Abstract
OBJECTIVES:
Term infants in resource-poor settings frequently develop hypothermia during the first hours after birth. Plastic bags or wraps are a low-cost intervention for the prevention of hypothermia in preterm and low birth weight infants that may also be effective in term infants. Our objective was to test the hypothesis that placement of term neonates in plastic bags at birth reduces hypothermia at 1 hour after birth in a resource-poor hospital.
METHODS:
This parallel-group randomized controlled trial was conducted at University Teaching Hospital, the tertiary referral center in Zambia. Inborn neonates with both a gestational age ≥37 weeks and a birth weight ≥2500 g were randomized 1:1 to either a standard thermoregulation protocol or to a standard thermoregulation protocol with placement of the torso and lower extremities inside a plastic bag within 10 minutes after birth. The primary outcome was hypothermia (<36.5°C axillary temperature) at 1 hour after birth.
RESULTS:
Neonates randomized to plastic bag (n = 135) or to standard thermoregulation care (n = 136) had similar baseline characteristics (birth weight, gestational age, gender, and baseline temperature). Neonates in the plastic bag group had a lower rate of hypothermia (60% vs 73%, risk ratio 0.76, confidence interval 0.60–0.96, P = .026) and a higher axillary temperature (36.4 ± 0.5°C vs 36.2 ± 0.7°C, P < .001) at 1 hour after birth compared with infants receiving standard care.
CONCLUSIONS:
Placement in a plastic bag at birth reduced the incidence of hypothermia at 1 hour after birth in term neonates born in a resource-poor setting, but most neonates remained hypothermic.
Keywords: infant, term; infant, newborn; infant, hypothermia/prevention and control; plastic bag; bedding and linens; body temperature, regulation; polyethylenes; delivery, obstetrics
What’s Known on This Subject:
Term neonates in resource-poor settings frequently develop hypothermia. Plastic bags or wraps are a low-cost intervention for the prevention of hypothermia in preterm and low birth weight infants that may also be effective in term infants.
What This Study Adds:
For term neonates born in a resource-poor health facility, placement in a plastic bag at birth can reduce the incidence of hypothermia at 1 hour after birth.
Approximately 3 million neonates die annually worldwide.1 Compared with the developed world, neonatal mortality in the developing world is 6 times higher.2 Hypothermia, in particular, is associated with increased neonatal mortality,3,4 and each 1°C decrease in axillary temperature is associated with a 75% increase in neonatal mortality.5 Hypothermia in newborns occurs in all climates, and the highest risk is within the first minutes to hours after birth as the newborn adjusts to the extrauterine environment.5,6
Standard thermoregulation World Health Organization (WHO) guidelines recommend comprehensive measures to prevent hypothermia, including warm delivery rooms, immediate drying, skin-to-skin contact, early breast feeding, postponed bathing and weighing, appropriate clothing and bedding, and warm transportation and resuscitation.7 However, hypothermia still occurs in spite of these techniques, as reported in a large study in rural India in which there was a 49% rate of hypothermia in low birth weight infants and a 43% rate in normal birth weight infants.8
In randomized controlled trials in developed countries, placement of very low birth weight and very preterm infants in occlusive polyethylene plastic bags or wraps decreased hypothermia.9–12 Systematic reviews of randomized controlled trials and historical controlled trials found that occlusive skin wrapping at delivery increases NICU admission temperature and reduces hypothermia in preterm infants.13,14 Plastic bags or wraps aid in thermal regulation by protecting infants from radiant, evaporative, and convective heat loss.15,16 Randomized trials of plastic bag use during the first hour after birth in larger preterm infants showed decreased rates of hypothermia.17–19 It is possible that the addition of the plastic bag to standard WHO thermoregulation care may be an effective intervention to decrease rates of hypothermia in term neonates. The current trial sought to determine if placing term infants in polyethylene plastic bags at birth reduces hypothermia 1 hour after birth.
Methods
Study Design
This randomized controlled trial was conducted at University Teaching Hospital, the tertiary referral center in Lusaka, Zambia. The trial was approved by the institutional review boards of University Teaching Hospital in Lusaka, Zambia, and the University of Alabama at Birmingham (clinicaltrials.gov identifier NCT01604460). Infants born at the hospital with both a gestational age of ≥37 weeks and a birth weight ≥2500 g were eligible for inclusion in the trial. Gestational age was determined by best obstetrical estimate, predominantly last menstrual period. Exclusion criteria included planned admission to the NICU, abdominal wall defect, myelomeningocele, major congenital anomaly, or blistering skin disorder. After arrival in the labor and delivery unit, mothers of potentially eligible infants were identified, and consent for the study was obtained either before delivery or within 10 minutes after birth by 4 of the authors (T.C.B., A.E.T., T.R.M., R.H.K.). Enrollment of participants took place from June through July 2012 and occurred during both day and night shifts.
After parental consent was obtained, infants were randomized by 4 of the authors (T.C.B., A.E.T., T.R.M., R.H.K.) within 10 minutes of birth using a 1:1 allocation parallel design to either a standard thermoregulation protocol or to a standard thermoregulation protocol with placement inside a plastic bag without drying. Sealed sequentially numbered envelopes with a computer-generated random allocation sequence were used for randomization to maintain allocation concealment. Multiple births were randomized individually. The 10-minute window for consent and enrollment allowed the inclusion of infants whose mothers delivered precipitously and infants when more than 1 birth occurred at once.
Control Group
Infants randomized to the control group received standard hospital care based on WHO thermoregulation of the newborn protocol.7 This included warm delivery room efforts, immediate drying, skin-to-skin contact, early and exclusive breast feeding, postponed bathing, bundling, and incubator or radiant warmer if the infant’s condition dictated. Kangaroo care with skin-to-skin, chest-to-chest contact occurred while the cord was clamped and the infant was dried. Infants were then transferred to the nursery at approximately 10 minutes after birth according to local practice. In the nursery, infants were weighed, and an initial axillary temperature was obtained. The infants were swaddled in blankets provided by the mother (usually a terry cloth and a fleece blanket), the head was covered with a hat, and the infants were placed either in an open crib or under a radiant warmer as necessary and available. On admission to the nursery, infants found to have an initial axillary temperature <36.0°C were placed under a radiant warmer for the first hour after birth, and those with temperatures ≥36.0°C were placed in an open crib. A repeat axillary temperature was taken at 1 hour after birth. Using a digital air-temperature thermometer, the room temperature was recorded at the time of each axillary temperature measurement. Efforts to maintain the air temperature included a space heater in each delivery room and one in the nursery.
Intervention Group
The intervention group infants were provided the same care as control infants except that their torso and lower extremities were placed in a clear polyethylene plastic bag (inexpensive [3 cents/bag] 10 × 8 × 24-in, 1.2-mil [thousandth of an inch]-thick nonmedical polyethylene bag) within 10 minutes after birth. The plastic bag was placed against the skin and was secured under the neonate’s arms and around the chest by tightly swaddling the neonate with a terry cloth towel or thin blanket provided by the infant’s mother. Care was exercised to prevent the bag from covering the mouth or nose. The intervention group infants remained in the plastic bag for the first hour after birth. The plastic bags were changed when soiled. Kangaroo care with skin-to-skin, chest-to-chest contact with the mother occurred with the infant remaining in the bag the first few moments after birth before transfer to the nursery.
Outcomes
The primary outcome measure was the presence of hypothermia (<36.5°C axillary temperature) at 1 hour after birth with hypothermia divided into the WHO categories of mild (36.0–36.4°C), moderate (32.0–35.9°C), and severe (<32.0°C) (WHO thermoregulation). The temperature was obtained using a single measurement in 1 axilla with a digital thermometer. Secondary outcome measures included hyperthermia (>38°C at 1 hour after birth) and death (before discharge).
Statistical Analysis
The sample size was calculated based on a predicted 15% prevalence of hypothermia in full-term infants at 1 hour after birth, assuming a lower prevalence of hypothermia than that found in preterm infants during a previous trial at the same hospital.18,20 A sample size of 276 was necessary to detect a 10% absolute risk reduction (67% relative reduction), with a 95% confidence level, 80% power, and continuity correction method.
The baseline characteristics of each group were compared using descriptive statistics. The Student’s t test and χ2 were used to compare continuous and categorical variables, respectively. Fisher’s exact test was used for events with low prevalences. Risk ratio (RR) and confidence intervals (CIs) were determined using contingency tables for risk analysis of the primary outcome. Correlations were calculated using simple linear regressions. All analyses were prespecified except for the exploratory subgroup analysis of neonates normothermic at 15 minutes after birth. P values <.05 were significant, and all tests were 2-tailed. SPSS 17.0 for Windows (IBM SPSS Statistics, IBM Corporation, Chicago, IL) was used to analyze all data by modified intention-to-treat excluding neonates admitted to the NICU during the first hour after birth from the primary outcome analysis.
Results
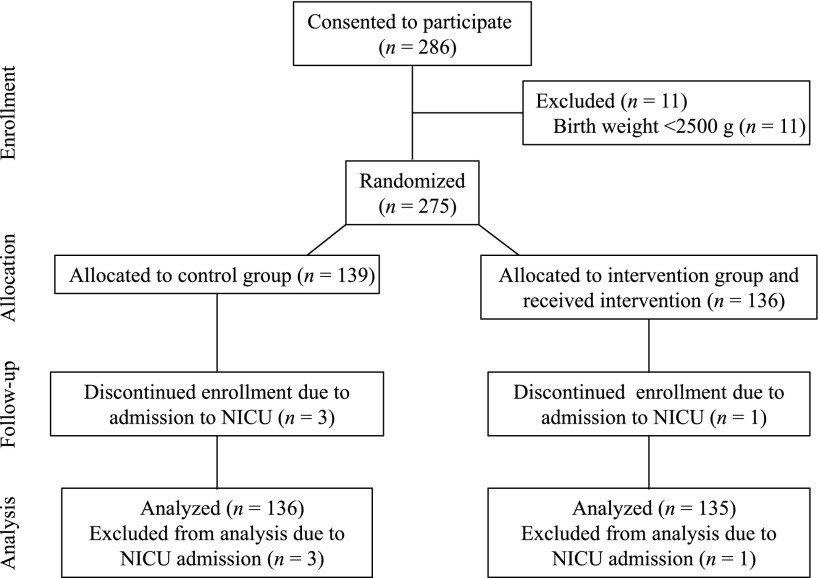
During the cold season from June 20 to July 12, 2012, 136 neonates were enrolled in the intervention group and 139 in the control group (Fig 1). Data from 3 infants (2 in the control and 1 in the intervention group) born after pregnancies initially thought to be term but with an estimated gestational age <37 weeks were included in the analyses. Four enrolled neonates (3 from the control and 1 from the intervention group) were admitted to the NICU before 1 hour after birth because of respiratory distress (n = 3) and hypoglycemia (n = 1) and were excluded from the analyses. Baseline characteristics of the control and intervention groups were similar except that the room temperature within 15 minutes of birth of the control group was higher (P = .023, Table 1).
FIGURE 1.
Flow of participants through enrollment, randomization, and analysis of the study.
TABLE 1.
Baseline Characteristics of the Intervention and Control Groups
| Intervention, n = 135 | Control, n = 136 | |
|---|---|---|
| Birth weight, kg, mean ± SD. | 3.18 ± 0.39 | 3.13 ± 0.43 |
| ≤2.50, n (%)a | 3 (2) | 11 (8) |
| 2.51–3.00, n (%) | 48 (36) | 45 (33) |
| 3.01–3.50, n (%) | 64 (47) | 59 (43) |
| 3.51–4.00, n (%) | 15 (11) | 18 (13) |
| >4.00, n (%) | 5 (4) | 3 (2) |
| Gestational age, wk, mean ± SDb | 39.0 ± 1.4 | 39.0 ± 1.9 |
| <37, n (%) | 1 (1) | 2 (1) |
| 37–38, n (%) | 54 (40) | 54 (40) |
| 39–40, n (%) | 65 (48) | 56 (41) |
| 41–42, n (%) | 13 (10) | 19 (14) |
| >42, n (%) | 2 (1) | 4 (3) |
| Male gender, n (%) | 71 (53) | 68 (50) |
| NICU admission, n (%) | 0 (0) | 1 (1) |
| Room temperature at 15 min, °C, mean ± SDb,c | 27.4 ± 1.5 | 27.8 ± 1.5 |
| Body temperature at 15 min, °C, mean ± SDb | 36.2 ± 0.7 | 36.3 ± 0.7 |
| Hypothermia (<36.5°C) at 15 min, n (%)b | 84 (63) | 81 (60) |
| Body temperature <36°C at 15 min, n (%)b | 44 (33) | 40 (30) |
P = .051.
May reflect as many as 5 missing values.
P = .023.
Neonates placed in a plastic bag had significantly less hypothermia at 1 hour after birth than infants receiving standard care (60% vs 73%, RR 0.76, confidence interval 0.60–0.96, P = .026, Table 2). The intervention group also had higher average axillary temperatures at 1 hour after birth, larger increases in axillary temperature over the first hour after birth, and lower rates of moderate hypothermia (all P < .001, Table 2). None of the infants in the trial developed severe hypothermia. The room temperature at 1 hour after birth did not differ between the intervention and control groups. There was a positive correlation between birth weight and temperature at 1 hour after birth among infants in the control group (R = 0.351, P < .001) but not in the intervention group infants (R = 0.109, P = .206). There was no correlation between the room temperatures and axillary temperatures at 15 minutes after birth or at 1 hour after birth.
TABLE 2.
Outcomes at 1 Hour After Birth
| Intervention, n = 135 | Control, n = 136 | P | RR (95% CI) | |
|---|---|---|---|---|
| Hypothermia (<36.5°C), n (%) | 81 (60) | 99 (73) | .026a | 0.824 (0.69–0.99) |
| Mild hypothermia (36.0–36.4°C), n (%) | 55 (41) | 48 (35) | .357a | 1.15 (0.84–1.60) |
| Moderate hypothermia (32.0–35.9°C), n (%) | 26 (19) | 51 (38) | <.001a | 0.51 (0.33–0.79) |
| Severe hypothermia (<32.0°C), n (%) | 0 | 0 | — | — |
| Temperature at 1 h, °C, mean ± SD. | 36.4 ± 0.5 | 36.2 ± 0.7 | <.001b | — |
| Change in temperature from 15 min to 1 h, °C, mean ± SDc | +0.2 ± 0.7 | −0.1 ± 0.6 | <.001b | — |
CI, confidence interval; NA, .
χ2.
Student’s t test.
Reflects 1 missing value in each group.
A subgroup analysis of infants normothermic (≥36.5°C) at 15 minutes after birth showed similar baseline characteristics (Table 3). There was no difference in the overall development of hypothermia at 1 hour after birth. The intervention group had a lower rate of moderate hypothermia (2% vs 18%, RR 0.17, confidence interval 0.03–1.14, P = .007, Table 3) and smaller decreases in axillary temperature over the first hour after birth (P = .021, Table 3).
TABLE 3.
Normothermic (>36.5°C) Neonates at 15 Minutes After Birth Subgroup Analysis
| Intervention, n = 51 | Control, n = 55 | P | RR (95% CI) | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Birth weight, kg, mean ± SD | 3.18 ± 0.36 | 3.17 ± 0.36 | — | — |
| Gestational age, wk, mean ± SDa | 39.0 ± 1.5 | 39.3 ± 1.9 | — | — |
| Male gender, n (%) | 24 (47) | 23 (42) | — | — |
| NICU admission, n (%) | 0 | 0 | — | — |
| Room temperature at 15 min, °C, mean ± SDa | 27.4 ± 1.4 | 27.9 ± 1.5 | — | — |
| Body temperature at 15 min, °C, mean ± SDa | 36.9 ± 0.3 | 36.9 ± 0.4 | — | — |
| Outcomes at 1 h after birthb | ||||
| Hypothermia (<36.5°C), n (%) | 17 (33) | 25 (45) | .205c | 0.73 (0.43–1.23) |
| Mild hypothermia (36.0–36.4°C), n (%) | 16 (31) | 15 (27) | .644c | 1.15 (0.60–2.22) |
| Moderate hypothermia (32.0–35.9°C), n (%) | 1 (2) | 10 (18) | .008d | 0.11 (0.01–0.76) |
| Severe hypothermia (<32.0°C), n (%) | 0 | 0 | NA | NA |
| Body temperature at 1 h, °C, mean ± SD | 36.7 ± 0.5 | 36.6 ± 0.6 | .106e | — |
| Avg. increase in temperature from 15 min to 1 h, °C, mean ± SDa | −0.2 ± 0.4 | −0.4 ± 0.5 | .021e | — |
CI, confidence interval; NA, .
May reflect as many as 1 missing value.
There was no observed difference in room temperature at 1 h after birth.
χ2.
Fisher’s exact test.
Student’s t test.
One infant in the intervention group developed hyperthermia (38.1°C) but became normothermic soon after removal from the radiant warmer. There were no deaths or other adverse events before discharge. The plastic bag was well accepted by the labor and delivery staff and did not interfere with neonatal resuscitation. Kangaroo care was done usually for less than 10 minutes as mothers were moved from the delivery beds to the recovery area because of the high demand for delivery beds. There were only 4 radiant warmers, which were insufficient at times, and windows were often open in the nursery and delivery rooms. Half of the enrollments occurred at night when the outside air temperature was much cooler. Despite this, including measurements in both the nursery and delivery rooms, the infants were predominantly in rooms within the WHO’s recommended room temperature range (mean 27.4°C, median 27.3°C, range 22.0–32.9°C).7
Discussion
In the current trial, placing neonates in a plastic bag during the first hour after birth reduced hypothermia and increased the axillary temperature when compared with standard thermoregulatory care alone. As shown in trials of preterm very low birth weight and low birth weight infants,10,17,18 there was a positive correlation between birth weight and temperature at 1 hour after birth, but the correlation was weaker in the intervention group, suggesting that the plastic bag has a greater effect on infants with a lower birth weight. Even neonates who were normothermic at 15 minutes after birth benefited from the plastic bags by developing a lower rate of moderate hypothermia and smaller decreases in axillary temperature over the first hour after birth. These data support the plastic bag as an inexpensive, effective, short-term intervention to reduce the rates of hypothermia in term infants that could be introduced in resource-poor settings because of its effectiveness and affordability.
Ideal thermoregulatory care was limited by various limitations in the hospital facilities and care that likely contributed to the high rates of overall hypothermia at 1 hour and the decrease in temperature at 1 hour in the infants who were initially normothermic. The delivery room and nursery were usually within the recommended minimal temperature range of 25 to 28°C,7 but other standard thermoregulatory practices were difficult to follow. The limited use of kangaroo care, insufficient radiant warmers, and air currents created by the open windows likely contributed to the high rate of hypothermia overall and drop in temperature of initially normothermic infants. Despite these difficulties, use of a plastic bag significantly decreased hypothermia, suggesting its applicability and effectiveness in less than ideal conditions. In resource-poor settings there is likely to be some delay before an infant is placed in a plastic bag, and the 10-minute window for placement in a plastic bag included in this trial shows that even with a delay, the plastic bag is effective at reducing hypothermia.
The average birth weight of the intervention group was 50 g heavier than that of the control group, potentially explaining a portion of the lower rate of hypothermia at 1 hour after birth seen in the intervention group. But because a categorical analysis of the birth weights shows no difference and there is no difference between the axillary temperatures at 15 minutes after birth, we believe that the 2 groups are comparable and the plastic bag is the primary reason for the lower rate of hypothermia seen in the intervention group at 1 hour after birth.
All infants are at increased risk for hypothermia because of their high body surface area–to-volume ratio. Although the incidence of hypothermia is lower in term infants than in preterm infants, term infants also experience hypothermia in settings where climate control is difficult.8,18,21,22 A study in Nepal found 46% of term infants (n = 353) to be hypothermic (<36°C) 24 hours after birth.21 Similarly, a study in Uganda showed that 79% of the 300 newborns (64% were >2500 g) became hypothermic (<36.5°C) 90 minutes after birth.22 The current trial showed a similar rate of hypothermia (73%) in infants in the control group receiving standard thermoregulatory care. Thus, term infants are at high risk for hypothermia in these settings, and the current trial indicates that term infants benefit from placement in plastic bags as also shown in preterm infants.
An earlier trial at the study site involving preterm infants had a lower rate of hypothermia in the control group than that seen in the current study (59% to 73%).18 Besides the current study occurring during a colder month and enrolling more infants at night, the difference in the rate of hypothermia may be because of the labor and delivery staff being more vigilant to prevent hypothermia in preterm infants. Most of the infants in the current trail had mild hypothermia; however, a previous report from this study site reported moderate rates of hypothermia of 44% in 0- to 7-day neonates admitted to the NICU.20
The plastic bag intervention is easy to implement, as it is inexpensive (3 cents/bag and ∼1 bag/infant) and readily accepted by the labor and delivery staff after demonstrating that term infants frequently develop hypothermia. Folding the bag underneath the infant’s axillae, leaving the arms outside of the bag, and tightly swaddling the infant reduce the possibility of suffocation. Mild hyperthermia occurred only once, but may be a risk.
In summary, in resource-poor settings where the availability of radiant warmers and incubators may be limited, plastic bags may be useful tools in reducing the rates of hypothermia. Further trials are needed to determine whether this low-cost intervention reduces morbidity and mortality associated with hypothermia.
Acknowledgments
We thank Monica Collins, RN, Med, and Becky Brazeel, CPS, CAP, from the University of Alabama at Birmingham, and the nurses and midwives from the University Teaching Hospital in Lusaka for their help with this project.
Glossary
- CI
confidence interval
- RR
risk ratio
- WHO
World Health Organization
Footnotes
Mr Belsches conceptualized and designed the study, coordinated and supervised data collection, and carried out the initial analyses; Ms Tilly assisted in study design, coordinated and supervised data collection, and drafted the initial manuscript; Ms Miller assisted in study design and data collection; Mr Kambeyanda conceptualized and designed the study and assisted in data collection; Drs Leadford and Carlo conceptualized and designed the study; Drs Manasyan and Chomba assisted in study design; Drs Ramani and Ambalavanan assisted in study design and performed the statistical analyses; and all authors reviewed and revised the manuscript, and approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifier NCT 01604460).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported in part by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Global Network for Women’s and Children’s Health Research (U01HD043464), the Perinatal Health and Human Development Research Program of the University of Alabama at Birmingham, and the Children’s of Alabama Centennial Scholar Fund. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr Carlo is on the Board of Directors of Mednax, Inc; the other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Liu L, Johnson HL, Cousens S, et al. Child Health Epidemiology Reference Group of WHO and UNICEF . Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–2161 [DOI] [PubMed] [Google Scholar]
- 2.You D, Jones G, Wardlaw T, United Nations Inter-agency Group for Child Mortality Estimation Levels and Trends in Child Mortality: Estimates Developed by the UN Inter-agency Group for Child Mortality Estimation. New York, NY: UNICEF; 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sodemann M, Nielsen J, Veirum J, Jakobsen MS, Biai S, Aaby P. Hypothermia of newborns is associated with excess mortality in the first 2 months of life in Guinea-Bissau, West Africa. Trop Med Int Health. 2008;13(8):980–986 [DOI] [PubMed] [Google Scholar]
- 4.Shah S, Zemichael O, Meng HD. Factors associated with mortality and length of stay in hospitalised neonates in Eritrea, Africa: a cross-sectional study. BMJ Open. 2012;2(5):e000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullany LC, Katz J, Khatry SK, LeClerq SC, Darmstadt GL, Tielsch JM. Risk of mortality associated with neonatal hypothermia in southern Nepal. Arch Pediatr Adolesc Med. 2010;164(7):650–656 [DOI] [PubMed] [Google Scholar]
- 6.Klaus MH, Fanaroff AA. Care of the High-Risk Neonate. 5th ed. St. Louis, MO: W. B. Saunders Company; 2001 [Google Scholar]
- 7.World Health Organization Thermal Protection of the Newborn: A Practical Guide. Geneva, Switzerland: World Health Organization; 1997 [Google Scholar]
- 8.Darmstadt GL, Kumar V, Yadav R, et al. Introduction of community-based skin-to-skin care in rural Uttar Pradesh, India. J Perinatol. 2006;26(10):597–604 [DOI] [PubMed] [Google Scholar]
- 9.Vohra S, Frent G, Campbell V, Abbott M, Whyte R. Effect of polyethylene occlusive skin wrapping on heat loss in very low birth weight infants at delivery: a randomized trial. J Pediatr. 1999;134(5):547–551 [DOI] [PubMed] [Google Scholar]
- 10.Vohra S, Roberts RS, Zhang B, Janes M, Schmidt B. Heat Loss Prevention (HeLP) in the delivery room: a randomized controlled trial of polyethylene occlusive skin wrapping in very preterm infants. J Pediatr. 2004;145(6):750–753 [DOI] [PubMed] [Google Scholar]
- 11.Carroll PD, Nankervis CA, Giannone PJ, Cordero L. Use of polyethylene bags in extremely low birth weight infant resuscitation for the prevention of hypothermia. J Reprod Med. 2010;55(1-2):9–13 [PubMed] [Google Scholar]
- 12.Knobel RB, Wimmer JE, Jr, Holbert D. Heat loss prevention for preterm infants in the delivery room. J Perinatol. 2005;25(5):304–308 [DOI] [PubMed] [Google Scholar]
- 13.Cramer K, Wiebe N, Hartling L, Crumley E, Vohra S. Heat loss prevention: a systematic review of occlusive skin wrap for premature neonates. J Perinatol. 2005;25(12):763–769 [DOI] [PubMed] [Google Scholar]
- 14.McCall EM, Alderdice F, Halliday HL, Jenkins JG, Vohra S. Interventions to prevent hypothermia at birth in preterm and/or low birthweight infants. Cochrane Database Syst Rev. 2010;(3):CD004210. [DOI] [PubMed] [Google Scholar]
- 15.Baumgart S, Engle WD, Fox WW, Polin RA. Effect of heat shielding on convective and evaporative heat losses and on radiant heat transfer in the premature infant. J Pediatr. 1981;99(6):948–956 [DOI] [PubMed] [Google Scholar]
- 16.Baumgart S. Reduction of oxygen consumption, insensible water loss, and radiant heat demand with use of a plastic blanket for low-birth-weight infants under radiant warmers. Pediatrics. 1984;74(6):1022–1028 [PubMed] [Google Scholar]
- 17.Rohana J, Khairina W, Boo NY, Shareena I. Reducing hypothermia in preterm infants with polyethylene wrap. Pediatr Int. 2011;53(4):468–474 [DOI] [PubMed] [Google Scholar]
- 18.Leadford AE, Warren JB, Manasyan A, et al. Plastic bags for prevention of hypothermia in preterm and low birth weight infants. Pediatrics. 2013;132(1). Available at: www.pediatrics.org/cgi/content/full/132/1/e128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chantaroj S, Techasatid W. Effect of polyethylene bag to prevent heat loss in preterm infants at birth: a randomized controlled trial. J Med Assoc Thai. 2011;94(suppl 7):S32–S37 [PubMed] [Google Scholar]
- 20.Christensson K, Bhat GJ, Eriksson B, Shilalukey-Ngoma MP, Sterky G. The effect of routine hospital care on the health of hypothermic newborn infants in Zambia. J Trop Pediatr. 1995;41(4):210–214 [DOI] [PubMed] [Google Scholar]
- 21.Johanson RB, Spencer SA, Rolfe P, Jones P, Malla DS. Effect of post-delivery care on neonatal body temperature. Acta Paediatr. 1992;81(11):859–863 [DOI] [PubMed] [Google Scholar]
- 22.Byaruhanga R, Bergstrom A, Okong P. Neonatal hypothermia in Uganda: prevalence and risk factors. J Trop Pediatr. 2005;51(4):212–215 [DOI] [PubMed] [Google Scholar]