Abstract
BACKGROUND:
After licensure of the 7-valent pneumococcal conjugate vaccine (PCV7) in the United States in 2000, the incidence of pediatric pneumococcal meningitis decreased significantly. However, cases continue to occur. It is unknown whether meningitis due to PCV7 and non-PCV7 serotypes causes similar morbidity and mortality.
METHODS:
We performed a retrospective cohort study of laboratory-confirmed pneumococcal meningitis among Utah children from 1997 to 2010. We reviewed medical records and obtained clinical data during the acute illness and follow-up data on neurologic sequelae.
RESULTS:
Sixty-eight cases of meningitis were identified. PCV7 serotypes caused 64% of cases before and 25% of cases after licensure of PCV7 (P < .01). The age range was similar before and after PCV7 licensure (P = .5). The overall case fatality rate was 13% and was similar among cases caused by PCV7 and non-PCV7 serotypes (P = .7). Children with PCV7 serotypes were more likely to require mechanical ventilation (68% vs 34%; P < .01). Of all survivors, 63% had neurologic sequelae, and the proportion was similar after infection with PCV7 or non-PCV7 serotypes (P = .1). More than one-half (54%) of all children who developed pneumococcal meningitis in the PCV7 period were eligible for PCV7 and had not been immunized.
CONCLUSIONS:
Pneumococcal meningitis continues to be associated with high mortality and morbidity; death and neurologic sequelae are common with both PCV7 and non-PCV7 serotype meningitis. The substantial burden of this disease and continued cases among unimmunized children reinforce the need for more effective immunization strategies and continued surveillance in the era of PCV13.
Keywords: bacterial meningitis, children, neurologic sequelae, Streptococcus pneumoniae
What’s Known on This Subject:
The incidence of pediatric pneumococcal meningitis has declined after introduction of the 7-valent pneumococcal conjugate vaccine (PCV7). It is unknown whether the frequency of severe neurologic sequelae and adverse outcomes has changed in the era of widespread PCV7 use.
What This Study Adds:
Pneumococcal meningitis continues to be associated with substantial mortality and long-term morbidity. Sixty-three percent of survivors had neurologic sequelae. More than one-half of the children who were eligible for PCV7 were unimmunized at the time that they developed pneumococcal meningitis.
Bacterial meningitis in children is associated with significant morbidity and mortality worldwide. In countries in which the Haemophilus influenzae type B vaccine is widely used, Streptococcus pneumoniae is the most common cause of bacterial meningitis in young children.1–5
The epidemiology of invasive pneumococcal disease in the United States changed after licensure of the 7-valent pneumococcal conjugate vaccine in 2000 (PCV7; Wyeth Lederle Vaccines/Pfizer; Philadelphia, PA).6 National guidelines recommend that children receive 4 doses of PCV7 at 2, 4, 6, and 12 to 15 months of age.7 Increasing immunization rates have been associated with marked reductions in the incidence of pneumococcal meningitis.8 The Centers for Disease Control and Prevention’s Active Bacterial Core surveillance sites reported a 62% decrease in the incidence of pneumococcal meningitis among children aged <2 years in 2006 and 2007 compared with 1998 and 1999.5 In Utah, the incidence of pneumococcal meningitis in this age group decreased by 52% between 1996–2000 and 2006–2009.9 The decrease in pneumococcal meningitis was primarily due to a decrease in PCV7 serotype disease. Before the introduction of PCV7, 64% of pneumococcal meningitis was attributable to PCV7 serotypes in Utah. However, from 2001 to 2010, PCV7 serotypes caused 22% of pneumococcal meningitis.10
Pneumococcal meningitis is frequently associated with poor neurologic outcomes as a consequence of cortical and subcortical injury. In 2 studies of bacterial meningitis before the widespread use of PCV7, neurologic sequelae associated with S pneumoniae were reported in 20% to 40% of children at the time of hospital discharge.11,12 It is unknown whether the frequency of severe neurologic sequelae and adverse outcomes has changed in the era of widespread PCV7 use as a consequence of the change in infecting serotypes. Our objective in the current study was to describe the epidemiology, serotypes, clinical outcomes, and sequelae of culture-confirmed pediatric pneumococcal meningitis before and after PCV7 licensure in Utah. These data have the potential to inform our understanding of how pneumococcal meningitis may evolve in the years after licensure of the 13-valent pneumococcal conjugate vaccine (PCV13) and aid in identifying targets for future pneumococcal vaccine research.
Methods
Human Subjects Protection
This study was approved and granted a waiver of informed consent by the institutional review boards of the University of Utah and Primary Children’s Medical Center (PCMC).
Setting
This study was performed at PCMC in Salt Lake City, Utah. PCMC is a children’s hospital that has grown from 207 beds in 1997 to 289 beds in 2010. PCMC serves as the community hospital for northern Utah and as a referral center for southern Utah, Idaho, Wyoming, Nevada, and Montana. Over the study period, 75% to 85% of all pediatric admissions in Utah were to PCMC; the proportion was higher for severe disease (J. Bradshaw, MPA, personal communication, 2012).
Identification of Children With Pneumococcal Meningitis
The study was divided into 2 periods: before licensure of PCV7 (pre-PCV7 period; January 1997–December 2000) and after licensure of PCV7 (PCV7 period; January 2001–December 2010). Pneumococcal meningitis cases were defined as isolation of S pneumoniae from a cerebrospinal fluid culture or the presence of cerebrospinal fluid pleocytosis (>10 cells/µL) in conjunction with isolation of S pneumoniae from blood in a child aged <18 years.13 PCMC has archived all pneumococcal isolates from children with invasive pneumococcal disease since 1996. Pneumococcal serotyping was performed (by Dr Mason) with the use of previously described capsular swelling methods.14
Medical Record Review
We reviewed the paper and electronic medical records of children with culture-confirmed pneumococcal meningitis cared for at PCMC. Measures of severity were collected; these included symptom duration, admission to the PICU, mechanical ventilation, total hospital length of stay, and death. The Utah Statewide Immunization Information System (https://apps.usiis.org/) was used to document PCV7 vaccination status at the time of hospitalization for pneumococcal meningitis. Children were considered to be fully immunized if they had received an age-appropriate number of doses, based on recommendations from the Advisory Committee on Immunization Practices, before their hospitalization.7
Neurologic outcomes of survivors were determined by manual review of the medical records for data associated with discharge, consultation with the neurology service, and any subsequent encounters in the Intermountain Healthcare system. All consulting pediatric neurologists involved in the care of these patients use this medical record system. To be consistent with previous literature,15 neurologic sequelae were defined as new evidence of cortical blindness, hydrocephalus, institutionalization, microcephaly, quadriplegia, severe developmental delay, seizures at 1 year postinfection, ataxia, sensorineural hearing loss, hemiparesis, hyperactivity, cranial nerve palsy, or cochlear implantation. The assessment of neurologic sequelae was performed by a pediatric neurologist (Dr Filloux) who was blinded to the results of pneumococcal serotype testing. For children with pre-existing neurologic conditions (n = 7), clinical judgment was used to determine whether abnormal neurologic findings noted at discharge or follow-up reflected sequelae of pneumococcal meningitis or previous impairment.
Statistical Analyses
We compared children with pneumococcal meningitis due to PCV7 serotypes versus children with pneumococcal meningitis caused by non-PCV7 serotypes. We also compared children who developed pneumococcal meningitis during the pre-PCV7 period of 1997–2000 versus those who developed meningitis during the PCV7 2001–2010 period. Lastly, during the PCV7 period, we compared children who were immunized with PCV7 versus those who were unimmunized. Categorical data were compared by means of the χ2 test or Fisher’s exact test, as appropriate. Continuous variables are reported as the median and interquartile range (IQR) and were compared by using the Wilcoxon rank-sum test. The age-specific incidence of pneumococcal meningitis was calculated by using data from Utah resident children with meningitis and population estimates from the Utah Population Estimates Committee and the Governor’s Office of Planning and Budget.16 Exact 95% confidence intervals (CIs) were calculated from a Poisson distribution for point estimates of the incidence of pneumococcal meningitis before and after PCV7 licensure. Statistical significance was set at P = .05, and all reported comparisons are 2-tailed. Analyses were performed by using Stata 11.2 (Stata Corp, College Station, TX).
Results
Incidence of Pneumococcal Meningitis and Vaccine Coverage
During the study period, 68 children with culture-confirmed pneumococcal meningitis were cared for at PCMC (22 before PCV7 licensure and 46 after). The incidence of pneumococcal meningitis decreased 26% from 0.78 case per 100 000 children (95% CI: 0.49–1.17) before PCV7 licensure to 0.57 case per 100 000 (95% CI: 0.42–0.77) after; however, this finding was not statistically significant (P = .3). Among children aged 0 to 23 months, the incidence decreased 29% from 6.26 cases per 100 000 children (95% CI: 3.92–9.48) to 4.47 per 100 000 (95% CI: 3.27–5.96). The decrease in pneumococcal meningitis was driven by a 72% decrease in meningitis due to PCV7 serotypes (0.49 per 100 000 [95% CI: 0.27–0.83] to 0.14 per 100 000 [95% CI: 0.07–0.25]). The incidence of meningitis due to non-PCV7 serotypes increased by 46% from 0.28 per 100 000 (95% CI: 0.12–0.56) to 0.41 per 100 000 (95% CI: 0.28–0.58).
The median age at diagnosis was 9 months (range: 6 days–16 years). The median age was 11 months before PCV7 licensure and 9 months after (P = .5). A comparison of demographic characteristics and PCV7 vaccination status according to PCV7 serotypes and non-PCV7 serotypes are presented in Table 1.
TABLE 1.
Demographic Characteristics and Vaccination Status of Children With Culture-Confirmed Pneumococcal Meningitis Caused by PCV7 and Non-PCV7 Serotypes From 1997–2010 in Utah
| Characteristic | PCV7 Serotypes (n = 25) | Non-PCV7 Serotypes (n = 41) | P |
|---|---|---|---|
| Age, mo | |||
| Median (IQR) | 13 (5–29) | 9 (3–76) | .400 |
| Age group, n (%) | |||
| <6 mo | 7 (28) | 19 (46) | .139 |
| 6–23 mo | 10 (40) | 9 (22) | .116 |
| 2–4 y | 3 (12) | 1 (2) | .148 |
| 5–9 y | 3 (12) | 5 (12) | 1.000 |
| 10–17 y | 2 (8) | 7 (17) | .464 |
| Male, n (%) | 17 (68) | 23 (56) | .337 |
| Race/ethnicity, n (%) | |||
| White | 18 (72) | 33 (80) | .425 |
| Black | 1 (4) | 0 (0) | .379 |
| Hispanic | 6 (24) | 3 (7) | .072 |
| Asian/Pacific Islander | 0 (0) | 4 (10) | .289 |
| Unknown | 0 (0) | 1 (2) | 1.000 |
| PCV7 vaccination status | |||
| No. eligible for PCV7 | 8 | 19 | .250 |
| Immunization records unavailable, n (%) | 1 (13) | 1 (5) | .513 |
| Not immunized, n (%) | 6 (75) | 9 (48) | .236 |
| Complete, n (%) | 2 (25) | 10 (53) | .236 |
Of 46 children with pneumococcal meningitis during the PCV7 period, 28 (61%) were eligible for PCV7 according to the American Academy of Pediatrics’ recommendations.7,17 The remainder were either too young to receive vaccine or were too old to be included in recommendations for catch-up immunization. Two (7%) children’s immunization records were unavailable for review. Of the remaining 26 (93%) children with complete immunization records, 12 (46%) had received an age-appropriate number of PCV7 doses. However, 14 (54%) children who developed pneumococcal meningitis in the PCV7 period were eligible for PCV7 and had not been immunized. Five (36%) of these children developed PCV7 serotype meningitis.
Four children developed pneumococcal meningitis after the approval of PCV13 in February 2010. All were unimmunized and had received no doses of either PCV7 or PCV13.
Serotype Distribution Before and After PCV7 Licensure
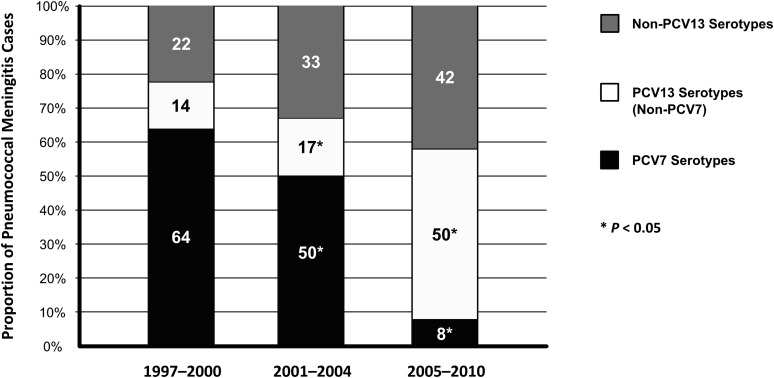
Sixty-six (97%) of 68 pneumococcal isolates were serotyped, and 27 different serotypes were identified (Table 2). In the pre-PCV7 period, 10 different serotypes were isolated from children with pneumococcal meningitis; 64% of these were PCV7 serotypes and 14% were the additional 6 pneumococcal serotypes included in PCV13 (Fig 1). The most common serotypes in the pre-PCV7 period were 14 (27% of isolates), 19F (18%), 6A (9%), and 22F (9%).
TABLE 2.
Serotypes Associated With Pediatric Pneumococcal Meningitis in Utah During the Pre-PCV7 and PCV7 Periods
| Serotype | Pre-PCV7 Perioda (n = 22) | PCV7 Period,a Unimmunized (n = 28) | PCV7 Period,a ≥1 Dose of PCV7 (n = 16) |
|---|---|---|---|
| PCV7 | |||
| Any PCV7 serotype | 14 (64) | 10 (36) | 2 (14) |
| 4 | 0 (0) | 1 (4) | 0 (0) |
| 6B | 1 (5) | 1 (4) | 0 (0) |
| 9V | 0 (0) | 0 (0) | 0 (0) |
| 14 | 6 (27) | 3 (11) | 0 (0) |
| 18 | 0 (0) | 1 (4) | 0 (0) |
| 18C | 1 (5) | 3 (11) | 0 (0) |
| 19F | 4 (18) | 1 (4) | 2 (13) |
| 23F | 2 (9) | 0 (0) | 0 (0) |
| PCV13 | |||
| Any PCV13 serotype | 3 (14) | 8 (29) | 7 (50) |
| 1 | 0 (0) | 0 (0) | 0 (0) |
| 3 | 0 (0) | 2 (7) | 0 (0) |
| 5 | 0 (0) | 0 (0) | 0 (0) |
| 6A | 2 (9) | 1 (4) | 0 (0) |
| 7F | 1 (5) | 5 (18) | 5 (31) |
| 19A | 0 (0) | 0 (0) | 3 (19) |
| Non-PCV | |||
| Any non-PCV13 serotype | 5 (23) | 10 (36) | 5 (36) |
| 2 | 1 (5) | 0 (0) | 0 (0) |
| 6C | 0 (0) | 1 (4) | 0 (0) |
| 8 | 0 (0) | 0 (0) | 1 (6) |
| 9N | 0 (0) | 0 (0) | 1 (6) |
| 10 | 0 (0) | 0 (0) | 0 (0) |
| 12 | 1 (5) | 0 (0) | 0 (0) |
| 15 | 0 (0) | 0 (0) | 1 (6) |
| 15A | 0 (0) | 1 (4) | 0 (0) |
| 16 | 0 (0) | 0 (0) | 1 (6) |
| 17 | 0 (0) | 1 (4) | 0 (0) |
| 20 | 0 (0) | 1 (4) | 0 (0) |
| 22F | 2 (9) | 3 (11) | 1 (6) |
| 23B | 0 (0) | 1 (4) | 0 (0) |
| 29, 35, 42 | 0 (0) | 1 (4) | 0 (0) |
| 33 | 0 (0) | 0 (0) | 1 (6) |
| NT | 1 (5) | 1 (4) | 0 (0) |
Data are presented as n (%).
Pre-PCV7 period spans 1997 to 2000, and the PCV7 period ranged from 2001 to 2010.
FIGURE 1.
Proportion of pneumococcal meningitis cases according to PCV serotype status and time period. Asterisks denote a significant decrease (P < .05) in PCV7 serotype meningitis and a significant increase in PCV13 serotype meningitis.
After PCV7 licensure, 24 different serotypes were isolated. The proportion of pneumococcal meningitis cases attributable to PCV7 serotypes fell to 25% (P < .01), and the proportion attributable to the additional 6 pneumococcal serotypes in PCV13 increased to 36% (P = .06). During this period, serotypes 7F (23%), 22F (9%), 19A (7%), and 14 (7%) were the most common.
Two (14%) children developed PCV7-serotype pneumococcal meningitis (serotype 19F) despite being completely immunized with PCV7. Serotypes 7F (29%) and 19A (21%) were also commonly isolated (21%) from immunized children. Of 28 unimmunized children in the PCV7 period, the most common serotypes were also non-PCV7 serotypes, which included serotypes 7F (18%) and 22F (11%). Nine unimmunized children (32%) developed PCV7-serotype pneumococcal meningitis.
Clinical Outcomes by PCV7 and Non-PCV7 Serotypes
All children with meningitis were admitted to PCMC; 58 (85%) were cared for in the ICU, and 32 (47%) required mechanical ventilatory support. Nine (13%) children died. The median hospital length of stay was 12 days (IQR: 7–19).
To test the hypothesis that the clinical characteristics of pneumococcal meningitis changed with the change in serotypes, we compared clinical outcomes among children with PCV7 and non-PCV7 serotype meningitis (Table 3). The proportion that required mechanical ventilation was higher among children infected with PCV7 serotypes (68% vs 34%; P < .01). However, other clinical outcomes, including the case fatality rate (16% vs 12%; P = .7), were similar among children with pneumococcal meningitis caused by PCV7 and non-PCV7 serotypes. We could not detect an association between mortality and individual serotypes (P > .5 for all).
TABLE 3.
Clinical Outcomes of Children With Culture-Confirmed PCV7 and Non-PCV7 Pneumococcal Meningitis in Utah
| Characteristic | PCV7 Serotypes | Non-PCV7 Serotypes | P |
|---|---|---|---|
| PICU admission | |||
| All children <18 y | 23/25 (92%) | 33/41 (80%) | .297 |
| Children <2 y | 15/17 (88%) | 22/28 (79%) | .690 |
| Mechanical ventilation | |||
| All children <18 y | 17/25 (68%) | 14/41 (34%) | .008 |
| Children <2 y | 11/17 (65%) | 11/28 (39%) | .098 |
| Death | |||
| All children <18 y | 4/25 (16%) | 5/41 (12%) | .721 |
| Children <2 y | 3/17 (18%) | 4/28 (14%) | 1.000 |
| Total hospital length of stay, days | |||
| All children <18 y | .777 | ||
| Median | 15 | 12 | |
| IQR | 10–20 | 7–18 | |
| Children <2 y | .807 | ||
| Median | 12 | 12.5 | |
| IQR | 8–18 | 8.5–18 |
Neurologic Sequelae
Seizures during the acute meningitis episode occurred among 48 (71%) of 68 children. Data on sequelae were obtained for 59 (100%) survivors. The median duration of follow-up was 3.1 years (IQR: 1.3–9.2). A new neurologic impairment was reported in 37 (63%) survivors. Developmental delay of any degree was the most common long-term sequelae, detected in 43% of children. New-onset seizure disorders, defined as the receipt of seizure medications or documented seizures at ≥12 months’ postinfection, were diagnosed in 19 (32%) of 59 survivors. Sensorineural hearing loss was documented in 17 children (29%). Eleven (65%) with hearing loss were fitted with and use cochlear implants.
The frequencies of individual neurologic sequelae according to age group are presented in Table 4. Age, gender, and race were not significantly associated with the development of a new neurologic impairment. However, sensorineural hearing loss was more common among children aged <24 months (41% vs 11%; P = .02).
TABLE 4.
Neurologic Sequelae of Culture-Confirmed Pneumococcal Meningitis in Utah Children
| Sequelaea | <2 y (n = 39) | 2–9 y (n = 11) | 10–17 y (n = 9) |
|---|---|---|---|
| Severe developmental delay | 18 (46) | 4 (36) | 1 (13) |
| Sensorineural hearing loss | 15 (38) | 2 (18) | 0 (0) |
| Persistent seizures at 1 year | 13 (33) | 6 (55) | 0 (0) |
| Cochlear implant | 9 (23) | 2 (18) | 0 (0) |
| Cranial nerve palsy | 9 (23) | 3 (27) | 2 (22) |
| Hydrocephalus | 8 (21) | 0 (0) | 0 (0) |
| Quadriplegia | 7 (18) | 0 (0) | 0 (0) |
| Cortical blindness | 6 (15) | 1 (9) | 0 (0) |
| Hemiparesis | 4 (10) | 1 (9) | 2 (22) |
| Ataxia | 2 (5) | 2 (18) | 1 (13) |
| Hyperactivity | 2 (5) | 2 (18) | 0 (0) |
| Microcephaly | 2 (5) | 0 (0) | 0 (0) |
| Any neurologic sequelae, including hearing loss and/or behavioral impairment | 26 (67) | 8 (73) | 3 (33) |
Data are presented as n (%).
Calculated for survivors only (n = 59).
Neurologic sequelae were diagnosed at discharge and/or follow-up.
Neurologic sequelae were reported among 16 (76%) survivors infected with PCV7 serotypes and 20 (56%) children infected with non-PCV7 serotypes (P = .1). Overall, there was no difference in the proportion of children who developed neurologic sequelae according to PCV7 immunization status (P = .7). However, cortical blindness (P = .07), hemiparesis (P = .09), and seizures >1 year after infection (P = .08) were more often associated with PCV7 serotypes (Table 5). Of the 14 unimmunized children who were eligible for PCV7, 5 had disease due to PCV7 serotypes and 4 (80%) had neurologic sequelae.
TABLE 5.
Neurologic Sequelae of Culture-Confirmed Pneumococcal Meningitis in Utah Children According to PCV7 Serotype Status
| Sequelaea | PCV7 Serotypes (n = 21)a | Non-PCV7 Serotypes (n = 36)a | P |
|---|---|---|---|
| Cortical blindness | 0 (0) | 6 (17) | .07 |
| Hydrocephalus | 4 (19) | 4 (11) | .4 |
| Institutionalization | 0 (0) | 1 (3) | .5 |
| Microcephaly | 1 (5) | 1 (3) | .7 |
| Quadriplegia | 1 (5) | 6 (17) | .2 |
| Severe developmental delay | 10 (48) | 13 (36) | .6 |
| Persistent seizures at 1 year | 10 (48) | 9 (25) | .08 |
| Ataxia | 3 (14) | 2 (6) | .3 |
| Sensorineural hearing loss | 4 (19) | 13 (36) | .2 |
| Hemiparesis | 5 (24) | 2 (6) | .05 |
| Hyperactivity | 3 (14) | 1 (3) | .09 |
| Cranial nerve palsy | 4 (19) | 9 (25) | .6 |
| Cochlear implant | 2 (10) | 9 (25) | .2 |
| Any neurologic sequelae, including hearing loss and/or behavioral impairment | 16 (76) | 20 (56) | .1 |
Data are presented as n (%).
Calculated for survivors with serotyping data available (n = 57).
Neurologic sequelae were diagnosed at discharge and/or follow-up.
Discussion
Licensure of PCV7 was associated with a 72% decrease in the incidence of pneumococcal meningitis attributable to PCV7 serotypes among children in Utah. More than one-half (54%) of all children who developed pneumococcal meningitis in the PCV7 period were eligible for PCV7 and had not been immunized. The case fatality rate was 13% among children with pneumococcal meningitis, and 63% of survivors developed a new neurologic impairment at discharge or follow-up. Overall severity, morbidity, and mortality during the acute phase were similar among children with PCV7 and non-PCV7 serotype meningitis. However, mechanical ventilation and some severe neurologic sequelae, including cortical blindness, hemiparesis, and continued seizure disorder, were slightly more common with meningitis due to PCV7 serotypes, although these did not reach the level of statistical significance.
Our finding of a decrease in meningitis due to PCV7 serotypes in all age groups is in accord with earlier reports from the 8 sites of the Centers for Disease Control and Prevention’s Active Bacterial Core surveillance program.8,13,18 Bingen et al19 also reported a 28% decline in the number of children aged 2 to 24 months with pneumococcal meningitis in France after PCV7 licensure. Similar to our study, the overall decrease in pneumococcal meningitis was associated with a modest increase in the incidence of disease caused by non-PCV7 serotypes; Bingen et al observed a 68% decrease in PCV7 serotype disease and a 16% to 31% increase in non-PCV7 serotype disease.
More than one-half of the study children who were eligible for PCV7 were unimmunized at the time that they developed pneumococcal meningitis, representing a tragic missed opportunity. In the United States, undervaccination due to parental choice is an increasing trend that has been associated with adverse health outcomes.20 In a case-control study, Whitney et al21 demonstrated that PCV7 was 96% effective against pneumococcal meningitis caused by a vaccine serotype among healthy children. A recent study found that children of parents who declined immunization with PCV7 were 6.5 times more likely to be hospitalized for invasive pneumococcal disease or lobar pneumonia, when compared with a cohort of immunized age-matched controls.22 When parents have been asked why they choose not to immunize their children, many state that their children are “not at risk for vaccine-preventable diseases” and that “vaccine-preventable diseases are not dangerous.”22,23 In stark contrast, our data show that pediatric pneumococcal meningitis is frequently associated with potentially devastating neurologic sequelae and a substantial risk of death. The severity of these outcomes underscores the important role that physicians have in helping to educate parents about the decision to immunize their children. These findings also highlight the public health imperative to foster community-based advocacy and immunization efforts.
We documented an overall case fatality rate of 13%, which is slightly higher than has been reported elsewhere in the United States (8%), Europe (8%), and Australia (9%).24–26 This finding may be due in part to delayed hospital presentation because of the large geographic referral area. PCMC’s catchment area is 400 000 square miles, and distance may result in delayed presentation. Our case fatality rate remained stable over time and did not significantly change after the introduction and widespread use of PCV7. The case fatality rate was also similar among children with PCV7 and non-PCV7 serotype meningitis. Jansen et al27 demonstrated that certain serotypes were independently associated with increased case fatality rates among adults in the Netherlands. In a pediatric population, Rückinger et al28 reported that serotype 7F infection had the highest case fatality rate (15%) and accounted for an increased risk of severe and fatal outcomes compared with other S pneumoniae serotypes. Interestingly, serotype 7F (17% of all isolates) was the most common serotype causing pediatric pneumococcal meningitis in Utah. Nine (90%) of 10 children with this serotype were admitted to the ICU, although none died.
We found that two-thirds of children who survived an episode of pneumococcal meningitis were diagnosed with a new neurologic impairment at discharge or follow-up. This high proportion of neurologic sequelae among children is similar to the proportion of sequelae reported among adults with pneumococcal meningitis (54%–75%).29,30 Previous pediatric studies have reported neurologic sequelae in 20% to 40% of children with bacterial meningitis.11,25,31 The higher incidence of neurologic sequelae documented in this cohort of children may be due to our ability to capture long-term follow-up data (median follow-up period: 3.1 years) through the shared electronic data warehouse of Intermountain Healthcare, which is used by all consulting pediatric neurologists for these patients. Developmental delay was documented in 37% of survivors, persistent seizures in 31%, and hearing loss in 29%. These data remind us of the significant human and economic burden of pneumococcal meningitis.
The current study has several limitations. First, our study was performed retrospectively, and the availability of clinical records may influence our results. Second, because we captured only 75% to 85% of Utah pediatric hospitalizations (although likely a higher proportion of hospitalizations for severe illness), our incidence estimates may be conservative. Third, vaccination records were not complete for all subjects in the post-PCV7 period, although 94% of subjects had immunization records available for review. Among children with immunization records available, it is possible that incomplete records could represent a potential source of misclassification. Fourth, 7 older children had premorbid neurologic conditions such as traumatic brain injury (n = 3), ventriculoperitoneal shunt (n = 2), or previous seizure disorder (n = 1), which complicated analyses of neurologic outcomes. This outcome may also contribute somewhat to the relatively higher rates of neurologic sequelae encountered in our study. Baseline parameters for hearing loss were infrequently obtained before the onset of meningitis, which may have influenced the interpretation of audiologic testing results. However, these are rarely available in any meningitis study.32 In addition, this study focused on neurologic sequelae, and other forms of sequelae (eg, orthopedic, psychological) were not captured. Finally, it was not possible to evaluate potentially important risk factors for pneumococcal meningitis, including household size and day care attendance.
Conclusions
Despite these limitations, the current study allows us to draw several conclusions. There was a decrease in the incidence of PCV7 serotype meningitis after licensure of the pneumococcal conjugate vaccine. However, non-PCV7 serotype disease continues to be associated with substantial mortality as well as long-term morbidity. Sixty-three percent of survivors had neurologic sequelae. Although there were trends suggesting decreased severity, the overall rates of sequelae were similar among PCV7 and non-PCV7 serotypes. Tragically, more than one-half of the children who were eligible for PCV7 were unimmunized at the time that they developed pneumococcal meningitis. Given the high rate of death and disability from pneumococcal meningitis, strategies to increase immunization rates and to address immunization hesitancy are urgently needed. More than one-third of pneumococcal meningitis cases from 2001 to 2010 were caused by serotypes contained within the recently licensed PCV13. Immunization with PCV13 will likely further reduce the burden of pediatric meningitis, although ongoing surveillance will be critical to document changes in serotype burden and associated sequelae.
Acknowledgment
We gratefully acknowledge the support of the PCMC Microbiology Laboratory in identifying and archiving all pneumococcal isolates.
Glossary
- CI
confidence interval
- IQR
interquartile range
- PCV7
7-valent pneumococcal conjugate vaccine
- PCV13
13-valent pneumococcal conjugate vaccine
- PCMC
Primary Children’s Medical Center
Footnotes
Mr Stockmann analyzed the data and drafted the initial manuscript; Dr Ampofo designed the study, created the data set, and critically reviewed and revised the manuscript; Dr Byington designed the study, maintains the pneumococcal archive, analyzed the data, and critically reviewed and revised the manuscript; Dr Filloux manually reviewed the medical records and contributed his expert clinical judgment in assessing neurologic sequelae after infection with pneumococcal meningitis, and critically reviewed and revised the manuscript; Drs Hersh and Blaschke designed the study, provided feedback on the data analyses, and critically reviewed and revised the manuscript; Mrs Cowan manually reviewed the medical records and collected study data, and critically reviewed and revised the manuscript; Mr Korgenski created the data set, analyzed the data, and edited the manuscript; Dr Mason serotyped Streptococcus pneumoniae isolates and critically reviewed and revised the manuscript; and Dr Pavia designed the study and critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by grants from the National Institute of Allergy and Infectious Diseases (grant U01A1082482 [to Drs Ampofo and Byington], grant U01 AI074419 [to Drs Byington and Pavia], grant U01AI082184 [to Drs Blaschke and Pavia], grant K23-AI079401 [to Dr Blaschke]) and the Centers for Disease Control Prevention (U18-IP000303 [to Mr Stockman and Drs Ampofo, Byington, Blaschke, and Pavia]). This project was further supported by the University of Utah, Department of Pediatrics, through the Children’s Health Research Center and the Pediatric Clinical and Translational Research Scholars Program, the H.A. and Edna Benning Presidential Endowment, and the Primary Children’s Medical Center Foundation. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Drs Ampofo, Byington, Hersh, Blaschke, and Pavia collaborate with BioFire Diagnostics, Inc. (formerly Idaho Technology, Inc.) on several projects funded by the National Institutes of Health and the Centers for Disease Control and Prevention (see Funding). Drs Byington and Blaschke have intellectual property in and receive royalties from BioFire Diagnostics, Inc. Dr Byington and Ampofo served as site Principal Investigators for clinical trials of PCV13 (Wyeth Pfizer from 2007–2010). The other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Schlech WF, III, Ward JI, Band JD, Hightower A, Fraser DW, Broome CV. Bacterial meningitis in the United States, 1978 through 1981. The National Bacterial Meningitis Surveillance Study. JAMA. 1985;253(12):1749–1754 [PubMed] [Google Scholar]
- 2.Haddy RI, Perry K, Chacko CE, et al. Comparison of incidence of invasive Streptococcus pneumoniae disease among children before and after introduction of conjugated pneumococcal vaccine. Pediatr Infect Dis J. 2005;24(4):320–323 [DOI] [PubMed] [Google Scholar]
- 3.Schuchat A, Robinson K, Wenger JD, et al. Active Surveillance Team . Bacterial meningitis in the United States in 1995. N Engl J Med. 1997;337(14):970–976 [DOI] [PubMed] [Google Scholar]
- 4.Chávez-Bueno S, McCracken GH, Jr. Bacterial meningitis in children. Pediatr Clin North Am. 2005;52(3):795–810, vii [DOI] [PubMed] [Google Scholar]
- 5.Thigpen MC, Whitney CG, Messonnier NE, et al. Emerging Infections Programs Network . Bacterial meningitis in the United States, 1998-2007. N Engl J Med. 2011;364(21):2016–2025 [DOI] [PubMed] [Google Scholar]
- 6.Whitney CG, Farley MM, Hadler J, et al. Active Bacterial Core Surveillance of the Emerging Infections Program Network . Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348(18):1737–1746 [DOI] [PubMed] [Google Scholar]
- 7.Recommended immunization schedules for persons aged 0 through 18 years—United States, 2012 [published correction appears in MMWR Morb Mortal Wkly Rep. 2012;61(8):147]. MMWR Morb Mortal Wkly Rep. 2012;61(5):1–4 [PubMed] [Google Scholar]
- 8.Tsai CJ, Griffin MR, Nuorti JP, Grijalva CG. Changing epidemiology of pneumococcal meningitis after the introduction of pneumococcal conjugate vaccine in the United States. Clin Infect Dis. 2008;46(11):1664–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ampofo K, Pavia AT, Stockmann CR, et al. Evolution of the epidemiology of pneumococcal disease among Utah children through the vaccine era. Pediatr Infect Dis J. 2011;30(12):1100–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ampofo K, Pavia AT, Chris S, Hersh AL, Bender JM, Blaschke AJ, et al. The changing epidemiology of invasive pneumococcal disease at a tertiary children's hospital through the 7-valent pneumococcal conjugate vaccine era: a case for continuous surveillance Pediatr Infect Dis J. 2012;31(3):228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kornelisse RF, Westerbeek CM, Spoor AB, et al. Pneumococcal meningitis in children: prognostic indicators and outcome. Clin Infect Dis. 1995;21(6):1390–1397 [DOI] [PubMed] [Google Scholar]
- 12.Ramakrishnan M, Ulland AJ, Steinhardt LC, Moïsi JC, Were F, Levine OS. Sequelae due to bacterial meningitis among African children: a systematic literature review. BMC Med. 2009;7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu HE, Shutt KA, Moore MR, et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med. 2009;360(3):244–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shutt CK, Samore M, Carroll KC. Comparison of the Denka Seiken slide agglutination method to the quellung test for serogrouping of Streptococcus pneumoniae isolates. J Clin Microbiol. 2004;42(3):1274–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herson VC, Todd JK. Prediction of morbidity in Hemophilus influenzae meningitis. Pediatrics. 1977;59(1):35–39 [PubMed] [Google Scholar]
- 16.Indicator-Based Information System for Public Health (IBIS-PH). Available at: http://ibis.health.utah.gov/query/result/pop/PopMain/Count.html. Accessed October 26, 2012
- 17.American Academy of Pediatrics . Committee on Infectious Diseases. Policy statement: recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics. 2000;106(2 pt 1):362–366 [DOI] [PubMed] [Google Scholar]
- 18.Pilishvili T, Lexau C, Farley MM, et al. Active Bacterial Core Surveillance/Emerging Infections Program Network . Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41 [DOI] [PubMed] [Google Scholar]
- 19.Bingen E, Levy C, Varon E, et al. Bacterial Meningitis Study Group . Pneumococcal meningitis in the era of pneumococcal conjugate vaccine implementation. Eur J Clin Microbiol Infect Dis. 2008;27(3):191–199 [DOI] [PubMed] [Google Scholar]
- 20.Glanz JM, Newcomer SR, Narwaney KJ, et al. A population-based cohort study of undervaccination in 8 managed care organizations across the United States. JAMA Pediatr. 2013;167(3):274–281 [DOI] [PubMed] [Google Scholar]
- 21.Whitney CG, Pilishvili T, Farley MM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368(9546):1495–1502 [DOI] [PubMed] [Google Scholar]
- 22.Glanz JM, McClure DL, O’Leary ST, et al. Parental decline of pneumococcal vaccination and risk of pneumococcal related disease in children. Vaccine. 2011;29(5):994–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salmon DA, Moulton LH, Omer SB, DeHart MP, Stokley S, Halsey NA. Factors associated with refusal of childhood vaccines among parents of school-aged children: a case-control study. Arch Pediatr Adolesc Med. 2005;159(5):470–476 [DOI] [PubMed] [Google Scholar]
- 24.Gil Prieto R, San Román Montero J, Gómez Alejandre C, Alvaro Meca LA, Rivero A, Gil de Miguel A. Epidemiology of pneumococcal meningitis hospitalizations in pediatric population in Spain (1998-2006). Vaccine. 2009;27(20):2669–2673 [DOI] [PubMed] [Google Scholar]
- 25.King BA, Richmond P. Pneumococcal meningitis in Western Australian children: epidemiology, microbiology and outcome. J Paediatr Child Health. 2004;40(11):611–615 [DOI] [PubMed] [Google Scholar]
- 26.Arditi M, Mason EO, Jr, Bradley JS, et al. Three-year multicenter surveillance of pneumococcal meningitis in children: clinical characteristics, and outcome related to penicillin susceptibility and dexamethasone use. Pediatrics. 1998;102(5):1087–1097 [DOI] [PubMed] [Google Scholar]
- 27.Jansen AG, Rodenburg GD, van der Ende A, et al. Invasive pneumococcal disease among adults: associations among serotypes, disease characteristics, and outcome. Clin Infect Dis. 2009;49(2):e23–e29 [DOI] [PubMed] [Google Scholar]
- 28.Rückinger S, von Kries R, Siedler A, van der Linden M. Association of serotype of Streptococcus pneumoniae with risk of severe and fatal outcome. Pediatr Infect Dis J. 2009;28(2):118–122 [DOI] [PubMed] [Google Scholar]
- 29.Bohr V, Paulson OB, Rasmussen N. Pneumococcal meningitis. Late neurologic sequelae and features of prognostic impact. Arch Neurol. 1984;41(10):1045–1049 [DOI] [PubMed] [Google Scholar]
- 30.Kastenbauer S, Pfister HW. Pneumococcal meningitis in adults: spectrum of complications and prognostic factors in a series of 87 cases. Brain. 2003;126(pt 5):1015–1025 [DOI] [PubMed] [Google Scholar]
- 31.Chandran A, Herbert H, Misurski D, Santosham M. Long-term sequelae of childhood bacterial meningitis: an underappreciated problem. Pediatr Infect Dis J. 2011;30(1):3–6 [DOI] [PubMed] [Google Scholar]
- 32.Østergaard C, Konradsen HB, Samuelsson S. Clinical presentation and prognostic factors of Streptococcus pneumoniae meningitis according to the focus of infection. BMC Infect Dis. 2005;5:93. [DOI] [PMC free article] [PubMed] [Google Scholar]