Abstract
Purpose.
We developed and validated a technique for measuring global motion perception in 2-year-old children, and assessed the relationship between global motion perception and other measures of visual function.
Methods.
Random dot kinematogram (RDK) stimuli were used to measure motion coherence thresholds in 366 children at risk of neurodevelopmental problems at 24 ± 1 months of age. RDKs of variable coherence were presented and eye movements were analyzed offline to grade the direction of the optokinetic reflex (OKR) for each trial. Motion coherence thresholds were calculated by fitting psychometric functions to the resulting datasets. Test–retest reliability was assessed in 15 children, and motion coherence thresholds were measured in a group of 10 adults using OKR and behavioral responses. Standard age-appropriate optometric tests also were performed.
Results.
Motion coherence thresholds were measured successfully in 336 (91.8%) children using the OKR technique, but only 31 (8.5%) using behavioral responses. The mean threshold was 41.7 ± 13.5% for 2-year-old children and 3.3 ± 1.2% for adults. Within-assessor reliability and test–retest reliability were high in children. Children's motion coherence thresholds were significantly correlated with stereoacuity (LANG I & II test, ρ = 0.29, P < 0.001; Frisby, ρ = 0.17, P = 0.022), but not with binocular visual acuity (ρ = 0.11, P = 0.07). In adults OKR and behavioral motion coherence thresholds were highly correlated (intraclass correlation = 0.81, P = 0.001).
Conclusions.
Global motion perception can be measured in 2-year-old children using the OKR. This technique is reliable and data from adults suggest that motion coherence thresholds based on the OKR are related to motion perception. Global motion perception was related to stereoacuity in children.
Keywords: motion coherence threshold, random-dot-kinematogram, vision, optokinetic nystagmus, dorsal stream
Global motion perception is susceptible to abnormal development and may provide a sensitive measure of visual cortex function. We describe and validate a technique for measuring global motion perception in 2-year-old children, and relate global motion perception to acuity and stereopsis.
Introduction
The dorsal visual cortical stream, which includes motion sensitive extrastriate area V5/MT, is thought to be particularly vulnerable to abnormal development.1–5 For example, specific deficits in the perception of global motion, which requires the integration of multiple local motion signals, have been reported in children with Williams syndrome,6 developmental dyslexia,7,8 cerebral palsy,9 and a history of preterm birth10,11 (see prior reviews1,3). Measurements of global motion perception typically use random dot kinematograms (RDK, Fig. 1). These stimuli consist of two populations of moving dots, a signal population that moves in a common, coherent direction and a noise population that moves randomly.12 The observer's task is to discriminate the direction of the signal dots and the signal-to-noise ratio is varied to estimate the percentage of signal dots required for a particular level of task performance. This is known as the motion coherence threshold. Evidence that this process involves dorsal extrastriate area V5/MT has been provided by neurophysiologic studies linking global motion perception to neural responses in primate MT.13–15 Relationships between V5/MT activity and global motion perception also have been found in humans using brain imaging techniques.16–21 Furthermore, studies in animals and humans have shown that lesions12,22–27 and stimulation28,29 of V5/MT can selectively influence global motion perception. Therefore, as global motion perception is likely to rely on processing in V5/MT, it may provide a useful measure of dorsal cortical stream development in children.
Figure 1.
The RDK stimulus. (A) A schematic of the RDK stimulus depicting 50% coherence. Open circles represent signal dots and filled circles represent noise dots. The arrows illustrate the motion direction of each dot. Half of the dots are signal dots (all moving to the right) and the other half are noise dots (moving in random directions). (B) A single frame of the RDK stimulus used in the study.
Global motion perception emerges at 1 to 3 months of age30–32 and continues to develop throughout childhood.9,10,33–36 However, the development of global motion perception in early childhood is not well understood and currently no data are available for children between the ages of 7 months and 3.5 years. This may be due to the difficulties involved in measuring visual function in children of this age. Preferential looking techniques, while still possible,37 can become more challenging as children reach 12 to 18 months of age,38–43 and tasks that require behavioral responses are feasible only when the requisite cognitive and language skills have developed, which typically is after the age of 36 months.44–49
The aim of our study was to develop and validate a technique for assessing global motion perception that could be applied to 2-year-old children. We used an approach that allowed for the optokinetic reflex (OKR) and behavioral responses to be recorded, and we assessed motion coherence thresholds using a modified method of constant stimuli. The OKR occurs in response to a continuously moving stimulus and is characterized by pursuit eye movements in one direction interleaved with saccadic refixation eye movements in the opposite direction.50 The OKR has been used previously to measure global motion perception in children aged 6 to 27 weeks.30,51 However, the use of this technique has not been reported for 2-year-old children, to our knowledge. Indeed, as mentioned above, there are no previous reports of global motion coherence thresholds in 2-year-olds, possibly due to the difficulties in obtaining reliable psychophysical measurements from children of this age.
Materials and Methods
Participants
Data were collected from 366 children at 24 ± 1 months of corrected age. The children were born with risk factors for neonatal hypoglycemia and were part of the longitudinal Children with Hypoglycaemia and their Later Development (CHYLD) study. Risk factors for neonatal hypoglycemia included being born small (<10th percentile or <2.5 kg) or large (>90th percentile or >4.5 kg), infant of a diabetic mother, preterm (<37 weeks' gestation) or other (e.g., poor feeding). Only half of the cohort actually had experienced neonatal hypoglycemia, and in most cases this was mild and transient.52 We currently are investigating whether neonatal hypoglycemia had any effect on visual development within this cohort as part of the longitudinal CHYLD study. However, the goal of this particular investigation was to assess whether global motion perception could be measured accurately in these children at 2 years of age. To determine test–retest reliability, 15 children aged 21 to 27 months, 11 of whom were not part of the CHYLD study cohort, were assessed on two occasions separated by at least one day (range, 1–114 days). Ten adults with normal vision (mean age, 29.7 years; range, 26–35 years) also were assessed. All study protocols were done in accordance with the Declaration of Helsinki, and were approved by the Northern Y Regional Health and Disability Ethics Committee or the University of Auckland Human Participants Ethics Committee.
Stimuli
The global motion stimulus (Fig. 1) was an RDK presented on a cathode ray tube monitor (Dell E772p [Dell, Plano, TX] or HP 7540 [Hewlett-Packard Company, Palo Alto, CA], 1280 × 1024 resolution, 60 Hz refresh rate) placed 60 cm from the observer. The stimuli were generated using MATLAB (MathWorks, Natick, MA) and the Psychophysics Toolbox.53–55 The RDK was presented within a circular stimulus aperture with a radius of 8.3°, and was made up of 250 white dots (138 cd/m2) presented on a grey background (42 cd/m2, dot density = 1.16 dots/deg2). Each dot had a diameter of 0.5°, a speed of 8°/s (generated by displacing each dot by 0.13° on every frame), and a limited lifetime defined as a 5% chance of disappearing on each frame and being redrawn in a random location. The noise dots had a constant speed and direction. The duration of each RDK presentation was 8 seconds (480 frames per trial). Pictures were positioned on each side of the RDK stimulus to facilitate behavioral responses.
Procedure
For children from the CHYLD study cohort, measurements of global motion perception were made as part of a comprehensive developmental assessment. All psychophysical measurements were conducted in a well-lit room with minimal distractions. Eye movements were recorded at 50 Hz using a digital high definition camera (Sony HDR-CX7EK; Sony Corporation, Tokyo, Japan) placed to the left of the monitor with the zoom set so that the participant's head filled the frame.
Before the threshold measurement, training was conducted in two stages. First, the child was asked to follow a single dot with a finger and indicate which of the pictures the dot was approaching. This procedure then was repeated with a 100% coherent RDK stimulus. During threshold measurement a flashing fixation point accompanied by a beeping sound was presented in the center of the screen to attract the child's attention. Each trial then was initiated by the experimenter when the child was looking at the screen. During a trial, the RDK stimulus was presented for 8 seconds at a fixed level of coherence. Signal dot direction (left or right) was randomized for each trial. Coherence levels of 100%, 84%, 68%, 52%, 36%, and 20% were presented in descending order across consecutive trials and the sequence was repeated until the child could no longer be encouraged to look at the monitor. Children were asked to indicate the direction in which most of the dots were moving by pointing at and/or naming one of the pictures. After every five trials, brightly colored animations accompanied by music were presented to retain attention. Coherence levels for adult testing were fixed at 20%, 12%, 8%, 6%, 4%, and 2% to avoid ceiling effects. Adults completed 10 trials per level of coherence (60 trials in total).
Threshold Calculation
Behavioral and OKR responses were collected for each trial. Video recordings of OKR responses were assessed offline by an experienced assessor (TYY) who graded the OKR for each trial as “leftward,” “rightward,” or “no OKR” following a three-alternative forced choice procedure. The child's behavioral responses also were recorded as either “left,” “right,” or “no response.” Trials were excluded if the child's eyes were not clearly visible (e.g., if the child had moved out of the video frame), the child was not looking at the screen, or the child's parent/guardian prompted the child with the correct answer. Adult participants provided behavioral responses following a two alternative forced choice procedure.
Motion coherence thresholds were calculated separately for OKR and behavioral responses. Only datasets with more than 15 observations (a minimum of 3 per level of coherence for a minimum of 5 levels of coherence) were analyzed. For the OKR data, a proportion correct was calculated for each coherence level based on the direction of the OKR reported by the video recording assessor. Proportion correct values then were fit with a Weibull function using the Palamedes toolbox for Matlab,56 which also provided estimates of threshold (63% correct), slope, and goodness of fit using a bootstrap procedure.
Optometric Assessment
The optometric assessment, performed after the motion coherence threshold measurement, included tests of ocular motility, ocular health, and visual function (see Table).
Table.
Tests and Testing Distances Used in the Optometric Assessment Along With Expected Norms for 2-Year-Old Children
|
Visual Attribute |
Test |
Testing Distance |
Expected Norms for 2-Year-Old Children |
| Ocular health screening | External ocular inspection | – | No ocular pathology |
| Red reflex testing | – | Normal red reflexes | |
| Pupillary reflex testing | – | Normal direct and consensual pupillary reflexes to light | |
| Ocular alignment | Cover test | 25 cm fixation target | No movement |
| Hirschberg's corneal reflex | – | Symmetrical corneal reflexes | |
| Ocular motility | Smooth pursuit to a moving target | 30 to 40cm | Smooth movement without restrictions |
| Near point of convergence | – | No established normative value | |
| Stereopsis | Frisby Near Stereotest (distributed by Haag-Streit UK, Ltd, Sheffield, UK) | 30 cm moving to 60 cm | No established normative value. Pass (demonstrates stereopsis) rate of 90 to 40%38,49 |
| LANG stereotest I and II (distributed by LANG-STEREO TESTAG, Forch, Switzerland) | 40 cm | No established normative value. Pass (demonstrates stereopsis) rate of 90 to 74%38,49,57,58 | |
| Monocular and binocular visual acuity | Cardiff Acuity Cards (distributed by Richmond Products, Albuquerque, NM) | 100 cm (or 50 cm if unsuccessful at 100 cm) | Monocular: 0.6–0.1 logMAR |
| Binocular: 0.5–0.0 logMAR | |||
| Interocular difference: <0.1 logMAR59 | |||
| Motor fusion and suppression | 20Δ Base out prism test | 25 cm fixation target | Fusional convergence eye movements |
| Refractive error screening (noncycloplegic) | Distance retinoscopy | ≥3 m fixation target | Mean sphere: ≥−3.0 D and ≤+1.5 D Anisometropia: <1.5 D in any meridian Astigmatism: <2.0 D any axis60–62 |
| Suresight handheld autorefractor (Welch Allyn, Skaneateles Falls, NY) |
The tests were attempted in the listed order when possible.
Statistical Analysis
Testability was calculated as the percentage of children for whom successful measurements were made with reference to the number of children for whom the test was attempted. Within-assessor reliability and test–retest reliability were evaluated using paired-samples t-tests and single measures intraclass correlations. Correlations between different visual function measurements were evaluated using Spearman's ρ or Pearson's R correlation coefficients if the assumptions for parametric tests were met. Means are reported with associated standard deviations. When a child failed to demonstrate stereopsis, we were unable to differentiate between whether the child had no stereopsis or could not perform the test. Therefore, only successful measures were included in the reported means and statistical tests.
Results
Testability and Reliability in 2-Year-Old Children
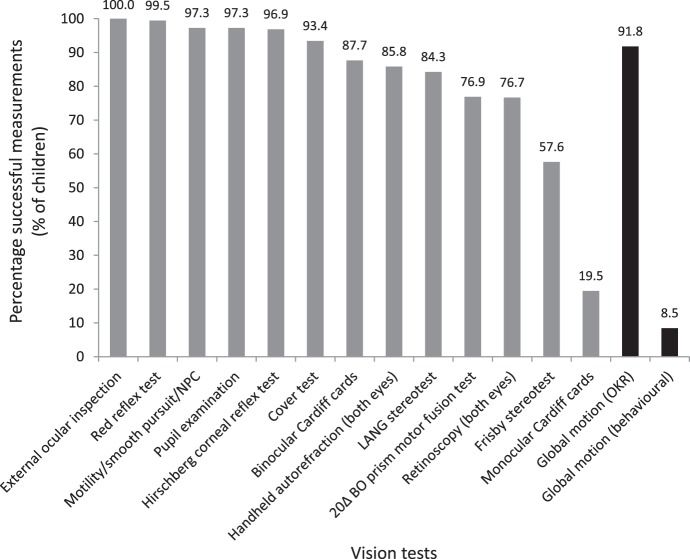
Motion coherence thresholds were measured successfully for 336 of 366 (91.8%) 2-year-old children using the OKR, which was higher than the optometry tests that required subjective responses from the child (Fig. 2). The average number of RDK trials completed was 59 ± 16 and the average duration of the measurement per child was 18.6 ± 7.6 min. Behavioral motion coherence thresholds could be estimated only for 31 (8.5%) children.
Figure 2.
Percentage of successful measurements for each of the clinical vision tests and global motion measurements for 2-year-old children.
To evaluate within-assessor reliability, 14 randomly selected OKR datasets were reanalyzed at least 6 months after the original analysis. The threshold results did not differ significantly between the first and second analysis (average difference = 1.47%, 95% confidence interval [CI] −3.56–0.62, t13 = 1.5, P = 0.15) and the single measures intraclass correlation was 0.96 (95% CI = 0.87–0.99, P < 0.001), reflecting “almost perfect” reliability.63
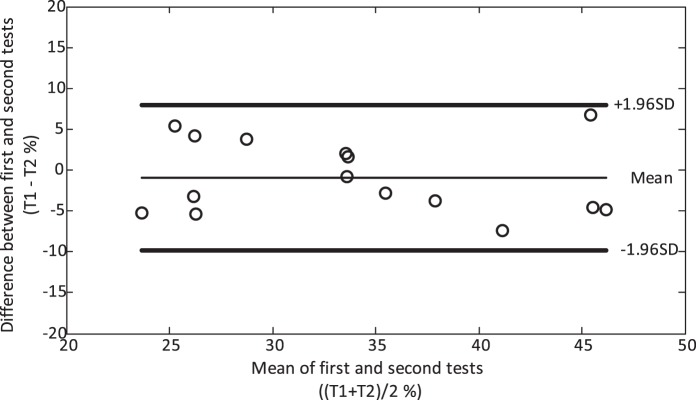
Test–retest reliability for the 15 children assessed on two separate days was analyzed using only the thresholds derived from OKR responses, as few of these children were able to provide behavioral responses. The average difference in motion coherence threshold was 0.94% (95% CI −3.45–1.57, t14 = −0.80, P = 0.44) and the single measures intraclass correlation was 0.85 (95% CI = 0.62–0.95, P < 0.001, Fig. 3). The threshold results between the first and second session of testing for children who were part of the CHYLD study cohort (n = 4) were not significantly different from those of the noncohort children (n = 11, 1.37 ± 4.05 vs. −1.78 ± 4.58, t13 = 1.21, P = 0.25).
Figure 3.
Bland-Altman plot of test–retest reliability for 2-year-old children who completed motion coherence threshold measurements on two separate days. T1, threshold from the first test; T2, threshold from the second test.
Motion Coherence Threshold Estimates in Children
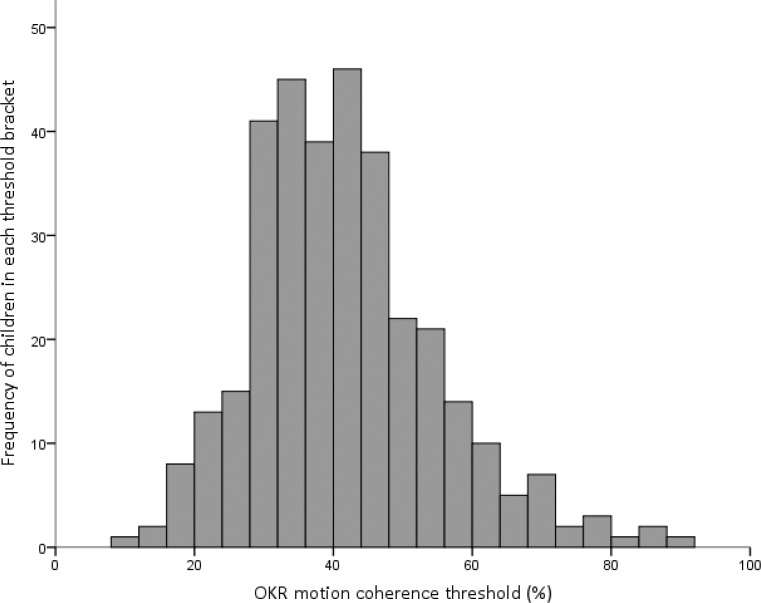
The average OKR motion coherence threshold was 41.7 ± 13.5% and the distribution was skewed to the right (P < 0.005 for comparison with a normal distribution, Fig. 4). Although the range of thresholds was large (9.0%–88.2%), 75% (252/336) of the children had thresholds ranging from 28% to 56% (the central region of the distribution shown in Fig. 4). The mean and standard deviation excluding children with abnormal findings for visual acuity, an inability to complete stereo testing, strabismus, and significant manifest refractive error (outside the expected norms listed in the Table) were very similar to the mean and standard deviation for the cohort as a whole at 41.6 ± 13.8% (n = 210). The examination of individual psychometric functions showed that confidence intervals were large due to the relatively small number of trials, but that the functions were reasonably monotonic indicating that the proportion of directional OKR responses scaled with coherence (Fig. 5).
Figure 4.
The distribution of OKR motion coherence thresholds for 336 2-year-old children.
Figure 5.
Examples of psychometric functions for 2-year-old children with associated 95% CIs for proportions. The vertical axis expresses the proportion of trials for which OKR was observed to be in the same direction as the stimulus. (A–C) Three examples of children whose thresholds fell above the 75th percentile (high thresholds). (D–F) Three examples of children whose thresholds were between the 40th and 60th percentile (moderate thresholds). (G–H) Three examples of children whose thresholds fell below the 25th percentile (low thresholds).
The Relationships Among Different Measures of Visual Function in Children
Mean Cardiff card binocular visual acuity was 0.05 ± 0.15 logMAR. Mean stereoacuity was 2.52 ± 0.20 log sec-of-arc using the LANG I & II stereo test and 2.46 ± 0.24 log sec-of-arc using the Frisby stereo test. Motion coherence thresholds were correlated positively with stereoacuity measured with the LANG I & II (Spearman's ρ = 0.28, P < 0.001) and the Frisby (ρ = 0.18, P = 0.013) stereo tests, but were not correlated significantly with visual acuity (ρ = 0.11, P = 0.065). When the results for children with abnormal findings for visual acuity, strabismus, and significant manifest refractive error (outside the expected norms listed in the Table) were excluded, the relationship between OKR motion coherence and measures of stereopsis became slightly stronger (LANG I & II, ρ = 0.34, P < 0.001; Frisby, ρ = 0.21, P = 0.012). Visual acuity measured using the Cardiff cards correlated with stereoacuity measured with the LANG I & II (ρ = 0.30, P < 0.001) and Frisby (ρ = 0.19, P = 0.02) stereo tests. The results of the two stereo tests (LANG I & II and Frisby) also were correlated positively (ρ = 0.18, P = 0.009).
Motion Coherence Thresholds in Adults
Motion coherence thresholds for OKR responses and behavioral responses were obtained for all 10 adults. The average OKR motion coherence threshold was 3.3 ± 1.2% (range, 1.7–5.6%) and was significantly lower than that based on behavioral data (5.8 ± 1.6%; range, 3.2–9.0%; t9 = 9.2, P < 0.001). However, the two measurements were correlated strongly (single measures intraclass correlation = 0.81, 95% CI = 0.41–0.95, P = 0.001).
Discussion
Evidence that global motion perception may be a sensitive measure of neurologic and visual cortex development1,3,5 motivated us to develop and validate a technique for assessing global motion perception in 2-year-old children. We found that video recordings of the optokinetic reflex in response to RDK stimuli with varying levels of coherence allowed for motion coherence thresholds to be calculated by fitting a psychometric function to the proportion of “correct” OKRs for each coherence level. This technique had high repeatability and test–retest reliability. Importantly, measurements were possible for over 90% of the 2-year-old children who took part in this study. This rate of successful measurements compared favorably to clinical tests of visual function performed on the same children. We cannot rule out an order effect as motion coherence thresholds were measured before clinical testing. However, across all tests, the success rates did not correspond to the preferred testing sequence. For example, stereopsis assessment was less successful than acuity assessment despite occurring earlier in the testing sequence. Only 8.5% of children were able to provide behavioral responses when viewing RDKs. This is in agreement with previous work indicating that reliable behavioral responses generally are not obtainable from children less than 3 to 4 years of age,45–49 and highlights the importance of using objective measures with young children. We found no obvious differences in age or visual performance between children who could and could not provide behavioral responses.
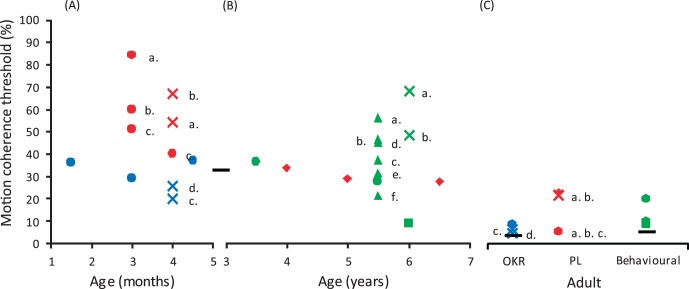
A comparison between the motion coherence thresholds measured in our study and those measured in previous studies shows a gradual improvement in global motion perception over the first 7 years of life, although thresholds do not reach adult levels (Fig. 6). While this developmental trend is consistent across studies, absolute thresholds vary. For children 1 to 3 months of age, the mean threshold was 60% coherence for studies using preferential looking30,32 and 30% for those using OKR-based measures.30,51 Thresholds were more stable on average for 3- to 7-year-old children, with preferential looking9 and behavioral34–36,64 techniques providing mean thresholds of 30% coherence. OKR techniques have not been used for this age group. Finally, studies that also included adult observers produced mean adult thresholds of 20% coherence for preferential looking paradigms,9,30,32 10% for OKR-based methods,30,51 and 10% for behavioral responses.34–36,64 However, as is evident from the variability in Figure 6, these comparisons should be interpreted with caution. In particular, the stimulus parameters and threshold criteria (i.e., whether thresholds reflect 50%, 63%, or 75% correct) differ across studies, which may lead to variations in absolute threshold (see Appendix for further details). For example, it recently has been shown that relatively small changes in dot speed can significantly affect global motion perception in older children.35 With this in mind, a comparison between child and adult thresholds for an identical stimulus may be more informative, as this gives an indication of how far along the developmental continuum or how “adult-like” children's thresholds are. We found that the mean adult threshold was more than 12 times lower than the mean for our specific cohort of 2-year-old children using the OKR technique. Even the children at the lower end of the distribution did not reach adult levels of performance, suggesting that development of global motion perception was incomplete in our cohort of 2-year-olds. This is in agreement with previous work demonstrating considerable differences between adults and children for the same psychophysical global motion task.30,34,51 Interestingly, the difference we reported for global motion discrimination is considerably larger than that found for the detection of moving dots within a field of static dots, which is only a factor of 3 to 4 times poorer than adult performance by 12 months of age.37
Figure 6.
A summary of mean motion coherence thresholds from previous studies of global motion perception in infants and children which used RDKs with dynamic signal and noise dots. (A) Results from studies of children aged 1 to 5 months. (B) Results from studies of children aged 3 to 7 years. (C) Results from studies of that also included adult participants. The color of the markers denotes the technique used to measure the motion coherence threshold; blue = OKR, red = preferential looking (PL), and green = behavioral responses. The shape of the markers in combination with the color of the marker denotes the study;  = Wattam-Bell,32
= Wattam-Bell,32
 = Bantan and Bertenthal,51
= Bantan and Bertenthal,51
 = Mason et al.,30
= Mason et al.,30
 = Ellemberg et al.,64
= Ellemberg et al.,64
 = Gunn et al.,9
= Gunn et al.,9
 = Parrish et al.,36
= Parrish et al.,36
 = Hadad et al.,34
= Hadad et al.,34
 and
and  = Narasimhan and Giaschi.35 The (a–f) labels are to be used in conjunction with Table A1 provided in the Appendix and identify different stimulus parameters used within individual studies. The black horizontal lines represent the average thresholds found in our study for 2-year-old children (OKR) and adults (OKR and behavioral).
= Narasimhan and Giaschi.35 The (a–f) labels are to be used in conjunction with Table A1 provided in the Appendix and identify different stimulus parameters used within individual studies. The black horizontal lines represent the average thresholds found in our study for 2-year-old children (OKR) and adults (OKR and behavioral).
The distribution of thresholds we report was relatively wide in comparison with previous studies of global motion perception in children. A number of factors may have contributed to this variability. It is possible that our test had greater variability than other techniques that have been used with children; however our test–retest reliability data indicated that this is unlikely to be the case. Previous studies have used considerably smaller sample sizes, often combined with stringent exclusion criteria. For example children with poor language skills, and children who were uncooperative, fussy, or inattentive have been excluded from prior studies.9,30,35,36,51,64 This presumably reduced the range of thresholds in these studies. However, our study is not the only one to find high variability in motion coherence thresholds. Hadad et al.34 also found considerable variability in motion coherence thresholds for children up to 13 years of age. In addition, no previous studies have reported global motion coherence thresholds for 2-year-old children and it is possible that there is considerable variability between children of this age.59
While area V5/MT is involved in mediating the OKR in the mature visual system,21,65–67 it has been suggested that in early infancy the OKR may be driven by subcortical mechanisms.68–71 Evidence for this includes a nasal/temporal asymmetry under monocular viewing conditions whereby only temporal-to-nasal motion can induce an OKR.69,71 This asymmetry could be due to a reliance on the nucleus of the optic tract, which contains directional neurons that only respond to nasal-ward motion. The gradual disappearance of monocular OKR asymmetry from the age of 2 months71,72 presumably is due to the development of cortical pathways that supersede the subcortical mechanism. The majority of studies indicate that the OKN becomes symmetrical before 2-years of age,69,71,73–76 (however, see the study of Lewis et al.72) suggesting that OKN responses involve cortical processing by the age of 2 years. Therefore, our recordings of OKR in 2-year-old children are likely to reflect cortical function. Furthermore, our use of RDKs with limited lifetime dots was designed to target dorsal extrastriate areas, such as V5/MT.12,16–20,24,77,78
Interestingly, we found that motion coherence thresholds measured using the OKR were lower than behavioral thresholds in adults whose visual cortex development is complete. One possible explanation is that the OKR reflects activity in the subconscious pathway that directly connects subcortical structures, such as the superior colliculus and pulvinar to V5.79,80 In fact, it recently was found that the early maturation of area MT likely is to be reliant on retinopulvinar input.81 An alternative explanation is that OKR is less susceptible to response errors. Whatever the reason for the absolute difference between behavioral and OKR responses in adults, the close correlation between the two measures suggests that they target a common neural mechanism, which is likely to be global motion processing within the extrastriate visual cortex.13,17,24,82
Our finding that motion coherence thresholds were correlated positively with stereopsis is consistent with previous work indicating that the development of motion perception and stereopsis are closely related.83–85 Cells in V5 are exclusively binocular86,87 and many are sensitive to the retinal disparity signals required for stereopsis.86,88,89 This suggests that the development of dorsal extrastriate areas, such as V5, influences global motion perception and stereopsis.90–92 By extension, a deficit in dorsal stream processing would be likely to affect abilities such as visually guided motor control,2,5,93–95 which rely on stereopsis and motion perception.96–98 There is a possibility that the correlation between motion coherence thresholds and stereopsis we observed is due to children with more advanced cognitive skills exhibiting superior performance on both tasks. While we cannot rule this out, we note that children who could not complete the stereo testing were not included in the analysis and that the OKR based measure of motion coherence threshold does not require cooperation other than looking at the screen, hence the high success rates in testing.
The narrow range of binocular visual acuities we measured is consistent with previous reports (e.g., the study of Adoh and Woodhouse59), and could explain why no relationship was found between binocular visual acuity and motion coherence thresholds. However, it may be the case that motion coherence thresholds are relatively independent of visual acuity. A number of previous studies have demonstrated that motion coherence thresholds are unaffected by optical defocus.99–101 For example, Trick et al.100 reported that optically induced blur of 4 diopters (D) or less had no effect on coherence thresholds. Therefore, global motion perception appears to be relatively robust to the spatial frequency content of RDKs when the stimuli are presented at high contrast. This suggests that an assessment of global motion perception could provide information relating to visual cortex development even in the presence of refractive error.
A major obstacle that we faced when assessing 2-year-olds was their limited attention span resulting in only short periods of cooperation. A number of previous studies have used staircase techniques when studying pediatric populations to allow for shorter testing times in an attempt to minimize this issue. However, staircase methods are particularly vulnerable to errors of inattentiveness early in the procedure102,103 and also require the child's responses to be interpreted on a trial-by-trial basis. This can be challenging, particularly when judging whether children are orienting towards a particular stimulus or exhibiting an OKR, and our pilot observations indicated that a staircase technique was not viable for 2-year-old children. We therefore chose to use a modified method of constant stimuli as this approach is less sensitive to lapses in concentration and allowed for offline analysis, whereby video footage could be examined carefully to ensure that the child was attending to the visual stimulus, and to assess the presence and direction of the OKR. Several other studies of children with developmental disorders have used a similar approach to measure visual function.104–107
One disadvantage of our OKR-based technique for measuring global motion perception is that it requires the subjective assessment of OKR eye movements by a trained observer, albeit offline. An automated, objective procedure for quantifying the OKR would be preferable. Such systems have been developed for adult subjects, for example Hyon et al.,108 but are not currently suitable for young children. With the availability of such a system, the assessment of OKR eye movement could allow for the accurate measurement of a range of visual functions in young children, such as grating acuity and contrast sensitivity.
In summary, the optokinetic reflex can be used to provide reliable and repeatable measures of global motion perception in 2-year old children, which are correlated with stereoacuity and robust to reduced levels of visual acuity. This technique has high testability compared to other age-appropriate pediatric vision tests and may provide a sensitive measure of visual cortex development in young children.
Acknowledgments
The authors thank all members of the CHYLD Study team for their contribution to the research.
Supported by the Health Research Council of New Zealand, Auckland Medical Research Foundation, University of Auckland, Waikato Medical Research Foundation, The New Zealand Association of Optometrists, and Grant R01HD069622 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and by a University of Auckland Doctoral Scholarship (T-YY). The authors alone are responsible for the content and writing of the paper.
Disclosure: T.-Y. Yu, None; R.J. Jacobs, None; N.S. Anstice, None; N. Paudel, None; J.E. Harding, None; B. Thompson, P
APPENDIX
The CHYLD Study Team
Aaron Le Compte, Anna Gsell, Anna Timmings, Arijit Chakraborty, Christina McQuoid, Coila Bevan, Deborah Harris, Ellen Campbell, Jessica Charlton, Geoff Chase, Grace McKnight, Greg Gamble, Heather Stewart, Jane Alsweiler, Janine Paynter, Jenny Rogers, Judith Ansell, Karen Frost, Kelly Fredell, Kelly Jones, Matthew Signal, Rebecca Young, Sapphire Martin, and Trecia Wouldes.
Table A1.
Summary of the RDK Stimulus Parameters and Response Methods for the Studies Included in Figure 6
Blank cells indicate that the information was not provided in the original report.
Footnotes
See the appendix for the members of the CHYLD Study Team.
References
- 1. Braddick O, Atkinson J, Wattam-Bell J. Normal and anomalous development of visual motion processing: motion coherence and ‘dorsal-stream vulnerability'. Neuropsychologia. 2003; 41: 1769–1784 [DOI] [PubMed] [Google Scholar]
- 2. Macintyre-Béon C, Ibrahim H, Hay I, et al. Dorsal stream dysfunction in children. A review and an approach to diagnosis and management. Curr Ped Rev. 2010; 6: 166–182 [Google Scholar]
- 3. Grinter EJ, Maybery MT, Badcock DR. Vision in developmental disorders: is there a dorsal stream deficit? Brain Res Bull. 2010; 82: 147–160 [DOI] [PubMed] [Google Scholar]
- 4. Dutton GN. ‘Dorsal stream dysfunction' and ‘dorsal stream dysfunction plus': a potential classification for perceptual visual impairment in the context of cerebral visual impairment? Dev Med Child Neurol. 2009; 51: 170–172 [DOI] [PubMed] [Google Scholar]
- 5. Atkinson J, Braddick O. From genes to brain development to phenotypic behavior. “Dorsal-stream vulnerability” in relation to spatial cognition, attention, and planning of actions in Williams syndrome (WS) and other developmental disorders. Prog Brain Res. 2011; 189: 261–283 [DOI] [PubMed] [Google Scholar]
- 6. Atkinson J, King J, Braddick O, Nokes L, Anker S, Braddick F. A specific deficit of dorsal stream function in Williams syndrome. Neuroreport. 1997; 8: 1919–1922 [DOI] [PubMed] [Google Scholar]
- 7. Raymond JE, Sorensen RE. Visual motion perception in children with dyslexia: normal detection but abnormal integration. Vis Cognit. 1998; 5: 389–404 [Google Scholar]
- 8. Benassi M, Simonelli L, Giovagnoli S, Bolzani R. Coherence motion perception in developmental dyslexia: a meta-analysis of behavioral studies. Dyslexia. 2010; 16: 341–357 [DOI] [PubMed] [Google Scholar]
- 9. Gunn A, Cory E, Atkinson J, et al. Dorsal and ventral stream sensitivity in normal development and hemiplegia. Neuroreport. 2002; 13: 843–847 [DOI] [PubMed] [Google Scholar]
- 10. Taylor NM, Jakobson LS, Maurer D, Lewis TL. Differential vulnerability of global motion, global form, and biological motion processing in full-term and preterm children. Neuropsychologia. 2009; 47: 2766–2778 [DOI] [PubMed] [Google Scholar]
- 11. Guzzetta A, Tinelli F, Del Viva M, et al. Motion perception in preterm children: role of prematurity and brain damage. Neuroreport. 2009; 20: 1339–1343 [DOI] [PubMed] [Google Scholar]
- 12. Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT). J Neurosci. 1988; 8: 2201–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Britten KH, Shadlen MN, Newsome WT, Movshon JA. Responses of neurons in macaque MT to stochastic motion signals. Vis Neurosci. 1993; 10: 1157–1169 [DOI] [PubMed] [Google Scholar]
- 14. Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci. 1992; 12: 4745–4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Newsome WT, Britten KH, Movshon JA. Neuronal correlates of a perceptual decision. Nature. 1989; 341: 52–54 [DOI] [PubMed] [Google Scholar]
- 16. McKeefry DJ, Watson JDG, Frackowiak RSJ, Fong K, Zeki S. The Activity in human areas V1/V2, V3, and V5 during the perception of coherent and incoherent motion. Neuroimage. 1997; 5: 1–12 [DOI] [PubMed] [Google Scholar]
- 17. Braddick OJ, O'Brien JM, Wattam-Bell J, Atkinson J, Hartley T, Turner R. Brain areas sensitive to coherent visual motion. Perception. 2001; 30: 61–72 [DOI] [PubMed] [Google Scholar]
- 18. Rees G, Friston KJ, Koch C. A direct quantitative relationship between the functional properties of human and macaque V5. Nat Neurosci. 2000; 3: 716–723 [DOI] [PubMed] [Google Scholar]
- 19. Helfrich RF, Becker HGT, Haarmeier T. Processing of coherent visual motion in topographically organized visual areas in human cerebral cortex. Brain Topogr. 2013; 26: 247–263 [DOI] [PubMed] [Google Scholar]
- 20. Giaschi D, Zwicker A, Young SA, Bjornson B. The role of cortical area V5/MT+ in speed-tuned directional anisotropies in global motion perception. Vision Res. 2007; 47: 887–898 [DOI] [PubMed] [Google Scholar]
- 21. Galati G, Pappata S, Pantano P, Lenzi GL, Samson Y, Pizzamiglio L. Cortical control of optokinetic nystagmus in humans: a positron emission tomography study. Exp Brain Res. 1999; 126: 149–159 [DOI] [PubMed] [Google Scholar]
- 22. Lauwers K, Saunders R, Vogels R, Vandenbussche E, Orban GA. Impairment in motion discrimination tasks is unrelated to amount of damage to superior temporal sulcus motion areas. J Comp Neurol. 2000; 420: 539–557 [DOI] [PubMed] [Google Scholar]
- 23. Rudolph K, Pasternak T. Transient and permanent deficits in motion perception after lesions of cortical areas MT and MST in the macaque monkey. Cereb Cortex. 1999; 9: 90–100 [DOI] [PubMed] [Google Scholar]
- 24. Newsome WT, Wurtz RH, Dursteler MR, Mikami A. Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J Neurosci. 1985; 5: 825–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marcar VL, Zihl J, Cowey A. Comparing the visual deficits of a motion blind patient with the visual deficits of monkeys with area MT removed. Neuropsychologia. 1997; 35: 1459–1465 [DOI] [PubMed] [Google Scholar]
- 26. Shipp S, De Jong BM, Zihl J, Frackowiak RSJ, Zeki S. The brain activity related to residual motion vision in a patient with bilateral lesions of V5. Brain. 1994; 117: 1023–1038 [DOI] [PubMed] [Google Scholar]
- 27. Vaina LM, Cowey A, Jakab M, Kikinis R. Deficits of motion integration and segregation in patients with unilateral extrastriate lesions. Brain. 2005; 128: 2134–2145 [DOI] [PubMed] [Google Scholar]
- 28. Salzman CD, Murasugi CM, Britten KH, Newsome WT. Microstimulation in visual area MT: effects on direction discrimination performance. J Neurosci. 1992; 12: 2331–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thakral PP, Slotnick SD. Disruption of MT impairs motion processing. Neurosci Lett. 2011; 490: 226–230 [DOI] [PubMed] [Google Scholar]
- 30. Mason AJS, Braddick OJ, Wattam-Bell J. Motion coherence thresholds in infants--different tasks identify at least two distinct motion systems. Vision Res. 2003; 43: 1149–1157 [DOI] [PubMed] [Google Scholar]
- 31. Manny RE, Fern KD. Motion coherence in infants. Vision Res. 1990; 30: 1319–1329 [DOI] [PubMed] [Google Scholar]
- 32. Wattam-Bell J. Coherence thresholds for discrimination of motion direction in infants. Vision Res. 1994; 34: 877–883 [DOI] [PubMed] [Google Scholar]
- 33. Ellemberg D, Lewis TL, Dirks M, et al. Putting order into the development of sensitivity to global motion. Vision Res. 2004; 44: 2403–2411 [DOI] [PubMed] [Google Scholar]
- 34. Hadad BS, Maurer D, Lewis TL. Long trajectory for the development of sensitivity to global and biological motion. Dev Sci. 2011; 14: 1330–1339 [DOI] [PubMed] [Google Scholar]
- 35. Narasimhan S, Giaschi D. The effect of dot speed and density on the development of global motion perception. Vision Res. 2012; 62: 102–107 [DOI] [PubMed] [Google Scholar]
- 36. Parrish EE, Giaschi DE, Boden C, Dougherty R. The maturation of form and motion perception in school age children. Vision Res. 2005; 45: 827–837 [DOI] [PubMed] [Google Scholar]
- 37. Bosworth RG, Birch EE. Motion detection in normal infants and young patients with infantile esotropia. Vision Res. 2005; 45: 1557–1567 [DOI] [PubMed] [Google Scholar]
- 38. Shute RH, Candy R, Westall CA, Woodhouse JM. Success rates in testing monocular acuity and stereopsis in infants and young children. Ophthalmic Physiol Opt. 1990; 10: 133–136 [DOI] [PubMed] [Google Scholar]
- 39. Thompson C, Drasdo N. Clinical experience with preferential looking acuity tests in infants and young children. Ophthalmic Physiol Opt. 1988; 8: 309–321 [PubMed] [Google Scholar]
- 40. Duckman RH, Selenow A. Use of forced preferential looking for measurement of visual acuity in a population of neurologically impaired children. Am J Optom Physiol Opt. 1983; 60: 817–821 [DOI] [PubMed] [Google Scholar]
- 41. McDonald M, Ankrum C, Preston K, Sebris S, Dobson V. Monocular and binocular acuity estimation in 18-to 36-month-olds: acuity card results. Am J Optom Physiol Opt. 1986; 63: 181–186 [DOI] [PubMed] [Google Scholar]
- 42. Awaya S, Sugawara M, Kodama A, Yagasaki T, Oishi F, Hirai T. The preferential looking method for visual acuity assessment in infants with special reference to normal curve, visual acuity difference between the two eyes and success rate in measurement. Folia Ophthol Jpn. 1983; 34: 1160–116l [Google Scholar]
- 43. Cavallini A, Fazzi E, Viviani V, et al. Visual acuity in the first two years of life in healthy term newborns: an experience with the teller acuity cards. Funct Neurol. 2002; 17: 87–92 [PubMed] [Google Scholar]
- 44. Woodhouse JM, Adoh TO, Oduwaiye KA, et al. New acuity test for toddlers. Ophthalmic Physiol Opt. 1992; 12: 249–251 [DOI] [PubMed] [Google Scholar]
- 45. Cyert L. Preschool visual acuity screening with HOTV and Lea symbols: testability and between-test agreement. Optom Vis Sci. 2004; 81: 678–683 [DOI] [PubMed] [Google Scholar]
- 46. Hered RW, Murphy S, Clancy M. Comparison of the HOTV and Lea Symbols charts for preschool vision screening. J Pediatr Ophthalmol Strabismus. 1997; 34: 24–28 [DOI] [PubMed] [Google Scholar]
- 47. McDonald MA. Assessment of visual acuity in toddlers. Surv Ophthalmol. 1986; 31: 189–210 [DOI] [PubMed] [Google Scholar]
- 48. Kay H. New method of assessing visual acuity with pictures. Br J Ophthalmol. 1983; 67: 131–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Broadbent H, Westall CA. An evaluation of techniques for measuring stereopsis in infants and young children. Ophthalmic Physiol Opt. 1990; 10: 3–7 [PubMed] [Google Scholar]
- 50. Büttner U, Kremmyda O. Smooth pursuit eye movements and optokinetic nystagmus. In: Straube A, Buttner U. eds Neuro-Ophthalmology Developments in Ophthalmology. Basel, Switzerland: S. Karger; 2007: 76–89 [DOI] [PubMed] [Google Scholar]
- 51. Banton T, Bertenthal BI. Infants' sensitivity to uniform motion. Vision Res. 1996; 36: 1633–1640 [DOI] [PubMed] [Google Scholar]
- 52. Harris DL, Weston PJ, Harding JE. Incidence of neonatal hypoglycemia in babies identified as at risk. J Ped. 2012; 161: 787–791 [DOI] [PubMed] [Google Scholar]
- 53. Brainard DH. The psychophysics toolbox. Spat Vis. 1997; 10: 433–436 [PubMed] [Google Scholar]
- 54. Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997; 10: 437–442 [PubMed] [Google Scholar]
- 55. Kleiner M, Brainard D, Pelli D, Ingling A, Murray R, Broussard C. What's new in Psychtoolbox-3. Perception. 2007; 36: 14 [Google Scholar]
- 56. Prins N. Psychophysics: a Practical Introduction. Salt Lake City, UT: Academic Press; 2009. [Google Scholar]
- 57. Birch E, Williams C, Hunter J, Lapa MC. Random dot stereoacuity of preschool children. J Pediatr Ophthalmol Strabismus. 1997; 34: 217–222 [DOI] [PubMed] [Google Scholar]
- 58. Lang JI. Eye screening with the Lang stereo test. Am Orthop J. 1988; 38: 48–50 [Google Scholar]
- 59. Adoh TO, Woodhouse JM. The Cardiff acuity test used for measuring visual acuity development in toddlers. Vision Res. 1994; 34: 555–560 [DOI] [PubMed] [Google Scholar]
- 60. Cordonnier M, Dramaix M. Screening for abnormal levels of hyperopia in children: a non-cycloplegic method with a hand held refractor. Br J Ophthalmol. 1998; 82: 1260–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cordonnier M, Dramaix M. Screening for refractive errors in children: accuracy of the hand held refractor Retinomax to screen for astigmatism. Br J Ophthalmol. 1999; 83: 157–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Atkinson J, Braddick O, Robier B, et al. Two infant vision screening programmes: prediction and prevention of strabismus and amblyopia from photo- and videorefractive screening. Eye. 1996; 10: 189–198 [DOI] [PubMed] [Google Scholar]
- 63. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33: 159–174 [PubMed] [Google Scholar]
- 64. Ellemberg D, Lewis TL, Maurer D, Brar S, Brent HP. Better perception of global motion after monocular than after binocular deprivation. Vision Res. 2002; 42: 169–179 [DOI] [PubMed] [Google Scholar]
- 65. Bucher SF, Dieterich M, Seelos KC, Brandt T. Sensorimotor cerebral activation during optokinetic nystagmus. Neurology. 1997; 49: 1370–1377 [DOI] [PubMed] [Google Scholar]
- 66. Bense S, Janusch B, Schlindwein P, et al. Direction-dependent visual cortex activation during horizontal optokinetic stimulation (fMRI Study). Hum Brain Mapp. 2006; 27: 296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dieterich M, Müller-Schunk S, Stephan T, Bense S, Seelos K, Yousry TA. Functional magnetic resonance imaging activations of cortical eye fields during saccades, smooth pursuit, and optokinetic nystagmus. Ann N Y Acad Sci. 2009; 1164: 282–292 [DOI] [PubMed] [Google Scholar]
- 68. Distler C, Hoffmann KP. Visual pathway for the optokinetic reflex in infant macaque monkeys. J Neurosci. 2011; 31: 17659–17668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Atkinson J, Braddick O. Development of optokinetic nystagmus in infants: an indicator of cortical binocularity?. In: Fisher DF, Monty RA, Senders JW. eds Eye Movements: Cognition and Visual Perception. Hillsdale, NJ: Lawrence Erlbaum Associates; 1981; 53–64 [Google Scholar]
- 70. Braddick O, Atkinson J. Development of human visual function. Vision Res. 2011; 51: 1588–1609 [DOI] [PubMed] [Google Scholar]
- 71. Lewis TL, Maurer D, Smith RJ, Haslip JK. The development of symmetrical optokinetic nystagmus during infancy. Clin Vis Sci. 1992; 7: 211–218 [Google Scholar]
- 72. Lewis TL, Maurer D, Chung JY, Holmes-Shannon R, Van Schaik CS. The development of symmetrical OKN in infants: quantification based on OKN acuity for nasalward versus temporalward motion. Vision Res. 2000; 40: 445–453 [DOI] [PubMed] [Google Scholar]
- 73. Atkinson J. Development of optokinetic nystagmus in the human infant and monkey infant: an analogue to development in kittens. In: Freeman RD. ed Developmental Neurobiology of Vision. New York, NY: Plenum Press; 1979; 277–287 [Google Scholar]
- 74. Van Hof-van Duin J, Mohn G Visual field measurements, optokinetic nystagmus and the visual threatening response: normal and abnormal development. Doc Ophthalmol Proc Ser. 1986; 45: 305–316 [Google Scholar]
- 75. Naegele JR, Held R. The postnatal development of monocular optokinetic nystagmus in infants. Vision Res. 1982; 22: 341–346 [DOI] [PubMed] [Google Scholar]
- 76. Roy MS, Lachapelle P, Lepore F. Maturation of the optokinetic nystagmus as a function of the speed of stimulation in fullterm and preterm infants. Clin Vis Sci. 1989; 4: 357–366 [Google Scholar]
- 77. Serences JT, Boynton GM. The representation of behavioral choice for motion in human visual cortex. J Neurosci. 2007; 27: 12893–12899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hesselmann G, Kell CA, Kleinschmidt A. Ongoing activity fluctuations in hMT+ bias the perception of coherent visual motion. J Neurosci. 2008; 28: 14481–14485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lyon DC, Nassi JJ, Callaway EM. A disynaptic relay from superior colliculus to dorsal stream visual cortex in macaque monkey. Neuron. 2010; 65: 270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gaglianese A, Costagli M, Bernardi G, Ricciardi E, Pietrini P. Evidence of a direct influence between the thalamus and hMT+ independent of V1 in the human brain as measured by fMRI. Neuroimage. 2012; 60: 1440–1447 [DOI] [PubMed] [Google Scholar]
- 81. Warner CE, Kwan WC, Bourne JA. The early maturation of visual cortical area MT is dependent on input from the retinorecipient medial portion of the inferior pulvinar. J Neurosci. 2012; 32: 17073–17085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nakamura H, Kashii S, Nagamine T, et al. Human V5 demonstrated by magnetoencephalography using random dot kinematograms of different coherence levels. Neurosci Res. 2003; 46: 423–433 [DOI] [PubMed] [Google Scholar]
- 83. Seymour KJ, Clifford CWG. Decoding conjunctions of direction-of-motion and binocular disparity from human visual cortex. J Neurophysiol. 2012; 107: 2335–2341 [DOI] [PubMed] [Google Scholar]
- 84. Wattam-Bell J. Stereo and motion Dmax in infants. J Vis. 2009; 9: 1–9 [DOI] [PubMed] [Google Scholar]
- 85. Qian N. Computing stereo disparity and motion with known binocular cell properties. Neural Comput. 1994; 6: 390–404 [Google Scholar]
- 86. Maunsell JHR, Van Essen DC. Functional properties of neurons in middle temporal visual area of the macaque monkey. II. Binocular interactions and sensitivity to binocular disparity. J Neurophysiol. 1983; 49: 1148–1167 [DOI] [PubMed] [Google Scholar]
- 87. Born RT, Bradley DC. Structure and function of visual area MT. Neuroscience. 2005; 28: 157–189 [DOI] [PubMed] [Google Scholar]
- 88. DeAngelis GC, Uka T. Coding of horizontal disparity and velocity by MT neurons in the alert macaque. J Neurophysiol. 2003; 89: 1094–1111 [DOI] [PubMed] [Google Scholar]
- 89. Krug K, Parker AJ. Neurons in dorsal visual area V5/MT signal relative disparity. J Neurosci. 2011; 31: 17892–17904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bradley DC, Qian N, Andersen RA. Integration of motion and stereopsis in middle temporal cortical area of macaques. Nature. 1995; 373: 609–611 [DOI] [PubMed] [Google Scholar]
- 91. DeAngelis GC, Newsome WT. Perceptual “read-out” of conjoined direction and disparity maps in extrastriate area MT. PLoS Biol. 2004; 2: E77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tailby C, Majaj NJ, Movshon JA. Binocular integration of pattern motion signals by MT neurons and by human observers. J Neurosci. 2010; 30: 7344–7349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Milne E, White S, Campbell RA, Swettenham J, Hansen PC, Ramus F. Motion and form coherence detection in autistic spectrum disorder: Relationship to motor control and 2:4 digit ratio. J Autism Dev Disord. 2006; 36: 225–237 [DOI] [PubMed] [Google Scholar]
- 94. Purcell C, Wann JP, Wilmut K, Poulter D. Reduced looming sensitivity in primary school children with Developmental Co-ordination Disorder. Developmental Science. 2012; 15: 299–306 [DOI] [PubMed] [Google Scholar]
- 95. Sigmundsson H, Hansen PC, Talcott JB. Do ‘clumsy' children have visual deficits. Behav Brain Res. 2003; 139: 123–129 [DOI] [PubMed] [Google Scholar]
- 96. Hrisos S, Clarke MP, Kelly T, Henderson J, Wright CM. Unilateral visual impairment and neurodevelopmental performance in preschool children. Br J Ophthalmol. 2006; 90: 836–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. O'Connor AR, Birch EE, Anderson S, Draper H. Relationship between binocular vision, visual acuity, and fine motor skills. Optom Vis Sci. 2010; 87: 942–947 [DOI] [PubMed] [Google Scholar]
- 98. O'Connor AR, Birch EE, Anderson S, Draper H. Group tFR. The functional significance of stereopsis. Invest Ophthalmol Vis Sci. 2010; 51: 2019–2023 [DOI] [PubMed] [Google Scholar]
- 99. Trick GL, Silverman SE. Visual sensitivity to motion: age-related changes and deficits in senile dementia of the Alzheimer type. Neurology. 1991; 41: 1437–1440 [DOI] [PubMed] [Google Scholar]
- 100. Trick GL, Steinman SB, Amyot M. Motion perception deficits in glaucomatous optic neuropathy. Vision Res. 1995; 35: 2225–2233 [DOI] [PubMed] [Google Scholar]
- 101. Zwicker AE, Hoag RA, Edwards VT, Boden C, Giaschi DE. The effects of optical blur on motion and texture perception. Optom Vis Sci. 2006; 83: 382–390 [DOI] [PubMed] [Google Scholar]
- 102. Roach NW, Edwards VT, Hogben JH. The tale is in the tail: an alternative hypothesis for psychophysical performance variability in dyslexia. Perception. 2004; 33: 817–830 [DOI] [PubMed] [Google Scholar]
- 103. Spry PGD, Johnson CA, McKendrick AM, Turpin A. Measurement error of visual field tests in glaucoma. Br J Ophthalmol. 2003; 87: 107–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bertone A, Mottron L, Jelenic P, Faubert J. Motion perception in autism: A “complex” issue. J Cogn Neurosci. 2003; 15: 218–225 [DOI] [PubMed] [Google Scholar]
- 105. Bertone A, Mottron L, Jelenic P, Faubert J. Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain. 2005; 128: 2430–2441 [DOI] [PubMed] [Google Scholar]
- 106. Kogan CS, Bertone A, Cornish K, et al. Integrative cortical dysfunction and pervasive motion perception deficit in fragile X syndrome. Neurology. 2004; 63: 1634–1639 [DOI] [PubMed] [Google Scholar]
- 107. Nakamura M, Kaneoke Y, Watanabe K, Kakigi R. Visual information process in Williams syndrome: intact motion detection accompanied by typical visuospatial dysfunctions. Eur J Neurosci. 2002; 16: 1810–1818 [DOI] [PubMed] [Google Scholar]
- 108. Hyon JY, Yeo HE, Seo JM, Lee IB, Lee JH, Hwang JM. Objective measurement of distance visual acuity determined by computerized optokinetic nystagmus test. Invest Ophthalmol Vis Sci. 2010; 51: 752–757 [DOI] [PubMed] [Google Scholar]