SUMMARY
Genetic manipulation of single-celled organisms such as the Leishmania parasite enables in depth analysis of the consequences of genotypic change on biological function. In probing the immune responses to infection, use of transgenic Leishmania has the potential to unravel both the contribution of the parasite to the infection process and the cellular interactions and mechanisms that characterize the innate and adaptive immune responses of the host. Here, we briefly review recent technical advances in parasite genetics and explore how these methods are being used to investigate parasite virulence factors, elucidate immune regulatory mechanisms and contribute to the development of novel therapeutics for the leishmaniases. Recent developments in imaging technology, such as bioluminescence and intravital imaging, combined with parasite transfection with fluorescent or enzyme-encoding marker genes, provides a rich opportunity for novel assessment of intimate, real-time host–parasite interactions at a previously unexplored level. Further advances in transgenic technology, such as the introduction of robust inducible gene cassettes for expression in intracellular parasite stages or the development of RNA interference methods for down-regulation of parasite gene expression in the host, will further advance our ability to probe host–parasite interactions and unravel disease-promoting mechanisms in the leishmaniases.
Keywords: imaging , immune responses , Leishmania, transgenesis
INTRODUCTION
The leishmaniases, a spectrum of infectious diseases caused by species of the kinetoplastid parasite Leishmania, affect man and other mammals in tropical and subtropical regions of the world (1). Transmitted by blood-feeding female sandflies, flagellated Leishmania metacyclic promastigotes are phagocytosed by host cells (usually macrophages) at cutaneous sites and differentiate into replicative amastigotes within intracellular phagolysosomal compartments. Maintenance of parasites at dermal sites or subsequent dispersal to internal tissues contributes to disease progression, resulting in the distinct pathologies associated with cutaneous, mucocutaneous, diffuse cutaneous and visceral leishmaniases (CL, MCL, DCL and VL, respectively) (2). While these diseases are often associated with particular parasite species, the immune response to infection in the host, influenced by genetic factors, is the dominant factor determining clinical outcome (3).
Our understanding of the innate and acquired response to infection with Leishmania has been recently reviewed (4,5). While many of these immunological studies have used strains of Leishmania major (causative agent of CL) as the infecting parasites, alternative Old World (e.g. L. donovani, L. infantum) and New World (e.g. L. mexicana, L. braziliensis) species have been used to unravel immune interactions associated with the different types of disease. Development of transgenic methods for the manipulation of parasite genotypes, together with the recent availability of Leishmania genome sequences, have both facilitated in depth analysis of the role of parasite genes in infection and provided new tools for probing immune responses in the host. In this review, we focus on how transgenic Leishmania have been used to date to elucidate immunological mechanisms underlying host responses and consider future prospects in this developing research area.
MANIPULATING THE PARASITE
Since the pioneering demonstration of gene targeting by homologous recombination in L. major (6), there has been steady progress in construction of a ‘genetic toolkit’ to facilitate research involving transgenic Leishmania [reviewed in (7)]. Thus, parasites can be transfected both transiently and stably, using a range of episomal and integrating expression constructs together with suitable markers for both positive and negative selection; genes can be targeted for disruption or replacement by homologous recombination; genetic screens can be facilitated by transposon mutagenesis or functional gene rescue. The development of robust in vitro culture systems for growth and differentiation of parasite life cycle stages and well-defined in vivo disease models have enhanced the opportunities to address fundamental questions relating to host–parasite interactions. To date, transgenic Leishmania have been used to explore three broad overlapping research areas relevant to this review: investigation of parasite gene function, including those sequences implicated in host interactions and virulence; generation of attenuated parasite lines suitable for vaccination studies; parasite ‘tagging’ (using fluorescent and biochemical reporter genes) for analysis post-infection, both in vitro and in vivo. Examples of each are included in Table 1 and discussed in more detail below.
Table 1. Key studies using transgenic Leishmania to study immune responses to infection.
| Category | Species | Gene/function | References |
|---|---|---|---|
| Parasite manipulation | L. major | Gene knockout methods | (6,77) |
| L. spp. (various) | Application to drug screening | (78) | |
| L. spp. (various) | Cosmid vector transfection | (79) | |
| L. chagasi | rRNA promoter regions | (80) | |
| L. donovani | LPG | (81,82) | |
| L. major | HSV-1 thymidine kinase expression | (83) | |
| L. spp. (various) | Luciferase | (65,66,84,86) | |
| L. donovani | Luciferase – inducible expression | (74) | |
| L. donovani | GFP for in vitro drug testing | (85) | |
| L. mexicana and major | Integrated GFP and β-gal | (100) | |
| L. infantum | GFP | (87) | |
| Parasite virulence factors | L. mexicana | Cysteine protease mutants | (21,22,88,89) |
| L. major | Leishmanolysin (Gp63) deletion | (23,90) | |
| L. donovani | A2 mutants | (25–27) | |
| L. tarentolae | Human tissue-type plasminogen activator expression |
(91) | |
| L. major | MAP-kinase overexpression | (92) | |
| L. mexicana | Lpg1 knockout | (17) | |
| L. major | UDP-gal transporter null | (18) | |
| L. major | T. cruzi trans-sialic acid expression | (93) | |
| L. donovani | LPG | (94,95) | |
| L. mexicana | GDP-mannose biosynthesis | (96,97) | |
| Parasite attenuation: Targets for vaccination | L. major | LPG | (16,30–32,98) |
| L. major | dhfr-ts | (33) | |
| L. chagasi and donovani | dhfr-ts | (34) | |
| L. major | LACK antigen mutants | (35,36) | |
| L. donovani | biopterin transporter (BT1) | (38) | |
| L. donovani | Centrin mutants | (39,99) | |
| Interactions with cells of the innate immune system | L. donovani | Luciferase | (42) |
| L. major | eGFP | (44) | |
| L. mexicana | Lpg knockout | (46) | |
| L. donovani | LPG1 and LPG2 | (41) | |
| Antigen processing and presentation | L. mexicana | β-galactosidase | (47) |
| L. major and donovani | OVA and β-galactosidase | (50,52) | |
| L. major and donovani | HASPB targeted expression of OVA | (51,56,68) | |
| L. major | Truncated OVA | (58,59) | |
| L. mexicana | Membrane bound acid-phosphatase (MAP) |
(55) | |
| Immune modulation | L. major | Transgenic IFN-γ | (60) |
| L. major | Transgenic for human and mouse GM- CSF |
(101) | |
| L. major | murine CD40 ligand (extracellular domain) expression |
(61) | |
| L. major and amazonensis | Murine chemokine monocyte chemoattractant protein 1 (MCP-1) |
(62) | |
| L. mexicana | Cysteine peptidase B (CPB) deletion mutant |
(63,102) | |
| In vivo imaging | L. amazonensis | Luciferase | (66) |
PARASITE VIRULENCE FACTORS
The main interface between the invading Leishmania promastigote and its phagocytic host cell is the parasite glycocalyx, a highly specialized surface coat composed of lipid-anchored glycoconjugates together with some key proteins [reviewed in (8)]. This coat provides a suitably charged surface for opsonization with complement components to facilitate uptake, utilizing a wide range of phagocytic receptors [reviewed in (9)]. The major component of the promastigote glycocalyx is lipophosphoglycan (LPG), a multifunctional molecule required for parasite survival during vector transmission and initial establishment of intracellular infection [reviewed in (10–14)]. Given these critical roles, LPG was one of the first targets for transgenic experimentation in Leishmania, an approach that initially required mutagenesis and biochemical screening to identify genes encoding the LPG biosynthetic enzymes (15). Once identified, mutants defective in specific components of the LPG synthetic pathway could be generated and used to probe immune responses to infection both in vitro and in vivo. For example, infection with L. major deficient in LPG1 (that codes for the galactofuranosyl transferase essential for synthesis of the LPG lipid core) results in poor parasite survival in vivo and attenuated virulence in macrophages in vitro (16). Using these robust genetic approaches, LPG has been classified as a promastigote virulence factor, although there appear to be some surprising functional differences between the LPGs of different Leishmania species. For example, in contrast to the situation in L. major, loss of LPG1 function does not affect disease outcome in L. mexicana, with lesion formation comparable to wild-type parasites (17). Interpretation of these and other data is complicated by the large diversity of Leishmania phosphoglycan (PG)-containing macromolecules that share structural domains with LPG, some of which have been characterized only relatively recently. This difficulty is exemplified in a recent paper analysing the virulence profiles of new L. major PG mutants null for UDP-galactose transporter genes that facilitate uptake of galactose precursors required for PG synthesis (18). In this study, loss of UDP-galactose transporter gene function did not compromise amastigote virulence, a phenotype similar to that observed with the L. major LPG1 mutants. In contrast, both L. major and L. mexicana LPG2 transgenic parasites (that are null for the Golgi nucleotide-sugar transporter required for uptake of GDP-mannose, another essential component of the LPG PG repeat units) are avirulent as amastigotes. Further work will be required to determine what other metabolites are compromised in their transport in these mutants and how these might affect parasite macromolecules that are critical for amastigote maintenance in the host. This type of analysis underlines the complexities in resolving the role of specific glycoconjugate species in parasite virulence, especially in intracellular amastigotes that down-regulate LPG but not the more abundant surface glycoinositol phospholipids [although these too are not essential for amastigote virulence (19,20)].
Another class of parasite virulence factors, the cysteine proteases, have been fully characterized using transgenic techniques in L. mexicana. Targeted deletion of CPB genes led to attenuated virulence in vivo (21), while a series of genetic complementation experiments demonstrated a requirement for multiple cysteine proteases for the full restoration of parasite virulence (22). Similarly, targeted deletion of the L. major GP63 gene locus (coding for the major lipid-anchored surface zinc metalloprotease of promastigote stages) resulted in increased parasite sensitivity to complement-mediated lysis and attenuated cutaneous lesion formation in BALB/c mice (23). Both effects could be complemented by episomal expression of a single GP63 gene, confirming GP63 as a virulence factor in Leishmania pathogenesis.
Do the factors affecting Leishmania virulence differ between parasite species causing different types of disease? Recent sequencing of three Leishmania species, L. major, L. infantum and L. braziliensis, has facilitated the comparison of three genomes which result in distinct clinical phenotypes: CL, VL and MCL, respectively (24). This analysis has revealed a striking level of genetic conservation between these three species, with an unexpectedly short list of species-specific genes. The application of targeted gene knockout and re-expression techniques may be useful in determining if any of these sequences play a role in host–parasite interactions and in particular, parasite migration and establishment at visceral sites. To date, only one gene target (A2) has been validated as contributing to viscero-tropism, although the function of the A2 protein is still poorly understood. The A2 gene family members were identified as virulence factors in L. donovani (25), with targeted deletion of the A2-A2rel gene cluster resulting in reduced infection of visceral organs (26). Interestingly, in L. major the A2 genes are only present in truncated form and are not expressed. Ectopic expression of a single A2 copy in L. major results in reduced cutaneous lesion formation, with enhanced splenomegaly and increased parasitization of the viscera (27).
PARASITE ATTENUATION: TARGETS FOR VACCINATION
Despite extensive international research efforts, leishmanization, a practice involving inoculation with viable Leishmania parasites, is the only vaccine with proven efficacy in humans (28). Whilst leishmanization has been largely abandoned due to safety concerns, the success of this approach suggests that the use of live attenuated parasite strains may be the way forward for the development of an effective vaccine in man. Parasite transgenesis has been used successfully to further this research goal, with the aim of producing immunogenic but attenuated organisms that stimulate protective immune responses and confer long-term protection against disease [reviewed in (29)]. Two general approaches have been taken: first, by deletion of genes encoding virulence factors (or their synthetic enzymes) or metabolic pathway components to produce attenuated infective parasites that are incapable of sustaining infection and causing pathology in the host; second, by creating parasites that secrete host immune mediators to boost antiparasite responses and facilitate parasite clearance.
A variety of parasites with attenuated virulence genes have been produced. In the case of L. major, these include the LPG2 null mutants described above that are able to infect, but not support lesion development in susceptible mice, and persist in low numbers at the site of infection (30). These same mutants are able to confer resistance to heterologous challenge in susceptible mouse strains (31) but can only induce a potent Th1 response when coadministered with CPG oligodeoxynucleotides (32). In terms of offering wider spectrum protection, inoculation with L. major dhfr-ts (dihydrofolate reductase-thymidylate synthase) null mutants has been shown to provide protection against re-challenge with L. major (33) and L. infantum (34). In contrast, mice infected with a L. major mutant defective in Leishmania homologue of receptors for activated C kinase (LACK) showed no increased protection against subsequent infection with wild-type parasites. This finding was not perhaps surprising given that mice infected with the mutant showed similar Th1/Th2 cytokine levels as mice that were infected with wild-type parasites (35,36), suggesting that the dominant LACK epitope does not play a role in the aberrant BALB/c response to L. major infection.
The CPB cysteine protease mutants of L. mexicana described earlier are attenuated in mice and also show decreased pathogenicity in hamsters, together with lower infectivity and growth in human mononuclear phagocytic host cells (37). In the hamster model, CPB-deficient transgenics give rise to significantly lower levels of the Th2-associated cytokines, IL-10 and TGF-β, when compared to wild-type parasites but give comparable protection against homologous challenge at low dose. These data support the feasibility of using these L. mexicana mutants to achieve protective immunity in vivo.
Only a small number of studies to date have focused on generating attenuated forms of L. donovani as a route to the production of an attenuated vaccine against VL. These include mutation of the essential biopterin transporter (BT1) gene in L. donovani, producing transgenic parasites with reduced infectivity, when compared to wild-type, that could confer protection to challenge with wild-type L. donovani in mice (38). In similar studies, deletion of centrin, a calcium-binding cytoskeletal protein, resulted in reduced viability of L. donovani promastigotes in vitro and an arrest of axenic amastigotes at the G2/M stage of the cell cycle, with a resultant inhibition in amastigote growth in macrophages (39). Given the importance of VL as a life-threatening infection in man, further studies in this area are clearly warranted.
INTERACTIONS WITH CELLS OF THE INNATE IMMUNE SYSTEM
Macrophages have long been regarded as the principle host cell for Leishmania and a range of studies has employed luminescent or fluorescent parasites to examine aspects of the host–parasite interaction in vitro (Table 1). In addition to macrophages, recent attention has focused on two other main cellular hosts, neutrophils and dendritic cells (DC).
In vitro, L. major (40) and L. donovani (41) promastigotes have both been shown to infect neutrophils. In the case of L. donovani, luciferase transgenic parasites have been used to demonstrate parasite retention within an ER-derived compartment, distinct from the classical phagolysosome (42), which retains metabolic activity (41). In addition to providing a potential means of entry into macrophages, under the guise of an apoptotic neutrophil, the extended life span of neutrophils infected with L. donovani promastigotes suggests an alternative means for dissemination from the site of initial infection. Whether this process occur in vivo remains to be explored, as does the extent to which Leishmania amastigotes interact with neutrophils. Immunohistochemical examination of mice harbouring long-term L. donovani infections failed to identify infected neutrophils in situ, although single cell ex vivo cultures revealed that granulocytes did indeed contain parasites (43).
Using transgenic L. major expressing enhanced green fluorescent protein (GFP), Misslitz et al. have demonstrated temporal differences in the appearance of parasite antigen in the draining LN (44) as well as the presence of viable intact parasites. DC isolated 16-h post-infection and carrying L. major antigen are an important source of IL-12/23p40 and functionally activate CD4+ Th2 cells. While the functional capacity of GFP+-infected DC was not explored, this study nevertheless highlights an important caveat when using fluorochrome transgenic parasites, namely the potential dissociation between immunologically relevant antigen and parasite localization. In addition, these authors also described a small number of GFP+ parasites apparently free within the subcapsular sinus, at 4-h post-infection. This is a somewhat surprising finding, given the presence of avidly phagocytic macrophages at this site (45), but one suggesting that direct lymphatic dissemination of L. major may occur, at least with high dose infection.
In related studies, the importance of LPG in the induction of DC pro-inflammatory responses has been investigated by transcriptional profiling of L. mexicana LPG1−/− mutants. The most striking observation from this work was the almost complete attenuation of interferon-induced and pro-inflammatory cytokine gene expression in the absence of LPG, a result that correlates with a similar lack of responsiveness to amastigote infection. Complementation of this phenotype could be achieved by episomal expression of LPG1 in LPG1−/− promastigotes (46).
ANTIGEN PROCESSING AND THE INFECTED ANTIGEN-PRESENTING CELL
As an intracellular parasite, Leishmania provides an interesting model to study the mechanisms that result in the cross-presentation of exogenous antigens to the Class I pathway for the stimulation of CD8+ T cells. Transgenic episomal expression of a reporter antigen, Escherichia coli β-galactosidase (β-gal), in L. mexicana was first described by Lopez et al. (47). Following infection of BALB/c mice, β-gal-specific cytotoxic T cells could be recovered from the spleen with potent killing activity directed towards β-gal-expressing tumour targets. However, specific in vitro lysis of macrophages infected with L. mexicana was not observed. Although this study was the first to demonstrate in vivo that cross presentation of Leishmania-derived antigen may occur, the underlying mechanism was not appreciated at the time. Although uptake of infected macrophages (and/or neutrophils) by DC might be involved, more recent identification of specific cross presentation pathways in DC (48) suggests that direct uptake of parasites by these cells may also play a role. The cellular decision controlling entry of ‘exogenous’ antigens into the cross-priming pathway (even in macrophages) may be made early during pathogen invasion (49).
In a similar series of experiments, Garcia et al. (50) also failed to demonstrate direct recognition of two reporter antigens, OVA and β-gal, this time in resting and interferon γ/LPS-activated bone marrow-derived macrophages infected with promastigotes of L. major and L. donovani. Although failure to enter an appropriate cross-priming pathway in these cells may also explain these results, the authors highlighted another potential mechanism, namely the cleavage of the reporter epitope by parasite-derive proteases (in this case, the major surface protease GP63). In more recent studies, OVA has again been used as a reporter system, with the OVA gene being integrated into the ribosomal DNA locus of L. donovani (51), allowing subsequent isolation of OVA-expressing amastigotes for infection in mice. In vivo recognition of OVA was confirmed by the capacity of adoptively transferred OVA-specific CD8+ OT-I T cells to cause a reduction in splenic and hepatic parasite burden in mice infected with OVA transgenic but not wild-type L. donovani amastigotes (51). Although these studies revealed kinetic differences in the function of naïve, central and effector memory and cytotoxic effector cells, it did not directly address the issue of which cells actually present OVA under these conditions and where these cells are located.
CD4+ T cells are known to be crucial in the clearance of Leishmania and antigen processing for CD4+ T cell recognition has also been examined using similar approaches to those described above. Kaye et al. (52) generated L. major transfectants expressing OVA and β-gal and as neither antigen was detectable as a secreted product, these parasites were used to test the efficacy of phagosomal-targeted antigen compared to that delivered as soluble antigen via endocytosis. Using in vivo-activated peritoneal macrophages, phagosomal delivery of OVA using L. major promastigotes was as efficient as soluble antigen in driving CD4+ T cell activation (once the response was adjusted for total antigen uptake). However, unlike macrophages pulsed with soluble OVA, which have a short-lived capacity for antigen presentation, presentation by OVA-L. major infected macrophages persisted through 24 h. The most likely explanation for these results was that slowly degraded parasites led to a slow-release of OVA into the MHCII processing pathway (52).
ANTIGEN LOCALIZATION AS A DETERMINANT OF PROCESSING EFFICIENCY
Early studies using E. coli and Salmonella down-played the importance of the site of antigen localization in the infecting micro-organism on the induction of CD4+ T cell responses, stressing antigen abundance as a more important response determinant (53). However, as both E. coli and S. tymphimurium are rapidly degraded in macrophages, with processing kinetics for CD4+ T cell recognition far faster than with Leishmania, the importance of antigen localization and hence accessibility to processing enzymes and/or antigen transport pathways for recognition of Leishmania remained unresolved.
In the first of two studies, Wolfram et al. (54) generated a T cell line against one of the L. mexicana cysteine proteases and used this to show that infected bone marrow-derived macrophages would only present the cysteine protease after intracellular amastigote killing (using either drugs or macrophage-activating cytokine cocktails). As with the studies of Kaye et al. (52), these data suggested that internal sequestration of antigen might limit availability to the MHCII processing pathway. To determine whether this was indeed the case, transgenic L. mexicana parasites in which the membrane acid phosphatase (MAP) was expressed either as a secreted or membrane antigen or retained in the parasite cytosol were generated. As predicted from their earlier work (54), cytosolic MAP was only recognized after parasites had died within the phagosome, but processing efficiency was strikingly enhanced when MAP was expressed in the membrane or better still, when secreted (55). These studies supported the importance of antigen localization for the recognition of intact amastigotes and suggested that antigen dose following degradation would be critical for effective recognition. This concept has recently been confirmed in vivo. Prickett et al. (56) utilized the N-terminal targeting sequence of L. major hydrophilic acylated surface protein B (HASPB) to generate parasites expressing OVA at equivalent levels in either the plasma membrane or in the cytosol. Surprisingly, given the complexity of potential processing options in vivo, only plasma membrane expression of OVA led to significant activation of antigen-specific CD4+ T cell responses.
In contrast to MHCII recognition, an extensive literature suggests that antigen secretion from intracellular pathogens facilitates MHCI loading, most notably in the presence of membrane lytic virulence factors such as listeriolysin (57). To examine this question in Leishmania, in which no known lysins have been described, extended peptides of OVA were fused to either the L. donovani 3′ nucleotidase/nuclease or the nucleotidase lacking its signal peptide and then expressed in L. major (58). Expression of OVA in L. major resulted in cross-presentation and priming of OVA-specific T cells by DC but not macrophages in vitro. In vivo, the transgenic L. major NT-OVA primed both CD4 and CD8 responses, demonstrating that this secreted product was able to be processed efficiently into both the Class I and Class II pathways. Secretion of the protein was demonstrated as a requirement of Class I processing, as priming of OT-I cells was significantly reduced in vitro when parasites could no longer secrete OVA. Secretion of antigens into the phagolysosome of infected cells therefore enhances the processing of antigens into the Class I pathway (58). Further studies using the NT-OVA L. major have examined which pathway of cross-presentation results in Leishmania proteins being presented in complex with Class I molecules. If the classical pathway is used, then trafficking of Leishmania antigens to the cytosol and the endoplasmic reticulum would occur, in a TAP dependant manner. Infection of DC with NT-OVA L. major, however, showed that TAP knockout DC were equally as efficient as wild-type DC in cross-presenting OVA to OT-I T cells in vitro. Processing of Leishmania antigens into the Class I pathway therefore occurs independently of the TAP pathway with peptides loaded directly into class I molecules within the phagolysosome of the infected cell. Infection of TAP knockout mice also demonstrated that TAP was dispensable for the efficient priming of CD8 responses in vivo (59).
IMMUNE MODULATION
Three different studies have focused on the use of transgenic L. major which express immunomodulatory proteins predicted to skew the host cytokine production towards a protective Th1 immune response and away from a pathogenic Th2 response. Transgenic L. major secreting biologically active murine interferon-γ failed to protect susceptible BALB/c mice from infection, despite a detectable increase in the amount of this cytokine in the infected tissues (60). In a similar study, transgenic L. major secreting biologically active GM-CSF showed more promise in promoting a protective host response (101). These GM-CSF expressing parasites survived poorly in macrophages in vitro, as a result of increased macrophage pro-inflammatory cytokine production (including IL-1β, IL-18 and IL-6) and infection in vivo showed delayed lesion development in susceptible mice.
In a third study, L. major engineered to express the extracellular domain of murine CD40-L caused smaller lesions in vivo with fewer parasites than controls in susceptible mice and reduced dissemination of transgenic parasites to lymph nodes (61). This protection was associated with reduced levels of IL-4 but similar levels of interferon-γ. However, the mechanism that facilitates access to and activation of host CD40 by parasite-derived CD40-L was not addressed.
Transgenic parasites have also been employed in boosting the innate immune response to infection. Transgenic L. major engineered to secrete monocyte chemoattractant protein-1 (MCP-1) have been used effectively to study the role of this chemokine in the early phase of the immune response to infection (62). In susceptible BALB/c mice, MCP-1-L. major showed reduced pathogenicity in vivo with reduced lesion sizes and smaller parasite burdens than those observed after infection with wild-type parasites. MCP-1 produced by the parasite was sufficient to mediate this effect, as MCP-1 knockout mice showed no difference in disease progression compared to wild-type infected mice. Parasite-produced MCP-1 led to recruitment of CCR2 positive macrophages but this innate immune-mediated effect was insufficient to protect the host against a subsequent challenge with wild-type parasites. It was therefore not surprising that little cell-mediated immune response was seen in lymphocytes from the MCP-1-L. major infected mice upon re-stimulation.
Very few studies to date have used transgenic Leishmania to investigate the immunomodulatory effects of parasite proteins on host immune responses. One such study examined cysteine protease B (CPB) derived from L. mexicana (63). Leishmania mexicana CPB deletion mutants showed similar kinetics of lesion development as wild-type parasites early in infection, but transgenic parasite-infected mice were able to clear the infection following the development of a strong STAT4 and IL-12-dependent Th1 response. CPB was confirmed to have a strong effect on the immune response in further studies where addition of the CPB gene array to L. major resulted in suppression of interferon-γ production as a result of degradation of components of the intracellular NF-κB signalling pathway resulting in lower IL-12 levels (63).
VISUALIZING THE IMMUNE RESPONSE
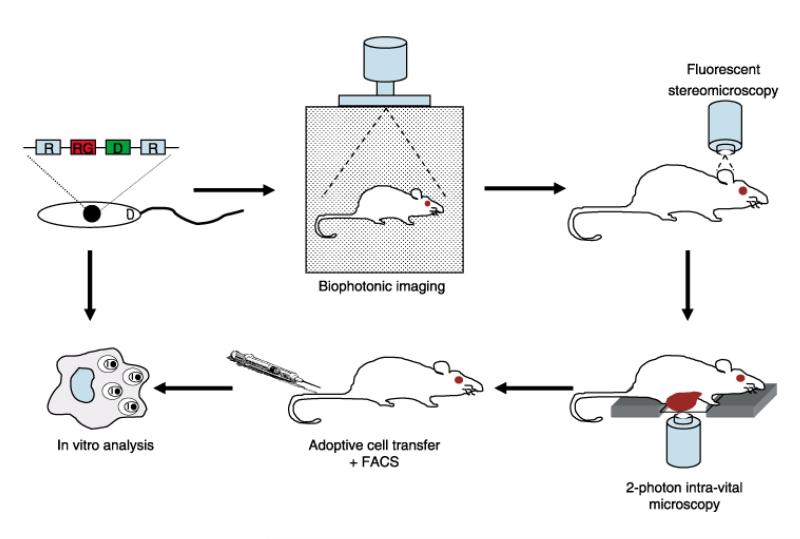
The development of transgenic Leishmania species expressing biochemical and fluorescent markers, combined with recent advances in imaging technology, is providing valuable opportunities to directly visualize Leishmania infection both in vitro and in vivo. A combination of approaches to study host–parasite interactions at a cellular, organ and whole body level can provide comprehensive and specific information regarding mechanisms of pathogenesis (Figure 1).
Figure 1. Approaches to analysing the immune response to infection with transgenic Leishmania.
Infection with transgenic Leishmania carrying one or more ‘reporter’ genes (RG; here coupled with a drug selection marker (D) and integrated into the ribosomal DNA locus (R) of the parasite nuclear genome) allows progressive in-depth analysis of the immune response to infection, from whole animal to specific tissue to cell-type to individual cell. For examples of the generation of transgenic parasites, see Figure 2 (a); Biophotonic imaging, see (b), (c); Fluorescent stereomicroscopy, see (d)–(f); Photon intravital microscopy, see (g); Adoptive cell transfer and FACS, see (h); Cell biology, see (i).
Bioluminescent imaging technology has enabled the study of infectious diseases in vivo in real time, utilizing transgenic pathogens expressing the firefly luciferase (LUC) gene [reviewed in Hutchens and Luker (64)]. This marker has been stably transfected into a number of Leishmania species, including L. major and L. donovani, and validated as a sensitive method for measuring parasite numbers in infected macrophages and tissue, as well as in drug screening (65,66).
To date, however, few studies using bioluminescent whole body imaging following Leishmania infection have been published. This non-invasive and nondestructive technique can provide real-time information about the kinetics of parasite growth and dissemination as well as quantitatively assessing parasite burdens in vivo. During infection with LUC-expressing L. amazonensis, Lang et al. (66) showed intensity of bioluminescent signal from the lesion correlated with parasite burden. Similarly, dissemination of L. major LUC parasites injected into the ear or footpad to the draining lymph node can be observed using the same approach (Figure 2b,c). In addition to the specificity and sensitivity of these experimental methods, they also require lower animal numbers to generate statistically significant data and allow non-invasive monitoring of infection progression, thereby supporting the reduction, refinement and replacement of animals in biomedical research.
Figure 2. Analysing the immune response to infection with transgenic Leishmania.
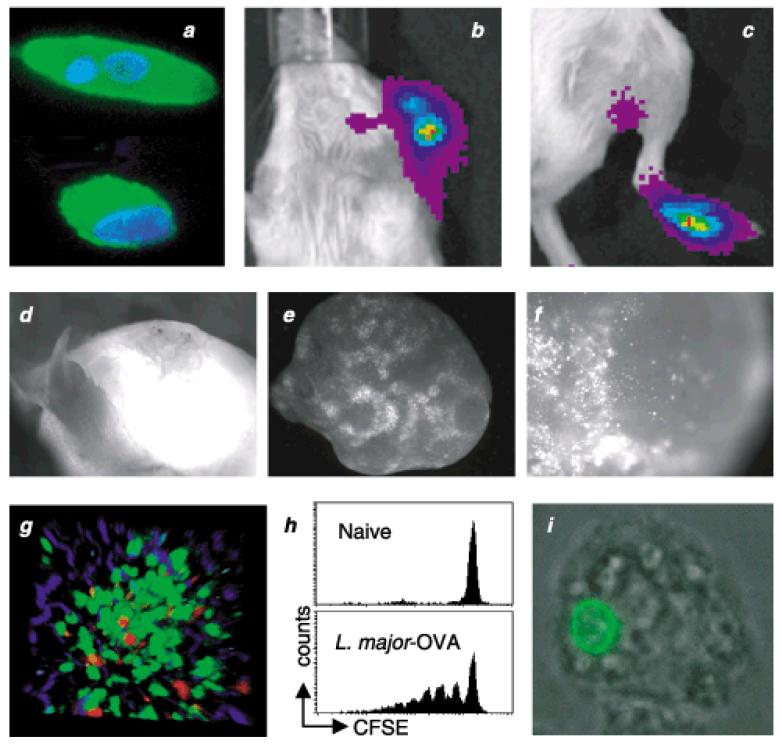
(a) Transgenic L. major promastigote (top)/amastigote (bottom) expressing HASPB18:OVA, labelled with goat anti-OVA followed by biotinylated rabbit antigoat F (ab′)2 and alexa-488-conjugated streptavidin (green). DAPI staining shows the nucleus and kinetoplast (blue). (b–c) BALB/c mice were infected intradermally in the ear, or subcutaneously in the footpad with 1 × 106 L major LUC promastigotes. Using a biophotonic imaging system, parasite luciferase signal was detected during the chronic stage of infection from both the lesion at the site of infection and from the draining lymph node, the auricular and popliteal lymph node, respectively. (d–f) Visualization of parasites in the primary lesion and the draining lymph node, by stereomicroscopy, during chronic infection with transgenic L. amazonensis expressing GFP. (g) Granuloma in the liver following infection with OVA transgenic L. donovani, in a transgenic mouse expressing GFP in T cells (green) with adoptively transferred in vitro activated OVA-specific OT-I effector memory cells labelled with CMTMR (red). Images were captured 12-h post-transfer by two-photon laser scanning microscopy. (h) CFSE-labelled OVA-specific CD4+ T cells were adoptively transferred into L. major-OVA infected mice and proliferation of the T cells monitored by CFSE dilution (unpublished data provided by P. Scott and P. Gray). (i) Transgenic L. major expressing a GFP:am1 gene fusion within a J774 macrophage. The am1 protein contains multiple transmembrane spanning domains that facilitate localization of the fusion protein at the parasite plasma membrane (103).
While bioluminescent imaging allows examination and quantification of parasite densities on a whole body level, it is limited in terms of resolution. Techniques such as stereomicroscopy allow visualization of both parasites and host cells at the level of individual organs. For example, GFP expressing L. amazonensis parasites can be visualized at the site of infection in the footpad (see Figure 2d) and are clearly detectable in the intact excised draining lymph node (see Figure 2e) and, at a higher resolution, within the paracortical area of the node itself (see Figure 2f).
Moving into methods that offer higher resolution of parasite interactions, intravital laser scanning microscopy, principally with 2-photon excitation, is a relatively new technique which has been applied very successfully to extend our understanding of how immune cells function [recently reviewed in (67)]. The availability of transgenic parasites expressing fluorophores and exogenous antigens for detection, coupled with the use of fluorochrome-labelled antigen-specific host cells, will provide increased power in the study of Leishmania immune responses, facilitating studies of parasite behaviour at the site of infection, interactions of immune cells with parasites (both at the initial sites of infection, as well as in visceral organs), interactions between antigen presenting cells and effector cell populations, and mechanisms that result in antiparasite effector responses (for example, see Figure 2g). This in situ information further extends the spatiotemporal data that can be obtained by ex vivo analysis of T cell responses to reporter antigens (such as analysis of replication by carboxyfluoroscein succinimidyl ester) dilution (56,68); and Figure 2(h).
At a cellular level, transgenic parasites expressing fluorophores and other exogenous proteins have contributed to our understanding of both parasite biology and primary cell biological events relevant to parasite persistence and/or elimination in the host (51,56,59,69–71). Tagging with GFP (or other fluorophores) allows protein detection within the parasite during live cell imaging analysis (see Figure 2i) and has the potential for real-time visualization of antigen shedding and host receptor interactions.
FUTURE DIRECTIONS
As described above, the development of transgenic techniques for manipulation of Leishmania has accelerated progress in our understanding of parasite biology and the host’s response to infection. Much has been achieved to date and further technical refinements can only increase the power and sensitivity of these approaches. Looking to the future, perhaps the most immediate need is for a robust and highly regulated system for inducible expression of parasite genes in the host. The ability to control the level and timing of transgenic gene expression experimentally would be invaluable in the dynamic study of host–parasite interactions during the time course of infection.
Tetracycline-inducible gene expression in the kinetoplastid parasites was originally pioneered in Trypanosoma brucei (72) and is now in widespread use, coupled with RNA interference to knock-down gene expression, to study parasite gene function both in culture and, most importantly, in susceptible hosts in vivo (73). Use of similar methods in Leishmania species has not been straightforward, not only due to the post-transcriptional regulatory mechanisms common to all kinetoplastids but also, to the lack of identifiable Leishmania promoters. The tetracycline-inducible systems developed so far are problematic with respect to background expression levels and low induction efficiencies (74,75), while components of an RNAi machinery are not encoded in the genomes of L. major and L. infantum. The recent sequencing of the L. braziliensis genome has revealed the potential for an active RNAi system however (24,76) and if active, this may be manipulable in the longer term to provide robust experimental methods for the manipulation of gene expression in vivo.
In terms of advancing imaging technologies, the ability to boost expression of reporter genes would increase both detection sensitivity and signal-to-noise ratios when examining host–parasite interactions. As an example, the activity of the LUC reporter gene when expressed in the 18 s ribosomal locus is greater than 10-fold down-regulated in L. amazonensis amastigotes as compared to metacyclic promastigotes (66), a degree of down-regulation which we have also observed in LUC-transfected L. major (K.J. Evans, unpublished data). The reasons for this down-regulation are currently unclear and could relate to metabolic differences between parasite stages, among other factors. Whatever the mechanism, the development of high level regulatable expression systems to boost transgenic protein levels in Leishmania amastigotes will be essential to further advance our understanding of host–parasite interactions during short and long-term infection. Exploiting the synergy between molecular and immunological approaches to the study of infection will lead to fully integrated studies that can significantly enhanced our knowledge and understanding of the leishmaniases and accelerate development of the next generation of therapeutics for management of these deadly diseases.
Acknowledgments
Research on the leishmaniases in the Immunology and Infection Unit is supported by programme grants from the UK Medical Research Council and the Wellcome Trust to PMK and DFS. We apologise to colleagues whose work has not been cited here due to space restrictions.
Abbreviations
- CL
cutaneous leishmaniasis
- MCL
mucocutaneous leishmaniasis
- DCL
diffuse cutaneous leishmaniasis
- VL
visceral leishmaniasis
- LPG
lipophosphoglycan
- PG
phosphoglycan
- OVA
ovalbumin
- LUC
luciferase
- MAP
membrane acid phosphatase
- DC
dendritic cells
- TAP
transporter associated with antigen processing
- CFSE
carboxyfluoroscein succinimidyl ester
REFERENCES
- 1.Desjeux P. Leishmaniasis. Nat Rev Microbiol. 2004;2:692. doi: 10.1038/nrmicro981. [DOI] [PubMed] [Google Scholar]
- 2.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 3.Lipoldova M, Demant P. Genetic susceptibility to infectious disease: lessons from mouse models of leishmaniasis. Nat Rev Genet. 2006;7:294–305. doi: 10.1038/nrg1832. [DOI] [PubMed] [Google Scholar]
- 4.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 5.Kaye PM, Svensson M, Ato M, et al. The immunopathology of experimental visceral leishmaniasis. Immunol Rev. 2004;201:239–253. doi: 10.1111/j.0105-2896.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 6.Cruz A, Beverley SM. Gene replacement in parasitic protozoa. Nature. 1990;348:171–173. doi: 10.1038/348171a0. [DOI] [PubMed] [Google Scholar]
- 7.Beverley SM. Protozomics: trypanosomatid parasite genetics comes of age. Nat Rev Genet. 2003;4:11–19. doi: 10.1038/nrg980. [DOI] [PubMed] [Google Scholar]
- 8.Naderer T, Vince JE, McConville MJ. Surface determinants of Leishmania parasites and their role in infectivity in the mammalian host. Curr Mol Med. 2004;4:649–665. doi: 10.2174/1566524043360069. [DOI] [PubMed] [Google Scholar]
- 9.Mosser DM, Brittingham A. Leishmania, macrophages and complement: a tale of subversion and exploitation. Parasitology. 1997;115:S9–S23. doi: 10.1017/s0031182097001789. [DOI] [PubMed] [Google Scholar]
- 10.Turco SJ. The leishmanial lipophosphoglycan: a multifunctional molecule. Exp Parasitol. 1990;70:241–245. doi: 10.1016/0014-4894(90)90105-l. [DOI] [PubMed] [Google Scholar]
- 11.Ilg T. Proteophosphoglycans of Leishmania. Parasitol Today. 2000;16:489–497. doi: 10.1016/s0169-4758(00)01791-9. [DOI] [PubMed] [Google Scholar]
- 12.Sacks D, Kamhawi S. Molecular aspects of parasite-vector and vector–host interactions in leishmaniasis. Annu Rev Microbiol. 2001;55:453–483. doi: 10.1146/annurev.micro.55.1.453. [DOI] [PubMed] [Google Scholar]
- 13.Descoteaux A, Turco SJ. Functional aspects of the Leishmania donovani lipophosphoglycan during macrophage infection. Microbes Infect. 2002;4:975–981. doi: 10.1016/s1286-4579(02)01624-6. [DOI] [PubMed] [Google Scholar]
- 14.Lodge R, Descoteaux A. Modulation of phagolysosome biogenesis by the lipophosphoglycan of Leishmania. Clin Immunol. 2005;114:256–265. doi: 10.1016/j.clim.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Beverley SM, Turco SJ. Lipophosphoglycan (LPG) and the identification of virulence genes in the protozoan parasite Leishmania. Trends Microbiol. 1998;6:35–40. doi: 10.1016/S0966-842X(97)01180-3. [DOI] [PubMed] [Google Scholar]
- 16.Spath GF, Epstein L, Leader B, et al. Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc Natl Acad Sci USA. 2000;97:9258–9263. doi: 10.1073/pnas.160257897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ilg T. Lipophosphoglycan is not required for infection of macrophages or mice by Leishmania mexicana. Embo J. 2000;19:1953–1962. doi: 10.1093/emboj/19.9.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capul AA, Hickerson S, Barron T, Turco SJ, Beverley SM. Comparisons of mutants lacking the Golgi UDP-Galactose or GDP-Mannose transporters establish that phosphoglycans are important for promastigote but not amastigote virulence in Leishmania major. Infect Immun. 2007 doi: 10.1128/IAI.00735-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garami A, Ilg T. The role of phosphomannose isomerase in Leishmania mexicana glycoconjugate synthesis and virulence. J Biol Chem. 2001;276:6566–6575. doi: 10.1074/jbc.M009226200. Epub 2000 November 6517. [DOI] [PubMed] [Google Scholar]
- 20.Zufferey R, Allen S, Barron T, et al. Ether phospholipids and glycosylinositolphospholipids are not required for amastigote virulence or for inhibition of macrophage activation by Leishmania major. J Biol Chem. 2003;278:44708–44718. doi: 10.1074/jbc.M308063200. Epub 42003 August 44727. [DOI] [PubMed] [Google Scholar]
- 21.Mottram JC, Souza AE, Hutchison JE, Carter R, Frame MJ, Coombs GH. Evidence from disruption of the lmcpb gene array of Leishmania mexicana that cysteine proteinases are virulence factors. Proc Natl Acad Sci U S A. 1996;93:6008–6013. doi: 10.1073/pnas.93.12.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denise H, McNeil K, Brooks DR, Alexander J, Coombs GH, Mottram JC. Expression of multiple CPB genes encoding cysteine proteases is required for Leishmania mexicana virulence in vivo. Infect Immun. 2003;71:3190–3195. doi: 10.1128/IAI.71.6.3190-3195.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi PB, Kelly BL, Kamhawi S, Sacks DL, McMaster WR. Targeted gene deletion in Leishmania major identifies leishmanolysin (GP63) as a virulence factor. Mol Biochem Parasitol. 2002;120:33–40. doi: 10.1016/s0166-6851(01)00432-7. [DOI] [PubMed] [Google Scholar]
- 24.Peacock CS, Seeger K, Harris D, et al. Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nat Genet. 2007;39:839–847. doi: 10.1038/ng2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang WW, Matlashewski G. Loss of virulence in Leishmania donovani deficient in an amastigote-specific protein, A2. Proc Natl Acad Sci U S A. 1997;94:8807–8811. doi: 10.1073/pnas.94.16.8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang WW, Matlashewski G. Characterization of the A2–A2rel gene cluster in Leishmania donovani: involvement of A2 in visceralization during infection. Mol Microbiol. 2001;39:935–948. doi: 10.1046/j.1365-2958.2001.02286.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang WW, Mendez S, Ghosh A, et al. Comparison of the A2 gene locus in Leishmania donovani and Leishmania major and its control over cutaneous infection. J Biol Chem. 2003;278:35508–35515. doi: 10.1074/jbc.M305030200. [DOI] [PubMed] [Google Scholar]
- 28.Tabbara KS, Peters NC, Afrin F, et al. Conditions influencing the efficacy of vaccination with live organisms against Leishmania major infection. Infect Immun. 2005;73:4714–4722. doi: 10.1128/IAI.73.8.4714-4722.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selvapandiyan A, Duncan R, Debrabant A, et al. Genetically modified live attenuated parasites as vaccines for leishmaniasis. Indian J Med Res. 2006;123:455–466. [PubMed] [Google Scholar]
- 30.Spath GF, Garraway LA, Turco SJ, Beverley SM. The role(s) of lipophosphoglycan (LPG) in the establishment of Leishmania major infections in mammalian hosts. Proc Natl Acad Sci USA. 2003;100:9536–9541. doi: 10.1073/pnas.1530604100. Epub 2003 July 9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uzonna JE, Spath GF, Beverley SM, Scott P. Vaccination with phosphoglycan-deficient Leishmania major protects highly susceptible mice from virulent challenge without inducing a strong Th1 response. J Immunol. 2004;172:3793–3797. doi: 10.4049/jimmunol.172.6.3793. [DOI] [PubMed] [Google Scholar]
- 32.Kebaier C, Uzonna JE, Beverley SM, Scott P. Immunization with persistent attenuated Delta lpg2 Leishmania major parasites requires adjuvant to provide protective immunity in C57BL/6 mice. Infect Immun. 2006;74:777–780. doi: 10.1128/IAI.74.1.777-780.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Titus RG, Gueiros-Filho FJ, de Freitas LA, Beverley SM. Development of a safe live Leishmania vaccine line by gene replacement. Proc Natl Acad Sci U S A. 1995;92:10267–10271. doi: 10.1073/pnas.92.22.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Streit JA, Recker TJ, Filho FG, Beverley SM, Wilson ME. Protective immunity against the protozoan Leishmania chagasi is induced by subclinical cutaneous infection with virulent but not avirulent organisms. J Immunol. 2001;166:1921–1929. doi: 10.4049/jimmunol.166.3.1921. [DOI] [PubMed] [Google Scholar]
- 35.Kelly BL, Stetson DB, Locksley RM. Leishmania major LACK antigen is required for efficient vertebrate parasitization. J Exp Med. 2003;198:1689–1698. doi: 10.1084/jem.20031162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly BL, Locksley RM. The Leishmania major LACK antigen with an immunodominant epitope at amino acids 156–173 is not required for early Th2 development in BALB/c mice. Infect Immun. 2004;72:6924–6931. doi: 10.1128/IAI.72.12.6924-6931.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saravia NG, Escorcia B, Osorio Y, et al. Pathogenicity and protective immunogenicity of cysteine proteinase-deficient mutants of Leishmania mexicana in non-murine models. Vaccine. 2006;24:4247–4259. doi: 10.1016/j.vaccine.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 38.Papadopoulou B, Roy G, Breton M, et al. Reduced infectivity of a Leishmania donovani biopterin transporter genetic mutant and its use as an attenuated strain for vaccination. Infect Immun. 2002;70:62–68. doi: 10.1128/IAI.70.1.62-68.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selvapandiyan A, Debrabant A, Duncan R, et al. Centrin gene disruption impairs stage-specific basal body duplication and cell cycle progression in Leishmania. J Biol Chem. 2004;279:25703–25710. doi: 10.1074/jbc.M402794200. [DOI] [PubMed] [Google Scholar]
- 40.Aga E, Katschinski DM, van Zandbergen G, et al. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J Immunol. 2002;169:898–905. doi: 10.4049/jimmunol.169.2.898. [DOI] [PubMed] [Google Scholar]
- 41.Gueirard P, Laplante A, Rondeau C, Milon G, Desjardins M. Trafficking of Leishmania donovani promastigotes in non-lytic compartments in neutrophils enables the subsequent transfer of parasites to macrophages. Cell Microbiol. 2007 doi: 10.1111/j.1462-5822.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 42.Duclos S, Diez R, Garin J, et al. Rab5 regulates the kiss and run fusion between phagosomes and endosomes and the acquisition of phagosome leishmanicidal properties in RAW 264.7 macrophages. J Cell Sci. 2000;113(Part 19):3531–3541. doi: 10.1242/jcs.113.19.3531. [DOI] [PubMed] [Google Scholar]
- 43.Lang T, Ave P, Huerre M, Milon G, Antoine JC. Macrophage subsets harbouring Leishmania donovani in spleens of infected BALB/c mice: localization and characterization. Cell Microbiol. 2000;2:415–430. doi: 10.1046/j.1462-5822.2000.00070.x. [DOI] [PubMed] [Google Scholar]
- 44.Misslitz AC, Bonhagen K, Harbecke D, Lippuner C, Kamradt T, Aebischer T. Two waves of antigen-containing dendritic cells in vivo in experimental Leishmania major infection. Eur J Immunol. 2004;34:715–725. doi: 10.1002/eji.200324391. [DOI] [PubMed] [Google Scholar]
- 45.Nossal GJ, Abbot A, Mitchell J. Antigens in immunity. XIV. Electron microscopic radioautographic studies of antigen capture in the lymph node medulla. J Exp Med. 1968;127:263–276. doi: 10.1084/jem.127.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aebischer T, Bennett CL, Pelizzola M, et al. A critical role for lipophosphoglycan in proinflammatory responses of dendritic cells to Leishmania mexicana. Eur J Immunol. 2005;35:476–486. doi: 10.1002/eji.200425674. [DOI] [PubMed] [Google Scholar]
- 47.Lopez JA, LeBowitz JH, Beverley SM, Rammensee HG, Overath P. Leishmania mexicana promastigotes induce cytotoxic T lymphocytes in vivo that do not recognize infected macrophages. Eur J Immunol. 1993;23:217–223. doi: 10.1002/eji.1830230134. [DOI] [PubMed] [Google Scholar]
- 48.Schnorrer P, Behrens GM, Wilson NS, et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci USA. 2006;103:10729–10734. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burgdorf S, Lukacs-Kornek V, Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J Immunol. 2006;176:6770–6776. doi: 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- 50.Garcia MR, Graham S, Harris RA, Beverley SM, Kaye PM. Epitope cleavage by Leishmania endopeptidase (s) limits the efficiency of the exogenous pathway of major histocompatibility complex class I-associated antigen presentation. Eur J Immunol. 1997;27:1005–1013. doi: 10.1002/eji.1830270430. [DOI] [PubMed] [Google Scholar]
- 51.Polley R, Stager S, Prickett S, et al. Adoptive immunotherapy against experimental visceral leishmaniasis with CD8+ T cells requires the presence of cognate antigen. Infect Immun. 2006;74:773–776. doi: 10.1128/IAI.74.1.773-776.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaye PM, Coburn C, McCrossan M, Beverley SM. Antigens targeted to the Leishmania phagolysosome are processed for CD4+ T cell recognition. Eur J Immunol. 1993;23:2311–2319. doi: 10.1002/eji.1830230939. [DOI] [PubMed] [Google Scholar]
- 53.Wick MJ, Pfeifer JD, Findlay KA, Harding CV, Normark SJ. Compartmentalization of defined epitopes expressed in Escherichia coli has only a minor influence on efficiency of phagocytic processing for presentation by class I and class II major histocompatibility complex molecules to T cells. Infect Immun. 1993;61:4848–4856. doi: 10.1128/iai.61.11.4848-4856.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolfram M, Ilg T, Mottram JC, Overath P. Antigen presentation by Leishmania mexicana-infected macrophages: activation of helper T cells specific for amastigote cysteine proteinases requires intracellular killing of the parasites. Eur J Immunol. 1995;25:1094–1100. doi: 10.1002/eji.1830250435. [DOI] [PubMed] [Google Scholar]
- 55.Wolfram M, Fuchs M, Wiese M, Stierhof YD, Overath P. Antigen presentation by Leishmania mexicana-infected macrophages: activation of helper T cells by a model parasite antigen secreted into the parasitophorous vacuole or expressed on the amastigote surface. Eur J Immunol. 1996;26:3153–3162. doi: 10.1002/eji.1830261248. [DOI] [PubMed] [Google Scholar]
- 56.Prickett S, Gray PM, Colpitts SL, Scott P, Kaye PM, Smith DF. In vivo recognition of ovalbumin expressed by transgenic Leishmania is determined by its subcellular localization. J Immunol. 2006;176:4826–4833. doi: 10.4049/jimmunol.176.8.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen H, Miller JF, Fan X, Kolwyck D, Ahmed R, Harty JT. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell. 1998;92:535–545. doi: 10.1016/s0092-8674(00)80946-0. [DOI] [PubMed] [Google Scholar]
- 58.Bertholet S, Debrabant A, Afrin F, et al. Antigen requirements for efficient priming of CD8+ T cells by Leishmania major-infected dendritic cells. Infect Immun. 2005;73:6620–6628. doi: 10.1128/IAI.73.10.6620-6628.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bertholet S, Goldszmid R, Morrot A, et al. Leishmania antigens are presented to CD8+ T cells by a transporter associated with antigen processing-independent pathway. In Vitro Vivo J Immunol. 2006;177:3525–3533. doi: 10.4049/jimmunol.177.6.3525. [DOI] [PubMed] [Google Scholar]
- 60.Tobin JF, Reiner SL, Hatam F, et al. Transfected Leishmania expressing biologically active IFN-γ. J Immunol. 1993;150:5059–5069. [PubMed] [Google Scholar]
- 61.Field AE, Wagage S, Conrad SM, Mosser DM. Reduced pathology following infection with transgenic Leishmania major expressing murine CD40 ligand. Infect Immun. 2007;75:3140–3149. doi: 10.1128/IAI.00160-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Conrad SM, Strauss-Ayali D, Field AE, Mack M, Mosser DM. Leishmania-derived murine monocyte chemoattractant protein 1 enhances the recruitment of a restrictive population of CC chemokine receptor 2-positive macrophages. Infect Immun. 2007;75:653–665. doi: 10.1128/IAI.01314-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cameron P, McGachy A, Anderson M, et al. Inhibition of lipopolysaccharide-induced macrophage IL-12 production by Leishmania mexicana amastigotes: the role of cysteine peptidases and the NF-κB signaling pathway. J Immunol. 2004;173:3297–3304. doi: 10.4049/jimmunol.173.5.3297. [DOI] [PubMed] [Google Scholar]
- 64.Hutchens M, Luker GD. Applications of bioluminescence imaging to the study of infectious diseases. Cell Microbiol. 2007 doi: 10.1111/j.1462-5822.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- 65.Roy G, Dumas C, Sereno D, et al. Episomal and stable expression of the luciferase reporter gene for quantifying Leishmania spp. infections in macrophages and in animal models. Mol Biochem Parasitol. 2000;110:195–206. doi: 10.1016/s0166-6851(00)00270-x. [DOI] [PubMed] [Google Scholar]
- 66.Lang T, Goyard S, Lebastard M, Milon G. Bioluminescent Leishmania expressing luciferase for rapid and high throughput screening of drugs acting on amastigote-harbouring macrophages and for quantitative real-time monitoring of parasitism features in living mice. Cell Microbiol. 2005;7:383–392. doi: 10.1111/j.1462-5822.2004.00468.x. [DOI] [PubMed] [Google Scholar]
- 67.Cahalan MD, Parker I. Imaging the choreography of lymphocyte trafficking and the immune response. Curr Opin Immunol. 2006;18:476–482. doi: 10.1016/j.coi.2006.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gray PM, Reiner SL, Smith DF, Kaye PM, Scott P. Antigen-experienced T cells limit the priming of naive T cells during infection with Leishmania major. J Immunol. 2006;177:925–933. doi: 10.4049/jimmunol.177.2.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Denny PW, Gokool S, Russell DG, Field MC, Smith DF. Acylation-dependent protein export in Leishmania. J Biol Chem. 2000;275:11017–11025. doi: 10.1074/jbc.275.15.11017. [DOI] [PubMed] [Google Scholar]
- 70.Burchmore RJ, Rodriguez-Contreras D, McBride K, et al. Genetic characterization of glucose transporter function in Leishmania mexicana. Proc Natl Acad Sci USA. 2003;100:3901–3906. doi: 10.1073/pnas.0630165100. Epub 2003 March 3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dubessay P, Blaineau C, Bastien P, et al. Cell cycle-dependent expression regulation by the proteasome pathway and characterization of the nuclear targeting signal of a Leishmania major Kin-13 kinesin. Mol Microbiol. 2006;59:1162–1174. doi: 10.1111/j.1365-2958.2005.05013.x. [DOI] [PubMed] [Google Scholar]
- 72.Wirtz E, Clayton C. Inducible gene expression in trypanosomes mediated by a prokaryotic repressor. Science. 1995;268:1179–1183. doi: 10.1126/science.7761835. [DOI] [PubMed] [Google Scholar]
- 73.Sheader K, Vaughan S, Minchin J, Hughes K, Gull K, Rudenko G. Variant surface glycoprotein RNA interference triggers a precytokinesis cell cycle arrest in African trypanosomes. Proc Natl Acad Sci USA. 2005;102:8716–8721. doi: 10.1073/pnas.0501886102. Epub 2005 June 8713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yan S, Myler PJ, Stuart K. Tetracycline regulated gene expression in Leishmania donovani. Mol Biochem Parasitol. 2001;112:61–69. doi: 10.1016/s0166-6851(00)00345-5. [DOI] [PubMed] [Google Scholar]
- 75.Yan S, Martinez-Calvillo S, Schnaufer A, Sunkin S, Myler PJ, Stuart K. A low-background inducible promoter system in Leishmania donovani. Mol Biochem Parasitol. 2002;119:217–223. doi: 10.1016/s0166-6851(01)00418-2. [DOI] [PubMed] [Google Scholar]
- 76.Smith DF, Peacock CS, Cruz AK. Comparative genomics: from genotype to disease phenotype in the leishmaniases. Int J Parasitol. 2007;37:1173–1186. doi: 10.1016/j.ijpara.2007.05.015. Epub 2007 June 1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cruz A, Coburn CM, Beverley SM. Double targeted gene replacement for creating null mutants. Proc Natl Acad Sci USA. 1991;88:7170–7174. doi: 10.1073/pnas.88.16.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Monte-Alegre A, Ouaissi A, Sereno D. Leishmania amastigotes as targets for drug screening. Kinetoplastid Biol Dis. 2006;5:6. doi: 10.1186/1475-9292-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ryan KA, Dasgupta S, Beverley SM. Shuttle cosmid vectors for the trypanosomatid parasite Leishmania. Gene. 1993;131:145–150. doi: 10.1016/0378-1119(93)90684-u. [DOI] [PubMed] [Google Scholar]
- 80.Gay LS, Wilson ME, Donelson JE. The promoter for the ribosomal RNA genes of Leishmania chagasi. Mol Biochem Parasitol. 1996;77:193–200. doi: 10.1016/0166-6851(96)02594-7. [DOI] [PubMed] [Google Scholar]
- 81.Ryan KA, Garraway LA, Descoteaux A, Turco SJ, Beverley SM. Isolation of virulence genes directing surface glycosylphosphatidylinositol synthesis by functional complementation of Leishmania. Proc Natl Acad Sci USA. 1993;90:8609–8613. doi: 10.1073/pnas.90.18.8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang C, Turco SJ. Defective galactofuranose addition in lipophosphoglycan biosynthesis in a mutant of Leishmania donovani. J Biol Chem. 1993;268:24060–24066. [PubMed] [Google Scholar]
- 83.Davoudi N, Tate CA, Warburton C, Murray A, Mahboudi F, McMaster WR. Development of a recombinant Leishmania major strain sensitive to ganciclovir and 5-fluorocytosine for use as a live vaccine challenge in clinical trials. Vaccine. 2005;23:1170–1177. doi: 10.1016/j.vaccine.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 84.Sereno D, Roy G, Lemesre JL, Papadopoulou B, Ouellette M. DNA transformation of Leishmania infantum axenic amastigotes and their use in drug screening. Antimicrob Agents Chemother. 2001;45:1168–1173. doi: 10.1128/AAC.45.4.1168-1173.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh N, Dube A. Short report: fluorescent Leishmania: application to anti-leishmanial drug testing. Am J Trop Med Hyg. 2004;71:400–402. [PubMed] [Google Scholar]
- 86.Ashutosh, Gupta S, Ramesh, Sundar S, Goyal N. Use of Leishmania donovani field isolates expressing the luciferase reporter gene in in vitro drug screening. Antimicrob Agents Chemother. 2005;49:3776–3783. doi: 10.1128/AAC.49.9.3776-3783.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kamau SW, Grimm F, Hehl AB. Expression of green fluorescent protein as a marker for effects of antileishmanial compounds in vitro. Antimicrob Agents Chemother. 2001;45:3654–3656. doi: 10.1128/AAC.45.12.3654-3656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bart G, Frame MJ, Carter R, Coombs GH, Mottram JC. Cathepsin B-like cysteine proteinase-deficient mutants of Leishmania mexicana. Mol Biochem Parasitol. 1997;88:53–61. doi: 10.1016/s0166-6851(97)00072-8. [DOI] [PubMed] [Google Scholar]
- 89.Alexander J, Coombs GH, Mottram JC. Leishmania mexicana cysteine proteinase-deficient mutants have attenuated virulence for mice and potentiate a Th1 response. J Immunol. 1998;161:6794–6801. [PubMed] [Google Scholar]
- 90.Joshi PB, Sacks DL, Modi G, McMaster WR. Targeted gene deletion of Leishmania major genes encoding developmental stage-specific leishmanolysin (GP63) Mol Microbiol. 1998;27:519–530. doi: 10.1046/j.1365-2958.1998.00689.x. [DOI] [PubMed] [Google Scholar]
- 91.Soleimani M, Mahboudi F, Davoudi N, et al. Expression of human tissue-type plasminogen activator (t-PA) in Leishmania tarentolae. Biotechnol Appl Biochem. 2007 doi: 10.1042/BA20060217. [DOI] [PubMed] [Google Scholar]
- 92.Morales MA, Renaud O, Faigle W, Shorte SL, Spath GF. Over-expression of Leishmania major MAP kinases reveals stage-specific induction of phosphotransferase activity. Int J Parasitol. 2007 doi: 10.1016/j.ijpara.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 93.Belen Carrillo M, Gao W, Herrera M, et al. Heterologous expression of Trypanosoma cruzi trans-sialidase in Leishmania major enhances virulence. Infect Immun. 2000;68:2728–2734. doi: 10.1128/iai.68.5.2728-2734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lodge R, Diallo TO, Descoteaux A. Leishmania donovani lipophosphoglycan blocks NADPH oxidase assembly at the phagosome membrane. Cell Microbiol. 2006;8:1922–1931. doi: 10.1111/j.1462-5822.2006.00758.x. Epub 2006 July 1911. [DOI] [PubMed] [Google Scholar]
- 95.Winberg ME, Rasmusson B, Sundqvist T. Leishmania donovani: inhibition of phagosomal maturation is rescued by nitric oxide in macrophages. Exp Parasitol. 2007;117:165–170. doi: 10.1016/j.exppara.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 96.Ilg T, Demar M, Harbecke D. Phosphoglycan repeat-deficient Leishmania mexicana parasites remain infectious to macrophages and mice. J Biol Chem. 2001;276:4988–4997. doi: 10.1074/jbc.M008030200. [DOI] [PubMed] [Google Scholar]
- 97.Garami A, Ilg T. Disruption of mannose activation in Leishmania mexicana: GDP-mannose pyrophosphorylase is required for virulence, but not for viability. Embo J. 2001;20:3657–3666. doi: 10.1093/emboj/20.14.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Spath GF, Lye LF, Segawa H, Turco SJ, Beverley SM. Identification of a compensatory mutant (lpg2-REV) of Leishmania major able to survive as amastigotes within macrophages without LPG2-dependent glycoconjugates and its significance to virulence and immunization strategies. Infect Immun. 2004;72:3622–3627. doi: 10.1128/IAI.72.6.3622-3627.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Selvapandiyan A, Duncan R, Debrabant A, et al. Expression of a mutant form of Leishmania donovani centrin reduces the growth of the parasite. J Biol Chem. 2001;276:43253–43261. doi: 10.1074/jbc.M106806200. [DOI] [PubMed] [Google Scholar]
- 100.Misslitz A, Mottram JC, Overath P, Aebischer T. Targeted integration into a rRNA locus results in uniform and high level expression of transgenes in Leishmania amastigotes. Mol Biochem Parasitol. 2000;107:251–261. doi: 10.1016/s0166-6851(00)00195-x. [DOI] [PubMed] [Google Scholar]
- 101.Dumas C, Muyombwe A, Roy G, et al. Recombinant Leishmania major secreting biologically active granulocyte-macrophage colony-stimulating factor survives poorly in macrophages in vitro and delays disease development in mice. Infect Immun. 2003;71:6499–6509. doi: 10.1128/IAI.71.11.6499-6509.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Buxbaum LU, Denise H, Coombs GH, Alexander J, Mottram JC, Scott P. Cysteine protease B of Leishmania mexicana inhibits host Th1 responses and protective immunity. J Immunol. 2003;171:3711–3717. doi: 10.4049/jimmunol.171.7.3711. [DOI] [PubMed] [Google Scholar]
- 103.Dyall SD, Denny PW, Seepersaud R, McGhie D, Smith DF. Expression of the AM gene locus in infective stages of Leishmania. Mol Biochem Parasitol. 2000;109:73–79. doi: 10.1016/s0166-6851(00)00235-8. [DOI] [PubMed] [Google Scholar]