Abstract
Human cytomegalovirus (HCMV) pathogenesis is characterized by multiple organ system involvement due to viral spread to host organs after a cell-associated viremia. The cell type responsible for HCMV dissemination is unknown. Monocytes are the most likely candidate since they are the predominant cell type infected in the blood. However, monocytes are not productive for viral replication and are abortively infected. The results presented here provide a potential answer to this conundrum. We report that primary HCMV infection of monocytes induces transendothelial migration and monocyte-to-macrophage differentiation and that these HCMV-differentiated macrophages are productive for viral replication. Together, our data suggest a novel mechanism for HCMV pathogenesis; HCMV induces cellular changes in monocytes to promote viral replication and spread to host organs.
Human cytomegalovirus (HCMV) is a leading cause of morbidity and mortality in immunocompromised hosts. It is the leading cause of congenital central nervous system damage and deafness in neonates (58), one of the most common viral opportunistic infections in AIDS patients (38), and an important infectious agent affecting organ transplant recipients (23). In immunocompetent hosts, HCMV causes some cases of infectious mononucleosis and is associated with cardiovascular disease (1, 17, 31, 33, 39, 46, 57, 60). A hallmark of HCMV infection is a broad range of pathological complications attributed to viral spread to virtually every organ in the host, including the gastrointestinal tract, liver, salivary glands, brain, central nervous system, kidney, retina, and lung (44, 53, 63).
Systemic distribution of HCMV occurs during symptomatic and asymptomatic infections (63) and is believed to be essential for HCMV survival through the establishment of persistent infection in host organs, with subsequent viral shedding and spread to additional hosts (53). Therefore, the ability of HCMV to persist in a host and in the general population is dependent on efficient viral spread to multiple organ systems, which in immunocompromised hosts leads to overt organ disease. During primary infection, HCMV spreads from the initial site of infection to the peripheral blood, from which dissemination to host organ tissue occurs (53). It is known that HCMV viremia is cell associated, suggesting that peripheral blood leukocytes are involved (51, 53). However, the mechanism by which viral spread to host organ tissue occurs is unresolved, and the cell type responsible is unknown (53).
Because HCMV infection of host organs causes severe disease in immunocompromised patients (24), the investigation of the mechanism(s) for hematogenous spread is of key importance for understanding HCMV pathogenesis. Monocytes have been proposed to be the cell type responsible for dissemination and disease for several reasons. First, monocytes are the primary cell type infected in the blood during acute HCMV infection, as determined by the detection of HCMV DNA and antigens in monocytes (51, 53, 61). Second, monocytes are the predominant infiltrating cell type found in infected organs (9). Third, infection of peripheral blood mononuclear cells is more closely associated with clinical manifestations of organ disease than infection of polymorphonuclear leukocytes (36, 49), a finding consistent with studies associating viral dissemination with HCMV-mediated organ disease (7, 36, 53). Fourth, the analysis of murine CMV, a related herpesvirus, suggests that monocyte-associated viremia is a prerequisite for viral pathogenesis (10, 48, 59). However, in vivo and in vitro studies have shown that monocytes are not productive for viral replication and are abortively infected (14, 19, 20, 26, 51, 53, 62). This is a puzzling scenario; monocytes are “at the right place at the right time” to serve as vectors for dissemination but are not productive for viral replication. The short life span of monocytes in the blood (25) further complicates this scenario, because HCMV has a slow replication cycle of several days to weeks in vivo (43). On the other hand, macrophages, the differentiated counterparts of monocytes, are long-lived cells and are productively infected in patients with disseminated HCMV disease (15, 54). HCMV replication in macrophages has been confirmed by using in vitro-derived macrophage model systems (13, 26, 32, 55). However, because macrophages are not blood-borne cells, they cannot be responsible for HCMV hematogenous dissemination to host organs.
We propose an answer to this conundrum. When considered collectively, monocytes and macrophages possess the necessary features for viral dissemination. As monocytes they have the capacity to be infected in the blood and to migrate into host organs, while as macrophages they have the capacity to replicate the virus within host tissue. We hypothesized, therefore, that primary infection of monocytes induces differentiation into permissive macrophages and promotes transendothelial migration. This strategy would allow the virus to overcome two key hurdles for HCMV dissemination: location and replication. For this viral strategy to be feasible, HCMV must exert cellular changes in monocytes in the absence of viral gene expression. In support of this concept, we previously showed that HCMV binding to the cell surface of monocytes initiated a cellular signal transduction event, which resulted in increased inflammatory cytokine production (65).
Here, we report that a primary HCMV infection of human peripheral blood monocytes promotes monocyte-to-macrophage differentiation independently of viral gene expression. These HCMV-differentiated macrophages exhibited immediate-early (IE) and late viral gene expression and released virus, indicating that a primary infection of monocytes is not abortive and specifically drives the differentiation of monocytes to generate a cellular environment supportive of viral replication. Consistent with the idea of monocytes serving as mobile vectors for viral dissemination, we further demonstrated that HCMV infection of monocytes promoted transendothelial migration, increased cell motility, and upregulated adhesion molecule expression. Together, the results of these experiments provide evidence for a mechanism of HCMV dissemination and a role for infected monocytes in HCMV pathogenesis.
MATERIALS AND METHODS
Monocyte isolation.
Human peripheral blood monocytes were purified by double-density gradient centrifugation (65, 68). Whole blood was taken from HCMV-seronegative donors by venipuncture and centrifuged through a Ficoll Histopaque 1077 (Sigma, St. Louis, Mo.) gradient. Mononuclear cells were washed to remove platelets. Monocytes were further purified by centrifugation through a Percoll (Pharmacia, Piscataway, N.J.) gradient. This procedure yields ca. 3 × 107 to 5 × 107 monocytes per donor at a purity of ca. 90%. Monocytes were plated on fibronectin (Calbiochem, San Diego, Calif.) or collagen type IV (Sigma)-coated plates at cell densities of 106 cells per 100-mm dish or 2.5 × 104 cells per well in 24-well plates. Monocytes were cultured in RPMI 1640 (Cellgro; Mediatech, Herndon, Va.) supplemented with 10% human serum (Sigma) at 37°C with 5% CO2. Remaining nonadherent lymphocytes were removed by washing the cells at 4 h and 24 h postadhesion. University Institutional Review Board and Health Insurance Portability and Accountability Act protocols were followed for these procedures.
HCMV infection.
HCMV (Towne/E strain; passages 41 to 44) was cultured in human embryonic lung (HEL) fibroblasts, gradient purified (65-67), and used to infect monocytes at a multiplicity of infection (MOI) of 15. UV-inactivated virus was prepared as previously described from gradient-purified virus (66) and used to treat monocytes at an equivalent MOI of 15. UV-inactivated virus was replication defective and did not express detectable IE gene products in HEL fibroblasts (data not shown).
Phagocytosis assays.
Nonopsonized fluorescein isothiocyanate (FITC)-conjugated heat-killed yeast particles (zymosan) were prepared as previously described (22). A total of 2.5 × 104 mock-infected or HCMV-infected monocytes were added to glass coverslips in 24-well plates and then incubated. At each time point, 2.5 × 105 FITC-zymosan were added per well, followed by incubation for 1 h. Coverslips were washed in ice-cold saline to stop phagocytosis and then stained for 10 s with fluorescence quenching dye (0.4 mg of crystal violet/ml). Coverslips were mounted on slides and examined by fluorescence microscopy to determine the total ingestion, representing the total number of zymosan internalized by 50 cells in random fields of view, and the percentage of cells positive for phagocytosis of one or more zymosan out of 50 cells in random fields of view. The data are plotted as the means ± the standard deviations (SD) of quadruplicate experiments from a single blood donor. The statistical significance between experimental means (P value) was determined by the Student t test.
Western blot analysis.
One-hundred millimeter dishes containing 106 monocytes were harvested in whole-cell extract (WCE) buffer containing 5 mM HEPES (pH 7.9), 250 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 10% glycerol, 0.1% NP-40, 1 mM dithiothreitol, 10 μM trichostatin A (Calbiochem), 1× protease inhibitor cocktail (Sigma), 1× phosphatase inhibitor cocktail I (Sigma), and 1× phosphatase inhibitor cocktail II (Sigma). Samples were incubated on ice for 30 min and then centrifuged. Supernatants were collected and stored at −70°C. Protein quantification was performed on the WCEs by using the DC protein assay (Bio-Rad, Hercules, Calif.). Prior to Western blot analysis, WCEs were added to Laemmli sample buffer (Bio-Rad), and the mixtures were boiled. Equal protein amounts of each sample were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose (Immobilon-P; Millipore, Billerica, Mass.). Blots were blocked in a solution of 5% skim milk, 0.1% Tween 20, and phosphate-buffered saline (PBS) solution, followed by incubation with anti-CD68 monoclonal antibody (MAb; Beckman Coulter, Brea, Calif.), anti-HLA-DR MAb (Beckman Coulter), anti-β1 β-subunit-specific MAb TS2/16 (ATCC, Manassas, Va.), anti-ZO-1 MAb (Zymed, San Francisco, Calif.), or anti-occludin MAb (Zymed) in blocking solution. Blots were washed, incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, Calif.), and detected by using the ECL Plus (Pharmacia) protocol.
Plaque assays.
A total of 106 HCMV-infected or mock-infected monocytes were plated on fibronectin- or collagen-coated 100-mm dishes. At weekly intervals, supernatants were harvested from mock-infected and infected monocytes. Cell supernatants were purified on a sucrose gradient as described above to isolate potential virus and to remove cellular contaminants. Serial dilutions of purified supernatants were added to HEL fibroblasts, and fibroblasts were overlaid with a 50% methyl cellulose-50% medium suspension. At 2 weeks after the addition of monocyte supernatants to fibroblasts, the plaques were counted for each experimental arm.
Immunofluorescence staining.
We plated 2.5 × 104 HCMV-infected or mock-infected monocytes on fibronectin-coated coverslips. At the indicated time points, cells were fixed in paraformaldehyde and permeabilized in a PBS solution containing 0.1% Triton X-100, 1% bovine serum albumin, and 10% normal goat serum. Cells were then washed and incubated with primary antibodies against either gH (8) or IE2 (27) in blocking solution (PBS containing 1% bovine serum albumin and 10% normal goat serum). Cells were washed, incubated with an FITC-conjugated anti-mouse secondary antibody, and examined by fluorescence microscopy.
Transendothelial migration assay.
Transendothelial migration assays were performed with human dermal microvascular endothelial cells (HMECs) cultured on cell culture inserts (BD Falcon, Bedford, Mass.) with an 8-μm pore size in 24-well plates (Corning Costar, Cambridge, Mass.) and grown to confluence. HMECs were incubated and grown to confluence in EGM-1 (Clonetics, Walkersville, Md.) supplemented with 10% heat-inactivated fetal bovine serum (Clonetics), hydrocortisone (1 μg/ml; Clonetics), human epidermal growth factor (10 ng/ml; Clonetics), bovine brain extract (12 μg/ml; Clonetics), and gentamicin sulfate-amphotericin B (Clonetics) at 37°C with 5% CO2. Isolated monocytes were labeled with CellTracker Green CMFDA (5-chloromethylfluorescein diacetate; Molecular Probes, Eugene, Oreg.) according to the manufacturer's protocol. The dyed monocytes were infected with HCMV (MOI of 15), treated with UV-inactivated HCMV (equivalent MOI of 15), treated with phorbol myristate acetate (PMA; 10 ng/ml; Sigma), or mock infected by rocking them in 15-ml conical tubes (Falcon) at 37°C for 3 h. Monocytes were pelleted by centrifugation and resuspended in RPMI medium supplemented with 10% human serum. Prior to the addition of monocytes to the transwells, HMECs in the upper compartment were washed, and the medium in the transwells was replaced with RPMI medium supplemented with 10% human serum. Then, 2.5 × 104 labeled monocytes were plated onto each transwell, followed by incubation at 37°C with 5% CO2. At 24 h after the addition of the monocytes, the ratios of cells undergoing diapedesis versus those cells that were stationary on the surface of the endothelial monolayer were determined by inverted fluorescence microscopy. The results are plotted as the means ± the SD of 10 random fields of view. At 96 h after addition of the monocytes, the number of monocytes that had migrated completely through the endothelial monolayer and the cell culture insert were determined by inverted fluorescence microscopy. The results are plotted as mean ± the SD of 10 random fields of view. To determine the percentage of monocytes that had undergone total migration at 96 h out of the total number of monocytes added per transwell, transwells were removed from the 24-well plates. Monocytes adhered to the bottom of each well were incubated with EDTA for 1 h at 37°C and removed from the wells by gentle scraping. Monocytes were washed and counted with a hemocytometer to determine the total number of monocytes per well. The results are plotted as means ± the SD of three counted wells per experimental arm. Statistical significance between experimental means (P value) was determined by using the Student t test.
Phagokinetic track motility assay.
Colloidal gold-coated coverslips were prepared as previously described (2, 50). Briefly, glass coverslips were immersed in a 300 bloom gelatin solution (0.5 g in 300 ml; Sigma), heated at 90°C for 10 min, and dried at 70°C for 45 min. A colloidal gold suspension was prepared by adding 11 ml of tissue culture water (Sigma) and 6 ml of Na2CO3 (36.5 mM) to 1.8 ml of AuHCl4 (14.5 mM; Fisher Scientific, Pittsburgh, Pa.), bringing the solution to a boil, and rapidly adding 1.8 ml of 0.1% formaldehyde (Fisher Scientific). While hot, 2 ml of the colloidal gold suspension was added to each coverslip, followed by incubation at 37°C for 1 h. The coverslips were washed and transferred to 24-well plates. Then, 103 mock-infected, HCMV-infected (MOI of 15), UV-inactivated HCMV-treated (equivalent MOI of 15), and PMA-treated (10 ng/ml) monocytes were added per well, followed by incubation for 12 h at 37°C with 5% CO2. Cells were fixed and mounted on slides. Track images of cells were video captured, and the average area cleared per cell out of 50 cells per sample was determined in square arbitrary units by using Scion Image software. The results are plotted as mean ± the standard error of the mean of 50 cells. The statistical significance between experimental means (P value) was determined by using the Student t test.
RESULTS
HCMV promotes monocyte-to-macrophage differentiation.
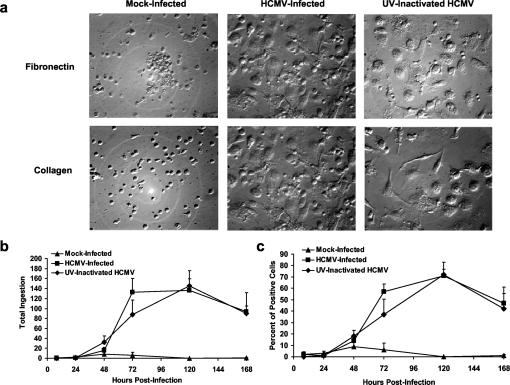
We hypothesized that HCMV infection promotes monocyte-to-macrophage differentiation as a strategy to promote viral replication. Monocytes lose their small, rounded morphology and exhibit increased size, cell spreading, and granularity as they differentiate into macrophages (5, 21). To determine whether HCMV infection of monocytes promotes differentiation, we first examined the morphology of mock-infected, HCMV-infected, and UV-inactivated HCMV-treated human peripheral blood monocytes over a 5-week time course. HCMV-infected and UV-inactivated HCMV-treated monocytes exhibited a maximal degree of cell spreading and granularity by 11 days postinfection when cultured on fibronectin or collagen (type IV)-coated plates (Fig. 1a), and this macrophage phenotype was maintained throughout the 5-week time course (data not shown). Mock-infected monocytes cultured on fibronectin or collagen (type IV)-coated plates exhibited a smaller, more rounded morphology at 11 days postinfection (Fig. 1a) and did not during the 5-week time course show the cell spreading and granularity seen in infected and UV-inactivated HCMV-treated monocytes (data not shown). UV-inactivated HCMV is incapable of de novo viral gene expression, suggesting that HCMV infection of monocytes can promote monocyte-to-macrophage differentiation independent of new viral gene expression.
FIG. 1.
HCMV infection promotes monocyte-to-macrophage differentiation. (a) HCMV promotes differentiation independently of viral gene expression as defined by the gain of macrophage morphology. Mock-infected, HCMV-infected, and UV-inactivated HCMV-treated monocytes were cultured on fibronectin and collagen for 11 days postinfection. Magnification, ×200 for all panels shown. The data shown are representative of five independent experiments from separate human blood donors. (b and c) HCMV promotes differentiation independently of viral gene expression as defined by the gain of macrophage function. FITC-zymosan was added to mock-infected, HCMV-infected, and UV-inactivated virus-treated monocytes at the indicated times postinfection, followed by incubation for 1 h. (b) Total ingestion represents the total number of FITC-zymosan internalized by 50 cells. (c) The percentage of cells positive for phagocytosis represents the percentage of cells positive for the internalization of one or more yeast particles out of 50 cells. The results are plotted as means ± the SD of quadruplicate experiments from a single blood donor. The data shown are representative of five independent experiments from separate blood donors. HCMV-infected and UV-inactivated HCMV-treated groups are significantly different (P < 0.01) from mock-infected groups at 48, 72, 120, and 168 h postinfection.
As controls, we showed that monocytes did not acquire macrophage morphology when treated with uninfected fibroblast supernatants or when HCMV was neutralized with neutralizing antibody prior to addition to monocyte cultures (data not shown). HCMV-induced differentiation was dependent, therefore, upon the specific interaction of HCMV with monocytes. Immunofluorescent staining of infected monocyte cultures for the HCMV tegument protein pp65 showed that ∼100% of monocytes contained pp65 at 4 h postinfection, indicating that the virus bound to and entered monocytes in our system (data not shown). Furthermore, the addition of infected monocyte supernatant to freshly isolated monocytes from the same donor did not promote morphological differentiation (data not shown). These data indicated that HCMV-induced differentiation was not a bystander effect and was not due solely to the release of soluble mediators.
As monocytes differentiate into functional macrophages, increased zymosan phagocytosis occurs (5, 21). To determine whether HCMV infection induces functional macrophage differentiation, mock-infected, HCMV-infected, and UV-inactivated virus-treated monocytes were cultured on glass coverslips and assayed for FITC-zymosan phagocytosis over a 1-week time course. To ensure that noninternalized zymosan were not scored, the technique of fluorescent dye quenching was used in our assay to quench the fluorescence of bound, noninternalized zymosan (22). HCMV-infected and UV-inactivated HCMV-treated monocytes exhibited a time-dependent increase in total ingestion (Fig. 1b), which represents the total number of zymosan ingested by 50 cells, and in the percentage of cells positive for phagocytosis (Fig. 1c). Mock-infected monocytes did not exhibit a time-dependent increase in either total ingestion (Fig. 1b) or in the percentage of cells positive for zymosan phagocytosis (Fig. 1c). Similar results were obtained when monocytes were cultured on fibronectin- or collagen-coated coverslips (data not shown). As an additional internalization control, cytochalasin D, an inhibitor of actin filament function that blocks ingestion but not the binding of zymosan (45, 64), was used. The pretreatment of monocyte cultures with cytochalasin D 1 h prior to the addition of zymosan abolished phagocytosis for HCMV-infected, UV-inactivated HCMV-treated, and mock-infected monocytes at each time point (data not shown), showing that washing steps sufficiently removed noninternalized zymosan in our assay system.
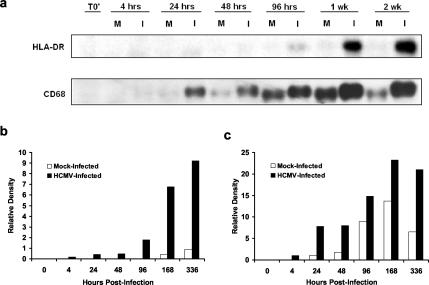
Human leukocyte-associated antigen-DR (HLA-DR) and CD68, a human pan-macrophage marker homologous to mouse macrosialin, are expressed at low levels by monocytes and are upregulated during monocyte-to-macrophage differentiation (6, 16, 37). To determine the temporal kinetics of HLA-DR and CD68 expression by mock and infected monocytes, Western blot analyses were performed over a time course of infection with equal loading of protein harvested from mock-infected and HCMV-infected monocytes cultured on fibronectin-coated plates (Fig. 2a). HLA-DR (Fig. 2b) and CD68 (Fig. 2c) expression was quantitated by densitometry. The expression of HLA-DR by infected monocytes was detected at 96 h postinfection and increased out to 2 weeks postinfection, whereas mock-infected monocytes did not express this marker at detectable levels throughout the 2-week time course (Fig. 2a and b). CD68 was expressed earlier and at higher levels in HCMV-infected monocytes than in mock-infected monocytes (Fig. 2a and c). The finding that low levels of CD68 were expressed by mock-infected monocytes suggests that these cells underwent some aspect of differentiation with reduced kinetics, a finding consistent with reports that adhesion events can promote various degrees of differentiation (29). A higher-molecular-weight form of CD68 was detected in HCMV-infected monocytes but not in mock-infected cells. A higher-molecular-weight glycoform of CD68 is reported to be expressed by macrophages exposed to inflammatory stimuli (47), a finding consistent with our previous findings that HCMV infection is an inflammatory stimulus (65). Increased expression of these macrophage markers was confirmed by immunofluorescent staining. All infected cells (∼100%) expressed high levels of HLA-DR and CD68, as well as the myeloid lineage marker CD14 (data not shown). Thus, infected cells were of myeloid origin (CD14+) and expressed differentiation markers (CD68 and HLA-DR). Together, these results suggest that HCMV promotes monocyte-to-macrophage differentiation as determined by morphological, functional, and phenotypic assays.
FIG. 2.
HCMV infection results in the upregulation of macrophage markers. (a) Western blot analyses of HLA-DR and CD68 were performed by using equal protein loading of mock-infected and HCMV-infected monocyte cell lysates harvested at the indicated times postinfection. Bands were analyzed by densitometry to determine relative levels of HLA-DR (b) and CD68 (c) expression. T0′ (time zero) represents cell lysates harvested from freshly isolated monocytes. The results are representative of three independent experiments from separate human blood donors.
HCMV-differentiated macrophages are permissive for viral replication.
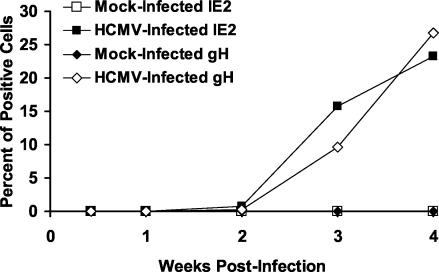
For HCMV-infected monocytes to serve as vectors for viral dissemination, they must overcome the block in viral gene expression and replication. To determine whether HCMV-differentiated macrophages become permissive for viral gene expression, immunofluorescent staining for the IE viral gene product, IE2, and the late viral gene product, gH, was performed over a 4-week time course on mock-infected and HCMV-infected monocytes cultured on fibronectin-coated coverslips. IE2 and gH were expressed by ca. 15 and 10% of HCMV-induced macrophages, respectively, at 3 weeks postinfection (Fig. 3). By 4 weeks postinfection, ca. 25% of HCMV-induced macrophages expressed IE2 and gH (Fig. 3), demonstrating that the process of HCMV-induced monocyte-to-macrophage differentiation allowed HCMV-infected cells to overcome the block in viral gene expression. Mock-infected monocytes did not express detectable levels of IE2 or gH over the time course (Fig. 3). Slot blot hybridization analysis showed that the viral genome was maintained in the infected monocyte cultures during the period of viral inactivity (data not shown). Infected cells expressing gH expressed high levels of CD68 and HLA-DR and exhibited macrophage morphology (data not shown), demonstrating that the cells replicating HCMV were true macrophages.
FIG. 3.
HCMV-induced macrophages become permissive for HCMV gene expression. Mock-infected and HCMV-infected monocytes were cultured on fibronectin-coated coverslips, and immunofluorescent staining for the IE viral gene product IE2 and the late viral gene product gH was performed at the indicated times postinfection. The data are presented as the percentage of cells positive for IE2 or gH expression. The results are representative of three independent experiments from separate human blood donors.
We next determined whether virus was released into cellular supernatants by performing plaque assays on supernatants harvested at weekly intervals from HCMV-infected and mock-infected monocytes. Consistent with the time course of viral gene expression (Fig. 3), infectious virus was first detected in supernatants harvested from HCMV-infected monocytes cultured on fibronectin at 3 weeks (donor 1) and 4 weeks (donor 2) postinfection and was still detectable in supernatants harvested at 5 weeks postinfection (Table 1). The highest titer of virus obtained over both time courses of infection was 103 PFU/ml at 4 weeks postinfection. HCMV-infected monocytes cultured on collagen (type IV) did not produce virus until 5 weeks postinfection (data not shown), suggesting that adhesion to extracellular matrix components influences viral replication kinetics in HCMV-induced macrophages. These data demonstrate that a primary infection of monocytes with HCMV is productive and is contingent upon an HCMV-induced differentiation event.
TABLE 1.
HCMV-induced macrophages release infectious HCMVa
| Donor | Presence (+) or absence (−) of PFU at:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 wk
|
2 wk
|
3 wk
|
4 wk
|
5 wk
|
||||||
| Mock | Infected | Mock | Infected | Mock | Infected | Mock | Infected | Mock | Infected | |
| 1 | − | − | − | − | − | + | − | + | − | + |
| 2 | − | − | − | − | − | − | − | + | − | + |
Mock-infected and HCMV-infected monocytes from two human donors were cultured separately on fibronectin-coated dishes. Cellular supernatants were harvested at weekly intervals, serially diluted, and subjected to plaque assays. The peak virus titer was 103 PFU/ml at 4 weeks postinfection for donors 1 and 2.
HCMV infection promotes transendothelial migration.
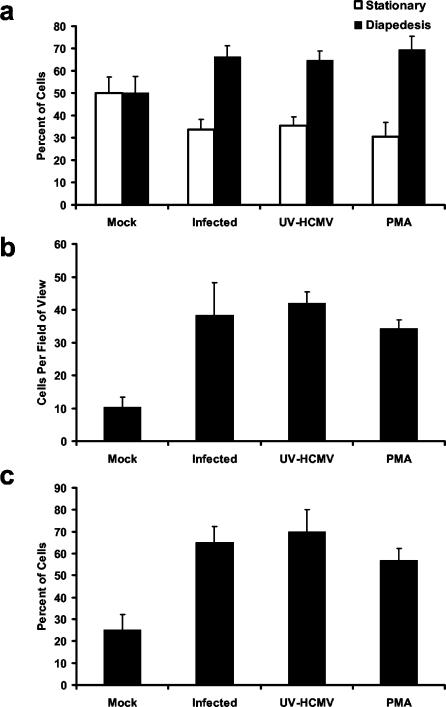
For HCMV-induced differentiation to be a mechanism of viral dissemination, the infected cells must leave the peripheral blood and enter various tissues as macrophages. We hypothesized that the activation of monocytes after HCMV infection promotes transendothelial migration as a means of driving infected cells into host organs. To test this hypothesis, we examined the potential of HCMV-infected and mock-infected monocytes to migrate, in the absence of a chemoattractant, through transwells containing monolayers of HMECs. At 24 h after the addition of monocytes to the upper chamber of the transwells, the ratio of monocytes that were undergoing diapedesis through the HMEC monolayer to those monocytes that were stationary on the surface of the HMEC monolayer was determined. Both HCMV-infected and UV-inactivated HCMV-treated monocytes exhibited a significantly (P < 0.01) higher percentage of cells undergoing diapedesis (∼65%) than the percentage of mock-infected monocytes undergoing diapedesis (∼50%; Fig. 4a). PMA treatment of monocytes was used as a positive control. At 96 h after the addition of monocytes to the upper chamber, we determined the average number of monocytes per field of view that had completely migrated through HMEC monolayers and through the pores in the transwell (total migration). HCMV-infected, UV-inactivated HCMV-treated, and PMA-treated monocytes exhibited an ∼4-fold increase in total migration compared to mock-infected monocytes after 96 h (Fig. 4b). The percentage of total monocytes added to the transwells that had undergone total migration was next determined at 96 h after the addition of monocytes to the transwells. Approximately 70% of the total number of HCMV-infected and UV-inactivated HCMV-treated monocytes added to the transwells had undergone total migration, whereas only 25% of the total number of mock-infected monocytes added to the transwells underwent total migration (Fig. 4c). HCMV infection also promoted transendothelial migration through immortalized human endothelial cells derived from microvascular endothelial cells of the brain and primary human umbilical vein endothelial cells of embryonic and macrovascular origin (data not shown). Together, these results indicate that HCMV promotes monocyte transendothelial migration independent of viral gene expression and are consistent with the proposed role of monocytes in viral dissemination.
FIG. 4.
HCMV infection of monocytes promotes transendothelial migration. (a) HCMV infection of monocytes is associated with an increase in diapedesis at 24 h postinfection. Cell tracker green-labeled mock-infected, HCMV-infected, UV-inactivated HCMV-treated, and PMA-treated monocytes were added to cell culture inserts containing confluent monolayers of HMECs. The ratios of cells undergoing diapedesis to those cells that were stationary at the monolayer surface were determined by fluorescence microscopy after 24 h in culture. The results are plotted as means ± the SD of 10 random fields of view at ×100 magnification. The results are representative of five independent experiments from separate human blood donors. HCMV-infected, UV-inactivated HCMV-treated, and PMA-treated groups are significantly different (P < 0.01) from the mock-infected group. (b) HCMV infection of monocytes is associated with increased total migration through endothelial monolayers. The number of monocytes that had migrated through endothelial monolayers and the cell culture insert filter was determined by fluorescence microscopy after 96 h in culture. The results are plotted as means ± the SD of 10 random fields of view at ×200 magnification. The results are representative of five independent experiments from separate human blood donors. HCMV-infected, UV-inactivated HCMV-treated, and PMA-treated groups are significantly different (P < 0.01) from the mock-infected group. (c) The majority of HCMV-infected and UV-inactivated HCMV-treated monocytes added to the transwells underwent complete transendothelial migration. At 96 h after the addition of monocytes to the transwells, monocytes adhered to the bottom of the wells were removed by using EDTA and then counted with a hemocytometer to determine the percentage of monocytes having undergone total migration out of the total number (2.5 × 104) of monocytes added per transwell. The results are plotted as means ± the SD of three wells per experimental arm. The results are representative of three independent experiments from separate blood donors. HCMV-infected, UV-inactivated HCMV-treated, and PMA-treated groups are significantly different (P < 0.01) from the mock-infected group.
Mechanisms of HCMV-mediated migration of infected monocytes.
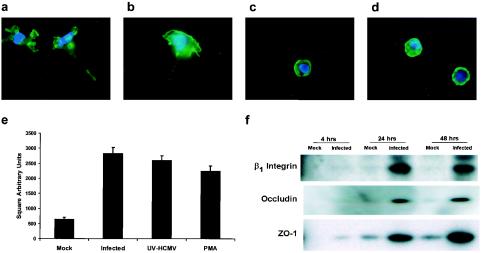
The increased percentage of HCMV-infected monocytes undergoing diapedesis (Fig. 4a) suggested that HCMV induced cellular changes in monocytes involved in the early events of transendothelial migration. Cell motility is a key factor regulating the early event of monocyte diapedesis (28). At early times postinfection, HCMV-infected monocytes were observed to exhibit a motile cell morphology compared to mock-infected monocytes. F-actin staining of HCMV-infected monocytes at 48 h postinfection revealed pronounced pseudopodia (Fig. 5a) and at 72 h postinfection revealed pronounced lamellipodium and retractile fiber formation (Fig. 5b), findings that are indicative of cellular motility (28). Mock-infected monocytes exhibited a phenotype contraindicative of a motile cell in that they exhibited a rounded morphology with staining of a cortical ring of F-actin at 48 h (Fig. 5c) and 72 h (Fig. 5d) postinfection. To quantitatively evaluate the induction of monocyte motility by HCMV infection, we performed phagokinetic track random motility assays by culturing mock-infected, HCMV-infected, UV-inactivated HCMV-treated, and PMA-treated monocytes on colloidal gold-coated coverslips for 12 h. At 12 h postadhesion, the average cleared area per cell in square arbitrary units was determined for mock-infected, HCMV-infected, UV-inactivated HCMV-treated, and PMA-treated monocytes. Both HCMV-infected and UV-inactivated HCMV-treated monocytes exhibited a similar degree of motility compared to the PMA-treated monocyte control and were approximately five times more motile than mock-infected monocytes (Fig. 5e), suggesting that HCMV promotes diapedesis by upregulating cell motility independently of viral gene expression.
FIG. 5.
HCMV infection of monocytes promotes cell motility and adhesion molecule expression. (a to d) HCMV-infected monocytes have a motile morphology, as illustrated by F-actin staining. HCMV-infected monocytes at 48 h (a) and 72 h (b) postinfection and mock-infected monocytes at 48 h (c) and 72 h (d) postinfection were stained with Alexa Fluor 594 phalloidin and DAPI (4′,6′-diamidino-2-phenylindole). The results are representative of three independent experiments from separate human blood donors. (e) HCMV infection of monocytes promotes cell motility independent of viral gene expression. Phagokinetic track motility assays were performed to determine the average area (in square arbitrary units) of colloidal gold cleared per monocyte by mock-infected, HCMV-infected, UV-inactivated HCMV-treated, and PMA-treated samples at 12 h postadhesion. The results are plotted as means ± the standard error of the mean of 50 cells. The results are representative of three independent experiments from separate human blood donors. HCMV-infected, UV-inactivated HCMV-treated, and PMA-treated groups are significantly different (P < 0.01) from the mock-infected group. (f) HCMV infection of monocytes upregulates the expression of adhesion molecules. Western blot analyses for the β1 integrin subunit, occludin, and ZO-1 were performed by using equal protein loading of mock-infected and HCMV-infected monocyte cell lysates harvested at the indicated times postinfection. The results are representative of two independent experiments from separate human blood donors.
Firm adhesion to endothelial cells is another factor regulating monocyte diapedesis through the endothelium. The β1 integrin VLA-4 binds to immunoglobulin superfamily counter-receptors expressed on the cell surface of endothelial cells (40) and promotes firm adhesion. Western blot analysis revealed that HCMV-infected monocytes expressed higher levels of the β1 integrin subunit than mock-infected monocytes (Fig. 5f), a finding consistent with the idea of HCMV promoting firm adhesion of monocytes to the endothelium. Increased expression of β1 integrins on the surface of monocytes was confirmed by immunofluorescent staining (data not shown).
The fourfold increase in total migration of HCMV-infected monocytes suggested that HCMV infection induces cellular changes in monocytes involved in the late stages of transendothelial migration. Occludin is a transmembrane protein that interacts in a zipper-like manner with copies of occludin on adjacent cells in endothelial monolayers to form tight junctions, which impose a barrier to leukocytes during the later stages of transendothelial migration (42). ZO-1 is a peripheral membrane protein that associates with the cytoplasmic tail of occludin linking this complex to the cytoskeleton (3, 4). Western blot analyses were performed to examine the expression of occludin and ZO-1 by mock-infected and HCMV-infected monocytes over a time course of infection (Fig. 5f). Infected monocytes expressed detectable levels of occludin at 24 h postinfection, whereas only trace amounts of occludin were expressed by mock-infected monocytes during the 48-h time course (Fig. 5f). ZO-1 expression was detected in infected monocytes at 4 h postinfection and increased over the 48-h time course (Fig. 5f). Mock-infected monocytes did not express detectable levels of ZO-1 until 24 h postinfection and expressed lower levels of this protein (Fig. 5f). The detection of occludin and ZO-1 expression by infected monocytes, coupled with the fourfold increase in infected monocyte total migration, suggests that expression of these gap junction proteins might allow integration into and transit through endothelial tight junctions during infected monocyte migration out of the peripheral blood.
DISCUSSION
Our study documents the first report of a virus inducing the differentiation of peripheral blood monocytes into macrophages for the promotion of viral replication. Furthermore, HCMV infection of monocytes promoted transendothelial migration, adhesion molecule expression, and cell motility, which we propose are mechanisms utilized by HCMV infection to promote infected cell migration from the peripheral blood into host organ tissue. Together, our data suggest that HCMV infection activates monocytes and induces the cellular changes required for its replication and dissemination to host tissue, both of which are required steps for HCMV pathogenesis, persistence, and transmission.
The results showed that the HCMV-induced macrophages possessed morphological, functional, and molecular markers of macrophages. In contrast, mock-infected cells did not possess the morphological or functional characteristics of macrophages, nor did they express high levels of HLA-DR. Mock-infected monocytes did express a lower-molecular-weight form of CD68 with delayed kinetics and at a reduced level. Adhesion to extracellular matrix components is known to promote some degree of differentiation (29), and our results are consistent with these findings. Based on our data, however, we propose that the HCMV-induced macrophages represent a more differentiated and distinct macrophage phenotype compared to mock-infected cells. Further supporting the idea of HCMV-induced macrophages having a distinct phenotype, gene chip array analysis revealed that at 2 weeks postinfection 228 genes were upregulated and 73 genes were downregulated in HCMV-induced macrophages at levels >2-fold in comparison to mock-infected monocytes (data not shown). This analysis revealed that HCMV-induced macrophages expressed unique cell surface markers, including E-cadherin and galectin 2. Furthermore, HCMV-induced macrophages exhibited an inflammatory macrophage phenotype with increased expression of numerous inflammatory cytokines and chemokines, including interleukin-8, monocyte chemoattractant protein 2, macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and MIP-3α. This inflammatory macrophage phenotype is consistent with both the link between HCMV infection and inflammatory cardiovascular diseases (12, 60) and with previous reports that HCMV infection of monocytes promotes inflammatory cytokine production (11, 65). It is intriguing to speculate that HCMV must utilize signal transduction pathways involved in inflammatory cytokine production to ensure a proper monocyte programming for both viral replication and dissemination and that HCMV-associated chronic inflammatory disease is in part a pathological consequence of this viral mechanism of spread. Consistent with the concept of HCMV programming monocytes along a specific differentiation pathway, Gredmark and Söderberg-Nauclér have shown that HCMV infection of monocytes blocks their differentiation into dendritic cells (18).
Supporting our hypothesis that HCMV infection promotes differentiation as a mechanism of viral spread, we showed in our system that a primary infection of monocytes is not abortive and that monocytes acquire permissiveness for viral replication following differentiation. More than 25% of HCMV-induced macrophages expressed the late viral gene product gH (Fig. 3), which is comparable to the degree of late viral gene expression seen after a primary infection of macrophages in vitro (13, 26). HCMV-induced macrophages also released a low titer of virus into cellular supernatants. Our findings are not the result of a low frequency of infection or the loss of the viral genome for two reasons. First, staining for the HCMV tegument protein pp65 at 4 h postinfection revealed that HCMV entered ∼100% of our monocytes (data not shown). Second, slot blot hybridization analysis showed that the viral genome was maintained in monocytes/macrophages prior to the detection of viral gene expression (data not shown). Because in vitro model systems do not entirely reproduce the complexities of an in vivo environment, the possibility exists that additional stimuli in vivo might enhance viral replication in HCMV-induced macrophages. Such in vivo stimuli could include interactions with multiple extracellular matrix components, which our data suggest affect the kinetics of viral replication. On the other hand, HCMV has developed a vast arsenal of immune evasion strategies to promote viral survival and persistence in the host (34). Perhaps a strict regulation of viral gene expression and/or virus release in HCMV-differentiated macrophages is an additional mechanism of immune evasion.
Consistent with our proposed role for monocytes as vectors of viral dissemination, HCMV infection promoted monocyte transendothelial migration independent of viral gene expression. This increase in infected monocyte migration occurred without the addition of chemoattractants and would therefore be mediated solely by cellular changes induced by virus-mediated cell signaling. Our previous report that HCMV binding to the cell surface induces NF-κB activation (65, 66) and promotes inflammatory cytokine production in monocytes (65) gives precedence for such a mechanism. In the present study, we showed HCMV-induced cellular changes in monocytes consistent with increased migration potential. These cellular changes included increased cell motility and the upregulation of occludin, ZO-1, and β1 integrin subunit expression. The mechanisms by which monocytes cross tight junctions during migration are currently not well understood. We present here the first evidence that occludin can be expressed by monocytes under appropriate stimuli. A hypothesis currently under investigation is that occludin expression by infected monocytes promotes migration by allowing monocytes to integrate into tight junctions and transit the endothelium.
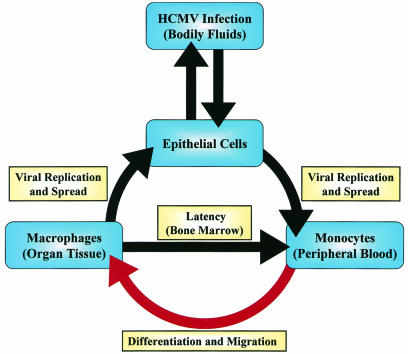
We developed a model for HCMV infection and dissemination in the host based on our findings (Fig. 6). Initial infection occurs naturally in the host when epithelial cells are infected by contact with HCMV-containing bodily fluids (53). The virus then replicates and spreads by unknown means to the peripheral blood, where a primary infection of monocytes occurs. Our data suggest that primary infection of monocytes promotes their differentiation into permissive macrophages and their migration into host tissue. The ensuing viral maturation could promote persistence in the host via two possible outcomes that are consistent with HCMV infection. First, it could allow the establishment of a chronic infection through the slow release of virus from these infected macrophages, which in immunocompromised hosts could lead to overt organ disease. Second, it could establish latency through infected monocyte migration into bone marrow, where evidence suggests that HCMV may establish latency in myeloid progenitor cells (30, 35, 41, 52, 56). It has been proposed that latently infected myeloid progenitor cells give rise to the pool of latently infected monocytes detected in the peripheral blood of seropositive hosts (51).
FIG. 6.
Model for HCMV infection and dissemination in the host. Epithelial cells of the host are infected by contact with HCMV-containing bodily fluids. HCMV then replicates and spreads to monocytes in the peripheral blood by an unknown mechanism. As indicated by the red arrow, our data suggest that primary infection of monocytes promotes both their migration into host organ tissue and their differentiation into permissive macrophages. These macrophages could become sites of persistent infection in the host tissue. The possibility also exists that HCMV-induced macrophages could migrate into the bone marrow and infect myeloid progenitor cells, which are believed to be sites of HCMV latency.
Acknowledgments
We thank R. S. Scott, M. Muggeridge, and W. Klimstra for careful reading of the manuscript. We also thank C. Moody for drawing blood and P. Haze for helpful discussions.
This study was supported by grants from the American Heart Association (0365207B and 0160239B), the Louisiana Board of Regents [LEQSF (2000-2003)-RD-A-19], the March of Dimes (1-FY01-332), and the National Institutes of Health (1-P20-RR018724-01).
REFERENCES
- 1.Adam, E., J. L. Melnick, J. L. Probtsfield, B. L. Petrie, J. Burek, K. R. Bailey, C. H. McCollum, and M. E. DeBakey. 1987. High levels of cytomegalovirus antibody in patients requiring vascular surgery for atherosclerosis. Lancet ii:291-293. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht-Buehler, G. 1977. The phagokinetic tracks of 3T3 cells. Cell 11:395-404. [DOI] [PubMed] [Google Scholar]
- 3.Alexander, J. S., J. W. Elrod, and J. H. Park. 2001. Roles of leukocyte and immune cell junctional proteins. Microcirculation 8:169-179. [DOI] [PubMed] [Google Scholar]
- 4.Aurrand-Lions, M., C. Johnson-Leger, and B. A. Imhof. 2002. The last molecular fortress in leukocyte trans-endothelial migration. Nat. Immunol. 3:116-118. [DOI] [PubMed] [Google Scholar]
- 5.Basta, S., S. M. Knoetig, M. Spagnuolo-Weaver, G. Allan, and K. C. McCullough. 1999. Modulation of monocytic cell activity and virus susceptibility during differentiation into macrophages. J. Immunol. 162:3961-3969. [PubMed] [Google Scholar]
- 6.Becker, S., M. K. Warren, and S. Haskill. 1987. Colony-stimulating factor-induced monocyte survival and differentiation into macrophages in serum-free cultures. J. Immunol. 139:3703-3709. [PubMed] [Google Scholar]
- 7.Bissinger, A. L., C. Sinzger, E. Kaiserling, and G. Jahn. 2002. Human cytomegalovirus as a direct pathogen: correlation of multiorgan involvement and cell distribution with clinical and pathological findings in a case of congenital inclusion disease. J. Med. Virol. 67:200-206. [DOI] [PubMed] [Google Scholar]
- 8.Bogner, E., M. Reschke, B. Reis, W. J. Britt, and K. Radsak. 1992. Recognition of compartmentalized intracellular analogs of glycoprotein H of human cytomegalovirus. Arch. Virol. 126:67-80. [DOI] [PubMed] [Google Scholar]
- 9.Booss, J., P. R. Dann, B. P. Griffith, and J. H. Kim. 1989. Host defense response to cytomegalovirus in the central nervous system. Predominance of the monocyte. Am. J. Pathol. 134:71-78. [PMC free article] [PubMed] [Google Scholar]
- 10.Collins, T. M., M. R. Quirk, and M. C. Jordan. 1994. Biphasic viremia and viral gene expression in leukocytes during acute cytomegalovirus infection of mice. J. Virol. 68:6305-6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudding, L. R., S. Haskill, B. D. Clark, P. E. Auron, S. Sporn, and E. S. Huang. 2003. Cytomegalovirus infection stimulates expression of monocyte-associated mediator genes. J. Immunol. 143:3343-3352. [PubMed] [Google Scholar]
- 12.Epstein, S. E., Y. F. Zhou, and J. Zhu. 1999. Infection and atherosclerosis: emerging mechanistic paradigms. Circulation 100:e20-e28. [DOI] [PubMed] [Google Scholar]
- 13.Fish, K. N., A. S. Depto, A. V. Moses, W. Britt, and J. A. Nelson. 1995. Growth kinetics of human cytomegalovirus are altered in monocyte-derived macrophages. J. Virol. 69:3737-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerna, G., D. Zipeto, E. Percivalle, M. Parea, M. G. Revello, R. Maccario, G. Peri, and G. Milanesi. 1992. Human cytomegalovirus infection of the major leukocyte subpopulations and evidence for initial viral replication in polymorphonuclear leukocytes from viremic patients. J. Infect. Dis. 166:1236-1244. [DOI] [PubMed] [Google Scholar]
- 15.Gnann, J. W., Jr., J. Ahlmen, C. Svalander, L. Olding, M. B. Oldstone, and J. A. Nelson. 1988. Inflammatory cells in transplanted kidneys are infected by human cytomegalovirus. Am. J. Pathol. 132:239-248. [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon, S. 1999. Macrophage-restricted molecules: role in differentiation and activation. Immunol. Lett. 65:5-8. [DOI] [PubMed] [Google Scholar]
- 17.Grattan, M. T., C. E. Moreno-Cabral, V. A. Starnes, P. E. Oyer, E. B. Stinson, and N. E. Shumway. 1989. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA 261:3561-3566. [PubMed] [Google Scholar]
- 18.Gredmark, S., and C. Söderberg-Nauclér. 2003. Human cytomegalovirus inhibits differentiation of monocytes into dendritic cells with the consequence of depressed immunological functions. J. Virol. 77:10943-10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grefte, A., M. C. Harmsen, G. M. van der, S. Knollema, W. J. van Son, and T. H. The. 1994. Presence of human cytomegalovirus (HCMV) immediate-early mRNA but not ppUL83 (lower matrix protein pp65) mRNA in polymorphonuclear and mononuclear leukocytes during active HCMV infection. J. Gen. Virol. 75:1989-1998. [DOI] [PubMed] [Google Scholar]
- 20.Grefte, J. M., B. T. van der Gun, S. Schmolke, G. M. van der, W. J. van Son, B. Plachter, G. Jahn, and T. H. The. 1992. The lower matrix protein pp65 is the principal viral antigen present in peripheral blood leukocytes during an active cytomegalovirus infection. J. Gen. Virol. 73:2923-2932. [DOI] [PubMed] [Google Scholar]
- 21.Hammerstrom, J. 1979. Human macrophage differentiation in vivo and in vitro: a comparison of human peritoneal macrophages and monocytes. Acta Pathol. Microbiol. Scand. 87C:113-120. [PubMed] [Google Scholar]
- 22.Hed, J. 1986. Methods for distinguishing ingested from adhering particles. Methods Enzymol. 132:198-204. [DOI] [PubMed] [Google Scholar]
- 23.Ho, M. 1977. Virus infections after transplantation in man: brief review. Arch. Virol. 55:1-24. [DOI] [PubMed] [Google Scholar]
- 24.Huang, E. S., and T. F. Kowalik. 1993. The pathogenicity of human cytomegalovirus: an overview, p. 1-45. In Y. Becker, G. Darai, and E. S. Huang (ed.), Molecular aspects of human cytomegalovirus diseases. Springer-Verlag, Berlin, Germany.
- 25.Hume, D. A., I. L. Ross, S. R. Himes, R. T. Sasmono, C. A. Wells, and T. Ravasi. 2002. The mononuclear phagocyte system revisited. J. Leukoc. Biol. 72:621-627. [PubMed] [Google Scholar]
- 26.Ibanez, C. E., R. Schrier, P. Ghazal, C. Wiley, and J. A. Nelson. 1991. Human cytomegalovirus productively infects primary differentiated macrophages. J. Virol. 65:6581-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson, R. A., A. D. Yurochko, E. E. Poma, L. Zhu, and E. S. Huang. 1999. Domain mapping of the human cytomegalovirus IE1-72 and cellular p107 protein-protein interaction and the possible functional consequences. J. Gen. Virol. 80:1293-1303. [DOI] [PubMed] [Google Scholar]
- 28.Jones, G. E. 2000. Cellular signaling in macrophage migration and chemotaxis. J. Leukoc. Biol. 68:593-602. [PubMed] [Google Scholar]
- 29.Juliano, R. L. 2002. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu. Rev. Pharmacol. Toxicol. 42:283-323. [DOI] [PubMed] [Google Scholar]
- 30.Kondo, K., H. Kaneshima, and E. S. Mocarski. 1994. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc. Natl. Acad. Sci. USA 91:11879-11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koskinen, P. K., M. S. Nieminen, L. A. Krogerus, K. B. Lemstrom, S. P. Mattila, P. J. Hayry, and I. T. Lautenschlager. 1993. Cytomegalovirus infection and accelerated cardiac allograft vasculopathy in human cardiac allografts. J. Heart Lung Transplant. 12:724-729. [PubMed] [Google Scholar]
- 32.Lathey, J. L., and S. A. Spector. 1991. Unrestricted replication of human cytomegalovirus in hydrocortisone-treated macrophages. J. Virol. 65:6371-6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loebe, M., S. Schuler, O. Zais, H. Warnecke, E. Fleck, and R. Hetzer. 1990. Role of cytomegalovirus infection in the development of coronary artery disease in the transplanted heart. J. Heart Transplant. 9:707-711. [PubMed] [Google Scholar]
- 34.Loenen, W. A., C. A. Bruggeman, and E. J. Wiertz. 2001. Immune evasion by human cytomegalovirus: lessons in immunology and cell biology. Semin. Immunol. 13:41-49. [DOI] [PubMed] [Google Scholar]
- 35.Maciejewski, J. P., E. E. Bruening, R. E. Donahue, E. S. Mocarski, N. S. Young, and S. C. St Jeor. 1992. Infection of hematopoietic progenitor cells by human cytomegalovirus. Blood 80:170-178. [PubMed] [Google Scholar]
- 36.Manez, R., S. Kusne, C. Rinaldo, J. M. Aguado, K. St George, P. Grossi, B. Frye, J. J. Fung, and G. D. Ehrlich. 1996. Time to detection of cytomegalovirus (CMV) DNA in blood leukocytes is a predictor for the development of CMV disease in CMV-seronegative recipients of allografts from CMV-seropositive donors following liver transplantation. J. Infect. Dis. 173:1072-1076. [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Pomares, L., N. Platt, A. J. McKnight, R. P. da Silva, and S. Gordon. 1996. Macrophage membrane molecules: markers of tissue differentiation and heterogeneity. Immunobiology 195:407-416. [DOI] [PubMed] [Google Scholar]
- 38.Masur, H., S. M. Whitcup, C. Cartwright, M. Polis, and R. Nussenblatt. 1996. Advances in the management of AIDS-related cytomegalovirus retinitis. Ann. Intern. Med. 125:126-136. [DOI] [PubMed] [Google Scholar]
- 39.McDonald, K., T. S. Rector, E. A. Braulin, S. H. Kubo, and M. T. Olivari. 1989. Association of coronary artery disease in cardiac transplant recipients with cytomegalovirus infection. Am. J. Cardiol. 64:359-362. [DOI] [PubMed] [Google Scholar]
- 40.Meerschaert, J., and M. B. Furie. 1995. The adhesion molecules used by monocytes for migration across endothelium include CD11a/CD18, CD11b/CD18, and VLA-4 on monocytes and ICAM-1, VCAM-1, and other ligands on endothelium. J. Immunol. 154:4099-4112. [PubMed] [Google Scholar]
- 41.Mendelson, M., S. Monard, P. Sissons, and J. Sinclair. 1996. Detection of endogenous human cytomegalovirus in CD34+ bone marrow progenitors. J. Gen. Virol. 77:3099-3102. [DOI] [PubMed] [Google Scholar]
- 42.Mitic, L. L., and J. M. Anderson. 1998. Molecular architecture of tight junctions. Annu. Rev. Physiol. 60:121-142. [DOI] [PubMed] [Google Scholar]
- 43.Mocarski, E. S., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2673. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 44.Myerson, D., R. C. Hackman, J. A. Nelson, D. C. Ward, and J. K. McDougall. 1984. Widespread presence of histologically occult cytomegalovirus. Hum. Pathol. 15:430-439. [DOI] [PubMed] [Google Scholar]
- 45.Newman, S. L., C. Bucher, J. Rhodes, and W. E. Bullock. 1990. Phagocytosis of Histoplasma capsulatum yeasts and microconidia by human cultured macrophages and alveolar macrophages: cellular cytoskeleton requirement for attachment and ingestion. J. Clin. Investig. 85:223-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nieto, F. J., E. Adam, P. Sorlie, H. Farzadegan, J. L. Melnick, G. W. Comstock, and M. Szklo. 1996. Cohort study of cytomegalovirus infection as a risk factor for carotid intimal-medial thickening, a measure of subclinical atherosclerosis. Circulation 94:922-927. [DOI] [PubMed] [Google Scholar]
- 47.Rabinowitz, S. S., and S. Gordon. 1991. Macrosialin, a macrophage-restricted membrane sialoprotein differentially glycosylated in response to inflammatory stimuli. J. Exp. Med. 174:827-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saederup, N., Y. C. Lin, D. J. Dairaghi, T. J. Schall, and E. S. Mocarski. 1999. Cytomegalovirus-encoded beta chemokine promotes monocyte-associated viremia in the host. Proc. Natl. Acad. Sci. USA 96:10881-10886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schafer, P., W. Tenschert, L. Cremaschi, M. Schroter, K. Gutensohn, and R. Laufs. 2000. Cytomegalovirus cultured from different major leukocyte subpopulations: association with clinical features in CMV immunoglobulin G-positive renal allograft recipients. J. Med. Virol. 61:488-496. [DOI] [PubMed] [Google Scholar]
- 50.Scott, W. N., K. McCool, and J. Nelson. 2000. Improved method for the production of gold colloid monolayers for use in the phagokinetic track assay for cell motility. Anal. Biochem. 287:343-344. [DOI] [PubMed] [Google Scholar]
- 51.Sinclair, J., and P. Sissons. 1996. Latent and persistent infections of monocytes and macrophages. Intervirology 39:293-301. [DOI] [PubMed] [Google Scholar]
- 52.Sindre, H., G. E. Tjoonnfjord, H. Rollag, T. Ranneberg-Nilsen, O. P. Veiby, S. Beck, M. Degre, and K. Hestdal. 1996. Human cytomegalovirus suppression of and latency in early hematopoietic progenitor cells. Blood 88:4526-4533. [PubMed] [Google Scholar]
- 53.Sinzger, C., and G. Jahn. 1996. Human cytomegalovirus cell tropism and pathogenesis. Intervirology 39:302-319. [DOI] [PubMed] [Google Scholar]
- 54.Sinzger, C., B. Plachter, A. Grefte, T. H. The, and G. Jahn. 1996. Tissue macrophages are infected by human cytomegalovirus in vivo. J. Infect. Dis. 173:240-245. [DOI] [PubMed] [Google Scholar]
- 55.Soderberg-Naucler, C., K. N. Fish, and J. A. Nelson. 1997. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J. Clin. Investig. 100:3154-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soderberg-Naucler, C., K. N. Fish, and J. A. Nelson. 1997. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 91:119-126. [DOI] [PubMed] [Google Scholar]
- 57.Sorlie, P. D., E. Adam, S. L. Melnick, A. Folsom, T. Skelton, L. E. Chambless, R. Barnes, and J. L. Melnick. 1994. Cytomegalovirus/herpesvirus and carotid atherosclerosis: the ARIC Study. J. Med. Virol. 42:33-37. [DOI] [PubMed] [Google Scholar]
- 58.Stagno, S., R. F. Pass, G. Cloud, W. J. Britt, R. E. Henderson, P. D. Walton, D. A. Veren, F. Page, and C. A. Alford. 1986. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA 256:1904-1908. [PubMed] [Google Scholar]
- 59.Stoddart, C. A., R. D. Cardin, J. M. Boname, W. C. Manning, G. B. Abenes, and E. S. Mocarski. 1994. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J. Virol. 68:6243-6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Streblow, D. N., S. L. Orloff, and J. A. Nelson. 2001. Do pathogens accelerate atherosclerosis? J. Nutr. 131:2798S-2804S. [DOI] [PubMed] [Google Scholar]
- 61.Taylor-Wiedeman, J., J. G. Sissons, L. K. Borysiewicz, and J. H. Sinclair. 1991. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J. Gen. Virol. 72:2059-2064. [DOI] [PubMed] [Google Scholar]
- 62.Taylor-Wiedeman, J., P. Sissons, and J. Sinclair. 1994. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J. Virol. 68:1597-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toorkey, C. B., and D. R. Carrigan. 1989. Immunohistochemical detection of an immediate-early antigen of human cytomegalovirus in normal tissues. J. Infect. Dis. 160:741-751. [DOI] [PubMed] [Google Scholar]
- 64.Yamaya, M., T. Fukushima, K. Sekizawa, T. Ohrui, and H. Sasaki. 1995. Cytoplasmic motility reflects phagocytic activity in alveolar macrophages from dog lungs. Respir. Physiol. 101:199-205. [DOI] [PubMed] [Google Scholar]
- 65.Yurochko, A. D., and E. S. Huang. 1999. Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J. Immunol. 162:4806-4816. [PubMed] [Google Scholar]
- 66.Yurochko, A. D., E. S. Hwang, L. Rasmussen, S. Keay, L. Pereira, and E. S. Huang. 1997. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-κB during infection. J. Virol. 71:5051-5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yurochko, A. D., T. F. Kowalik, S. M. Huong, and E. S. Huang. 1995. Human cytomegalovirus upregulates NF-κB activity by transactivating the NF-κB p105/p50 and p65 promoters. J. Virol. 69:5391-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yurochko, A. D., D. Y. Liu, D. Eierman, and S. Haskill. 1992. Integrins as a primary signal transduction molecule regulating monocyte immediate-early gene induction. Proc. Natl. Acad. Sci. USA 89:9034-9038. [DOI] [PMC free article] [PubMed] [Google Scholar]