Abstract
The metabolism of cancer cells is reprogrammed by oncogene signaling and/or mutations in metabolic enzymes. These metabolic alterations support cell proliferation and survival, but leave cancer cells dependent on continuous support of the nutrients that fuel their altered metabolism. Thus, in addition to core oncogenic pathways, many metabolic enzymes have become targets for novel therapies. Two novel processes- isoform-specific expression of metabolic enzymes and autophagy- have recently been shown to play critical roles in the adaptation of tumor cells to changes in nutrient availability and the cell's ability to sense and adapt to depletion of critical nutrients. These findings suggest that a better understanding of the molecular basis of cancer-associated metabolic changes has the potential to provide insights to enhance cancer therapy.
In the past decades, the development of genomic screening has contributed to the identification of many oncogenes and tumor suppressors which are frequently altered in a variety of tumors. A significant portion of these oncogenic abnormalities are associated with growth signaling pathways. Recently, increasing evidence has suggested that growth signaling pathways directly control cell metabolism, growth and proliferation by regulating metabolic enzymes. Furthermore, individual metabolic enzymes have been reported to be mutated or amplified during tumor progression. Since most cancer cells display and rely on enhanced nutrient utilization, this feature can potentially be explored from a therapeutic perspective. Understanding how metabolic pathways are altered in tumors and how cancer cells benefit from tumor-specific metabolic changes may contribute to the identification of novel therapeutic targets and the development of more effective cancer therapies.
In this review, we discuss studies that elucidate the enzymes contributing to the metabolic reprogramming in cancer cells and their potential as therapeutic targets. In addition, we discuss how cancer cells adapt to bioenergetic challenges by using autophagy as a cell survival strategy and summarize the ongoing efforts to target autophagy in combination with conventional chemotherapy. This review proposes new avenues for cancer therapies in light of the recent progress in understanding tumor metabolism.
Metabolic targets responsible for reprogrammed cancer metabolism
Cancer cells maintain their growth advantage through persistent activation of growth signaling pathways and inactivation of tumor suppressors. Canonical oncogenic signaling pathways such as PI3K/AKT and mTOR directly reprogram the core carbon metabolism in cancer, leading to increased nutrient uptake, which favors increased macromolecular biosynthesis to support cell proliferation. Indeed, a number of enzymes involved in metabolic alterations are direct targets of oncogenic transcription factors such as Myc and Hypoxia-inducible Factor 1α (HIF-1α). Various approaches to target oncogenic signaling pathways have been actively explored and showed great success in clinical trials. The detailed regulatory connection between signaling pathways and metabolic enzymes have been the topic of numerous reviews1,2.
Moreover, emerging evidence suggests that the metabolites derived from altered metabolism also influence oncogenic signaling pathways in a reciprocal manner, and such interaction may be the basis for tumor progression and/or resistance to conventional chemotherapeutic approaches. Accordingly, metabolic alterations involved in cancer progression can become an attractive target for cancer therapy. Table 1 summarizes the enzymes contributing to the metabolic reprogramming in cancer which are targets for clinical trials. In addition, additional metabolic enzymes are being actively investigated for their roles in the progression of various cancers and their potential as therapeutic targets (Figure 1).
Table 1. Potential therapeutic compounds targeting metabolic enzymes of tumors.
| Compound | Target | Tumor type/Cancer cell types | Clinical stages |
|---|---|---|---|
|
| |||
| Glucose metabolism | |||
|
| |||
| 2-DG | Glucose transporter | Prostate cancer | Phase I (terminated) |
| Silybin/silibinin | Prostate cancer | Phase I/II | |
|
| |||
| 2-DG | HK2 | Prostate cancer | Phase I (terminated) |
| Lonidamine | Phase I (Europe) | ||
|
| |||
| TLN-232/CAP-23 | PKM2 | Metastatic RCC, melanoma | Phase II |
|
| |||
| Gossypol/AT-101 | LDHA | Multiple cancers | Phase I/II |
|
| |||
| Dichloroacetate (DCA) | PDK | Brain cancer | Phase I |
| Non-small cell lung cancer (NSCLC) | Phase II | ||
| Head and neck cancer | |||
|
| |||
| AZD3965 | MCT1 | Advanced solid tumors | Phase I/II |
|
| |||
| Nucleic acid metabolism | |||
|
| |||
| Methotrexate | Folate cycle (DHFR) | Multiple cancers | Registered |
| Pemetrexel | |||
|
| |||
| 5-Fluorouracil | Thymidine synthesis (TYMS) | Multiple cancers | Registered |
|
| |||
| Hydroxyurea | Deoxynucleotide synthesis (Ribonucleotide reductase: RNR) | Multiple cancers | Registered |
|
| |||
| Gemcitabine, Fludarabine | Nucleotide incorporation (DNA polymerase/RNR) | Multiple cancers | Registered |
|
| |||
| Amino acid metabolism | |||
|
| |||
| L-Asparginase | Asparagine | Leukemia | Registered |
|
| |||
| Arginine deaminase (ADI-PEG conjugated) | Arginine | Multiple cancers | Phase II |
|
| |||
| NAD metabolism | |||
|
| |||
| FK866/APO866 | Nicotinamide phosphoribosyl-transferase (NAMPT) | Cutaneous T cell lymphoma (CTCL), B-Cell Chronic Lymphocytic Leukemia(CLL), melanoma | Phase II |
| CHS828/GMX1777 | Metastatic Melanoma, Solid Tumors, Lymphomas | Phase II | |
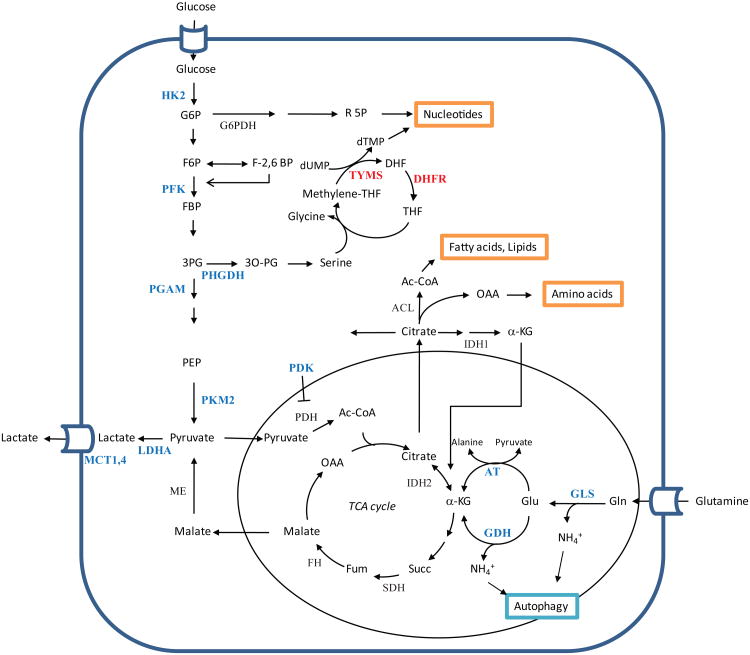
Figure 1. Core metabolic pathways and metabolic enzymes suitable as cancer therapeutic targets.
Active metabolic pathways in proliferating cells involving glucose and glutamine catabolism are interconnected and linked to macromolecular synthesis and energy balance. Key metabolic enzymes discussed in the text (shown in blue) are actively investigated as therapeutic targets for cancer treatment. Metabolic enzymes targeted by registered agents are shown in Red.
ACL, ATP citrate lyase; αKG, α-ketoglutarate; DHFR, dehydrofolate reductase;; dTMP, deoxythymidine monophosphate; dUMP, deoxyuridine monophosphate; F-2,6-BP, fructose-2,6-bisphosphate; F6P, fructose-6-phosphate; FBP, fructose-1,6-bisphosphate; FH, fumarate hydratase; G6P, glucose-6-phosphate; GLS, glutaminase; HK2, hexokinase 2; IDH, isocitrate dehydrogenase; LDHA, lactate dehydrogenase A; MCT1,4, monocarboxylate transporter 1,4; OAA, oxaloacetate; PDH, pyruvate dehydrogenase complex; PDK, pyruvate dehydrogenase kinase; PEP, phosphoenolpyruvate; PFK1, phosphofructokinase 1; PFK2, phosphofructokinase 2; PGAM, phosphoglycerate mutase; PHGDH, phosphoglycerate dehydrogenase; PKM2, pyruvate kinase M2 isoform; R5P, ribose-5-phosphate; SDH, succinate dehydrogenase; THF, tetrahydrofolate; TYMS, thymidylate synthase
Glucose metabolism
As early as in the 1920s the German biochemist Otto Warburg made the observation that tumor cells utilize glucose in a substantially distinct way from normal cells, probably the first evidence for metabolic alterations in cancer3. While normal cells direct glucose to mitochondrial oxidative phosphorylation to generate ATP when oxygen is abundant, tumor cells generally exhibit increased glucose uptake, glycolytic flux and lactate secretion regardless of the oxygen availability. This phenomenon, termed aerobic glycolysis, formed the basis for the development of 18F-deoxyglucose positron emission tomography (FDG-PET) to image tumor development and regression in patients. The exact role and regulation of aerobic glycolysis is still not fully understood, however it has been suggested that its major benefit to cancer cells is to supply more favorable forms of energetic and anabolic substrates required for massive macromolecular synthesis. The Warburg effect also led to attempts to target glucose metabolism to preferentially eliminate cancer cells.
As a first step, glucose enters the cancer cell via specific transporters. Therefore a straightforward strategy would be to block glucose uptake by cancer cells through inhibition of glucose transporters. However, as there are several isoforms of glucose transporters and most of them have been shown to be highly expressed in cancer cells, thus it will be challenging to develop inhibitors that target all isoforms with an acceptable therapeutic window. At least one small molecule inhibitor (i.e. Silybin/silibinin) of glucose transporters has been developed and clinical trials are ongoing to test its toxicity and efficacy4, 5.
As an alternative strategy, 2-deoxy-D-glucose (2DG), an analogue of glucose, has been tested as an anti-tumor agent. 2DG enters the cells via glucose transporters but is trapped as 2-deoxyglucose-6-phosphate after phosphorylation by hexokinase (HK) and cannot be further metabolized. The accumulation of 2-deoxyglucose-6-phosphate was thought to inhibit glycolytic enzymes and glucose catabolism. Although 2DG was shown to be effective in pre-clinical and clinical studies, the treatment was never fully explored because of concerns related to potential toxicity at high doses6. In contrast, recently it was discovered that cancer cells seem to preferentially rely on specific isoforms of glycolytic enzymes. Therefore attention has turned to developing isoform-specific inhibitors which are expected to increase drug specificity to cancer cells and avoid unwanted toxicity to normal cells. Figure 1 describes key metabolic pathways and enzymes being investigated as potential targets for cancer therapy. Examples include the muscle-specific isoform of hexokinase (HK2) and phosphofructokinase 2 (PFK2). Tumor-specific expression of these isoforms led to the identification of isoform-specific inhibitors which showed significant suppression of tumor growth in pre-clinical studies7, 8 (Table 1).
Pyruvate kinase (PK) converts phosphoenolpyruvate (PEP) to pyruvate and generates ATP, which is a rate-limiting step in glycolysis. A splicing variant PKM2, the muscle form of PK, is predominantly expressed in embryonic cells and tumor cells. The glycolytic intermediate, fructose-1,6-bisphosphate (FBP), binds to PKM2 and converts it to an enzymatically active form, whereas the tyrosine kinase signaling pathway inactivates PKM2 through the release of FBP from PKM29. Because most cancer cells exhibit constitutively active tyrosine kinase signaling, the enzyme activity of PKM2 is decreased in cancer cells. This has been proposed to be important for cell proliferation as low activity of PKM2 leads to the accumulation of glycolytic intermediates, many of which are precursors of macromolecular synthesis such as nucleotides and amino acids or can be used for the generation of NADPH10. Later studies suggested that PEP acts as a phosphate donor for an upstream enzyme phosphoglycerate mutase (PGAM1) in PKM2-expressing cells which further promotes glycolysis as well as biosynthetic processes11. Indeed, it was recently reported that PKM2 cells maintain higher flux to the serine synthetic pathway12, 13. The unique regulation of PKM2-mediated serine synthesis was suggested to be important for tumor growth and mTORC1 signaling, which will be discussed later. Based on these data, a variety of PKM2-specific small-molecule inhibitors or activators have been tested for their therapeutic potential in pre-clinical studies14-16 and some of them are being tested in clinical trials6(Table 1).
As the end product of glycolysis, the regulation of lactate generation and disposal has been extensively studied. Since lactate dehydrogenase (LDH) converts pyruvate to lactate while oxidizing NADH to NAD+ to support continued glycolytic flux, this enzyme has been considered as one of the critical targets for suppressing elevated glycolysis in cancer.
An isoform of LDH, LDHA is preferentially expressed in many cancers and is a transcriptional target of Myc and HIF-1α. RNAi knock-down experiments in various cancer cells showed that LDHA plays an important role in tumor growth17-19. A natural phenol derivative, Gossypol and its derivatives have been shown to compete with NADH binding to LDH and inhibit LDH activity. Despite the lack of specificity, this agent is being tested in clinical trials for various cancers20. The analogues of Gossypol have been screened for small molecule inhibitors specific for LDHA. Among them, FX11 (3-dihydroxy-6-methyl-7-(phenylmethyl)-4-propylnaphthalene-1-carboxylic acid) was shown to effectively inhibit cancer cell growth in vitro and in vivo by increasing the levels of oxidative stress21. More recently, N-hydroxyindole-based compounds have been identified as isoform-specific inhibitors of LDHA which compete with its substrates pyruvate and the cofactor, NADH22.
Pyruvate dehydrogenase kinase 1 (PDK1) is another transcriptional target of Myc and HIF1α which appears to play a critical role in many cancers. It inactivates pyruvate dehydrogenase (PDH), which converts pyruvate to acetyl-CoA in the mitochondria. As a result, pyruvate is shuttled from the TCA cycle to produce lactate. Accordingly, specific inhibitors of PDK can block aerobic glycolysis and increase the rate of oxidative phosphorylation. For example, dichloroacetate (DCA), which is widely used for the treatment of lactic acidosis, has been tested in some pre-clinical cancer models and yielded promising results in clinical trials23,24 (Table 1), although its mechanism of action requires further investigation as it appears that this pyruvate mimetic compound lacks isoform selectivity among PDKs.
Lactate accumulated inside the cancer cells is exported through the monocarboxylate transporters (MCT) family. Impaired function of MCT leads to significant defects in cancer cell proliferation and tumor growth, indicating that efficient lactate secretion is required for the optimal growth and proliferation of cancer cells25,26. Moreover, a recent report showed that secreted lactate can be taken up by an isoform of MCT (MCT1) and used as fuel source to support proliferation of neighboring cells which are relatively oxidative, suggesting that in the same tumor, cancer cells with diverse metabolicprofiles exchange their metabolites for survival and growth in a symbiotic manner. Several compounds that block the function of MCT 1 are being developed as potential cancer therapeutics27-28 (Table 1).
Many of these glycolytic enzyme-targeted compounds have showed synergistic anti-cancer effect in combination with conventional chemo/radiotherapy as well as with pathway targeted agents. At present, although some agents targeting glucose metabolism have shown promise in clinical trials, various enzymes involved in the reactions branching from glucose metabolism which are altered in cancer cells are also actively investigated as novel targets with a great therapeutic potential30 (Figure 1).
Glutamine metabolism
Although the initial investigation of cancer cell metabolism focused primarily on glucose metabolism, it has become appreciated that the metabolism of amino acids and fatty acids is also reprogrammed to provide the building blocks for cancer cell growth and proliferation. Glutamine is the most abundant amino acid in the blood and it is a major source of nitrogen for nucleotides, amino acids and glutathione synthesis. Moreover, in highly proliferative cells glutamine is utilized as a carbon source to replenish the TCA cycle to support cell bioenergetics and anabolic reactions. Moreover, several groups recently proposed that cancer cells grown in hypoxia increase their dependency on glutaminolysis since glutamine-derived α-ketoglutarate (αKG) can undergo reductive carboxylation to produce citrate and lipids31-33. Therefore, glutamine metabolism in these cancer cells seems to be an essential pathway and an attractive therapeutic target.
After being taken up, glutamine is converted to glutamate by a mitochondrial enzyme, glutaminase (GLS). It is subsequently converted to αKG by either glutamate dehydrogenase (GDH) or aminotransferases. As an intermediate of the TCA cycle, αKG can function to provide carbon backbones for cellular anabolic reactions34 (Figure 1). Although oncogenic signaling pathways involved in rewiring the glutamine metabolism are not fully understood, multiple reports have suggested that the oncogenic transcription factor Myc controls glutamine catabolism by regulating the expression of glutamine transporters and the enzymes involved in glutaminolysis34,35. In addition, Myc-overexpressing cells are remarkably sensitive to glutamine deprivation, suggesting that these cells are addicted to glutamine34,36. Recently, Myc-induced tumorigenesis was associated with glutamine metabolism as shown by the correlation between Myc levels and metabolic profiling of mouse tumors37.
As a potential target, GLS has two isoforms: glutaminase 1 (GLS1) is thought to be the primary enzyme involved in glutaminolysis, while GLS2 seems to have a different function related to the antioxidant system 34, 35, 38. Recently, GLS1 was identified as a target of the small molecule inhibitor which blocks Rho-GTPase-driven transformation using unbiased high throughput screening39. Moreover, an isoform of GLS1, glutaminase C (GAC) has been considered an important target due to its elevated level in tumors showing glutamine addiction. Recent structure-based studies suggest that its activity is regulated by inorganic phosphate which is highly enriched in mitochondria under hypoxia40, 41.
Accordingly, several glutamine analogs including the compound 6-diazo-5-oxo-L-norleucine (L-DON) have been tested as therapeutic agents pre-clinically and clinically. Although the treatment with these agents led to significant inhibition of cancer cell growth in vitro and in mouse xenograft studies, these compounds show a high rate of toxicity due to their lack of specificity42. Recently, a GLS1 specific inhibitor, bis-2-(5-phenylacetimido-1,2,4,thiadiazol-2-yl)ethyl sulfide (BPTES) has been identified43 and shown to significantly inhibit cancer cell growth in vitro and in mouse tumor model37, 44. As a selective therapeutic target, BPTES may achieve a greater therapeutic window than glutamine analogues as specific inhibition of GLS1 would suppress the tumor relying on glutaminolysis without affecting other important functions of glutamine in normal tissues.
Similarly, another glutaminolysis enzyme, glutamate dehydrogenase (GDH) was shown to be important for glioblastoma cell survival under conditions where glucose catabolism is impaired or the Akt pathway is inhibited45. Epigallocatechin gallate (EGCG), a potent and specific inhibitor of GDH, has been shown to block glutaminolysis and sensitize glioblastoma cells to glucose deprivation45, 46. Since glutamine can support cancer cell survival under glucose limitation and hypoxia, the suppression of glutamine catabolism would be synergistic with inhibition of growth signaling pathways related to glucose catabolism.
Serine synthetic pathways
Glycolytic intermediates derived from enhanced glycolysis in cancer cells can be shunted to generate non-essential amino acids, lipids and nucleotides which facilitate cancer cell growth and proliferation. For example, cells expressing PKM2 accumulate the glycolytic intermediate 3-phosphoglycerate (3-PG) which can be shunted to the serine synthetic pathway. As a result, PKM2 cells (i.e. cancer cells) can maintain mTORC1 activity and proliferation in serine-depleted medium while PKM1 cells (i.e. normal cells) cannot12, 13. Interestingly, the rate-limiting enzyme involved in branching glycolysis to the serine synthetic pathways; phosphoglycerate dehydrogenase (PHGDH) was recently found to be amplified in a subset of melanoma and ER-negative breast cancer, suggesting that increased flux into the serine synthetic pathway provides selective advantage to tumor cells. Consistently, overexpression of this gene leads to cellular transformation and RNAi suppression of PHGDH leads to inhibition of cancer cell proliferation and transformation47, 48. Therefore PHGDH is an attractive metabolic target for cancer therapeutics together with other enzymes involved in the serine synthetic pathway.
Serine is an important nonessential amino acid which can be used for the synthesis of other amino acids such as glycine and cysteine and the generation of phospholipids. In addition, when serine is metabolized to glycine, tetrahydrofolate (THF) is converted to 5,10-methylene-THF which is a critical step in maintaining folate cycle for nucleotide synthesis49 (Figure 1). The dependency of cancer cells to the folate cycle has been demonstrated by the successful development of anti-folate drugs, many of which are widely used in clinics to treat various types of cancer (Table 1). Examples include 5-fluorouracil (5-FU) which inhibits thymidine synthesis and methotrexate which blocks purine synthesis by inhibiting dihydrofolate reductase (DHFR) which converts dihydrofolate (DHF) to tetrahydrofolate (THF) (Table 1).
Autophagy
Context-dependent role of autophagy in cancer
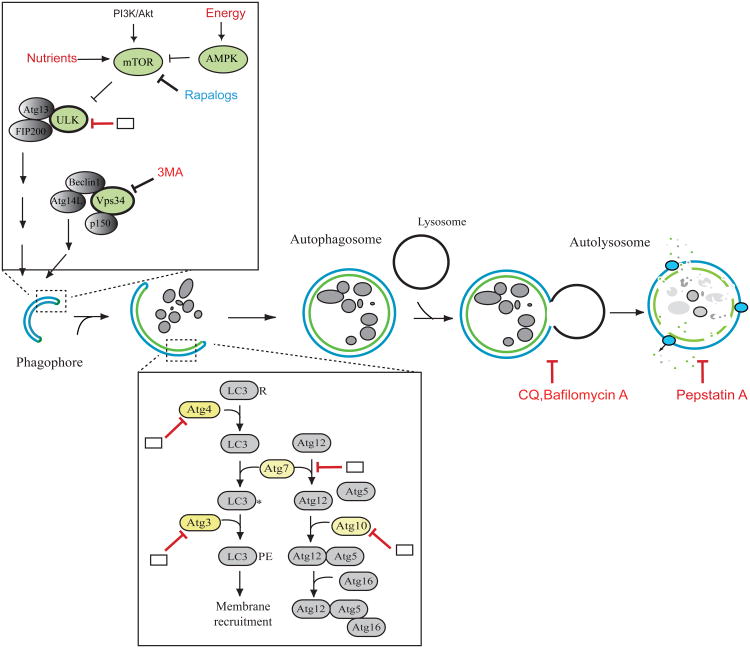
Autophagy is a self-degradation process where cytosolic components and organelles are sequestered in double membrane-bound vesicles and delivered to lysosomes for degradation and recycling (Figure 2). In normal tissue, autophagy maintains cellular homeostasis by clearing damaged organelles or misfolded proteins.
Figure 2. Modulators of the autophagy pathway.
Various growth and nutrient signaling pathways are associated with regulation of autophagy. Following inhibition of mTOR, the ULK/Atg13/FIP200 complex is activated and initiate autophagosome/phagophore formation. The class III PI3 kinase (Vps34)-Atg14L-Beclin 1 complex also regulates the autophagosome nucleation step. To expand the autophagosome membrane, two ubiquitin-like conjugation systems are required for conjugation of LC3 and Atg12 to phosphatidyl ethanolamine (PE) on the autophagosome membrane and Atg5, respectively. Further, the Atg12-Atg5 conjugate interacts with Atg16, presumably located on the surface of the autophagosome membrane. The complete autophagosome fuse with the lysosome to form the autolysosome and cargo molecules enwrapped by autophagosomes are degraded by lysosomal hydrolases and recycled back to the cytoplasm. As shown in red, a variety of pharmacological inhibitors are depicted which modulate distinct steps of autophagy. Some autophagy proteins which enzymatic activity (shown in green and yellow) could be crucial target proteins for modulation of autophagy activity. Potential points where inhibitors could be potentially developed are shown in blank boxes.
The role of autophagy in cancer is complex and paradoxical. Defects in autophagy such as those resulting from deletions of autophagy genes lead to increased tumorigenesis. Early studies found mono-allelic deletion of the autophagy gene beclin1 in various human cancers and mice heterozygous for beclin1 developed multiple spontaneous malignancies50, 51. Moreover, some gastric cancers were shown to have nonsense mutations of UVRAG (ultraviolet radiation resistance-associate gene), a beclin1-binding autophagy regulator52. More recently, mice with systemic mosaic deletion of atg5 or liver-specific deletion of atg7 showed high incidence of benign liver adenoma development, suggesting that autophagy serves to suppress tumor initiation53. Furthermore, it was reported that p62, an autophagy substrate protein, is accumulated in autophagy-deficient cells following metabolic stress, which leads to accumulation of damaged mitochondria and elevated oxidative stress and DNA damage54.
In contrast, autophagy has been proposed to be a pro-survival process in cells exposed to metabolic stress. It was shown that when growth factor dependent cells were subjected to metabolic stress following growth factor deprivation, these cells utilize autophagy to generate additional bioenergetic substrates to support cell survival55. Similar results were seen in established tumors where autophagy was shown to promote cell survival in the center of the tumor, which is likely to be deprived of nutrients and oxygen56. In addition, cells genetically deficient for autophagy genes such as beclin 1 or atg5 are sensitive to metabolic stress57, 58. Based on recent immunohistochemical analysis of tumor samples, the expression of mammalian homologues of Atg8, GABARAP or LC3 was increased in a variety of tumor tissues compared to normal tissues, and their expression levels correlated with tumor progression and poor diagnostic rate59, 60, indicating that autophagy is actively induced in advanced stages of tumorigenesis.
In addition to the adaptation to primary tumor microenvironment, autophagy has been shown to promote cell survival in extracellular matrix-detached conditions using a three dimensional (3D) mammary cell culture model, suggesting that autophagy is also a critical process for tumor survival during metastasis61.
Studies using genetically-engineered mouse models of cancer suggested that autophagy plays an indispensible role in tumor development and maintenance, regardless of nutrient availability. Targeted deletion of an autophagy gene FIP200 resulted in significant delay of mammary tumor progression in a mouse breast cancer model induced by the polyoma middle T(PyMT) oncogene62. Multiple studies have suggested that autophagy is required for Ras-induced transformation in vitro and Ras-driven tumorigenesis 63-65. Moreover, pancreatic cancers known to have Kras mutation showed elevated levels of autophagy necessary for cancer cell growth in an in vitro setting66.
Despite the increasing evidence supporting the pro-survival role of autophagy in established cancer, the exact mechanism of how autophagy supports energy-demanding cancer cell metabolism still remains largely unknown. One hypothesis is that metabolites derived from autophagic degradation of cellular components might be utilized for the adaptation and growth of cancer cells as bioenergetic and anabolic substrates. Indeed, metabolic analysis showed that autophagy was required to maintain full activities of mitochondrial TCA cycle and oxidative phosphorylation after Ras activation65. Moreover, melanoma cell lines established from clinical samples have shown a high dependency on extracellular leucine which could be rescued by autophagy activation67.
Another remaining question in the field of autophagy research is what triggers the autophagic response in cells. It is well established that deprivation of essential amino acids activates autophagy through the inhibition of the mTOR pathway. Intriguingly, we and others have described that ammonia, a metabolic byproduct derived from amino acid catabolism including glutaminolysis, also acts as an autophagy inducer. Interestingly, this form of autophagy is independent of the mTOR-ULK pathway68, 69. These findings suggest that autophagy induction is linked to either the relative lack of nitrogen or excess nitrogen mediated by distinct sensing mechanisms. It remains to be elucidated what the sensor of increased intracellular nitrogen levels is and its implication in cancer development. Since tumors with activated oncogenes such as Myc or Ras have been reported to rely on glutaminolysis to generate bioenergetic fuel34, 70, we can speculate that elevated autophagy shown in these oncogene-driven tumors might be initiated through excess nitrogen sensing mechanisms.
As evident from the above discussion, the role of autophagy in tumor initiation and progression is complex. Autophagy is more likely to have a tumor-suppressing role in the tumor initiation stage, whereas in established tumors, it may function to fulfill the metabolic needs of cancer cells during oncogene activation or nutrient limitation by providing a mechanism to recycle intracellular carbon and nitrogen and thus support tumor cell survival. Clearly, the cellular context of tumors (origin, stage, genetic make-up) plays a role in the outcome of the autophagy response and must be taken into consideration when using autophagy modulation in cancer therapy.
Autophagy as a therapeutic target
Given the critical role of autophagy in tumor progression and maintenance, various pre-clinical and clinical studies have been undertaken or are in progress to develop therapeutic agents targeting autophagy.
As most cancer therapeutic agents inevitably cause cellular stress, autophagy is often activated in cancer cells after drug treatment. Indeed, many of the therapies targeting growth factor signaling- either as single targeted treatment or combinatorial treatment with two agents targeting different pathways -clearly result in autophagy induction (Table 2). For example, several allosteric and catalytic inhibitors of mTOR, PI3K/AKT, and tyrosine kinases signaling and activators of energy sensing pathway (e.g. AMPK), induce autophagy in cells. In addition, various agents targeting cellular processes such as inhibitors of histone deacetylase (HDAC), proteasome, apoptosis and glycolysis have also been suggested to trigger autophagy (Table 2). It was originally proposed that autophagic cell death may be part of the mechanism of action of anti-cancer drugs. Indeed, some combinatorial treatments with the above drugs showed synergistic effect on the induction of cell death which was reduced by knock-down of autophagy genes, suggesting that autophagy induced by chemotherapy contributes to its anti-cancer effect (Table 2). However, it has been suggested that the increased autophagy seen during anti-cancer drug treatment could also be a survival response of the dying cells rather than cause of cell death71. As many of autophagy inducers have shown limited success in clinical trials, it is necessary to assess the contribution of autophagy in the mechanism of actions of these drugs. Moreover, a growing body of evidence has indicated that stress-induced autophagy in tumor cells might drive cellular resistance against chemotherapy and facilitate tumor dormancy72, 73.
Table 2. Autophagy inducers and inhibitors for cancer treatment.
| Compound | Target | Tumor type/Cancer cell types | References |
|---|---|---|---|
|
| |||
| Autophagy inducers | |||
|
| |||
| Temsirolimus (CCI-779)* | mTORC1 inhibitors | Renal cancer*, Mantle cell lymphoma | 79 |
| Everolimus(RAD-001)* | Renal cancer*, Acute lymphoblastic leukemia (ALL) | 80 | |
| Rapamycin | Glioma, malignant | 81 | |
| Chronic myeloid leukemia (CML)* | 82 | ||
|
| |||
| Imatinib* | Tyrosine kinases Inhibitors; KIT, BCR-Abl, PDGFR, | Gastrointestinal stromal tumor*, Chronic myeloid leukemia (CML)* | 83 |
| Dasatinib* | EGFR | Glioma* | 84 |
| Erlotinib* | Non-small cell lung cancer (NSCLC)* | 85 | |
|
| |||
| Bortezomib* | Proteasome inhibitors | Multiple myeloma* | 86 |
| NPI-0052 | Prostate cancer | 87 | |
|
| |||
| Vorinostat* | HDAC inhibitors | Cutaneous T cell lymphoma (CTCL)* | |
| Butyrate, suberoylanilide hydroxamic acid (SAHA) | Multiple cancers, CML | 88, 89 | |
| LAQ824, Panobinostat (LBH589) | Lymphoma | 90 | |
|
| |||
| Temezolamide* | DNA alkylating agent | Glioblastoma, Metastatic melanoma* | 91 |
|
| |||
| GX15-070 | Bcl2 inhibitor | Pancreatic carcinoma | 92 |
| Leukemia | 93 | ||
|
| |||
| Arsenic trioxide* | Acute promyelocytic leukemia* | 94 | |
| Glioma, malignant | 95 | ||
|
| |||
| Resveratrol | Antioxidant | Ovarian cancer | 96 |
|
| |||
| Autophagy Inhibitors | |||
|
| |||
| 3-methyladenine (3-MA) | Class III PI3 kinase inhibitor | 97-99 | |
| Colorectal cancer, | 100 | ||
|
| |||
| Chloroquine* | Lysosomal pH | Malaria* | |
| Glioma, malignant | 89, 101 | ||
| Lymphoma | 77, 78 | ||
|
| |||
| Hydroxychloroquine* | Lysosomal pH | Malaria, Lupus, Rheumatoid arthritis* | |
| Breast cancer | 102 | ||
| 98 | |||
|
| |||
| Bafilomycin A1 | Vacuolar-ATPase | 98, 103 | |
| Glioma, malignant | 91 | ||
| Breast cancer | 104 | ||
|
| |||
| Monensin | Lysosomal pH | 98, 105 | |
| Glioma, malignant | 131 | ||
|
| |||
| Pepstatin A | Lysosomal protease (Cathepsin inhibitor) | Cervical cancer | 106 |
Compounds with an asterisk, are registered by FDA for designated diseases.
In light of the above data, inhibition of autophagy activity could be a useful approach in combination with conventional chemo/radiotherapy. Indeed, a series of studies have reported that the treatment with targeted agents which induce autophagy, synergized with autophagy inhibitors for anti-cancer effect in in vitro settings using numerous cancer cell lines as well as in xenograft models74, 75. Moreover, combination therapies with chemotherapy and autophagy inhibitor are being tested in clinical trials and have showed promising results.
The most used autophagy inhibitors in clinical trials are chloroquine (CQ) and hydroxychloroquine (HCQ). These agents are registered anti-malarial drugs which inhibit lysosomal acidification and eventually impair autophagosome fusion with lysosomes and degradation76. Pre-clinical studies using a Myc-induced mouse lymphoma model suggested that the inhibition of autophagy by treatment with HCQ increased cell death and significantly impaired recurrence of tumors following the treatment with chemotherapy77, 78. Clinical trials involving CQ- and HCQ-mediated autophagy inhibition for cancer therapy are ongoing and listed at http://www.clinicaltrial.gov.
In addition to CQ derivatives, a variety of other putative autophagy inhibitors have also been studied for their anti-tumor effect in vitro and in vivo (Table 2). However, these potential autophagy inhibitors have nonspecific effects in other cellular functions such as endocytosis (e.g. 3-MA) or lysosomal function (e.g. bafilomycin A).Targeting key autophagy proteins would be a more potent and specific approach. Several components of the autophagic machinery are druggable and serve as potential therapeutic targets. These include ULK1/2 serine/threonine protein kinases, the enzymes involved in ubiquitin-like conjugation systems (e.g. E1-like ubiquitination enzyme, Atg7 and E2- like enzymes, Atg3 or Atg10) and cysteine protease, Atg4. Moreover, the class III phosphatidyl inositol 3 (PI3) kinase, Vps34 could be targeted as it is a key autophagy regulator, although normal endocytosis is also regulated by this protein. In addition to pharmacological inhibitors, increasing pre-clinical evidence have supported that gene interference using siRNA against various autophagy proteins can have a synergistic effect in combination with other treatments. Accordingly, deeper understanding of the molecular basis of autophagy induction and execution could identify more cancer therapeutic targets that can be drugged alone or in combination.
Concluding Remarks
As discussed in this review, cancer cells display multiple layers of metabolic alterations affecting cell proliferation and survival. Cancer cell metabolism is reprogrammed by multiple factors including mutations of core oncogenic pathways and tumor suppressors as well as alterations in the microenvironment and stromal cells.
In addition, direct amplification of metabolic enzymes can influence the regulation of cancer growth, indicating that metabolic deregulation can contribute to tumor development. Therefore, defining the mechanism of how cancer cells coordinate their metabolism to support cancer growth and how cancer cells adapt to the tumor microenvironment can provide insights to understanding cancer development.
Understanding the multiple layers of defects in cancer cell metabolism, ranging from oncogene dysregulation, to mutations in specific metabolic enzymes has the potential to identify new targets for cancer therapy. Moreover, investigating the regulatory network of these cellular mechanisms will allow for the development of more effective combinatorial therapeutic approaches.
References
- 1.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 2.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warburg O, Wind F, Negelein E. The Metabolism of Tumors in the Body. J Gen Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhan T, Digel M, Kuch EM, Stremmel W, Fullekrug J. Silybin and dehydrosilybin decrease glucose uptake by inhibiting GLUT proteins. J Cell Biochem. 2011;112:849–859. doi: 10.1002/jcb.22984. [DOI] [PubMed] [Google Scholar]
- 5.Cheung CW, Gibbons N, Johnson DW, Nicol DL. Silibinin--a promising new treatment for cancer. Anticancer Agents Med Chem. 2010;10:186–195. doi: 10.2174/1871520611009030186. [DOI] [PubMed] [Google Scholar]
- 6.Porporato PE, Dhup S, Dadhich RK, Copetti T, Sonveaux P. Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front Pharmacol. 2011;2:49. doi: 10.3389/fphar.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathupala SP, Ko YH, Pedersen PL. Hexokinase-2 bound to mitochondria: cancer's stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin Cancer Biol. 2009;19:17–24. doi: 10.1016/j.semcancer.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Telang S, et al. Ras transformation requires metabolic control by 6-phosphofructo-2-kinase. Oncogene. 2006;25:7225–7234. doi: 10.1038/sj.onc.1209709. [DOI] [PubMed] [Google Scholar]
- 9.Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 10.Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 11.Vander Heiden MG, et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329:1492–1499. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Q, et al. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci U S A. 2011;108:4129–4134. doi: 10.1073/pnas.1014769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye J, et al. Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation. Proc Natl Acad Sci U S A. 2012;109:6904–6909. doi: 10.1073/pnas.1204176109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vander Heiden MG, et al. Identification of small molecule inhibitors of pyruvate kinase M2. Biochem Pharmacol. 2010;79:1118–1124. doi: 10.1016/j.bcp.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boxer MB, et al. Evaluation of substituted N,N′-diarylsulfonamides as activators of the tumor cell specific M2 isoform of pyruvate kinase. J Med Chem. 2010;53:1048–1055. doi: 10.1021/jm901577g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang JK, et al. Evaluation of thieno[3,2-b]pyrrole[3,2-d]pyridazinones as activators of the tumor cell specific M2 isoform of pyruvate kinase. Bioorg Med Chem Lett. 2010;20:3387–3393. doi: 10.1016/j.bmcl.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shim H, et al. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci U S A. 1997;94:6658–6663. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Qing G, et al. Combinatorial regulation of neuroblastoma tumor progression by N-Myc and hypoxia inducible factor HIF-1alpha. Cancer Res. 2010;70:10351–10361. doi: 10.1158/0008-5472.CAN-10-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Y, et al. Selective active site inhibitors of human lactate dehydrogenases A4, B4, and C4. Biochem Pharmacol. 2001;62:81–89. doi: 10.1016/s0006-2952(01)00636-0. [DOI] [PubMed] [Google Scholar]
- 21.Le A, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granchi C, et al. Discovery of N-hydroxyindole-based inhibitors of human lactate dehydrogenase isoform A (LDH-A) as starvation agents against cancer cells. J Med Chem. 2011;54:1599–1612. doi: 10.1021/jm101007q. [DOI] [PubMed] [Google Scholar]
- 23.Michelakis ED, et al. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010;2:31ra34. doi: 10.1126/scitranslmed.3000677. [DOI] [PubMed] [Google Scholar]
- 24.Bonnet S, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 25.Dimmer KS, Friedrich B, Lang F, Deitmer JW, Broer S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;350 Pt 1:219–227. [PMC free article] [PubMed] [Google Scholar]
- 26.Gallagher SM, Castorino JJ, Wang D, Philp NJ. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res. 2007;67:4182–4189. doi: 10.1158/0008-5472.CAN-06-3184. [DOI] [PubMed] [Google Scholar]
- 27.Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71:2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- 28.Bueno V, et al. The specific monocarboxylate transporter (MCT1) inhibitor, AR-C117977, a novel immunosuppressant, prolongs allograft survival in the mouse. Transplantation. 2007;84:1204–1207. doi: 10.1097/01.tp.0000287543.91765.41. [DOI] [PubMed] [Google Scholar]
- 29.Sonveaux P, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov. 2011;10:671–684. doi: 10.1038/nrd3504. [DOI] [PubMed] [Google Scholar]
- 31.Wise DR, et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A. 2011;108:19611–19616. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metallo CM, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mullen AR, et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481:385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wise DR, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao P, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuneva MO, et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 2012;15:157–170. doi: 10.1016/j.cmet.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu W, et al. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Natl Acad Sci U S A. 2010;107:7455–7460. doi: 10.1073/pnas.1001006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang JB, et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18:207–219. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cassago A, et al. Mitochondrial localization and structure-based phosphate activation mechanism of Glutaminase C with implications for cancer metabolism. Proc Natl Acad Sci U S A. 2012;109:1092–1097. doi: 10.1073/pnas.1112495109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kita K, Suzuki T, Ochi T. Diphenylarsinic acid promotes degradation of glutaminase C by mitochondrial Lon protease. J Biol Chem. 2012 doi: 10.1074/jbc.M112.362699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahluwalia GS, Grem JL, Hao Z, Cooney DA. Metabolism and action of amino acid analog anti-cancer agents. Pharmacol Ther. 1990;46:243–271. doi: 10.1016/0163-7258(90)90094-i. [DOI] [PubMed] [Google Scholar]
- 43.Robinson MM, et al. Novel mechanism of inhibition of rat kidney-type glutaminase by bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES) Biochem J. 2007;406:407–414. doi: 10.1042/BJ20070039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le A, et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15:110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang C, et al. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 2009;69:7986–7993. doi: 10.1158/0008-5472.CAN-09-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng G, Dixon DA, Muga SJ, Smith TJ, Wargovich MJ. Green tea polyphenol (-)-epigallocatechin-3-gallate inhibits cyclooxygenase-2 expression in colon carcinogenesis. Mol Carcinog. 2006;45:309–319. doi: 10.1002/mc.20166. [DOI] [PubMed] [Google Scholar]
- 47.Possemato R, et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Locasale JW, et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43:869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tibbetts AS, Appling DR. Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- 50.Liang XH, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 51.Qu X, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang C, et al. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat Cell Biol. 2006;8:688–699. doi: 10.1038/ncb1426. [DOI] [PubMed] [Google Scholar]
- 53.Takamura A, et al. Autophagy-deficient mice develop multiple liver tumors. Genes Dev. 2011;25:795–800. doi: 10.1101/gad.2016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mathew R, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lum JJ, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 56.Degenhardt K, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathew R, et al. Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev. 2007;21:1367–1381. doi: 10.1101/gad.1545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karantza-Wadsworth V, et al. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev. 2007;21:1621–1635. doi: 10.1101/gad.1565707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miao Y, Zhang Y, Chen Y, Chen L, Wang F. GABARAP is overexpressed in colorectal carcinoma and correlates with shortened patient survival. Hepatogastroenterology. 2010;57:257–261. [PubMed] [Google Scholar]
- 60.Fujii S, et al. Autophagy is activated in pancreatic cancer cells and correlates with poor patient outcome. Cancer Sci. 2008;99:1813–1819. doi: 10.1111/j.1349-7006.2008.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fung C, Lock R, Gao S, Salas E, Debnath J. Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol Biol Cell. 2008;19:797–806. doi: 10.1091/mbc.E07-10-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei H, et al. Suppression of autophagy by FIP200 deletion inhibits mammary tumorigenesis. Genes Dev. 2011;25:1510–1527. doi: 10.1101/gad.2051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lock R, et al. Autophagy facilitates glycolysis during Ras-mediated oncogenic transformation. Mol Biol Cell. 2011;22:165–178. doi: 10.1091/mbc.E10-06-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim MJ, et al. Involvement of autophagy in oncogenic K-Ras-induced malignant cell transformation. J Biol Chem. 2011;286:12924–12932. doi: 10.1074/jbc.M110.138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo JY, et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25:460–470. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang S, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sheen JH, Zoncu R, Kim D, Sabatini DM. Defective regulation of autophagy upon leucine deprivation reveals a targetable liability of human melanoma cells in vitro and in vivo. Cancer Cell. 2011;19:613–628. doi: 10.1016/j.ccr.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eng CH, Yu K, Lucas J, White E, Abraham RT. Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci Signal. 2010;3:ra31. doi: 10.1126/scisignal.2000911. [DOI] [PubMed] [Google Scholar]
- 69.Cheong H, Lindsten T, Wu J, Lu C, Thompson CB. Ammonia-induced autophagy is independent of ULK1/ULK2 kinases. Proc Natl Acad Sci U S A. 2011;108:11121–11126. doi: 10.1073/pnas.1107969108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weinberg F, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu Z, et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J Clin Invest. 2008;118:3917–3929. doi: 10.1172/JCI35512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen N, Karantza V. Autophagy as a therapeutic target in cancer. Cancer Biol Ther. 2011;11:157–168. doi: 10.4161/cbt.11.2.14622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Janku F, McConkey DJ, Hong DS, Kurzrock R. Autophagy as a target for anticancer therapy. Nat Rev Clin Oncol. 2011;8:528–539. doi: 10.1038/nrclinonc.2011.71. [DOI] [PubMed] [Google Scholar]
- 76.Poole B, Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol. 1981;90:665–669. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Amaravadi RK, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117:326–336. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maclean KH, Dorsey FC, Cleveland JL, Kastan MB. Targeting lysosomal degradation induces p53-dependent cell death and prevents cancer in mouse models of lymphomagenesis. J Clin Invest. 2008;118:79–88. doi: 10.1172/JCI33700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yazbeck VY, et al. Temsirolimus downregulates p21 without altering cyclin D1 expression and induces autophagy and synergizes with vorinostat in mantle cell lymphoma. Exp Hematol. 2008;36:443–450. doi: 10.1016/j.exphem.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 80.Crazzolara R, et al. Potentiating effects of RAD001 (Everolimus) on vincristine therapy in childhood acute lymphoblastic leukemia. Blood. 2009;113:3297–3306. doi: 10.1182/blood-2008-02-137752. [DOI] [PubMed] [Google Scholar]
- 81.Takeuchi H, et al. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65:3336–3346. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- 82.Carayol N, et al. Critical roles for mTORC2- and rapamycin-insensitive mTORC1-complexes in growth and survival of BCR-ABL-expressing leukemic cells. Proc Natl Acad Sci U S A. 2010;107:12469–12474. doi: 10.1073/pnas.1005114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ertmer A, et al. The anticancer drug imatinib induces cellular autophagy. Leukemia. 2007;21:936–942. doi: 10.1038/sj.leu.2404606. [DOI] [PubMed] [Google Scholar]
- 84.Milano V, Piao Y, LaFortune T, de Groot J. Dasatinib-induced autophagy is enhanced in combination with temozolomide in glioma. Mol Cancer Ther. 2009;8:394–406. doi: 10.1158/1535-7163.MCT-08-0669. [DOI] [PubMed] [Google Scholar]
- 85.Gorzalczany Y, et al. Combining an EGFR directed tyrosine kinase inhibitor with autophagy-inducing drugs: a beneficial strategy to combat non-small cell lung cancer. Cancer Lett. 2011;310:207–215. doi: 10.1016/j.canlet.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 86.Ding WX, et al. Oncogenic transformation confers a selective susceptibility to the combined suppression of the proteasome and autophagy. Mol Cancer Ther. 2009;8:2036–2045. doi: 10.1158/1535-7163.MCT-08-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu K, Dunner K, Jr, McConkey DJ. Proteasome inhibitors activate autophagy as a cytoprotective response in human prostate cancer cells. Oncogene. 2010;29:451–462. doi: 10.1038/onc.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shao Y, Gao Z, Marks PA, Jiang X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2004;101:18030–18035. doi: 10.1073/pnas.0408345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carew JS, et al. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood. 2007;110:313–322. doi: 10.1182/blood-2006-10-050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ellis L, et al. The histone deacetylase inhibitors LAQ824 and LBH589 do not require death receptor signaling or a functional apoptosome to mediate tumor cell death or therapeutic efficacy. Blood. 2009;114:380–393. doi: 10.1182/blood-2008-10-182758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kanzawa T, et al. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11:448–457. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- 92.Martin AP, et al. BCL-2 family inhibitors enhance histone deacetylase inhibitor and sorafenib lethality via autophagy and overcome blockade of the extrinsic pathway to facilitate killing. Mol Pharmacol. 2009;76:327–341. doi: 10.1124/mol.109.056309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wei Y, et al. The combination of a histone deacetylase inhibitor with the Bcl-2 homology domain-3 mimetic GX15-070 has synergistic antileukemia activity by activating both apoptosis and autophagy. Clin Cancer Res. 2010;16:3923–3932. doi: 10.1158/1078-0432.CCR-10-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qian W, Liu J, Jin J, Ni W, Xu W. Arsenic trioxide induces not only apoptosis but also autophagic cell death in leukemia cell lines via up-regulation of Beclin-1. Leuk Res. 2007;31:329–339. doi: 10.1016/j.leukres.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 95.Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003;63:2103–2108. [PubMed] [Google Scholar]
- 96.Opipari AW, Jr, et al. Resveratrol-induced autophagocytosis in ovarian cancer cells. Cancer Res. 2004;64:696–703. doi: 10.1158/0008-5472.can-03-2404. [DOI] [PubMed] [Google Scholar]
- 97.Seglen PO, Gordon PB. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boya P, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelarova H, Meijer AJ. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem. 1997;243:240–246. doi: 10.1111/j.1432-1033.1997.0240a.x. [DOI] [PubMed] [Google Scholar]
- 100.Petiot A, Ogier-Denis E, Blommaart EF, Meijer AJ, Codogno P. Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem. 2000;275:992–998. doi: 10.1074/jbc.275.2.992. [DOI] [PubMed] [Google Scholar]
- 101.Degtyarev M, et al. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J Cell Biol. 2008;183:101–116. doi: 10.1083/jcb.200801099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rahim R, Strobl JS. Hydroxychloroquine, chloroquine, and all-trans retinoic acid regulate growth, survival, and histone acetylation in breast cancer cells. Anticancer Drugs. 2009;20:736–745. doi: 10.1097/CAD.0b013e32832f4e50. [DOI] [PubMed] [Google Scholar]
- 103.Yamamoto A, et al. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- 104.Kanematsu S, et al. Autophagy inhibition enhances sulforaphane-induced apoptosis in human breast cancer cells. Anticancer Res. 2010;30:3381–3390. [PubMed] [Google Scholar]
- 105.Fan QW, et al. Akt and autophagy cooperate to promote survival of drug-resistant glioma. Sci Signal. 2010;3:ra81. doi: 10.1126/scisignal.2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hsu KF, et al. Cathepsin L mediates resveratrol-induced autophagy and apoptotic cell death in cervical cancer cells. Autophagy. 2009;5:451–460. doi: 10.4161/auto.5.4.7666. [DOI] [PubMed] [Google Scholar]