Abstract
OBJECTIVE:
To assess the implications of autistic traits (ATs) in youth with attention-deficit/hyperactivity disorder (ADHD) without a diagnosis of autism.
METHODS:
Participants were youth with (n = 242) and without (n = 227) ADHD and controls without ADHD in whom a diagnosis of autism was exclusionary. Assessment included measures of psychiatric, psychosocial, educational, and cognitive functioning. ATs were operationalized by using the withdrawn + social + thought problems T scores from the Child Behavior Checklist.
RESULTS:
A positive AT profile was significantly overrepresented among ADHD children versus controls (18% vs 0.87%; P < .001). ADHD children with the AT profile were significantly more impaired than control subjects in psychopathology, interpersonal, school, family, and cognitive domains.
CONCLUSIONS:
A substantial minority of ADHD children manifests ATs, and those exhibiting ATs have greater severity of illness and dysfunction.
Keywords: ADD, ADHD, attention deficit disorder, attention-deficit/hyperactivity disorder, AT, autistic traits, autism traits, comorbidity, social disability
What’s Known on This Subject:
Studies examining the prevalence and associated features of autistic traits (ATs) in children with ADHD with exclusionary autism spectrum disorders suggest that children with ATs exhibit more severe social and interpersonal dysfunction reminiscent of the deficits in children with autism spectrum disorders.
What This Study Adds:
Our results suggest that ATs are overrepresented in ADHD children when compared with control subjects. They also suggest that the presence of ATs is associated with more severe psychopathology as well as more impaired interpersonal, school, family, and cognitive functioning.
Twin, family, and linkage studies indicate that attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorders (ASDs) share a portion of their heritable etiology.1–4 Genome-wide association studies found rare copy number variants shared between the 2 disorders,5 raising the possibility that some children with ADHD may manifest symptoms of autism even in the absence of a diagnosis of ASD. Recent studies have identified that symptoms of autism or autistic traits (ATs) appear in 20% to 30% of children with ADHD4,6,7 and that such children are more impaired than other children with ADHD, particularly in the domains of interpersonal communication and empathy. However, these findings require replication.
The main aim of the current study was to examine the prevalence and correlates of ATs in youth with ADHD by using data from an existing, large-scale sample of referred youth with and without ADHD in whom the diagnosis of autism was exclusionary. We hypothesized that ATs would be prevalent in children with ADHD and that their presence would be associated with higher levels of morbidity and disability.
Methods
Subjects
Subjects were youth of both genders derived from longitudinal, case-control family studies conducted at Massachusetts General Hospital (MGH).8,9 These studies included participants aged 6 to 18 years with (n = 280) and without (n = 242) Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition (DSM-III-R), ADHD ascertained from pediatric clinics at a large health maintenance organization and referrals to a pediatric psychopharmacology clinic. Within each setting, we selected non-ADHD normal controls from pediatric medical clinics based on structured interview diagnoses. We did not exclude controls having other psychiatric disorders.
ADHD cases were identified from either a major academic medical center, in which we selected ADHD subjects from consecutive referrals to its pediatric psychopharmacology clinic and from a major health maintenance organization, in which ADHD subjects were selected from consecutively ascertained pediatric clinic outpatients. Healthy controls were ascertained from outpatients referred for routine physical examinations to its pediatric medical clinics at each setting identified from their computerized records as not having ADHD. Further information on the ascertainment of the sample have been published in detail elsewhere.10–13 In previous articles,14,15 we reported that the rates of other psychiatric disorders in the control sample were low and consistent with expectations from population studies.
Participants had a mean ± SD age of 11.3 ± 3.2 years, were 99% white, and had a mean socioeconomic status (SES) score of 1.7 ± 0.9. The sample included 274 children (52%) who reported that he or she was tutored (ADHD: 172 [61%]; controls: 54 [22%]), repeated a grade (ADHD: 69 [25%]; controls: 18 [7%]), or took a special class (ADHD: 74 [26%]; controls: 5 [2%]). Of the control children with academic difficulty (27% [n = 65]), 34% (n = 22) met DSM-III-R criteria for ≥1 psychiatric disorder versus 28% (n = 49) of those without academic difficulty (n = 177). Of the ADHD children with academic difficulty (75% [n = 209]), 81% (n = 169) met DSM-III-R criteria for ≥1 psychiatric disorder versus 73% (n = 52) of those without academic difficulty (n = 71). These numbers suggest that the presence of a psychiatric illness may account for the increased prevalence of academic functioning difficulties. Adoption, unavailable nuclear family, major sensorimotor handicaps, psychosis, autism, language barriers, or an estimated IQ <80 were exclusionary for both ADHD and control participants. Parents provided written informed consent, and children and adolescents provided written assent. The institutional review board at MGH approved the study.
Assessment Procedures
Psychiatric assessments relied on the Kiddie Schedule for Affective Disorders and Schizophrenia–Epidemiologic Version,16,17 conducted directly and individually with the mothers and the children. For children aged <12 years who could not provide reliable self-reports of their symptoms, interviews were conducted with their mothers (indirect interviews). Combining data from direct and indirect interviews, we considered a diagnosis positive if it was endorsed in either interview. Social class was assessed by using the Hollingshead and Redlich scale18
Interviews were administered by highly trained and supervised psychometricians, blinded to referral source or diagnostic status (ADHD or control). Based on 500 assessments from interviews of children and adults, the median κ coefficient of agreement between a psychometrician and an experienced clinician was .98.
We used an empirically derived profile from the Child Behavior Checklist (CBCL) to define ATs (CBCL-AT) by using a cutoff of 195 from the combined T scores of the withdrawn, social problems, and the thought problems subscales19 that correctly classified 78% of all subjects with ASD from a psychiatrically referred sample with and without ASD. Two subscales were created from the CBCL by summing the anxiety/depression, aggression, and attention scales (severe dysregulation: sum of T scores ≥210; deficient emotional self-regulation: sum of T scores of 180–210).20
Psychosocial functioning was assessed by using the Social Adjustment Inventory for Children and Adolescents (SAICA).21 Using methodology recommended by Reynolds22 and used previously by this team,23 we identified children who were socially disabled on the basis of the discrepancy between the expected SAICA scaled score (derived from the estimated Full Scale IQ) and the actual SAICA scaled score. We first converted the estimated Full Scale IQ and SAICA scores to the Z scores ZIQ and ZS. We then estimated the expected SAICA score, ZES, by the regression equation ZES = rIQS × ZIQ, where rIQS is the correlation between the IQ and SAICA scores. We used the value from our control sample (r = 0.25, P < .05). We then calculated the discrepancy score as ZES – ZS and its SD, √(1 – r2IQS). We defined as socially disabled any subject who had a value >1.65 on the standardized discrepancy score, ZES – ZS/√(1– r2IQS).
Family functioning was assessed by using the Moos Family Environment Scale.24 Mothers provided information regarding their child’s history of school problems (ie, grade retention, special placements, remedial assistance) and treatment history (ie, counseling, medication, hospitalization). For analytic purposes, we treated this information as follows: “counseling” classified those who had received any type of psychosocial treatment for their ADHD; “counseling + medication” classified those who had received any type of psychosocial treatment for their ADHD as well as any medication treatment for their ADHD; and others were classified as “no treatment.” Mothers also provided information regarding their history of pregnancy, delivery, and their child’s infancy.
Intellectual functioning was assessed through the vocabulary, block design, digit span, digit symbol, digit coding, and arithmetic subtests of the Wechsler Intelligence Scale for Children–Revised (WISC-R)25 and the perseverative errors subtest of the Wisconsin Card Sorting Test.26 Using procedures suggested by Sattler,27 we estimated Full Scale IQ from the block design and vocabulary subtests of the WISC-R by using age-corrected scaled scores. We computed the Freedom From Distractibility IQ by using the digit span, digit coding, and oral arithmetic subscales of the WISC-R. Reading and arithmetic achievement was assessed by using subtests of the Wide Range Achievement Test–Revised.28
Statistical Analysis
We used t tests, Pearson’s χ2 test, Wilcoxon rank-sum test, and analysis of variance as indicated. We controlled for any demographic confounder that reached significance at an α level of .05 (ie, age, SES). Logistic and linear regression was used in the adjusted analyses. Given the many statistical tests computed, the .01 α level was used to assert statistical significance for omnibus comparisons. All tests were 2-tailed. Bonferroni corrections were used to control for chance findings in pairwise comparisons.
Results
Because 53 participants did not have CBCL information, our final sample included 227 controls and 242 ADHD subjects. More ADHD than control participants had a positive AT profile (44 [18.18%] vs 2 [0.87%]; Fisher’s exact test, P < .001). Because there were only 2 control subjects with an AT profile, we did not include these in analyses. Comparisons were made between ADHD subjects with (ADHD + CBCL-AT, n = 44) and without (ADHD, n = 198) a CBCL-AT profile and controls without ADHD or the CBCL-AT profile (controls, n = 227).
Sociodemographic Characteristics
ADHD + CBCL-AT participants were slightly younger than control subjects and were of a more disadvantageous SES status, scoring an average of 2.18 on the Hollingshead measure of SES, than ADHD participants (1.8 on the Hollingshead) and controls (1.6) (Table 1). Therefore, all subsequent analyses controlled for age and family SES.
TABLE 1.
Sociodemographic and Clinical Characteristics
| Characteristic | Controls (n = 227) | ADHD (n = 198) | ADHD + CBCL-AT (n = 44) | Test Statistic | P |
|---|---|---|---|---|---|
| Age [age range], y | 11.9 ± 3.4 [6–18] | 11.0 ± 3.14a [6–18] | 9.8 ± 2.7a,b [6–18] | F(2466) = 9.8 | <.001 |
| Male | 117 (52) | 107 (54) | 26 (59) | χ2(2) = 0.9 | .6 |
| White | 213 (94) | 193 (97) | 44 (100) | χ2(2) = 5.7 | .06 |
| SES | 1.59 ± 0.74 | 1.8 ± 0.95 | 2.18 ± 1a,b | χ2(2) = 13.1 | .001 |
| Intactness of family (divorced/separated) | 40 (18) | 50 (25) | 14 (32)a | χ2(2) = 6.2 | .045 |
| IQ | 114.2 ± 11.6 | 108.5 ± 12.6a | 102 ± 14.9a,b | F(2, 466) = 23.2, χ2(2) = 6.2 | <.001, .045 |
| ADHD characteristics | |||||
| Age of onset | – | 2.9 ± 2.4 | 3.0 ± 1.8 | F(3, 238) = 1.90, t = –0.3 | .7, .97 |
| ADHD impairment | |||||
| Moderate or severec | – | 185 (94) | 43 (98) | χ2(3) = 6.7, Fisher’s exact test | .083 |
| Mean no. of symptoms | – | 11 ± 2 | 12 ± 2 | F(3, 238) = 0.8, t = –2.1 | .5, .04 |
| Treatment history | |||||
| Medication (any treatment) | 0 (0) | 546 (278)a | 10 (23)a | Fisher’s exact test | .04 |
| Counseling + medication | 0 (0) | 91 (46)a | 298 (664)a,b | Fisher’s exact test | <.001 |
| School functioning | |||||
| Tutoring | 52 (23) | 122 (62)a | 24 (55)a | χ2(42) = 69.768.8 | <.001 |
| Placement in special class | 5 (2) | 45 (23)a | 22 (50)a,b | χ2(42) = 79.281.1 | <.001 |
| Repeated grade | 17 (7) | 53 (27)a | 8 (18)a | χ2(42) = 31.328.4 | <.001 |
| Family functioning (FES) | |||||
| Expression | 50.2 ± 14.6 | 47.8 ± 14.0 | 42.5 ± 14.0a,b | F(24, 4579) = 4.45.4 | .0025 |
| Conflict | 50.6 ± 12.5 | 56.9 ± 12.3a | 61.5 ± 11.2a,b | F(42, 45760) = 10.921.3 | <.001 |
| Cohesion | 54.2 ± 16.6 | 44.2 ± 20.9a | 39.4 ± 21.6a | F(42, 45760) = 13.019.8 | <.001 |
Data are presented as mean ± SD or n (%). FES, Family Environment Scale.
Compared with the control group.
Compared with the ADHD group.
Severity reflected the number of symptoms endorsed and the associated difficulties with them at home, in school, and in the social context.
Clinical Correlates of ADHD
ADHD + CBCL-AT and ADHD participants had similar ages of ADHD onset, similar proportions of ADHD-associated level of impairment (ie, classified as “mild” and “moderate or severe” as defined by the structured interview procedure and relating to the impairment caused by ADHD in multiple areas of functioning as perceived by the child, or the mother, if an indirect interview was conducted), similar rates of ADHD symptoms, similar proportions of any medication treatment for ADHD, similar family impairments (ie, expression, conflicts, cohesion as measured by the Moos Family Environment Scale), and similar school functioning (eg, tutoring, repeated grades) (all P > .01). ADHD + CBCL-AT participants had increased rates of placement in a special class (2.2% controls; 22.8% ADHD; 50% ADHD + CBCL-AT; χ2[4] = 81.1, P < .001) (Table 1). ADHD + CBCL-AT participants experienced more family conflict as measured by the Moos Family Environment Scale than control subjects (ADHD + CBCL-AT: 61.5 ±11.2; ADHD: 56.9 ± 12.3; F[4, 257] = 10.9; P < .001; Table 1).
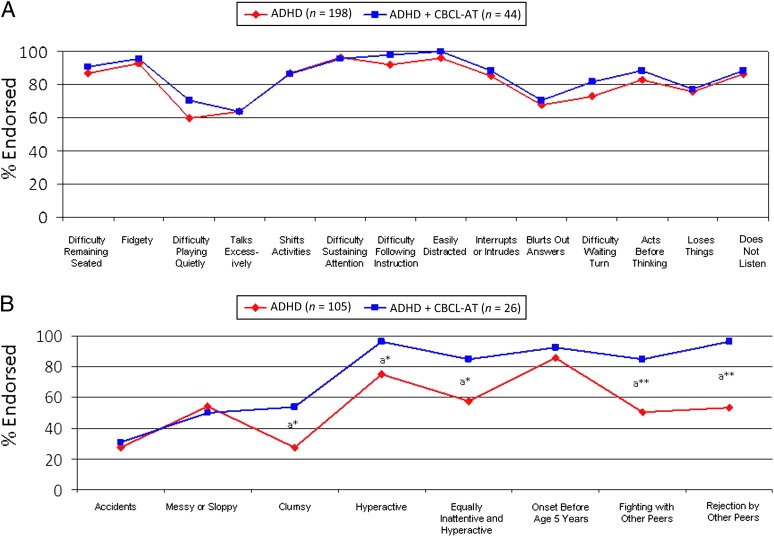
There were no differences between the ADHD + CBCL-AT and ADHD groups in individual DSM-III-R ADHD symptoms (Fig 1A), but ADHD + CBCL-AT participants had higher rates of additional ADHD-related symptoms captured during the structured interview, including “clumsiness” (odds ratio [OR]: 2.9, P = .02), “an illness equal in part inattention and hyperactivity” (OR: 3.7, P = .03), “hyperactivity” (OR: 8.9, P = .04), “fights with peers” (OR: 6.8, P = .002), and “rejection by peers” (OR: 24, P = .003) (Fig 1B).
FIGURE 1.
ADHD symptoms in the ADHD and ADHD + CBCL-AT groups. A, DSM-III-R symptoms. B, Additional related symptoms. aCompared with the ADHD group. *P < .05; **P < .005.
Pregnancy, Delivery, and Infancy Complications
ADHD + CBCL-AT mothers reported more infections during pregnancy (ADHD + CBCL-AT: 28%; ADHD: 11%; controls: 8%; χ2[4] = 10.7, P = .001), switching formulas during their children’s infancies (ADHD + CBCL-AT: 25%; ADHD: 9%; controls: 6%; χ2[4] = 13.6, P < .001), and described their infants as “stiffened” during infancy (ADHD + CBCL-AT: 25%; ADHD: 5%; controls: 1%; χ2[4] = 32, P < .001) (Table 2).
TABLE 2.
Pregnancy and Infancy Characteristics (Adjusting for Age and SES)
| Characteristic | Controls (n = 225) | ADHD (n = 188) | ADHD + CBCL-AT (n = 40) | Test Statistic | P |
|---|---|---|---|---|---|
| Pregnancy characteristics | |||||
| Excessive nausea | 25 (11) | 40 (21)a | 10 (25)a | χ2(4) = 10.4 | .03 |
| Infection | 19 (8) | 20 (11) | 11 (28)a,b | χ2(4) = 10.7 | .03 |
| High blood pressure | 24 (11) | 39 (21)a | 11 (28)a | χ2(4) = 14.1 | .007 |
| Accidents | 2 (1) | 9 (5)a | 4 (10)a | χ2(4) = 11 | .003 |
| Family problems | 20 (9) | 39 (21)a | 12 (30)a | χ2(4) = 21.2 | .003 |
| Medications | 47 (21) | 58 (31)a | 18 (45)a | χ2(4) = 13.8 | .008 |
| Smoking (3 mo at gestation) | 16 (7) | 27 (14)a | 9 (23)a | χ2(4) = 22.2 | .002 |
| Infancy characteristics | |||||
| Switch formulas | 13 (6) | 16 (9) | 10 (25)a,b | χ2(4) = 13.6 | .009 |
| Crying infant | 29 (13) | 48 (25)a | 12 (30)a | χ2(4) = 13.7 | .008 |
| Stiffened infant | 2 (1) | 9 (5)a | 10 (25)a,b | χ2(4) = 32.0 | <.001 |
Data are presented as n (%).
Compared with the control group.
Compared with the ADHD group.
Patterns of Psychiatric Comorbidity
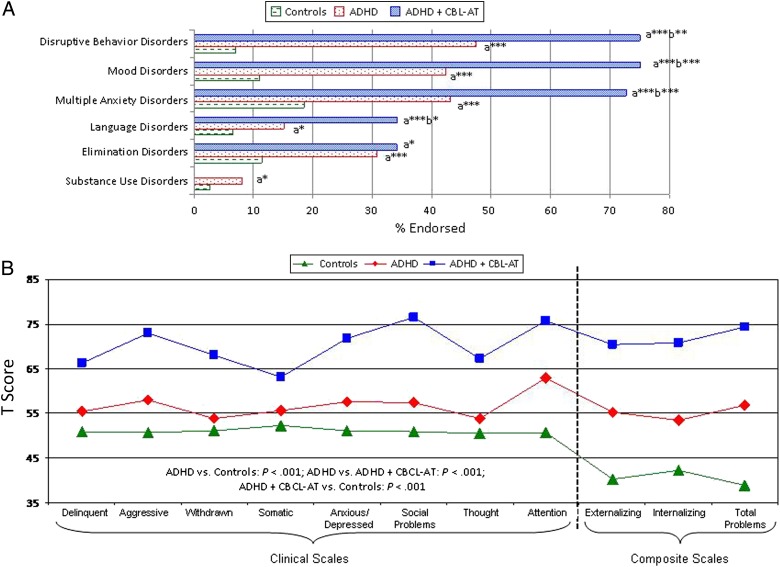
ADHD participants with and without ATs had significantly higher prevalence of all comorbid psychiatric disorders versus control subjects (ie, disruptive behavior disorders, χ2 = 142.4, P < .001; mood disorders, χ2 = 132.5, P < .001; multiple anxiety disorders, χ2 = 63.9, P < .001; language disorders, χ2 = 25.5, P < .001; elimination disorders, χ2 = 40.5, P < .001; and substance use disorders, χ2 = 20.9, P < .001). Compared with ADHD participants, ADHD + CBCL-AT participants had a significantly higher prevalence of disruptive behaviors (OR: 3.7, P = .001), mood disorders (OR: 5.4, P < .001), multiple anxiety disorders (>2) (OR: 3.7, P < .001), and language disorders (OR: 2.6, P = .01) (Fig 2A).
FIGURE 2.
Additional related symptoms in the ADHD, ADHD + CBCL-AT, and control groups. A, Prevalence of psychiatric disorders (lifetime). B, CBCL profile. aCompared with the control group. bCompared with the ADHD group. *P < .05; **P < .005; ***P < .001.
ADHD participants with and without ATs had significantly more impaired scores on each CBCL clinical and composite scale compared with controls (all P < .001). ADHD + CBCL-AT participants had significantly more impaired scores on all CBCL clinical and composite scales than ADHD participants, including scales that were not used to define ATs (all P < .001) (Fig 2B).
Deficits in Emotion Regulation and Social Disability
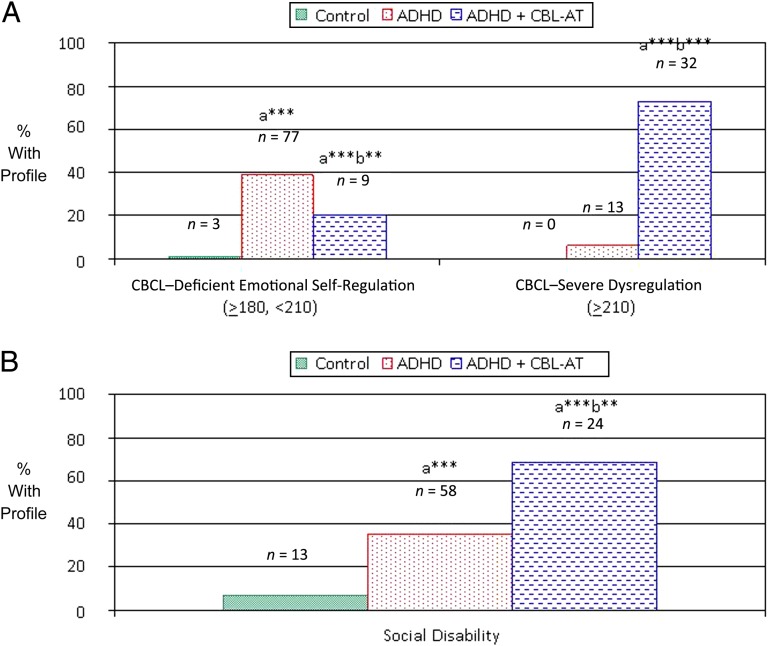
ADHD + CBCL-AT participants had a significantly higher prevalence of the CBCL– severe emotional dysregulation profile than both ADHD and control participants (controls: 0%; ADHD: 6.57%; ADHD + CBCL-AT: 72.73% [all P < .001]). In contrast, ADHD and ADHD + CBCL-AT participants did not differ from each other in the prevalence of the CBCL–deficient emotional self-regulation profile, which assesses a lower level of emotional dysregulation compared with the CBCL–severe emotional dysregulation profile. However, both groups had a significantly higher prevalence of the CBCL–deficient emotional self-regulation profile than control subjects (controls: 1.32%; ADHD: 38.89%; ADHD + CBCL-AT: 20.45% [all P < .001]) (Fig 3A).
FIGURE 3.
Clinical Features in the ADHD, ADHD + CBCL-AT, and control groups. A, Emotion dysregulation: CBCL AAA profile (attention + aggression + anxiety/depressed T scores). B, Social disability. aCompared with the control group. bCompared with the ADHD group. *P < .005; ***P < .0001.
Social Functioning
Both ADHD groups had a significantly higher prevalence of social disability as defined by the SAICA (all P < .01) than control subjects. However, rates were significantly higher in ADHD + CBCL-AT participants versus ADHD participants (controls: 6.77%; ADHD: 34.94%; ADHD-AT: 68.57%) (Fig 3B).
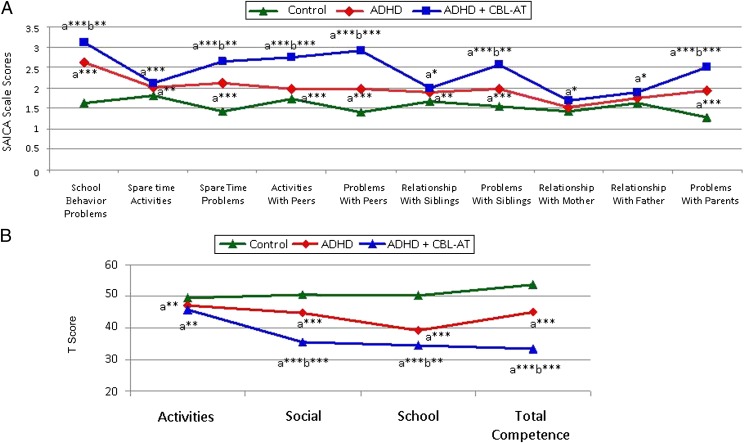
ADHD + CBCL-AT participants had significantly more impaired SAICA scaled scores than ADHD participants in measures of school behavior, spare time problems, activities and problems with peers, and problems with siblings and parents (all P < .001) (Fig 4A). A similar pattern was observed when analyzing findings from the CBCL social functioning scales, which consist of the activities, social, and total competence scales. Of these, only the social problems scale was used to define ATs. ADHD + CBCL-AT participants had significantly more impaired scores than ADHD participants on the CBCL social and total competence scales (Fig 4B).
FIGURE 4.
Social functioning in the ADHD, ADHD + CBCL-AT, and control groups. A, SAICA individual item scores. B, CBCL social functioning scales. aCompared with the control group. bCompared with the ADHD group. *P < .05; **P < .005; ***P < .0001.
Cognitive Findings
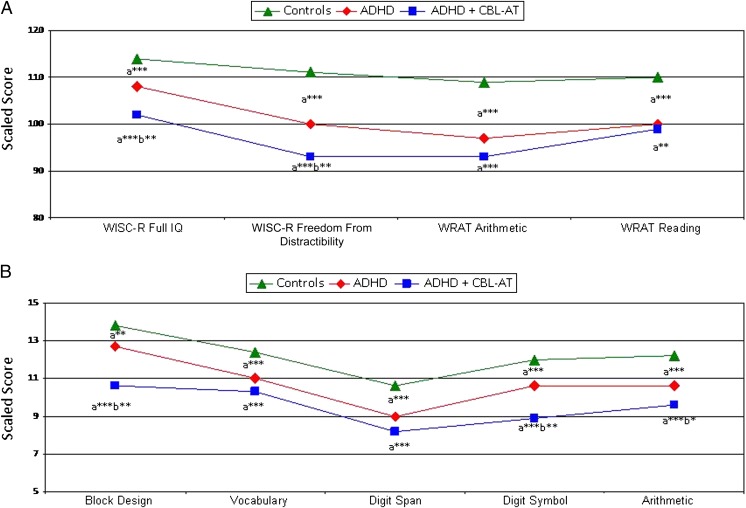
ADHD + CBCL-AT participants scored significantly worse than ADHD participants on the WISC-R Full IQ (ADHD + CBCL-AT: 101.96 ± 14.90; ADHD: 108.54 ± 12.55), freedom from distractibility (ADHD + CBCL-AT: 92.71 ± 16.11; ADHD: 100.28 ± 14.35), block design (ADHD + CBCL-AT: 10.61 ± 3.82; ADHD: 12.70 ± 3.48), digit symbol scaled scores (ADHD + CBCL-AT: 8.86 ± 3.99; ADHD: 10.57 ± 3.13) (all P < .05), and Wisconsin Card Sorting Test perseverative errors subtest (T scores: ADHD + CBCL-AT: 28.86 ± 16.88; ADHD: 17.71 ± 12.53; controls: 15.84 ± 10.92) (Fig 5).
FIGURE 5.
Neuropsychological functioning in the ADHD, ADHD + CBCL-AT, and control groups. A, WISC-R and Wide Range Achievement Test (WRAT). B, WISC-R. aCompared with the control group. bCompared with the ADHD group. *P < .05; **P < .005; ***P < .0001.
Discussion
Our findings revealed that ATs are present in children with ADHD, and their presence heralds a significantly more compromised clinical presentation characterized by higher rates of psychopathological, neuropsychological, and interpersonal deficits. These results are highly consistent with those of 3 previous reports4,6,7 that examined ATs in children with ADHD.
The current study found that ADHD children with a positive AT profile do not differ from other ADHD children in the core symptoms of ADHD, but they do present with a more severe clinical picture when additional and ADHD-relevant symptoms in the diagnostic criteria are considered, including clumsiness, messiness, and social difficulties with peers. Because these symptoms are commonly reported in children with ASDs, it is possible that they reflect ASD tendencies as well as ADHD ones.29–32 Of note, the presence of clumsiness in children with ADHD and ATs may be closely related to the disordered movement kinetics observed in Asperger’s syndrome33–36 and what has been previously described in children with the neuropsychological profile of deficits in attention, motor control, and perception.37–39
Also consistent with the extant literature are our findings on the Kiddie Schedule for Affective Disorders and Schizophrenia–Epidemiologic Version showing that ADHD + AT children exhibited significantly higher rates of comorbid psychiatric disorders versus ADHD children, especially in the domain of disruptive behavior disorders, which included the diagnoses of conduct and oppositional defiant disorder.4,6 Also reflecting the impairment observed in children with ASDs40,41 is the finding that ADHD + CBCL-AT children were more likely to have a positive CBCL–severe dysregulation profile versus ADHD children, indicating that these children experience very severe behavioral, emotional, and educational problems.20,42 The high rates of mood dysregulation in the ADHD + CBCL-AT children are also consistent with an emerging body of literature documenting high rates of mood disorders in children with ASDs.43 Further research is needed to better understand the role ATs confer on emotion regulation in children with ADHD.
ADHD + CBCL-AT children also had significantly lower Full IQ, freedom from distractibility, block design, and digit symbol WISC-R scores as well as differences in perseverative errors on the Wisconsin Card Sorting Test than ADHD children. These findings suggest impairments in executive functioning and cognitive flexibility, a pattern observed in other populations positive for ATs,44,45 as well as in children with ASDs.46–48
Also consistent with the extant literature are our findings showing that ADHD + AT children were more likely than ADHD children to fight with and be rejected by peers, to have more school behavior problems, more difficulties utilizing their spare time, and more problems with siblings. Considering the well-established evidence that social difficulties are a core component of ASDs,49–51 our findings also suggest that the social disability observed in the ADHD + CBCL-AT group may be more a reflection of underlying ATs than the presence of ADHD itself. The higher rates of mood, anxiety, disruptive, and substance use disorders,52 and school failure, school dropout, and delinquent offenses53 in ADHD + AT children is particularly worrisome.
Our findings that ADHD children with ATs had a higher rate of pregnancy and infancy complications than other ADHD children could suggest that prenatal and perinatal complications alone or in combination with genetic risk factors could account for the development of ATs in some children with ADHD. These findings are intriguing in light of previous reports yielding support for the role of maternal infection during pregnancy54–57 and the behavioral characteristics of infants later classified with ASDs.58
The ability to identify a subgroup of ADHD children with ATs may facilitate the development of more individualized clinical interventions. For instance, special care could be made to target treatment on the domain of social difficulties by focusing and expanding on the development of social skills training in addition to contingency management typically used for the psychosocial treatment of ADHD. Scientifically, this research will help inform future work targeted at identifying biomarkers for a potentially distinct subtype of ADHD. For example, twin, family, linkage, and genome-wide association studies already suggest that ADHD and ASD may share a common heritable etiology.1–4,59 Of note, Williams et al5 found that rare copy number variants identified in ADHD subjects were significantly enriched for loci implicated in autism.
Our findings need to be viewed in light of some limitations. Our sample did not contain a comparison group of children with a diagnosed ASD. Such a group would be useful to determine the degree to which our ADHD + CBCL-AT cohort exhibits features that are similar to the diagnostic class. However, because our primary diagnosis of interest was ADHD impacted by traits of autism, the absence of an autism-only control group does not detract from the finding that ADHD children with ATs exhibit more impairments than those with ADHD only. Although autism was excluded, it was done by using subject history as opposed to validated measures such as the Autism Diagnostic Observation Schedule, Social Communication Questionnaire, or the Social Responsiveness Scale, thus allowing for the possibility that some children with undiagnosed ASDs could have been included in our sample. However, considering that the mean age of our sample at baseline was 11 years, it is not very likely that children with a clear diagnosis of ASD would have remained undiagnosed. Because our measure of ATs has not been validated, it is possible that its external validity may be compromised because findings may not generalize to other samples. Finally, because our sample was referred and largely white, our findings may not generalize to community samples or other ethnic groups.
Conclusions
Despite these limitations, our work found that the CBCL-AT profile identifies a sizeable minority of ADHD children at high risk for significant morbidity and disability. More work is needed to replicate these findings in children with and without ADHD and to further examine their prognostic utility.
Glossary
- ADD
attention deficit disorder
- ADHD
attention-deficit/hyperactivity disorder
- ASD
autism spectrum disorder
- ATs
autistic traits
- CBCL
Child Behavior Checklist
- DSM-III-R
Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition
- MGH
Massachusetts General Hospital
- OR
odds ratio
- SAICA
Social Adjustment Inventory for Children and Adolescents
- SES
socioeconomic status
- WISC-R
Wechsler Intelligence Scale for Children–Revised
Footnotes
Dr Kotte was involved significantly in the acquisition of data, analysis and interpretation of data, in the drafting and revising of the article, and in the final approval of the to-be-published version; Drs Joshi, Fried, Uchida, and Spencer were involved in the interpretation of data, in revising it critically for important intellectual content, and final approval of the version to be published; Ms Woodworth and Ms Kenworthy were involved in the drafting and revising of the manuscript, analysis and interpretation of the data, and final approval of the version to be published; Dr Faraone was significantly involved in the interpretation of data, in the drafting and revising of the article, and in the final approval of the to-be-published article; and Dr Biederman made a substantial contribution to the conception and design of the study, was significantly involved in the acquisition and interpretation of data, in the revision of the manuscript, and in the final approval of the to-be-published version.
FINANCIAL DISCLOSURE: Dr Biederman is currently receiving research support from the following sources: American Professional Society of ADHD and Related Disorders, Department of Defense, ElMindA, Janssen, McNeil, Shire, and Vaya Pharma/Enzymotec. In 2012, Dr Biederman received an honorarium from the Massachusetts General Hospital (MGH) Psychiatry Academy and The Children’s Hospital of Southwest Florida/Lee Memorial Health System for tuition-funded continuing medical education (CME) courses. In 2011, Dr Biederman gave a single unpaid talk for Juste Pharmaceutical Spain, received honoraria from the MGH Psychiatry Academy for a tuition-funded CME course, and received honoraria for presenting at an international scientific conference on attention-deficit/hyperactivity disorder (ADHD). He also received an honorarium from Cambridge University Press for a chapter publication. Dr Biederman received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Eli Lilly, Shire, and AstraZeneca; these royalties are paid to the Department of Psychiatry at MGH. In 2010, Dr Biederman received a speaker’s fee from a single talk given at Fundación Dr. Manuel Camelo A.C. in Monterrey, Mexico. Dr Biederman provided single consultations for Shionogi Pharma Inc and Cipher Pharmaceuticals Inc; the honoraria for these consultations were paid to the Department of Psychiatry at MGH. Dr Biederman received honoraria from the MGH Psychiatry Academy for a tuition-funded CME course. In previous years, Dr Biederman received research support, consultation fees, or speaker’s fees for/from the following additional sources: Abbott, Alza, AstraZeneca, Boston University, Bristol-Myers Squibb, Celltech, Cephalon, Eli Lilly and Co, Esai, Fundacion Areces (Spain), Forest, GlaxoSmithKline, Gliatech, Hastings Center, Janssen, McNeil, Medice Pharmaceuticals (Germany), Merck, MMC Pediatric, National Alliance for Research on Schizophrenia and Depression, National Institute on Drug Abuse, New River, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Mental Health, Novartis, Noven, Neurosearch, Organon, Otsuka, Pfizer, Pharmacia, Phase V Communications, Physicians Academy, The Prechter Foundation, Quantia Communications, Reed Exhibitions, Shire, the Spanish Child Psychiatry Association, The Stanley Foundation, UCB Pharma Inc, Veritas, and Wyeth. Dr Joshi receives research support from Forest Research Laboratories and Duke University as a site principal investigator for multisite clinical trials. He is a co-investigator for clinical trials sponsored by Schering-Plough Corporation, Shire, ElMindA, and the US Department of Defense. In 2011, Dr Joshi received research support from Shire, Johnson & Johnson Pharmaceutical Research and Development, Eli Lilly, Forest Research Laboratories, Schering-Plough Corporation, ElMindA, and the National Institute of Mental Health. In the past year, Dr Faraone received consulting income and/or research support from Shire, Otsuka, and Alcobra and research support from the National Institutes of Health. He is also on the clinical advisory board for Akili Interactive Laboratories. In previous years, he received consulting fees or was on advisory boards or participated in continuing medical education programs sponsored by the following: Shire, McNeil, Janssen, Novartis, Pfizer, and Eli Lilly. Dr Faraone receives royalties from books published by Guilford Press (Straight Talk About Your Child’s Mental Health) and Oxford University Press (Schizophrenia: The Facts). Dr Fried has previously received honoraria from Shire. Drs Kotte, Uchida, Spencer, Ms Kenworthy, and Ms Woodworth have no financial relationships relevant to this article to disclose.
FUNDING: The data acquisition from which this analysis was derived was funded by National Institute of Mental Health grants MH-41314, HD036317, and MH050657 to Dr Biederman. The manuscript and analysis of the data were indirectly supported by the Pediatric Psychopharmacology Research Council Fund. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Rommelse NN, Franke B, Geurts HM, Hartman CA, Buitelaar JK. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry. 2010;19(3):281–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lichtenstein P, Carlström E, Råstam M, Gillberg C, Anckarsäter H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry. 2010;167(11):1357–1363 [DOI] [PubMed] [Google Scholar]
- 3.Nijmeijer JS, Arias-Vásquez A, Rommelse NN, et al. Identifying loci for the overlap between attention-deficit/hyperactivity disorder and autism spectrum disorder using a genome-wide QTL linkage approach. J Am Acad Child Adolesc Psychiatry. 2010;49(7):675–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulligan A, Anney RJ, O’Regan M, et al. Autism symptoms in attention-deficit/hyperactivity disorder: a familial trait which correlates with conduct, oppositional defiant, language and motor disorders. J Autism Dev Disord. 2009;39(2):197–209 [DOI] [PubMed] [Google Scholar]
- 5.Williams NM, Franke B, Mick E, et al. Genome-wide analysis of copy number variants in attention deficit/hyperactivity disorder: the role of rare variants and duplications at 15q13.3. Am J Psychiatry. 2012;169(2):195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grzadzinski R, Di Martino A, Brady E, et al. Examining autistic traits in children with ADHD: does the autism spectrum extend to ADHD? J Autism Dev Disord. 2011;41(9):1178–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kochhar P, Batty MJ, Liddle EB, et al. Autistic spectrum disorder traits in children with attention deficit hyperactivity disorder. Child Care Health Dev. 2011;37(1):103–110 [DOI] [PubMed] [Google Scholar]
- 8.Biederman J, Monuteaux MC, Mick E, et al. Young adult outcome of attention deficit hyperactivity disorder: a controlled 10-year follow-up study. Psychol Med. 2006;36(2):167–179 [DOI] [PubMed] [Google Scholar]
- 9.Biederman J, Monuteaux MC, Mick E, et al. Psychopathology in females with attention-deficit/hyperactivity disorder: a controlled, five-year prospective study. Biol Psychiatry. 2006;60(10):1098–1105 [DOI] [PubMed] [Google Scholar]
- 10.Biederman J, Faraone SV, Petty C, Martelon M, Woodworth KY, Wozniak J. Further evidence that pediatric-onset bipolar disorder comorbid with ADHD represents a distinct subtype: results from a large controlled family study. J Psychiatr Res. 2013;47(1):15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biederman J, Petty C, Spencer TJ, et al. Is ADHD a risk for posttraumatic stress disorder (PTSD)? Results from a large longitudinal study of referred children with and without ADHD [published online ahead of print April 23, 2013]. World J Biol Psychiatry. [DOI] [PubMed] [Google Scholar]
- 12.Biederman J, Petty CR, O’Connor KB, Hyder LL, Faraone SV. Predictors of persistence in girls with attention deficit hyperactivity disorder: results from an 11-year controlled follow-up study. Acta Psychiatr Scand. 2012;125(2):147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biederman J, Petty CR, Woodworth KY, Lomedico A, Hyder LL, Faraone SV. Adult outcome of attention-deficit/hyperactivity disorder: a controlled 16-year follow-up study. J Clin Psychiatry. 2012;73(7):941–950 [DOI] [PubMed] [Google Scholar]
- 14.Biederman J, Faraone SV, Keenan K, et al. Further evidence for family-genetic risk factors in attention deficit hyperactivity disorder. Patterns of comorbidity in probands and relatives psychiatrically and pediatrically referred samples. Arch Gen Psychiatry. 1992;49(9):728–738 [DOI] [PubMed] [Google Scholar]
- 15.Biederman J, Faraone SV, Mick E, et al. Clinical correlates of ADHD in females: findings from a large group of girls ascertained from pediatric and psychiatric referral sources. J Am Acad Child Adolesc Psychiatry. 1999;38(8):966–975 [DOI] [PubMed] [Google Scholar]
- 16.Orvaschel H. Schedule for Affective Disorders and Schizophrenia for School-Age Children Epidemiologic Version. 5th ed. Ft. Lauderdale, FL: Nova Southeastern University, Center for Psychological Studies; 1994 [Google Scholar]
- 17.Orvaschel H. Psychiatric interviews suitable for use in research with children and adolescents. Psychopharmacol Bull. 1985;21(4):737–745 [PubMed] [Google Scholar]
- 18.Hollingshead AB. Four Factor Index of Social Status. New Haven: Yale Press; 1975 [Google Scholar]
- 19.Biederman J, Petty CR, Fried R, et al. Child behavior checklist clinical scales discriminate referred youth with autism spectrum disorder: a preliminary study. J Dev Behav Pediatr. 2010;31(6):485–490 [DOI] [PubMed] [Google Scholar]
- 20.Biederman J, Petty CR, Day H, et al. Severity of the aggression/anxiety-depression/attention Child Behavior Checklist profile discriminates between different levels of deficits in emotional regulation in youth with attention-deficit hyperactivity disorder. J Dev Behav Pediatr. 2012;33(3):236–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John K, Gammon GD, Prusoff BA, Warner V. The Social Adjustment Inventory for Children and Adolescents (SAICA): testing of a new semistructured interview. J Am Acad Child Adolesc Psychiatry. 1987;26(6):898–911 [DOI] [PubMed] [Google Scholar]
- 22.Reynolds CR. Critical measurement issues in learning disabilities. J Spec Educ. 1984;18(4):451–475 [Google Scholar]
- 23.Faraone SV, Biederman J, Lehman BK, et al. Intellectual performance and school failure in children with attention deficit hyperactivity disorder and in their siblings. J Abnorm Psychol. 1993;102(4):616–623 [DOI] [PubMed] [Google Scholar]
- 24.Moos RH, Moos BS. Manual for the Family Environment Scale. Palo Alto, CA: Consulting Psychologists Press; 1974 [Google Scholar]
- 25.Wechsler D. Manual for the Wechsler Intelligence Scale for Children–Revised. New York, NY: The Psychological Corporation; 1974 [Google Scholar]
- 26.Grant DA, Berg EA. The Wisconsin Card Sorting Test. Odessa, FL: Psychological Assessment Resources; 1948 [Google Scholar]
- 27.Sattler J. Psychological Assessment. 4th ed. New York, NY: McGraw-Hill; 1988 [Google Scholar]
- 28.Jastak JF, Jastak S. The Wide Range Achievement Test–Revised. Wilmington, DE: Jastak Associates; 1985 [Google Scholar]
- 29.Dewey D, Cantell M, Crawford SG. Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. J Int Neuropsychol Soc. 2007;13(2):246–256 [DOI] [PubMed] [Google Scholar]
- 30.Green D, Charman T, Pickles A, et al. Impairment in movement skills of children with autistic spectrum disorders. Dev Med Child Neurol. 2009;51(4):311–316 [DOI] [PubMed] [Google Scholar]
- 31.Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. J Autism Dev Disord. 2010;40(10):1227–1240 [DOI] [PubMed] [Google Scholar]
- 32.Jansiewicz EM, Goldberg MC, Newschaffer CJ, Denckla MB, Landa R, Mostofsky SH. Motor signs distinguish children with high functioning autism and Asperger’s syndrome from controls. J Autism Dev Disord. 2006;36(5):613–621 [DOI] [PubMed] [Google Scholar]
- 33.Green D, Baird G, Barnett AL, Henderson L, Huber J, Henderson SE. The severity and nature of motor impairment in Asperger’s syndrome: a comparison with specific developmental disorder of motor function. J Child Psychol Psychiatry. 2002;43(5):655–668 [DOI] [PubMed] [Google Scholar]
- 34.Rinehart NJ, Bradshaw JL, Brereton AV, Tonge BJ. Movement preparation in high-functioning autism and Asperger disorder: a serial choice reaction time task involving motor reprogramming. J Autism Dev Disord. 2001;31(1):79–88 [DOI] [PubMed] [Google Scholar]
- 35.Rinehart NJ, Bellgrove MA, Tonge BJ, Brereton AV, Howells-Rankin D, Bradshaw JL. An examination of movement kinematics in young people with high-functioning autism and Asperger’s disorder: further evidence for a motor planning deficit. J Autism Dev Disord. 2006;36(6):757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weimer AK, Schatz AM, Lincoln A, Ballantyne AO, Trauner DA. “Motor” impairment in Asperger syndrome: evidence for a deficit in proprioception. J Dev Behav Pediatr. 2001;22(2):92–101 [DOI] [PubMed] [Google Scholar]
- 37.Díaz-Lucero AH, Melano CA, Etchepareborda MC. Deficits in attention, motor control and perception (DAMP) syndrome: neuropsychological profile [in Spanish]. Rev Neurol. 2011;52(suppl 1):S71–S75 [PubMed] [Google Scholar]
- 38.Hellgren L, Gillberg C, Gillberg IC, Enerskog I. Children with deficits in attention, motor control and perception (DAMP) almost grown up: general health at 16 years. Dev Med Child Neurol. 1993;35(10):881–892 [DOI] [PubMed] [Google Scholar]
- 39.Landgren M, Kjellman B, Gillberg C. Attention deficit disorder with developmental coordination disorders. Arch Dis Child. 1998;79(3):207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hutton J, Goode S, Murphy M, Le Couteur A, Rutter M. New-onset psychiatric disorders in individuals with autism. Autism. 2008;12(4):373–390 [DOI] [PubMed] [Google Scholar]
- 41.Simonoff E, Jones CR, Pickles A, Happé F, Baird G, Charman T. Severe mood problems in adolescents with autism spectrum disorder. J Child Psychol Psychiatry. 2012;53(11):1157–1166 [DOI] [PubMed] [Google Scholar]
- 42.Volk HE, Todd RD. Does the Child Behavior Checklist juvenile bipolar disorder phenotype identify bipolar disorder? Biol Psychiatry. 2007;62(2):115–120 [DOI] [PubMed] [Google Scholar]
- 43.Joshi G, Wozniak J, Petty C, et al. Psychiatric comorbidity and functioning in a clinically referred population of adults with autism spectrum disorders: a comparative study. J Autism Dev Disord. 2013;43(6):1314–1325 [DOI] [PubMed] [Google Scholar]
- 44.Ridley NJ, Homewood J, Walters J. Cerebellar dysfunction, cognitive flexibility and autistic traits in a non-clinical sample. Autism. 2011;15(6):728–745 [DOI] [PubMed] [Google Scholar]
- 45.van Rijn S, Bierman M, Bruining H, Swaab H. Vulnerability for autism traits in boys and men with an extra X chromosome (47,XXY): the mediating role of cognitive flexibility. J Psychiatr Res. 2012;46(10):1300–1306 [DOI] [PubMed] [Google Scholar]
- 46.Corbett BA, Constantine LJ, Hendren R, Rocke D, Ozonoff S. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Res. 2009;166(2–3):210–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Vries M, Geurts HM. Cognitive flexibility in ASD; task switching with emotional faces. J Autism Dev Disord. 2012;42(12):2558–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sachse M, Schlitt S, Hainz D, et al. Executive and visuo-motor function in adolescents and adults with autism spectrum disorder. J Autism Dev Disord. 2013;43(5):1222–1235 [DOI] [PubMed] [Google Scholar]
- 49.Alessandri M, Mundy P, Tuchman RF. [The social deficit in autism: focus on joint attention]. Rev Neurol. 2005;40(suppl 1):S137–S141 [PubMed] [Google Scholar]
- 50.Sasson NJ, Nowlin RB, Pinkham AE. Social cognition, social skill, and the broad autism phenotype [published online ahead of print September 17, 2012]. Autism. [DOI] [PubMed] [Google Scholar]
- 51.Sato W, Toichi M, Uono S, Kochiyama T. Impaired social brain network for processing dynamic facial expressions in autism spectrum disorders. BMC Neurosci. 2012;13:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greene RW, Biederman J, Faraone SV, Sienna M, Garcia-Jetton J. Adolescent outcome of boys with attention-deficit/hyperactivity disorder and social disability: results from a 4-year longitudinal follow-up study. J Consult Clin Psychol. 1997;65(5):758–767 [DOI] [PubMed] [Google Scholar]
- 53.Ollendick TH, Greene RW, Francis G, Baum CG. Sociometric status: its stability and validity among neglected, rejected and popular children. J Child Psychol Psychiatry. 1991;32(3):525–534 [DOI] [PubMed] [Google Scholar]
- 54.Abdallah MW, Larsen N, Grove J, et al. Amniotic fluid chemokines and autism spectrum disorders: an exploratory study utilizing a Danish Historic Birth Cohort. Brain Behav Immun. 2012;26(1):170–176 [DOI] [PubMed] [Google Scholar]
- 55.Atladóttir HO, Thorsen P, Østergaard L, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40(12):1423–1430 [DOI] [PubMed] [Google Scholar]
- 56.Goines P, Van de Water J. The immune system’s role in the biology of autism. Curr Opin Neurol. 2010;23(2):111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17(7):389–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karmel BZ, Gardner JM, Meade LS, et al. Early medical and behavioral characteristics of NICU infants later classified with ASD. Pediatrics. 2010;126(3):457–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor MJ, Charman T, Robinson EB, et al. Developmental associations between traits of autism spectrum disorder and attention deficit hyperactivity disorder: a genetically informative, longitudinal twin study. Psychol Med. 2012;(Nov 16):1–12 [DOI] [PubMed] [Google Scholar]