performed in the presence of ENF (5 μg/ml). Recombinant viruses carrying ENF resistance mutations at gp41 I37T (triangles), V38M (squares), and G36S/V38M (diamonds) were competed against recombinants carrying WT gp41 (circles; A, C, and E, respectively) or against each other (B and D). Recombinants carried hisD or PLAP sequence tags.
Relative replicative fitness of HIV-1 recombinants carrying ENF resistance mutations selected in vivo.
A similar series of experiments was performed to test the replicative fitness of HIV-1 recombinants carrying ENF resistance mutations selected in vivo. These experiments used the D36G variant of HIV-1NL4-3 as WT for the reasons described above. (Reciprocal growth competition experiments between recombinant viruses carrying D or G at codon 36 showed a small growth advantage for the 36G variant, whether linked to hisD or PLAP [data not shown].) Recombinant viruses carrying the single mutations V38A or N42T were each less fit than WT HIV-1NL4-3 when tested in the absence of ENF (Fig. 5). Likewise, the double mutants V38A/N42D, V38A/N42T, N42T/N43K, N42T/N43S, and 38E/42S were each less fit than WT (Fig. 5). Similar results were obtained whether the mutant env gene was linked to the hisD or PLAP sequence tag (data not shown).
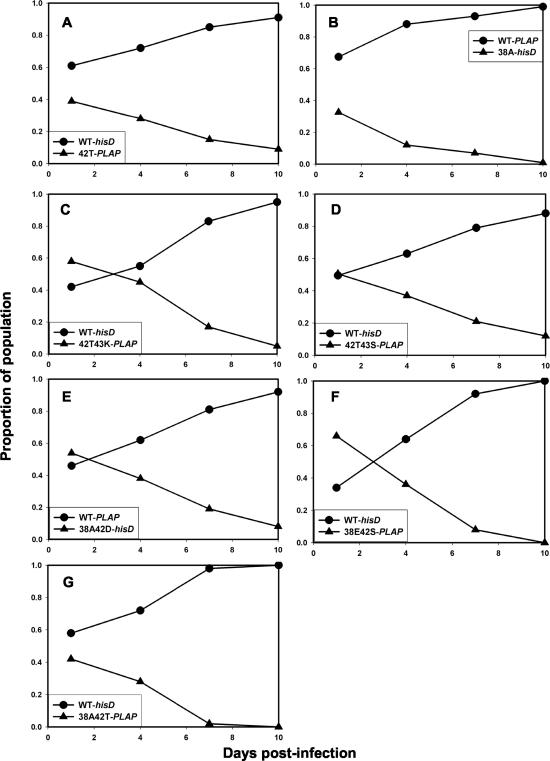
FIG. 5.
Growth competition assays between HIV-1NL4-3 recombinants carrying WT gp41 (circles) or ENF resistance mutations identified from ENF-resistant clinical isolates (triangles) linked to hisD or PLAP sequence tags. WT and mutant viruses were inoculated at a ratio of 1:1. Mutants tested were N42T (A), V38A (B), 42T43K (C), 42T43S (D), 38A42D (E), 38E42S (F), and 38A42T (G).
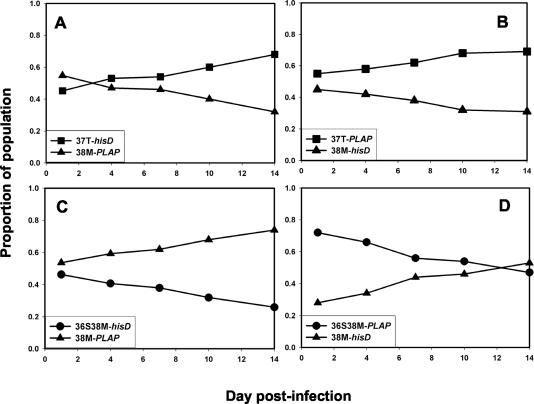
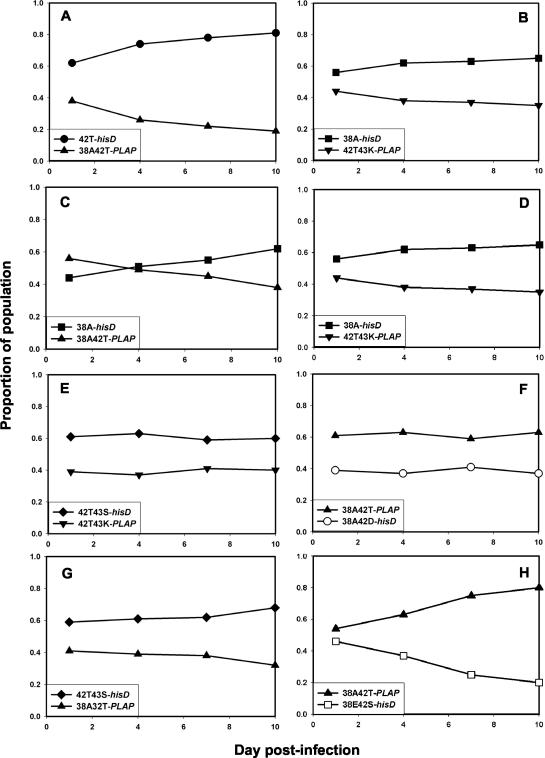
To determine the relative order of fitness, pairwise growth competition assays were performed with each of the mutant viruses. Figure 6 shows the results of these experiments. The N42T mutant was more fit than V38A, which in turn was fitter than the V38A/N42T double mutant. The V38A mutant also was more fit than the N42T/N43K double mutant, which had comparable fitness to the N42T/N43S mutant. The N42T/N43S double mutant was slightly fitter than the V38A/N42T mutant, which had comparable fitness to the V38A/N42D mutant; the V38A/N42T mutant was fitter than V38E/N42S. From these data, the relative order of fitness was deduced to be WT > N42T > V38A > N42T/N43K ≈ N42T/N43S > V38A/N42D ≈ V38A/N42T > V38E/N42S. This order was inversely correlated with resistance to ENF compared to that of WT HIV-1NL4-3 (r = −0.988; P < 0.001). When tested in the presence of ENF, resistant viruses were more fit than WT, as expected (data not shown).
FIG. 6.
Growth competition assays comparing ENF-resistant recombinants of HIV-1NL4-3 carrying ENF resistance mutations, identified from ENF-resistant clinical isolates, linked to a hisD or PLAP sequence tag. Viruses were inoculated at a ratio of 1:1. Mutant N42T (filled circles) was competed against the V38A/N42T double mutant (upright triangles) (A) and against V38A (filled squares) (B); V38A was tested against V38A/N42T (C) and against N42T/N43K (inverted triangles) (D); N42T/N43K was also tested against N42T/N43S (diamonds) (E); V38A/N42T was tested against V38A/N42D (open circles) (F), 42T/N43S (G), and V38E/N42S (open squares) (H).
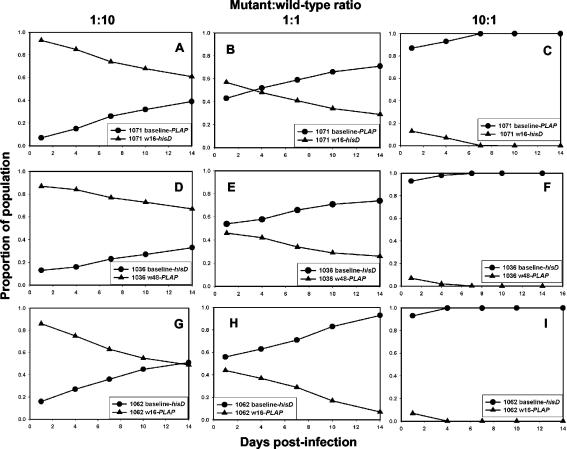
To verify that ENF resistance mutations produced the same effects on viral fitness when tested in the context of env genes derived from patient isolates, growth competition assays were performed on PBMC using full-length env sequences cloned from paired baseline and posttreatment isolates obtained from three subjects participating in the T20-205 trial. To ensure that env clones used for these experiments were representative of the predominant sequence in the virus population, several clones were isolated from each isolate. Clones with sequences that matched most closely the env sequences determined by bulk sequencing of the isolate were used to construct recombinant viruses for use in growth competition assays. Results of these experiments were consistent with those obtained with the site-directed mutants using HIV-1NL4-3 env (Fig. 7). Similar results were obtained when these same recombinant viruses were tested on MT-2 cells (data not shown).
FIG. 7.
Growth competition assays comparing HIV-1NL4-3 recombinants expressing cloned full-length env from patient samples obtained at baseline (circles) or after ENF treatment (triangles) and linked to a hisD or PLAP sequence tag. ENF resistance mutations in HR-1 present in the posttreatment isolates were V38A (A to C), V38A/N42T (D to F), and V38A/N42D (G to I). Mutant and WT viruses were inoculated at ratios of 1:10 (A, D, and G), 1:1 (B, E, and H), or 10:1 (C, F, and I).
DISCUSSION
Replication of HIV-1 in the presence of partially suppressive antiretroviral regimens leads rapidly to the accumulation of drug resistance mutations. Although such mutant viruses have a relative growth advantage over WT in the presence of drug, resistance mutations nevertheless may impair key viral functions, such as the activity of protease or the processivity of reverse transcriptase, resulting in virus with diminished replication capacity (3, 15, 18, 19). This reduction in viral replication capacity can have two consequences: (i) plasma HIV-1 RNA levels may remain significantly below baseline, despite the emergence of high-level drug resistance, and (ii) residual WT virus (possibly archived in the pool of latently infected resting CD4+ memory cells [4]) rapidly overgrows the mutant virus when treatment with the selecting drug is interrupted.
In this study we showed that recombinant HIV-1 isolates carrying HR-1 mutations associated with ENF resistance in vitro and in vivo had reduced replication rates compared to WT and were less fit than WT when tested in growth competition experiments using a recombinant marker virus assay in MT-2 cells and in PBMC. Consistent results were obtained regardless of which sequence tag was linked to the env gene being tested, providing confidence that observed fitness differences were indeed due to HR-1 mutations and not a function of the sequence tag. Linear regression analysis showed that viral doubling time in vitro was highly correlated with increasing IC50 for ENF (R2 = 0.859). Similarly, the relative order of fitness was inversely correlated with the reduction in ENF susceptibility (r = −0.988) and was reversed in the presence of drug. Double mutants that included a V38A mutation, which is the most commonly selected mutation in vivo (M. L. Greenberg, T. Melby, P. Sista, R. DeMasi, N. Cammack, M. Salgo, J. Whitcomb, C. Petropoulos, and T. J. Matthews, Abstr. 10th Conf. Retrovir. Opportunistic Infect., abstr. 141, p. 108, 2003), were among the least fit viruses in this study.
Preliminary results from clinical studies have provided additional evidence that ENF-resistant viruses are less fit than WT in the absence of drug. Virus isolates from 15 of 63 patients who received ENF in a dose-ranging study showed evidence of ENF resistance-associated mutations in HR-1 at the end of the 28-day dosing period (T. Melby, P. Sista, E. Nelson, S. Mosier, M. Mink, M. Greenberg, L. Fang, N. Cammack, M. Salgo, T. Matthews, abstr. 70, Antivir. Ther. 7:S58, 2002). Virus that was WT in HR-1 reemerged in all 15 patients during an average follow-up period of 4 months after stopping ENF. These observations provide additional evidence in support of the biological relevance of our in vitro data. Whether the kinetics of resistance reversal will be the same in patients receiving chronic ENF therapy remains to be determined, highlighting the need for further studies in those clinical settings.
In certain cases, compensatory mutations can emerge in the genetic background of drug-resistant viruses that improve replicative capacity without substantially increasing the level of drug resistance. For example, emergence of virus carrying protease mutations 36I, 54V, and 82T in a patient receiving ritonavir resulted in a ritonavir-resistant virus that was significantly less fit than WT (15). Subsequent emergence of a 71V mutation improved viral fitness without a further increase in ritonavir resistance. Similarly, accumulation of mutations at reverse transcriptase codons 62, 77, and 116 compensates for the loss of fitness conferred by the multinucleoside resistance mutation Q151M (9).
In the case of ENF, mutations in HR-2 or in gp120 could compensate for the fitness loss conferred by HR-1 mutations. Cells expressing envelope glycoproteins from HIV-1HXBc2 and several primary HIV-1 isolates bind labeled ENF in the presence of soluble CD4 or in the presence of CD4-expressing cells (5). Isolates that utilize CCR5 exclusively expose the ENF binding domain in the presence of cells that express only CD4 and CXCR4 (5). Conversely, preincubation of ENF with HIV-1 in the absence of CD4 fails to block subsequent virus entry (14). These observations suggest that the time window during which HR-1 is available for binding by ENF is opened upon CD4 binding. The precise timing of the closing of this window has not been established, but it may last until engagement of the chemokine coreceptor (16). HR-1 mutations that reduce affinity for ENF (and HR-2) should increase the length of this time window but slow the kinetics of virus entry, thereby resulting in reduced viral fitness. Conversely, mutations that shorten the time window would accelerate virus entry, possibly leading to an increase in relative fitness.
A number of limitations may apply to our results. The mutants examined in this study arose after relatively brief periods of ENF treatment and were restricted to the HR-1 domain of gp41. Additional resistance and/or compensatory mutations might arise after longer treatment with ENF. A second limitation is that the envelopes examined in this study came from X4 viruses; different results might be obtained with R5 viruses. However, as the X4 viruses are commonly found in patients with advanced HIV disease (1), our results are likely to be relevant in those patients most in need of ENF treatment. Recombination between viral genomes at sites between env and the sequence tag inserted into nef occurring during the growth competition assays could render results of these experiments difficult to interpret. However, our investigators have documented very low rates of recombination (5% or less) under the experimental conditions used in these assays (12). This low rate may be due in part to the low multiplicity of infection (0.001) used in our experiments. Because recombination between RNA genomes carrying different sequence tags in nef would tend to minimize the fitness differences between viruses carrying different env genes, the fitness differences observed in this study most likely represent minimum fitness differences.
In conclusion, the presence of ENF resistance mutations in the HR-1 domain of gp41 reduced the fitness of HIV-1 compared to that of WT. The relative fitness loss was correlated with the degree of ENF resistance. Whether the reduced replicative capacity of ENF-resistant viruses will contribute to persistent antiviral activity of ENF in the setting of ENF resistance requires further study in randomized clinical trials.
Acknowledgments
This study was supported by Public Health Service grants RR16482, AI41536, AI055357, and CA46934 from the National Center for Research Resources, the National Institute of Allergy and Infectious Diseases, and the National Cancer Institute. Additional support was provided by a Virology Support Laboratory contract from the Adult AIDS Clinical Trials Group (AI38858) and the Harvard Medical School Center for AIDS Research Virology Core (AI42851).
D. R. Kuritzkes is a consultant to Trimeris and Roche Pharmaceuticals.
We thank Malcolm Martin and the AIDS Research and Reference Reagent Program for providing HIV proviral clone pNL4-3, Uma Pugazenthi of the University of Colorado Cancer Center PCR Core for performing real-time PCR, Christopher Korch and Efang Li at the University of Colorado Cancer Center DNA Sequencing and Analysis Core for performing automated DNA sequencing, and Michael Farzan, Brigham and Women's Hospital, for helpful discussions.
REFERENCES
- 1.Bozzette, S. A., J. A. McCutchan, S. A. Spector, B. Wright, and D. D. Richman. 1993. A cross-sectional comparison of persons with syncytium- and non-syncytium-inducing human immunodeficiency virus. J. Infect. Dis. 168:1374-1379. [DOI] [PubMed] [Google Scholar]
- 2.Chan, D., D. Fass, J. Berger, and P. Kim. 1997. Core structure of gp41 from the envelope glycoprotein. Cell 89:263-273. [DOI] [PubMed] [Google Scholar]
- 3.Deeks, S. G., T. Wrin, T. Liegler, R. Hoh, M. Hayden, J. D. Barbour, N. S. Hellmann, C. J. Petropoulos, J. M. McCune, M. Hellerstein, and R. M. Grant. 2001. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N. Engl. J. Med. 344:472-480. [DOI] [PubMed] [Google Scholar]
- 4.Finzi, D., J. Blankson, J. D. Siliciano, J. Margolick, K. Chadwick, T. Pierson, K. Smith, J. Lisziewicz, F. Lori, C. Flexner, T. C. Quinn, R. E. Chaisson, E. Rosenberg, B. Walker, S. Gange, J. Gallant, and R. F. Siliciano. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 5:512-525. [DOI] [PubMed] [Google Scholar]
- 5.Furuta, R. A., C. T. Wild, Y. Weng, and C. D. Weiss. 2002. Capture of an early fusion-active conformation of HIV-1 gp41. Nat. Struct. Biol. 5:276-279. [DOI] [PubMed] [Google Scholar]
- 6.Japour, A. J., D. L. Mayers, V. A. Johnson, D. R. Kuritzkes, L. A. Beckett, J. M. Arduino, J. Lane, R. J. Black, P. S. Reichelderfer, R. T. D'Aquila, C. S. Crumpacker, et al. 1993. Standardized peripheral blood mononuclear cell culture assay for the determination of drug susceptibilities of clinical human immunodeficiency virus 1 isolates. Antimicrob. Agents Chemother. 37:1095-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilby, J. M., S. Hopkins, T. Venetta, B. DiMassimo, G. Cloud, J. Y. Lee, L. Alldredge, E. Hunter, D. Lambert, D. Bolognesi, T. Matthews, M. R. Johnson, M. A. Nowak, G. M. Shaw, and M. S. Saag. 1998. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat. Med. 4:1302-1307. [DOI] [PubMed] [Google Scholar]
- 8.Kimpton, J., and M. Emerman. 1992. Detection of replication competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J. Virol. 66:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosalaraksa, P., M. F. Kavlick, V. Maroun, R. Le, and H. Mitsuya. 1999. Comparative fitness of multi-dideoxynucleoside-resistant human immunodeficiency virus type 1 (HIV-1) in an in vitro competitive HIV-1 replication assay. J. Virol. 73:5356-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lalezari, J., J. Eron, M. Carlson, C. Cohen, E. DeJesus, R. Arduino, J. Gallant, P. Volberding, R. Murphy, F. Valentine, E. Nelson, P. Sista, A. Dusek, and J. Kilby. 2003. A phase II clinical study of the long-term safety and antiviral activity of enfuvirtide-based antiretroviral therapy. AIDS 17:691-698. [DOI] [PubMed] [Google Scholar]
- 11.Lalezari, J., K. Henry, M. O'Hearn, J. Montaner, P. Piliero, B. Trottier, S. Walmsley, C. Cohen, D. Kuritzkes, J. J. Eron, J. Chung, R. DeMasi, L. Donatacci, C. Drobnes, J. Delehanty, M. Salgo, et al. 2003. Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N. Engl. J. Med. 348:2175-2185. [DOI] [PubMed] [Google Scholar]
- 12.Lu, J., and D. R. Kuritzkes. 2001. A novel recombinant marker virus assay for comparing the relative fitness of HIV-1 reverse transcriptase variants. J. Acquir. Immune Defic. Syndr. 27:7-13. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Picado, J., A. V. Savara, L. Sutton, and R. T. D'Aquila. 1999. Replicative fitness of protease inhibitor-resistant mutants of human immunodeficiency virus type 1. J. Virol. 73:3744-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melikyan, G. B., R. M. Markosyan, H. Hemmati, M. K. Delmedico, D. Lambert, and F. S. Cohen. 2000. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J. Cell Biol. 151:413-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nijhuis, M., R. Schuurman, D. de Jong, J. Erickson, E. Gustchina, J. Albert, P. Schipper, S. Gulnik, and C. A. B. Boucher. 1999. Increased fitness of drug resistant HIV-1 protease as a result of acquisition of compensatory mutations during suboptimal therapy. AIDS 13:2349-2360. [DOI] [PubMed] [Google Scholar]
- 16.Reeves, J., S. Gallo, N. Ahmad, J. Miamidian, P. E. Harvey, M. Sharron, S. Pohlmann, J. N. Sfakianos, C. A. Derdeyn, R. Blumenthal, E. Hunter, and R. W. Doms. 2002. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc. Natl. Acad. Sci. USA 99:16249-16254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rimsky, L. T., D. C. Shugars, and T. J. Matthews. 1998. Determinants of human immunodeficiency virus type 1 resistance to gp41-derived inhibitory peptides. J. Virol. 72:986-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma, P. L., and C. Crumpacker. 1999. Decreased processivity of human immunodeficiency virus type 1 reverse transcriptase (RT) containing didanosine-selected mutation Leu74Val: a comparative analysis of RT variants Leu74Val and lamivudine-selected Met184Val. J. Virol. 73:8448-8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakefield, J. K., S. A. Jablonski, and C. D. Morrow. 1992. In vitro enzymatic activity of human immunodeficiency virus type 1 reverse transcriptase mutants in the highly conserved YMDD amino acid motif correlates with the infectious potential of the proviral genome. J. Virol. 66:6806-6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei, X., J. M. Decker, H. Liu, Z. Zhang, R. Arani, J. M. Kilby, M. Saag, S. Wu, and J. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wild, C. T., D. Shugars, T. Greenwell, C. McDanal, and T. Matthews. 1994. Peptides corresponding to a predictive α-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc. Natl. Acad. Sci. USA 91:9770-9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wild, C. T., C. Oas, D. McDanal, D. Bolognesi, and T. Matthews. 1992. A synthetic peptide inhibitor of human immunodeficiency virus replication: correlation between solution structure and viral inhibition. Proc. Natl. Acad. Sci. USA 89:10537-10541. [DOI] [PMC free article] [PubMed] [Google Scholar]