Abstract
OBJECTIVE:
To characterize urine test use in ambulatory, antibiotic-treated pediatric urinary tract infection (UTI).
METHODS:
We studied children <18 years who had an outpatient UTI and a temporally associated antibiotic prescription from 2002 through 2007 by using a large claims database, Innovus i3. We evaluated urine-testing trends and performed multivariable logistic regression to assess for factors associated with urine culture use.
RESULTS:
Of 40 603 treated UTI episodes in 28 678 children, urinalysis was performed in 76%, and urine culture in 57%; 32% of children <2 years had no urinalysis or culture performed for an antibiotic-treated UTI episode. Urine culture use decreased during the study period from 60% to 54% (P < .001). We observed variation in urine culture use with age (<2 years: odds ratio [OR] 1.0, 95% confidence interval [CI] 0.9–1.1; 2–5 years: OR 1.3, 95% CI 1.2–1.4; 6–12 years: OR 1.3, 95% CI 1.2–1.4, compared with 13–17 years); gender (boys: OR 0.8, 95% CI 0.8–0.9); and specialty (pediatrics: OR 2.6, 95% CI 2.5–2.8; emergency medicine, OR 1.2, 95% CI 1.1–1.3; urology: OR 0.5, 95% CI 0.4–0.6, compared with family/internal medicine). Recent antibiotic exposure (OR 1.1, 95% CI 1.1–1.2) and empirical broad-spectrum prescription (OR 1.2, 95% CI 1.1–1.2) were associated with urine culture use, whereas previous UTI and urologic anomalies were not.
CONCLUSIONS:
Providers often do not obtain urine tests when prescribing antibiotics for outpatient pediatric UTI. Variation in urine culture use was observed based on age, gender, and physician specialty. Additional research is necessary to determine the implications of empirical antibiotic prescription for pediatric UTI without confirmatory urine testing.
Keywords: urinary tract infection, urinalysis, urine culture, antibiotic prescription, pediatric
What’s Known on This Subject:
The diagnosis of urinary tract infection (UTI) is confirmed by urine testing with urinalysis and culture. No study has characterized the use of urine testing in the setting of empirical antibiotic prescription for outpatient UTI in children.
What This Study Adds:
Urine tests are not performed in a substantial percentage of antibiotic-treated pediatric UTIs. Additional research is necessary to determine whether empirical antibiotic prescription for UTI in children without urine testing is safe and effective.
Despite the fact that urinalysis and culture are recommended in all children aged 2 to 24 months suspected of having urinary tract infection (UTI),1,2 previous research has shown that practitioners are often more selective with urine testing than guidelines suggest.3 Moreover, there are no US-promulgated guidelines for the pediatric patient aged >2 years; European guidelines offer the option for more selective testing, with urinalysis alone, in a child aged >3 years with low risk of serious illness and no history of UTI.1 No guidelines endorse empirically prescribing antibiotics without conducting either urinalysis or culture.
The goals of the current study were to characterize trends in urine testing and factors associated with utilization of urine cultures when empiric antibiotics are prescribed for presumed UTI in children in the outpatient setting.
Methods
Institutional review board approval was granted at participating institutions.
Study Design and Data Source
We retrospectively evaluated urine test utilization for children aged <18 years who were prescribed antibiotics for UTI during the period 2002 through 2007. We analyzed data from Innovus i3, a private claims database that collects United Healthcare insurance enrollment information and submitted medical and prescription reimbursement claims from ∼39 million insured individuals and their dependents from all 50 states. Available data include patient demographics such as age and gender, as well as procedure and diagnosis codes, dates of service, and information regarding the treatment facility and provider. Reimbursement claims for completed laboratories and filled prescriptions are recorded. The prescription claims consist of prescription fill date, refills, brand name, and therapeutic class.
Study Population
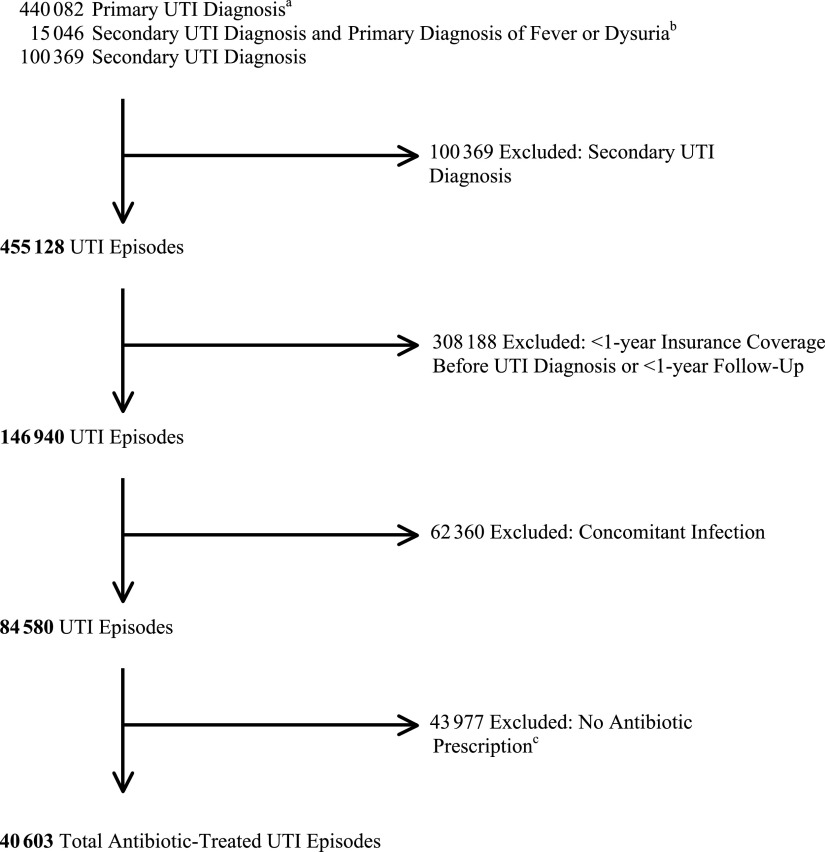
The study population comprised patients <18 years of age who had an outpatient visit with a primary diagnosis of UTI (International Classification of Diseases, Ninth Revision [ICD-9] codes 595.0, 595.2, 593.3, 595.81, 595.89, 595.9, 5980.0×, 590.1×, 590.2, 590.3, 590.80, 599.0, and 771.8) and had an antibiotic prescribed within 3 days before, and 5 days after the UTI visit (Fig 1). Patients with UTI as a secondary diagnosis were included if fever (780.6) or dysuria (788.1) was the primary diagnosis. We allowed the date of antibiotic prescription to precede the UTI visit date because patients may initially be prescribed antibiotics after phone consultation and subsequently follow-up in the clinical setting. Antibiotic prescriptions for UTI were limited to those prescriptions with <30-days’ supply to exclude prescriptions written for antibiotic prophylaxis. Visits were excluded in which patients were diagnosed with a UTI and another infection for which antibiotics are commonly prescribed to ensure that UTI was the primary reason for the visit and resulting antibiotic prescription.4,5 To capture comorbidities and recurrent UTI events more accurately, eligible subjects were required to have 1 year of follow-up after the first UTI visit and either 1 year of enrollment before the UTI visit or coverage since birth.
FIGURE 1.
Study population: pediatric antibiotic-treated UTI episodes. A UTI episode includes the 14-day period after initial visit date of the UTI. One patient may have multiple UTI episodes, and therefore, the total number of episodes (40 603) is greater than the total number of patients (28 678) in the study. a Diagnosis of UTI by ICD-9 codes 595.0, 595.2, 593.3, 595.81, 595.89, 595.9, 5980.0×, 590.1×, 590.2, 590.3, 590.80, 599.0, and 771.8. b Diagnosis of fever or dysuria by ICD-9 codes 780.6 and 788.1, respectively. c Seventy-one percent of episodes that were excluded for no antibiotic prescription had urine testing performed.
Antibiotic Classification
Antibiotics were categorized based on codes from the National Drug Code Directory and the American Hospital Formulary Service.6,7 We included the following antibiotic categories: penicillins cephalosporins, sulfonamides (trimethoprims and sulfonamides), urinary anti-infective agents (nitrofurantoins and methenamine mandelates), aminoglycosides, tetracyclines, macrolides, quinolones, lincosamides, and carbepenems. Broad-spectrum antibiotics were defined as broad-spectrum penicillins (antipseudomonal penicillins and β-lactamase/β-lactam inhibitor combination penicillins); second-, third-, or fourth-generation cephalosporins; macrolides; quinolones; lincosamides; and carbapenems. Drug formulations not applicable to UTI treatment, such as topical formulations, were excluded. Pharmacy claims were used to capture oral antibiotic prescriptions. Parenteral antibiotic administration was captured by J code classification from the Healthcare Common Procedure Coding System, which is used to report injectable drugs.8
Definitions and Measurements
A UTI episode was defined as the 14-day period after the initial visit date of the UTI. A recurrent UTI was defined as a visit for UTI occurring after the 14-day window of the preceding UTI. If multiple antibiotics were prescribed during a UTI episode, the first antibiotic prescribed during the episode was defined as the empirically prescribed antibiotic. Urine testing was captured by current procedural terminology codes (Urinalysis: 81000, 81001, 81002, 81003, 81004, 81005, 81007, and 81015; Urine Culture: 87086, 87088, 87181, 87184, 87185, 87186, and 87188). We defined urine test use associated with empiric antibiotic prescription as a urine test that occurred within 3 days before or after the first antibiotic prescribed in a UTI episode. We used an extended window of time to capture claims for urine testing to ensure testing was not overlooked due to delay in claims processing. Because providers may be more likely to perform urine testing based on patient age and likelihood for UTI, age was partitioned into categories: <2 years, 2 to 5 years, 6 to 12 years, and 13 to 17 years.4 Geographic region was based on US Census definitions of Northeast, Midwest, South, and West. Physician specialty was divided into 5 main categories: family/internal medicine, pediatrics, emergency medicine, urology, and other.
We identified patients with complex, chronic medical conditions by using the ICD-9 codes previously proposed by Feudtner et al.9 We assessed for the presence of the following conditions because of their association with an increased risk for resistant UTI: history of previous UTI, recent antibiotic exposure, recent hospitalization, and presence of genitourinary anomaly.10–12 Recent antibiotic exposure was classified as an antibiotic prescribed within 30 days before the UTI visit date. Recent hospitalization was defined as hospitalization within 30 days before UTI visit date. The presence of a urologic anomaly was defined by ICD-9 codes for the following conditions: hydronephrosis, renal/ureteral stones, vesicoureteral reflux, bladder stone, neurogenic bladder, ureteropelvic junction obstruction/other obstruction, ureterovesical junction obstruction, ureterocele, posterior urethral valves, ectopic ureter/other ureteral anomalies, spina bifida, and bladder exstrophy.
Statistical Analysis
Summary statistics were performed by using frequencies and proportions for categorical variables. Unadjusted associations were tested between predictor variables and the outcome variable (urine culture utilization with empirical antibiotic prescription) by using the χ2 test. We evaluated trends in urine test utilization and performed multivariable logistic regression to identify demographic and clinical factors associated with urine culture utilization with antibiotic-treated UTI. The unit of analysis for bivariate and multivariable analyses was a “UTI episode.” In the multivariable modeling, we clustered at the patient level to account for multiple observations in some patients who had recurrent UTI. Covariates with P < .2 on univariate analysis were included in the final model. We performed a stratified multivariable analysis by age <13 years and age 13 to 17 years because providers may be more likely to perform urine testing based on the age of the patient and likelihood for UTI. All analyses were 2-sided; we established P < .05 as statistically significant.
Results
Patient Population
There were 40 603 antibiotic-treated UTI episodes among the 28 678 patients from 2002 through 2007 (Table 1). The majority of the UTI episodes occurred in females (90%). Approximately 16% of the UTI episodes occurred in patients who had a urologic anomaly, and <2% occurred in patients with other nonurologic comorbidities. Twenty-nine percent of UTI episodes were recurrent UTIs.
TABLE 1.
Demographic Characteristics of Antibiotic-Treated UTI Episodes
| Characteristic | UTI Episodes, n (%) |
|---|---|
| Overall | 40 603 |
| Age, y | |
| <2 | 5890 (14) |
| 2–5 | 11 677 (29) |
| 6–12 | 13 867 (34) |
| 13–17 | 9169 (23) |
| Gender | |
| Male | 3917 (10) |
| Female | 36 686 (90) |
| Regiona | |
| Northeast | 3936 (10) |
| Midwest | 14 239 (35) |
| South | 5634 (14) |
| West | 16 776 (41) |
| Recurrent UTI | 11 925 (29) |
| Urologic anomalies | 6546 (16) |
| Nonurologic comorbidities | 669 (2) |
Region was missing for 18 UTI episodes.
Antibiotic Prescription and Urine Testing
Empiric antibiotics were broad-spectrum in 29% of the total antibiotic-treated UTI episodes (11 652 of 40 603). Urinalysis was performed in 76% and urine culture was performed in 57% of the total UTI episodes, respectively (Table 2). The percentage of urine cultures obtained among all UTI episodes decreased during the study period from 60% to 54% (P < .0001). Urine testing (either urinalysis or culture) was performed in ∼80% of UTI episodes. The lowest percentage of urine testing among UTI episodes occurred in children <2 years at 68% compared with all other age groups and urine testing in this age group also declined during the study period (69% to 65%, P < .001). The majority of UTI episodes were treated by pediatricians (46%) and family/internal medicine physicians (27%). Emergency department physicians (10%) and urologists (2%) treated a smaller percentage of UTI episodes (Table 3).
TABLE 2.
Urine Tests Performed for Antibiotic-Treated Pediatric UTI Episodesa
| Urine Test Performed | UTI Episodes, n (%) | Urine Test Use, % | |||
|---|---|---|---|---|---|
| All Years | Study Period 1b | Study Period 2b | Pc | ||
| All UTI episodes | 40 603 | ||||
| Culture performed | 57 | 60 | 54 | <.0001 | |
| Urinalysis performed | 76 | 77 | 75 | <.0001 | |
| Culture or urinalysis performed | 81 | 82 | 79 | <.0001 | |
| UTI episodes for age <2 y | 5890 (14) | ||||
| Culture performed | 57 | 58 | 52 | <.0001 | |
| Urinalysis performed | 61 | 62 | 58 | <.01 | |
| Culture or urinalysis performed | 68 | 69 | 65 | <.001 | |
| UTI episodes for age 2–5 y | 11 677 (29) | ||||
| Culture performed | 61 | 65 | 56 | <.0001 | |
| Urinalysis performed | 76 | 78 | 73 | <.0001 | |
| Culture or urinalysis performed | 81 | 83 | 78 | <.0001 | |
| UTI episodes for age 6–12 y | 13 867 (34) | ||||
| Culture performed | 60 | 62 | 57 | <.0001 | |
| Urinalysis performed | 80 | 82 | 78 | <.0001 | |
| Culture or urinalysis performed | 85 | 87 | 82 | <.0001 | |
| UTI episodes for age 13–17 y | 9169 (23) | ||||
| Culture performed | 50 | 51 | 48 | .03 | |
| Urinalysis performed | 80 | 81 | 78 | <.001 | |
| Culture or urinalysis performed | 84 | 85 | 82 | <.0001 | |
A UTI episode includes the 14-day period after initial visit date of the UTI. One patient may have multiple UTI episodes, and therefore the total number of UTI episodes (40 603) is greater than the total number of patients (28 678) in the study.
Study period 1 includes 2002 through 2004. Study period 2 includes 2005 to 2007.
The P value is the difference in proportion of urine test use between study period 1 and study period 2 using the χ2 test.
TABLE 3.
Factors Associated With Urine Culture Use in Antibiotic-Treated Pediatric UTI
| Factor | UTI Episodesa n = 40 603 (%) | Proportion Urine Culture Use | Bivariate P | Adjusted ORb (95% CI) | Multivariate P |
|---|---|---|---|---|---|
| Age, y | |||||
| <2 | 5890 (14) | .57 | <.0001 | 1.0 (0.9–1.1) | .89 |
| 2–5 | 11 677 (29) | .61 | 1.3 (1.2–1.4) | <.0001 | |
| 6–12 | 13 867 (34) | .60 | 1.3 (1.2–1.4) | <.0001 | |
| 13–17 | 9169 (23) | .50 | Reference | ||
| Gender | |||||
| Male | 3917 (10) | .53 | <.0001 | 0.8 (0.8–0.9) | <.0001 |
| Female | 36 686 (90) | .58 | Reference | ||
| Physician specialtyc | |||||
| Pediatrics | 18 653 (46) | .72 | <.0001 | 2.6 (2.5–2.8) | <.0001 |
| ER | 4008 (10) | .54 | 1.2 (1.1–1.3) | <.0001 | |
| Urology | 970 (2) | .34 | 0.5 (0.4–0.6) | <.0001 | |
| Other | 1480 (4) | .46 | 0.9 (0.8–1.0) | .08 | |
| Family/internal medicine | 10 753 (27) | .49 | Reference | ||
| Regiond | |||||
| Northeast | 3936 (10) | .59 | <.0001 | 0.9 (0.8–0.9) | .004 |
| Midwest | 14 239 (35) | .56 | 1.0 (0.9–1.0) | .95 | |
| West | 5634 (14) | .53 | 0.8 (0.8–0.9) | <.0001 | |
| South | 16 776 (41) | .59 | Reference | ||
| Recurrent UTI | |||||
| Yes | 11 925 (29) | .56 | <.001 | 1.0 (0.9–1.0) | .83 |
| No | 28 678 (71) | .58 | Reference | ||
| Recent hospitalization | |||||
| Yes (past 30 d) | 1187 (3) | .44 | <.0001 | 0.5 (0.5–0.6) | <.0001 |
| No | 39 416 (97) | .58 | Reference | ||
| Recent antibiotic exposure | |||||
| Yes (past 30 d) | 8142 (20) | .60 | <.0001 | 1.1 (1.1–1.2) | .0002 |
| No | 32 461 (80) | .57 | Reference | ||
| Urologic anomaly | |||||
| Yes | 6546 (16) | .57 | .9 | 1.1 (0.9–1.2) | .13 |
| No | 34 057 (84) | .57 | Reference | ||
| Nonurologic comorbidities | |||||
| Yes | 669 (2) | .57 | .02 | 1.0 (0.8–1.2) | .77 |
| No | 39 934 (98) | .57 | Reference | ||
| Empiric antibiotic treatment | |||||
| Broad spectrum | 11 652 (29) | .63 | <.0001 | 1.2 (1.1–1.2) | <.0001 |
| Narrow spectrum | 28 951 (71) | .55 | Reference |
The unit of analysis for bivariate and multivariable analyses is a “UTI episode.” A UTI episode includes the 14-day period after initial visit date of the UTI. One patient may have multiple UTI episodes, and therefore the total number of UTI episodes (40 603) is greater than the total number of study patients (28 678).
Multivariable logistic regression adjusted for all factors listed in the table.
11% of UTI episodes were missing physician specialty.
<0.05% of UTI episodes were missing region.
Factors Associated With Urine Culture Use in Antibiotic-treated UTI
See Table 3. On multivariable analysis, age (<2 years: odds ratio [OR] 1.0, 95% confidence interval [CI] 0.9–1.1; 2–5 years: OR 1.3, 95% CI 1.2–1.4; 6–12 years: OR 1.3, 95% CI 1.2–1.4, compared with age 13–17 years); physician specialty (emergency medicine physicians: OR 1.2, 95% CI 1.1–1.3, and pediatricians: OR 2.6, 95% CI 2.5–2.8 compared with family physicians/internists); recent antibiotic exposure in the past 30 days (OR 1.1, 95% CI 1.1–1.2); and broad-spectrum antibiotic prescription (OR 1.2, 95% CI 1.1–1.2) were independent predictors of urine culture utilization. Children were less likely to have urine cultures performed if they were male (OR 0.8, 95% CI 0.8–0.9), treated by a urologist (OR 0.5, 95% CI 0.4–0.6), lived in the Northeast (OR 0.9, 95% CI 0.8–0.9) or West (OR 0.8, 95% CI 0.8–0.9) compared with the South, or were hospitalized within the past 30 days (OR 0.5, 95% CI 0.5–0.6). Previous UTI, urologic anomalies, and nonurologic comorbidities were not associated with urine culture utilization. We found analogous results on age-stratified analysis (<13 years and 13–17 years), with no substantial changes in magnitude or direction of adjusted ORs compared with the overall model.
Discussion
To our knowledge, this is the first large-scale, claims-based study in children evaluating practice patterns surrounding urine testing in antibiotic-treated UTIs. Our study had 3 major findings: (1) urine tests are not performed in a substantial percentage of antibiotic-treated UTI episodes, (2) there was considerable variation in urine culture utilization among different groups of patients and physicians, and (3) factors that place a patient at increased risk for resistant UTI (history of previous UTI, recent antibiotic exposure, recent hospitalization, and presence of genitourinary anomaly)10–12 were not strongly associated with urine culture use.
We found not only that urine cultures were performed in <60% of antibiotic-treated UTI episodes, but also that in ∼20% of antibiotic-treated UTI episodes no urine testing (urinalysis or urine culture) was done. The question raised is: should all children who are prescribed antibiotic therapy for a presumed UTI undergo a urine culture or, at least, a urinalysis? Current American Academy of Pediatrics guidelines which focus on the febrile infant from 2 to 24 months suggest that a urine specimen for urinalysis and culture be collected before antibiotic administration.2 Similarly, the National Institute for Health and Clinical Excellence guidelines recommend that infants and children 3 to 36 months with fever or symptoms and signs suggestive of UTI have a specimen collected for urinalysis and culture.1 These recommendations are reasonable for young pediatric patients who present with nonspecific symptoms, such as fever, which the majority of the time are symptomatic of viral illnesses and not UTI.13,14 Nonetheless, we found that 32% of children <2 years with an antibiotic-treated UTI had neither a urinalysis nor culture performed.
Because there are no US-based guidelines for the management of a child >2 years with UTI, for purposes of discussion, we extrapolate findings from the adult literature to this population; this body of evidence suggests that it is debatable whether every child <18 years with signs and symptoms of UTI requires a urinalysis or culture if an antibiotic is to be started. A treatment algorithm based on meta-analytic data from Bent and colleagues suggests that women >18 years undergo empiric antibiotic treatment of UTI without urinalysis or culture if they have signs and symptoms consistent with UTI and they are without fever, back pain, and risk factors for complicated infection.15 One clear benefit to this approach is cost savings and previous research demonstrated that such a treatment algorithm in the adult female population does not increase adverse outcomes.16 Although comparable pediatric literature is lacking, the National Institute for Health and Clinical Excellence guidelines recommend urinalysis alone to establish the diagnosis and initiate treatment of a standard UTI in a child >3 years.
That said, there are implications associated with treating a presumed UTI without culture confirmation in the pediatric population. First, patients are frequently referred to specialists for UTI evaluation and management even though they have never had a UTI confirmed by urinalysis or culture.17 Specifically, toilet-trained children with bladder and bowel dysfunction can exhibit signs and symptoms similar to UTI but often do not have infection.18 Improperly labeling a child with a UTI can lead to unnecessary testing, which may be traumatic, may expose the child to needless radiation, and may potentially incite infection.19,20 An additional issue is that lack of culture-proven UTI may delay evaluation for the presence of concomitant urologic abnormality until symptoms recur and cultures are obtained. This argument is heavily debated and contingent on the ability of intervention for the urologic abnormality to reduce reinfection and its long-term sequelae.21–23
When urine cultures were performed in antibiotic-treated UTIs, we identified considerable variability in utilization among different groups of patients and physicians. We did not observe an association with urine culture utilization in children <2 years despite the fact that children this age cannot effectively communicate and often present with nonspecific symptoms that the majority of the time are attributable to an etiology other than UTI. Moreover, we observed that males had decreased odds of urine culture utilization with similar results on age-stratified analysis despite the fact that males, in general, are less likely to have a UTI compared with females.24 However, circumcision status was unknown, and because being uncircumcised can be a risk factor for UTI, it may influence a provider’s decision to obtain a culture. Conversely, patients who were prescribed empiric broad-spectrum antibiotic treatment were more likely to have a urine culture performed. These may be patients for whom the prescribing physician has concern for a resistant uropathogen; therefore, it would be important to obtain a urine culture to tailor empiric therapy.
In addition, the odds of urine culture utilization varied significantly by provider type, with pediatricians most likely to obtain urine cultures, followed by emergency department physicians. Pediatricians may be more likely to obtain urine cultures compared with other physicians due to familiarity with the current US guidelines on urine testing that are generated by a pediatrician-based organization, the American Academy of Pediatrics. In contrast, urologists had 50% lower odds of obtaining a urine culture. This finding is unanticipated, given that urologists are more likely to manage patients with urologic abnormalities, and research shows that these patients are at greater risk for resistant UTI.10 We also saw variation in utilization based on region. Although resistance patterns are known to vary at the regional level, the size of the regions evaluated in our study were likely too broad to identify differences based on regional resistance patterns. Therefore, the observed discrepancies in practice patterns might be influenced by nonclinical factors.
Previous research supports that history of UTI, presence of genitourinary anomaly, recent antibiotic exposure, and recent hospitalization are associated with an increased risk for resistant UTI.10–12 It is indicated to perform urine cultures in patients with these risk factors so that treatment of UTIs that prove to be resistant to empiric antibiotic therapy can be appropriately tailored. Nonetheless, we found no association with urine culture utilization in patients with previous UTI or genitourinary anomaly, only a weak association in patients with previous antibiotic exposure, and decreased odds of utilization in patients who had been recently hospitalized. Given that the current study is a retrospective claims analysis, one explanation for these findings is that past medical history was not elicited by the treating physician and therefore not considered in the decision-making. Also, recent antibiotic exposure may have been weakly associated with urine culture use because there may not have been widespread awareness that it was a risk factor for resistant UTI. Few studies had been published regarding previous antibiotic exposure and risk of resistant UTI in the pediatric literature previous to the study period.10,25,26 Finally, it is unclear as to why children who were recently hospitalized had a lower odds of being cultured. Conceivably, if a urine culture was collected during recent hospital admission, the physician may have decided not to repeat the culture. However, if the child was well enough to be discharged and then re-presented in the ambulatory setting with UTI, cultures performed during the admission might not accurately reflect the present infection.
Our study should be interpreted with limitations in mind. First, the lack of distinction between coding for “rule-out UTI” and actual UTI may explain why a large proportion of patients with a UTI ICD-9 code did not receive antibiotics. Most UTI patients without an antibiotic prescription had urine testing (71%), which likely represents patients evaluated for rule-out UTI who were not prescribed antibiotics because of negative urine testing. The remaining 29% of UTI patients who were not prescribed antibiotics and had no urine testing may represent patients with a remote UTI history, rule-out UTI visits without urine testing, and coding inaccuracies. However, our study population likely consists of patients with true UTI because we based patient selection on a combination of UTI ICD-9 code, antibiotic prescription, and lack of concomitant infection. Also, antibiotic prescription and urine testing information is likely robust because it is generated by submitted insurance claims from pharmacies and laboratories, respectively. Second, the claims database analyzed for this study represents a select population that is privately insured and does not include Medicaid or those without insurance. Consequently, these results may not apply to all children. Third, the database does not include certain demographic and clinical information that allows assessment of the appropriateness of urine test utilization.3,27 For example, we were unable to account for factors that affect the likelihood for UTI and may influence a provider’s decision to obtain a urine test such as race, circumcision status, and presence of lower urinary tract symptoms or fever. Finally, there is a risk for underestimating urine test utilization by incompletely capturing all urine studies performed. To minimize this, we used an extended window of time before and after antibiotic prescription to ensure maximum capture of urine testing claims. Also, some urine testing done by dipstick in the clinical setting may not have been captured because it requires that the provider bill for this test. The remaining urine studies would be captured with a claims analysis because formal laboratory testing is required for microscopic urinalysis and culture.
Conclusions
Our findings reveal that antibiotics are empirically prescribed for a significant number of UTI episodes without urine testing. There may be clinical situations and populations of children in which this practice is appropriate; however, additional research is necessary to determine whether this approach is safe and effective.
Acknowledgment
We thank Amy J. Markowitz, JD, for her critical review of the manuscript.
Glossary
- CI
confidence interval
- ICD-9
International Classification of Diseases, Ninth Revision
- OR
odds ratio
- UTI
urinary tract infection
Footnotes
Dr Copp was responsible for the study conception and design, analysis and interpretation of data, and manuscript drafting and revision; Dr Yiee contributed to the study design, data interpretation, and editing; Ms Smith was responsible for data acquisition and data analysis; Ms Hanley supervised the data analysis; and Dr Saigal supervised the study design and analysis and assisted with data interpretation. All authors approved the final article.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Copp is supported by the University of California, San Francisco KURe Career Development Program (National Institutes of Health grant 1-K12-DK-083021). This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases as a part of the Urologic Diseases in America Project (grant N01 DK012460). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.National Institute for Health and Clinical Excellence. Urinary tract infection in children. London, England: National Institute for Health and Clinical Excellence; 2007. Available at: http://guidance.nice.org.uk/CG054. Accessed July 15 2012
- 2.Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595–610 [DOI] [PubMed]
- 3.Newman TB, Bernzweig JA, Takayama JI, Finch SA, Wasserman RC, Pantell RH. Urine testing and urinary tract infections in febrile infants seen in office settings: the Pediatric Research in Office Settings’ Febrile Infant Study. Arch Pediatr Adolesc Med. 2002;156(1):44–54 [DOI] [PubMed] [Google Scholar]
- 4.Copp HL, Shapiro DJ, Hersh AL. National ambulatory antibiotic prescribing patterns for pediatric urinary tract infection, 1998–2007. Pediatrics. 2011;127(6):1027–1033 [DOI] [PMC free article] [PubMed]
- 5.Kallen AJ, Welch HG, Sirovich BE. Current antibiotic therapy for isolated urinary tract infections in women. Arch Intern Med. 2006;166(6):635–639 [DOI] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration. National Drug Code Directory. Available at: www.accessdata.fda.gov/scripts/cder/ndc/default.cfm. Accessed November 15, 2011
- 7.The American Hospital Formulary Service. The AHFS Pharmacologic-Therapeutic Classification System. Available at: www.ahfsdruginformation.com/class/index.aspx. Accessed November 15, 2011
- 8.The Healthcare Common Procedure Coding System. HCPCS J Codes. Available at: www.medicarenhic.com/dme/medical_review/mr_hcpcs/2011_hcpcs_j_codes.pdf. Accessed November 15, 2011
- 9.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107(6). Available at: www.pediatrics.org/cgi/content/full/107/6/e99 [DOI] [PubMed] [Google Scholar]
- 10.Allen UD, MacDonald N, Fuite L, Chan F, Stephens D. Risk factors for resistance to “first-line” antimicrobials among urinary tract isolates of Escherichia coli in children. CMAJ. 1999;160(10):1436–1440 [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng CH, Tsai MH, Huang YC, et al. Antibiotic resistance patterns of community-acquired urinary tract infections in children with vesicoureteral reflux receiving prophylactic antibiotic therapy. Pediatrics. 2008;122(6):1212–1217 [DOI] [PubMed] [Google Scholar]
- 12.Paschke AA, Zaoutis T, Conway PH, Xie D, Keren R. Previous antimicrobial exposure is associated with drug-resistant urinary tract infections in children. Pediatrics. 2010;125(4):664–672 [DOI] [PubMed] [Google Scholar]
- 13.Craig JC, Williams GJ, Jones M, et al. The accuracy of clinical symptoms and signs for the diagnosis of serious bacterial infection in young febrile children: prospective cohort study of 15 781 febrile illnesses. BMJ. 2010;340:c1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudinsky SL, Carstairs KL, Reardon JM, Simon LV, Riffenburgh RH, Tanen DA. Serious bacterial infections in febrile infants in the post-pneumococcal conjugate vaccine era. Acad Emerg Med. 2009;16(7):585–590 [DOI] [PubMed] [Google Scholar]
- 15.Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287(20):2701–2710 [DOI] [PubMed] [Google Scholar]
- 16.O’Connor PJ, Solberg LI, Christianson J, Amundson G, Mosser G. Mechanism of action and impact of a cystitis clinical practice guideline on outcomes and costs of care in an HMO. Jt Comm J Qual Improv. 1996;22(10):673–682 [DOI] [PubMed] [Google Scholar]
- 17.Koyle MA, Shifrin D. Issues in febrile urinary tract infection management. Pediatr Clin North Am. 2012;59(4):909–922 [DOI] [PubMed]
- 18.Franco I. Functional bladder problems in children: pathophysiology, diagnosis, and treatment. Pediatr Clin North Am. 2012;59(4):783–817 [DOI] [PubMed]
- 19.Lohr JA, Downs SM, Dudley S, Donowitz LG. Hospital-acquired urinary tract infections in the pediatric patient: a prospective study. Pediatr Infect Dis J. 1994;13(1):8–12 [DOI] [PubMed] [Google Scholar]
- 20.Merritt KA, Ornstein PA, Spicker B. Children’s memory for a salient medical procedure: implications for testimony. Pediatrics. 1994;94(1):17–23 [PubMed] [Google Scholar]
- 21.Craig JC, Irwig LM, Knight JF, Roy LP. Does treatment of vesicoureteric reflux in childhood prevent end-stage renal disease attributable to reflux nephropathy? Pediatrics. 2000;105(6):1236–1241 [DOI] [PubMed] [Google Scholar]
- 22.Doganis D, Siafas K, Mavrikou M, et al. Does early treatment of urinary tract infection prevent renal damage? Pediatrics. 2007;120(4). Available at: www.pediatrics.org/cgi/content/full/120/4/e922 [DOI] [PubMed] [Google Scholar]
- 23.Peters CA, Skoog SJ, Arant BS Jr, et al. Summary of the AUA guideline on management of primary vesicoureteral reflux in children. J Urol. 2010;184(3):1134–1144 [DOI] [PubMed]
- 24.Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27(4):302–308 [DOI] [PubMed] [Google Scholar]
- 25.Ashkenazi S, Even-Tov S, Samra Z, Dinari G. Uropathogens of various childhood populations and their antibiotic susceptibility. Pediatr Infect Dis J. 1991;10(10):742–746 [DOI] [PubMed] [Google Scholar]
- 26.McLoughlin TG, Jr, Joseph MM. Antibiotic resistance patterns of uropathogens in pediatric emergency department patients. Acad Emerg Med. 2003;10(4):347–351 [DOI] [PubMed] [Google Scholar]
- 27.Shaikh N, Morone NE, Lopez J, et al. Does this child have a urinary tract infection? JAMA. 2007;298(24):2895–2904 [DOI] [PubMed] [Google Scholar]