Abstract
γ-Hydroxybutyrate (GHB), a common drug of abuse, is often coingested with ethanol. Increasing renal clearance via monocarboxylate transporter (MCT) inhibition represents a potential therapeutic strategy in GHB overdose, as does inhibition of GABAB receptors. In this study, we investigate toxicokinetic/toxicodynamic interactions between GHB-ethanol and efficacy of treatment options for GHB-ethanol intoxication in rats. Sedation was assessed using the endpoint of return-to-righting reflex. Respiration was assessed using plethysmography. Coadministration of 2.0 g/kg ethanol i.v. with 600 mg/kg GHB i.v. increased sleep time compared with GHB alone. Administration of ethanol to steady-state concentrations of 0.1–0.2% and 0.3–0.4% (w/v) did not affect toxicokinetics of 600 mg/kg GHB i.v., or respiratory rate, but did result in significantly lower peak tidal volumes compared with GHB alone. Oral administration of 2.5 g/kg ethanol had no significant effect on toxicokinetics of 1500 mg/kg orally administered GHB. Pretreatment with specific receptor inhibitors indicated no effect of GABAA receptor inhibition on sleep time or respiratory depression in GHB-ethanol intoxication. GABAB receptor inhibition partially prevented sedation and completely prevented respiratory depression. Ethanol increased fatality when administered at 0.1–0.2% (4 of 10) and 0.3–0.4% (9 of 10) versus 1500 mg/kg GHB i.v. alone (0 of 10). Treatment with the MCT inhibitor, l-lactate, significantly decreased sleep time after GHB-ethanol and decreased fatality at 0.1–0.2% (0 of 10) and 0.3–0.4% ethanol (5 of 10). Treatment with a GABAB receptor antagonist completely prevented fatality at 0.3–0.4% (0 of 10). These data indicate that ethanol potentiates the sedative and respiratory depressant effects of GHB, increasing the risk of fatality. MCT and GABAB receptor inhibition represent potentially effective treatments in GHB-ethanol intoxication.
Introduction
Abuse of γ-hydroxybutyrate (GHB) and its precursors has become well recognized in the United States and other countries. GHB overdose results in 1000–2000 emergency department visits annually in the United States alone (Substance Abuse and Mental Health Services Administration, 2011). GHB intoxication can result in adverse effects including bradycardia, hypothermia, coma, and respiratory depression/arrest, which has led to GHB-associated fatalities (Chin et al., 1998; Caldicott et al., 2004; Zvosec et al., 2011). In overdose cases, other drugs of abuse are commonly coingested with GHB, and numerous reports indicate that ethanol is the most common of these, with some reports indicating ethanol coingestion in the majority of GHB overdose cases (Mason and Kerns, 2002; Galicia et al., 2011; Zvosec et al., 2011).
Although GHB abuse has been recognized as a significant issue in public health, both alone and with ethanol coingestion, there currently exists no pharmacologic treatment for GHB overdose. In our laboratory, we evaluated a novel therapeutic strategy for the treatment of GHB overdose by increasing GHB elimination via inhibition of its active renal reabsorption. Renal reabsorption of GHB is saturable, which can be attributed to transport by the group of transporters known as monocarboxylate transporters (MCTs) (Morris et al., 2005; Wang et al., 2006). We previously demonstrated the utility of MCT inhibition as a therapeutic strategy for overdose of GHB alone in animal studies, in which administration of MCT inhibitors increased GHB total and renal clearance at high GHB doses, improving toxicodynamic endpoints (Wang et al., 2008a,b; Morse et al., 2012). Similarly, in our pilot clinical study, MCT inhibition with l-lactate administered with osmotic diuresis increased the renal clearance of GHB in humans (Morris et al., 2011). Along with alteration of GHB toxicokinetics, direct inhibition of GHB toxicodynamics also represents a potential treatment strategy for GHB intoxication. The involvement of GABAB receptors in many GHB toxicodynamic endpoints has been demonstrated (Carai et al., 2001, 2005; Kaupmann et al., 2003; Morse et al., 2012), indicating that GABAB receptor antagonists may also be effective as a treatment for GHB overdose. However, unlike MCT inhibition with l-lactate, treatment with GABAB receptor antagonists is not currently a clinically available therapy, although the GABAB receptor antagonist 3-aminopropyl-n-butyl-phosphinic acid (SGS742) has undergone phase II clinical trials for indications other than GHB intoxication (Froestl et al., 2004).
Considering the high rate of GHB-ethanol coingestion, the toxicokinetic/toxicodynamic interactions between these two drugs are of interest, as is the treatment of GHB overdose in the presence of ethanol. Available literature supports minimal toxicokinetic interaction between GHB and ethanol when the two are administered at high intravenous doses or at low oral doses (Van Sassenbroeck et al., 2003; Thai et al., 2006; Fung et al., 2008). In contrast, significant toxicodynamic interactions between GHB and ethanol have been reported with an array of toxicodynamic endpoints (Van Sassenbroeck et al., 2003; Cook et al., 2006; Thai et al., 2006). These toxicodynamic interactions may be attributed to different pharmacological actions; whereas GHB’s effects are attributed to action at GABAB receptors, effects of ethanol are mainly attributed to action at GABAA receptors (Adinoff et al., 1988; Carai et al., 2001; Morse et al., 2012). Clinically, overdose of both GHB and ethanol can produce profound sedation and respiratory depression, potentially resulting in fatality (Altose and Hudgel, 1986; Adinoff et al., 1988; Zvosec et al., 2011). Although multiple studies have assessed the effect of GHB-ethanol administration on sedation (McCabe et al., 1971; Van Sassenbroeck et al., 2003; Cook et al., 2006), the effect of ethanol on respiration during GHB overdose has not been evaluated. We predict that, like sedation, GHB-induced respiratory depression is potentiated by the presence of ethanol. In addition, although the toxicological effects of these agents alone may primarily involve different receptors, the identification of specific receptors involved in these effects with GHB-ethanol coadministration has not been assessed. Due to their different pharmacological actions, it is likely that multiple mechanisms are involved in the toxicodynamics of GHB-ethanol intoxication. Finally, information is lacking on the efficacy of potential treatment options for GHB-ethanol intoxication using relevant toxicodynamic endpoints. We predict that, as with GHB alone, both MCT and GABAB receptor inhibition represent potential treatment strategies for improving sedation, respiratory depression, and mortality in GHB-ethanol intoxication.
Materials and Methods
Chemicals and Reagents
Sodium GHB and SGS742 were provided by the National Institute on Drug Abuse. Sodium l-lactate was obtained from Sigma-Aldrich (St. Louis, MO). Ethyl alcohol USP (200 proof) was purchased from PharmcoAAPER (Brookfield, CT). (2S)-(+)-5,5-Dimethyl-2-morpholineacetic acid (SCH50911) was purchased from R&D Systems (Minneapolis, MN).
Animals and Animal Surgery
Male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing 260–340 g were used for all experiments. Animals were housed under controlled temperature and humidity with an artificial 12-hour light/dark cycle and food was available ad libitum. All animal protocols were approved by the Institutional Animal Care and Use Committee at the University at Buffalo. Animals were allowed to acclimate to their environment for 1 week prior to surgical implantation of jugular and femoral vein cannulae under anesthesia with ketamine/xylazine. Cannulae were flushed daily with 40 IU/ml heparinized saline to maintain patency. Animals were allowed a minimum of 72 hours for recovery from surgery before drug administration.
Toxicokinetic/Toxicodynamic Interaction Studies
Sedation Studies.
The sedative effects of GHB and ethanol were measured using the endpoint of righting reflex, as in our previous studies (Wang et al., 2008a,b; Felmlee et al., 2010b). To determine the effect of ethanol on the sedative effect of GHB, rats were administered 2.0 g/kg ethanol or 2.0 g/kg ethanol plus 600 mg/kg GHB i.v. over 5 minutes (n = 3–4 in each group). This experiment was performed at a similar time and in a similar manner to our previous study assessing sedative effects of GHB alone (Felmlee et al., 2010b); therefore, the same data at 600 mg/kg GHB were used from the previous publication for comparison with GHB alone. To assess MCT inhibition as a treatment strategy for improving sedation in GHB-ethanol intoxication, the MCT inhibitor l-lactate (66 mg/kg bolus followed by 302.5 mg/kg per hour infusion) was administered 5 minutes after GHB-ethanol. This dose of l-lactate was chosen to increase plasma lactate concentrations by 1–2 mM (Morse et al., 2012). To assess the receptors involved in the sedative effect of GHB-ethanol intoxication, GHB and ethanol were administered alone and concomitantly, and specific receptor inhibitors were also administered immediately prior to the concomitant administration of GHB-ethanol. Bicuculline (1 mg/kg) was used to assess the role of GABAA receptors. SGS742 (500 and 1000 mg/kg) and the more potent GABAB receptor antagonist SCH50911 (100 and 200 mg/kg) were used to assess the role of GABAB receptors. SCH50911 at the lower dose has been demonstrated to completely prevent the sedative effect of GHB alone in mice (Carai et al., 2001). In all groups, the time of loss-of-righting reflex (LRR) and time of return-to-righting reflex (RRR) were recorded and sleep time determined as RRR − LRR. LRR was determined as the time at which the animal could not right itself after being placed on its back. Animals were left on their back after LRR and RRR was defined as the time at which the animal could right itself on its own. All animals were euthanized at RRR, at which time blood and brain samples were collected. Brain samples were immediately frozen in liquid nitrogen upon collection and all samples were stored at −80°C until analysis. In these studies, GHB was administered as a 200 or 300 mg/ml solution in sterile water and ethanol as a 50% (v/v) solution in sterile water. Bicuculline was dissolved in HCl, and then diluted in saline to 1 mg/ml and pH 5.0. SCH50911 and SGS742 were administered as 100 mg/ml solutions in saline. All bolus doses were administered via the jugular vein cannula. l-Lactate was administered as a 40 mg/ml solution in sterile water via the femoral vein cannula.
Respiratory Depression/Fatality Studies.
The effect of GHB-ethanol administration on respiration was measured using whole-body plethysmography, as in our previous study (Morse et al., 2012). Rats were placed in plethysmography chambers 1 hour prior to drug administration and were allowed to acclimate to the chambers for 45 minutes before five baseline recordings were collected over 15 minutes. In all studies, GHB administration was considered time 0 and respiration recordings were taken at 2.5, 5, 7.5, 10, 15, 20, 25, and 30 minutes and every 15 minutes thereafter for 6 hours. Measurements for the parameters of respiratory frequency (rate), tidal volume, and minute volume (rate ⋅ tidal volume) were quantitated at each recording.
To assess the effect of ethanol on intravenous GHB toxicokinetics and GHB-induced respiratory depression, 600 mg/kg GHB was administered alone and concomitantly with ethanol administered to target moderate and high steady-state concentrations of 0.1–0.2% and 0.3–0.4% (w/v), respectively (n = 5 in each group). Ethanol was administered as a 1.0 or 2.0 g/kg i.v. bolus over 5 minutes, right after the collection of baseline respiratory measurements. To maintain target steady-state concentrations, we used a strategy previously described by Boje and Fung (1989), in which an infusion of ethanol was initiated 30 minutes after the intravenous bolus at a rate of 1.85 mg/min, the average Vmax rate of ethanol metabolism in male Sprague-Dawley rats of this weight. Five respiratory measurements were again taken over 15 minutes starting 45 minutes after the ethanol bolus, to assess the effect of targeted ethanol concentrations alone on respiration. GHB was then administered intravenously at time 0 (60 minutes after the ethanol bolus). To determine the receptors involved in respiratory depression in GHB-ethanol intoxication, bicuculline, SCH50911, and SGS742 were administered immediately prior to GHB, at the higher ethanol dose (n = 3–4 in each group). In all respiratory depression experiments, blood and urine samples were collected for 6 hours after GHB administration. GHB was administered as a 300 mg/ml solution in sterile water via the jugular vein cannula. The ethanol bolus was given as a 50% (v/v) solution in sterile water via the jugular vein cannula and ethanol infusion as a 20% (v/v) solution in sterile water via the femoral vein cannula. Bicuculline, SCH50911, and SGS742 were administered in saline as above.
To assess the effects of ethanol on GHB-associated fatality and the effects of potential treatment strategies for preventing fatality due to respiratory arrest in GHB-ethanol intoxication, 1500 mg/kg i.v. GHB was administered alone and with the same ethanol regimens as above (n = 10 in each group). Treatment groups received 5 mg/kg SCH50911 or l-lactate (66 mg/kg bolus and 302.5 mg/kg per hour infusion), given 5 minutes after GHB. This dose of SCH50911 was the lowest dose demonstrated to significantly improve respiratory depression with GHB alone in our previous study (Morse et al., 2012). The dose of l-lactate was chosen to increase plasma lactate concentrations by 1–2 mM, as above. Animals were pronounced dead when respiration was ceased for several minutes. In animals alive at 8 hours, ethanol and l-lactate infusions were discontinued and animals were monitored up to 24 hours after GHB administration. For these experiments, GHB and ethanol were administered in a similar manner as in the respiratory depression experiments. SCH50911 was administered as a 5 mg/ml solution in saline via the jugular vein cannula. l-Lactate was administered as a 40 mg/ml solution in sterile water via the femoral vein cannula. For the simultaneous infusion of ethanol/l-lactate, the concentration of l-lactate was maintained at 40 mg/ml and ethanol was added to the solution to maintain a 1.85 mg/min infusion of ethanol in each animal.
Oral Toxicokinetic Study.
Because the effect of high oral doses of ethanol on the toxicokinetics of high oral doses of GHB have not been previously evaluated, this potential interaction was assessed by administration of 1500 mg/kg GHB to rats, with and without 2.5 g/kg ethanol, by oral gavage (n = 4–5). Rats were fasted for a minimum of 12 hours before drug administration. Blood and urine samples were collected for up to 15 hours after drug administration. GHB alone was administered as a 300 mg/ml solution in water. For combined GHB-ethanol administration, ethanol was added to 300 mg/ml GHB to result in an ethanol concentration of approximately 40% (v/v).
Sample Analysis
GHB plasma and urine concentrations were determined using previously validated liquid chromatography coupled to tandem mass spectrometry assays (Felmlee et al., 2010a; Morse et al., 2012). Serum ethanol concentrations were determined using an enzymatic assay (Ethanol Assay Kit A-111; Biomedical Research Service Center, University at Buffalo, Buffalo, NY).
Data/Statistical Analysis
To determine GHB toxicokinetic parameters, noncompartmental analysis was performed using WinNonLin 5.2 software (Pharsight, Palo Alto, CA). Area under the plasma concentration-time curve (AUC) was determined using the trapezoidal method. Total clearance (CL) or oral clearance (CL/F) was determined as dose/AUC. Renal clearance (CLR) was determined as Ae/AUC, where Ae represents the total amount of GHB excreted in the urine. Metabolic or nonrenal clearance (CLm) was determined as CL − CLR. To assess the effect of ethanol on GHB-induced respiratory depression, the pharmacodynamic descriptors of area below the effect curve (ABEC) and maximum effect (Emax) were used. ABEC was determined using WinNonLin software. In all studies, mean values were compared using t tests or one-way analysis of variance followed by Tukey’s or Dunnett’s post hoc tests for the detection of statistically significant differences in toxicokinetic/toxicodynamic parameters. Differences resulting in P < 0.05 were considered significant.
Results
Effect of Ethanol on GHB Toxicokinetics.
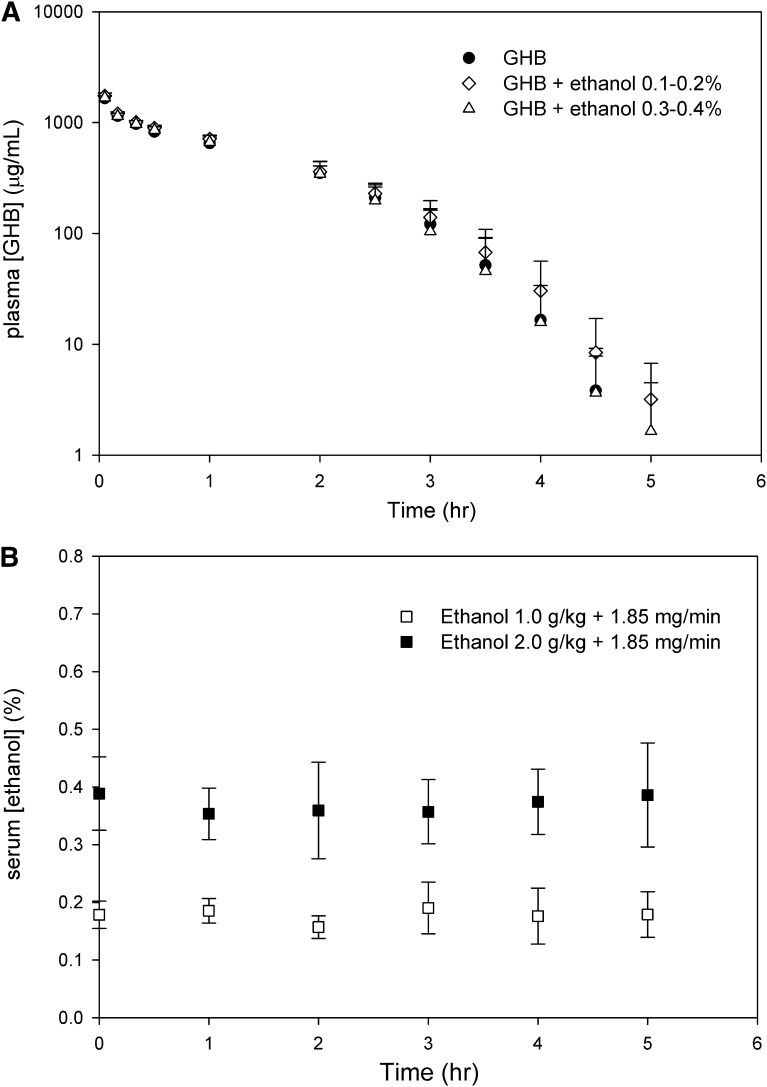
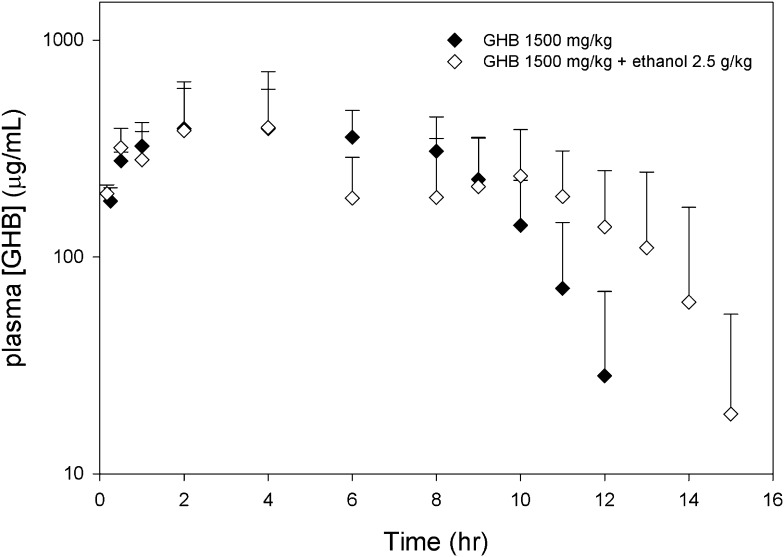
GHB plasma concentrations after the administration of 600 mg/kg GHB i.v. in the presence and absence of ethanol are displayed in Fig. 1A. Serum ethanol concentrations obtained in this experiment are shown in Fig. 1B. Mean steady-state ethanol concentrations were 0.18 ± 0.03% and 0.36 ± 0.06%, for the moderate and high doses of ethanol, respectively, indicating that the targeted steady-state ethanol concentrations were achieved with the doses administered. As shown in Table 1, ethanol coadministration did not result in a significant difference in GHB total, renal, or metabolic clearance at either dose compared with GHB alone. GHB plasma concentrations after the oral administration of GHB with and without ethanol are displayed in Fig. 2. As shown in Table 2, no significant differences in mean toxicokinetic parameters were detected between groups.
Fig. 1.
GHB and ethanol concentrations after administration of 600 mg/kg GHB i.v. with and without ethanol. (A) Plasma GHB concentrations. (B) Serum ethanol concentrations. Ethanol was administered i.v. as a 1.0 or 2.0 g/kg bolus to reach target concentrations of 0.1–0.2% and 0.3–0.4% (w/v), followed by a 1.85 mg/min infusion for 6 hours after GHB administration. GHB was administered 60 minutes after the ethanol bolus, at time 0. Data are presented as mean ± S.D. (n = 5).
TABLE 1.
Toxicokinetics of intravenous GHB with and without ethanol coadministration
Ethanol was administered as a 1.0 or 2.0 g/kg i.v. bolus injection (followed by an infusion of 1.85 mg/min), resulting in target concentrations of 0.1–0.2 and 0.3–0.4% (w/v), respectively. GHB was administered 60 minutes after ethanol bolus administration. Results of one-way analysis of variance followed by Dunnett’s post hoc test indicated no statistically significant differences between mean toxicokinetic parameters in either ethanol-treated group compared with GHB alone. Data are presented as the mean ± S.D. (n = 5).
| Dose Administered | CL | CLR | CLm |
|---|---|---|---|
| ml/kg per minute | |||
| GHB 600 mg/kg i.v. | 5.84 ± 0.64 | 1.61 ± 0.48 | 4.22 ± 0.44 |
| GHB 600 mg/kg i.v. + ethanol 0.1–0.2% | 5.30 ± 0.58 | 1.76 ± 0.22 | 3.54 ± 0.46 |
| GHB 600 mg/kg i.v. + ethanol 0.3–0.4% | 5.91 ± 1.03 | 2.09 ± 0.98 | 3.82 ± 0.47 |
Fig. 2.
Plasma GHB concentrations after administration of 1500 mg/kg GHB PO with and without ethanol. GHB and ethanol (2.5 g/kg) were administered concomitantly to rats by oral gavage. Data presented as mean ± S.D. (n = 4–5).
TABLE 2.
Toxicokinetics of oral GHB with and without ethanol coadministration
GHB and ethanol were administered concomitantly by oral gavage. t tests indicated no statistically significant differences in mean toxicokinetic parameters between groups. Data are presented as the mean ± S.D. (n = 4–5).
| Dose Administered | CL/F | Cmax | Tmax |
|---|---|---|---|
| ml/kg per minute | μg/ml | h | |
| GHB 1500 mg/kg PO | 7.49 ± 1.1 | 492 ± 200 | 6.0 ± 2.5 |
| GHB 1500 mg/kg PO + ethanol 2.5 g/kg | 7.46± 1.6 | 489 ± 240 | 3.88 ± 4.4 |
CL/F, total clearance/bioavailability (oral clearance).
Effect of Ethanol, Specific Receptor Inhibitors, and Treatment with l-Lactate on the Sedative Effect of GHB.
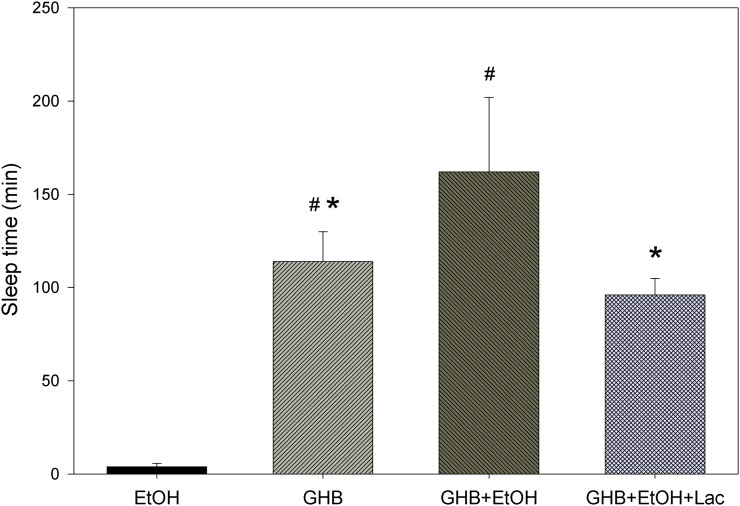
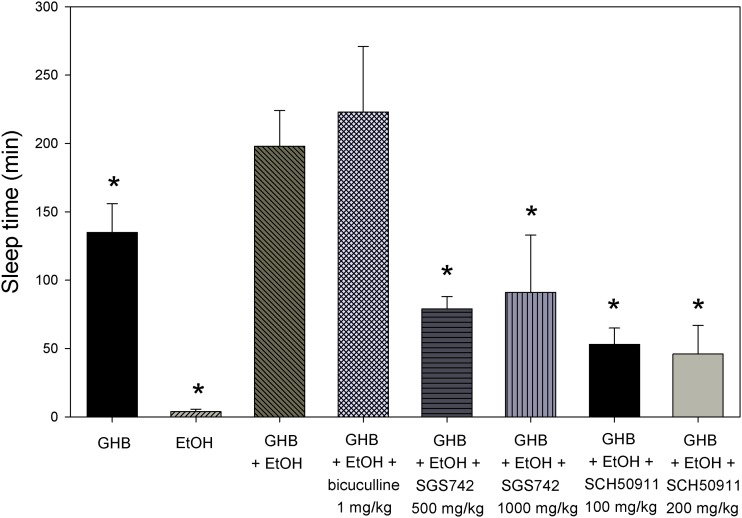
As displayed in Fig. 3, coadministration of 2.0 g/kg ethanol significantly increased the sleep time observed with 600 mg/kg GHB. Treatment with l-lactate 5 minutes after GHB-ethanol significantly decreased the sleep time compared with GHB-ethanol alone. As shown in Table 3, significantly lower GHB plasma and brain concentrations were determined at RRR with GHB-ethanol administration compared with GHB alone; however, the brain/plasma ratio was unchanged. With l-lactate administration, brain concentrations of GHB were similar at RRR compared with GHB-ethanol alone; however, the brain/plasma ratio was significantly decreased. Effects of pretreatment with specific receptor inhibitors are shown in Fig. 4. Pretreatment with 1 mg/kg bicuculline resulted in similar sleep times as that of GHB-ethanol alone. (Higher doses of this agent were also evaluated, but resulted in fatalities.) Pretreatment with both SCH50911 and SGS742 significantly decreased the sleep time compared with GHB-ethanol alone; however, there were no significant differences between the higher and lower dose groups of these agents. Receptor inhibitors had no effect on the blood-brain partitioning of GHB (data not shown).
Fig. 3.
Effect of ethanol (EtOH) coadministration and treatment with l-lactate (Lac) on the sedative effect of GHB. We administered 600 mg/kg GHB and 2.0 g/kg ethanol i.v.; 66 mg/kg l-lactate plus 302.5 mg/kg per hour infusion was administered 5 minutes after GHB-ethanol administration and continued until animals were euthanized at RRR. One-way analysis of variance followed by Tukey’s post hoc test was used to determine statistically significant differences in sleep time between groups. Data presented as mean ± S.D. (n = 3–8). Data from 600 mg/kg GHB alone were used from a previous study (Felmlee et al., 2010b). *P < 0.05, significantly different from GHB-ethanol; #P < 0.05, significantly different from ethanol alone.
TABLE 3.
Effect of ethanol and l-lactate treatment on GHB plasma and brain concentrations at RRR
GHB (600 mg/kg) and ethanol (2.0 g/kg) were administered intravenously, and l-lactate (66 mg/kg plus 302.5 mg/kg per hour) was given intravenously 5 minutes after GHB/ethanol. Animals were euthanized at RRR. One-way analysis of variance followed by Dunnett’s post hoc test was used to determine statistically significant differences compared with GHB plus ethanol. Data from 600 mg/kg alone GHB were used from a previous study (Felmlee et al., 2010b). Data are presented as the mean ± S.D. (n = 3–8).
| Treatment Administered | Time of RRR | Cplasma | Cbrain | Brain/Plasma Ratio |
|---|---|---|---|---|
| min | μg/ml | μg/ml | ||
| GHB | 110 ± 17a | 352 ± 64a | 72.3 ± 12a | 0.20 ± 0.03 |
| GHB + ethanol | 162 ± 38 | 178 ± 76 | 38.7 ± 19 | 0.21 ± 0.03 |
| GHB + ethanol + l-lactate | 93 ± 8a | 257 ± 61 | 37.8 ± 6.3 | 0.15 ± 0.02a |
Cbrain, brain GHB concentration at RRR; Cplasma, plasma GHB concentration at RRR.
Significantly different from GHB plus ethanol (P < 0.05).
Fig. 4.
Effect of pretreatment with specific receptor inhibitors on sedation in GHB-ethanol (EtOH) intoxication. We administered 600 mg/kg GHB and 2.0 g/kg i.v. ethanol. Receptor inhibitors were administered intravenously just prior to GHB-ethanol. One-way analysis of variance followed by Tukey’s post hoc test was used to determine statistically significant differences in sleep time between groups. Data presented as mean ± S.D. (n = 3–4). *P < 0.05, significantly different from GHB-ethanol.
Effect of Ethanol, Specific Receptor Inhibitors, and Treatment with l-Lactate on Respiratory Depression and Fatality in GHB Overdose.
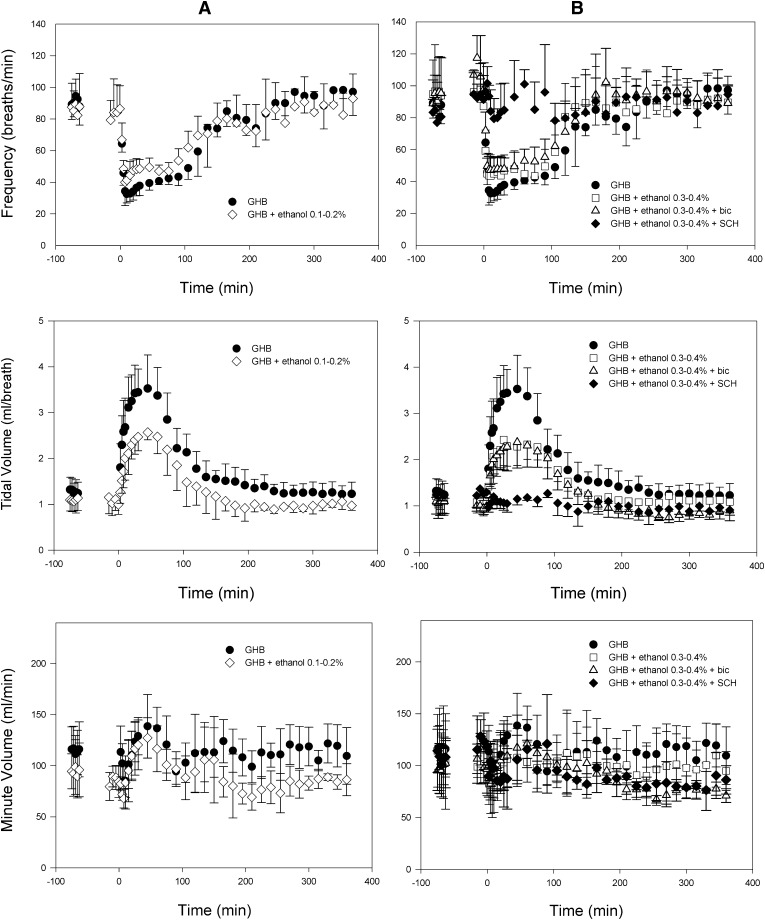
Plethysmography results after administration of 600 mg/kg GHB i.v. with and without ethanol are displayed in Fig. 5. Targeted ethanol concentrations of 0.1–0.2% and 0.3–0.4% were maintained in this experiment (Fig. 1B). No significant differences were detected for any respiratory parameter when assessed with ethanol alone at either dose compared with baseline. As shown in Table 4, the administration of ethanol with GHB did not significantly affect respiratory rate at either dose of ethanol compared with GHB alone; however, both doses of ethanol significantly decreased the ABEC and Emax for tidal volume after GHB administration. No significant differences in minute volume Emax were detected at this GHB dose. Pretreatment with bicuculline did not affect the respiratory rate or the tidal volume compared with GHB-ethanol alone. Pretreatment with 100 mg/kg SCH50911 completely prevented any decrease in respiratory rate. The compensatory increase in tidal volume in the SCH50911-treated group was also absent. Mean steady-state ethanol concentrations were maintained at target concentrations and were similar between control, bicuculline, and SCH50911 groups (0.36 ± 0.06, 0.31 ± 0.04, and 0.38 ± 0.07, respectively; P > 0.05). Bicuculline and SCH50911 had no effect on GHB clearance. Results after pretreatment with SGS742 were highly variable, completely preventing respiratory depression in some animals, whereas some animals still displayed effects similar to the control; therefore, these data are not shown. Administration of the same ethanol doses with 1500 mg/kg GHB resulted in increased rates of fatality with both ethanol doses compared with GHB alone, as shown in Table 5. Administration of l-lactate completely prevented fatality with 0.1–0.2% GHB-ethanol, and also decreased the rate of fatality observed with 0.3–0.4% GHB-ethanol compared with that without treatment. Administration of 5 mg/kg SCH50911 completely prevented fatality at the higher ethanol concentration of 0.3–0.4%.
Fig. 5.
Effect of ethanol and pretreatment with specific receptor antagonists on respiratory depression after administration of 600 mg/kg GHB i.v. Ethanol was administered intravenously as a 1.0 or 2.0 g/kg bolus at −60 minutes to reach target concentrations of 0.1–0.2% (w/v) (A) and 0.3–0.4% (w/v) (B), followed by a 1.85 mg/min infusion at −30 minutes, which continued until 360 minutes. GHB was administered at time 0. Receptor antagonists were administered in animals receiving 0.3–0.4% ethanol, just prior to GHB administration. Baseline measurements at −75 minutes represent baseline prior to any drug administration (measurements were taken at −15 minutes for GHB alone and are plotted at −75 minutes for comparison with ethanol-treated groups). Measurements at −15 minutes represent those in the presence of ethanol alone. Data presented as mean ± S.D. (n = 3–5). bic, bicuculline; SCH, SCH50911.
TABLE 4.
Effect of ethanol and pretreatment with specific receptor antagonists on GHB-induced respiratory depression
Dashes indicate that no significant changes in any respiratory parameters were determined compared with baseline with pretreatment of SCH50911. Ethanol was administered as a 1.0 or 2.0 g/kg i.v. bolus, followed by a 1.85 mg/min infusion. We administered 600 mg/kg GHB i.v. 60 minutes after ethanol bolus administration. One-way analysis of variance followed by Tukey’s post hoc test was used to determine statistically significant differences in mean toxicodynamic parameters between groups. Data are presented as the mean ± S.D. (n = 3–5).
| Toxicodynamic Parameter | GHB | GHB + 0.1–0.2% Ethanol | GHB + 0.3–0.4% Ethanol | GHB + 0.3–0.4% Ethanol + Bicuculline | GHB + 0.3–0.4% Ethanol + SCH50911 |
|---|---|---|---|---|---|
| Frequency ABEC (breaths) | 5540 ± 1000 | 4090 ± 1300 | 5440 ± 2100 | 5430 ± 1500 | —— |
| Frequency Emax (breaths/min) | 31 ± 5 | 38 ± 6 | 39 ± 11 | 45 ± 7 | —— |
| Tidal volume ABEC (ml/breath*min) | 207 ± 56 | 132 ± 49a | 119 ± 32a | 123 ± 11a | —— |
| Tidal volume Emax (ml) | 3.63 ± 0.69 | 2.65 ± 0.31a | 2.60 ± 0.38a | 2.60 ± 0.36a | —— |
| Minute volume Emax (ml/min) | 81 ± 16 | 64 ± 11 | 69 ± 23 | 65 ± 5 | —— |
Significantly different from GHB alone (P < 0.05).
TABLE 5.
Effect of ethanol and potential treatment strategies on fatality after administration of 1500 mg/kg GHB i.v.
Ethanol was administered intravenously as a 1.0 or 2.0 g/kg bolus to reach target concentrations of 0.1–0.2% and 0.3–0.4% (w/v), followed by a 1.85 mg/min infusion until 8 hours after GHB administration. GHB administration was given 60 minutes after the ethanol bolus and was considered time 0. l-Lactate (66 mg/kg, followed by an infusion of 302.5 mg/kg per hour for 8 hours) and SCH50911 (5 mg/kg) were administered 5 minutes after GHB. Data are presented as the number of fatalities/total animals evaluated.
| Dose Administered | No Treatment | l-Lactate | SCH50911 |
|---|---|---|---|
| GHB | 0 of 10 | —— | —— |
| GHB + ethanol 0.1–0.2% | 4 of 10 | 0 of 10 | —— |
| GHB + ethanol 0.3–0.4% | 9 of 10 | 5 of 10 | 0 of 10 |
Discussion
Although GHB abuse has been recognized as a significant public health issue, no specific treatment option exists for GHB overdose. Due to the high rate of ethanol coingestion in GHB overdose, evaluation of the toxicokinetic/toxicodynamic interaction between these agents is necessary to inform potential treatment of GHB intoxication. In this study, we aimed to determine the nature of the toxicokinetic/toxicodynamic interactions between GHB and ethanol, using clinically relevant endpoints, and to assess the potential of a clinically available treatment, l-lactate, along with that of receptor inhibition, for improvement of these toxicodynamic measures in GHB-ethanol intoxication.
Regarding the toxicokinetic interaction between GHB and ethanol, our study indicates no effect of intravenous ethanol administration on the clearance of GHB, as previously reported (Van Sassenbroeck et al., 2003; Fung et al., 2008). A lack of effect of GHB on ethanol toxicokinetics was also confirmed in these previous studies and was therefore not evaluated in the current study. GHB itself demonstrates several nonlinear pharmacokinetic properties, and previous research indicates oral GHB absorption to be dose dependent and saturable at high oral doses (Lettieri and Fung, 1979; Arena and Fung, 1980). Our study using CaCo-2 cells also indicates saturable transport of GHB, and suggests this process to be MCT mediated (Lam et al., 2010). It was reported that transport of the MCT substrate, butyrate, in rat intestinal epithelial cells could be inhibited by high concentrations of ethanol, as well as its metabolite, acetaldehyde, which may be present in the intestine after high oral doses of ethanol (Gonçalves et al., 2011). Due to a potential MCT-mediated intestinal interaction, we evaluated the effect of a high oral dose of ethanol on a high oral dose of GHB; however, our results from this experiment did not result in any significant effect on GHB oral clearance, although absorption appeared to be prolonged. It is possible that inhibition of MCTs by ethanol simply delays GHB absorption without affecting total bioavailability, due to the continuous expression of MCTs throughout the gastrointestinal tract, and therefore does not affect total oral GHB clearance.
Results from our sedation study indicate that ethanol increases the sedative effect observed with GHB, which also concurs with previous reports. McCabe et al. (1971) reported a longer sleep time with GHB-ethanol coadministration than the sum of the sleep times with either agent alone, as we observed in our study. Van Sassenbroeck et al. (2003) reported similar effects on sleep time, concluding that there was a synergistic interaction between the two drugs at high concentrations of ethanol. Studies in rodents have demonstrated the involvement of GABAA receptors in the sedative effect of ethanol and the involvement of GABAB receptors in the sedative effect of GHB (Liljequist and Engel, 1982; Beleslin et al., 1997; Carai et al., 2001). Interestingly, in the current study, pretreatment with even the highest possible bicuculline dose did not significantly decrease sleep time compared with GHB-ethanol alone. This indicates that although the sedative effects of ethanol alone may be partially attributed to action at GABAA receptors, this receptor does not appear to be involved in the potentiation of GHB’s sedative effect by ethanol. In addition, although pretreatment with both GABAB receptor antagonists significantly decreased sleep time compared with GHB-ethanol alone, GABAB inhibition was not completely effective in preventing sleep, and there was no difference between dose groups of either GABAB receptor antagonist, suggesting maximal inhibition of GABAB-mediated effects. In this experiment, coadministration of ethanol increased sleep time by approximately 60 minutes compared with GHB alone, which is similar to the sleep times observed with GHB-ethanol administration after GABAB receptor inhibition. This suggests that GABAB receptor inhibition prevents the sedative effect of GHB, but not the potentiating effect of ethanol on this endpoint, and indicates effects of GHB-ethanol coadministration on sedation other than that mediated through direct action at GABAB receptors. It has also been postulated that the increased effect of ethanol on GHB-induced sedation may be due to an increase in GHB concentrations at the effect site (i.e., an effect of ethanol on GHB blood-brain barrier permeability) (Van Sassenbroeck et al., 2003). When we measured GHB plasma and brain concentrations at RRR, both were lower with ethanol administration compared with GHB alone, but the brain/plasma ratio was maintained. These data indicate that ethanol coadministration alters the sedation concentration-effect relationship of GHB, but that this effect is not due to an increase in GHB brain partitioning. Interestingly, with administration of l-lactate after GHB-ethanol, the brain/plasma ratio was significantly lower compared with GHB-ethanol alone. Whereas the primary effect of l-lactate on GHB toxicokinetics is mediated through inhibition of renal reabsorption, as shown in our previous publications (Morris et al., 2005; Wang et al., 2008a), this decreased brain partitioning, also presumably due to inhibition of MCTs at the blood-brain barrier, may play a role in the improvement of sedation with l-lactate treatment in the current study.
To assess the effect of ethanol on GHB-induced respiratory depression, we chose two steady-state ethanol concentrations, a moderate, clinically relevant concentration of 0.1–0.2% (w/v), similar to blood alcohol levels reported in GHB overdose cases (Caldicott et al., 2004; Couper et al., 2004), and a high concentration of 0.3–0.4%. Our previous research evaluating the effect of GHB alone on respiration indicates the primary effect of GHB to be a decrease in respiratory rate, which is accompanied by a compensatory increase in tidal volume, allowing minute volume to be maintained until doses approach lethality (Morse et al., 2012). The decrease in respiratory rate in our previous study was demonstrated to be completely prevented by GABAB receptor inhibition. The current data indicate that the effect of ethanol on the concentration-effect relationship of GHB-induced respiratory depression prevents this normally observed increase in tidal volume. Similar to results from our sedation study, this effect of ethanol on respiration does not appear to be mediated through the GABAA receptor, because pretreatment with bicuculline did not prevent the inhibition of tidal volume induced by ethanol. However, similar to our previous study with GHB alone, pretreatment with the GABAB antagonist, SCH50911, was able to completely prevent any significant decrease in respiratory rate, precluding the need for the compensatory increase in tidal volume. Preclinical studies using the same dose of SCH50911 have reported no effect of this inhibitor alone on respiration (Bolser et al., 1995), indicating that the complete lack of respiratory depression with SCH50911 pretreatment is due to prevention of GHB GABAB-mediated effects.
The prevention of the compensatory increase in tidal volume observed with ethanol administration would be expected to translate into a lower minute volume with ethanol administration compared with GHB alone; however, at a GHB dose of 600 mg/kg, no significant difference in minute volume could be detected. Based upon our previous data, minute volume represents an insensitive measure of GHB-induced respiratory depression, due to little change in this measure before death (Morse et al., 2012). Therefore, to determine the overall effect of ethanol on GHB-induced respiratory depression, we chose to directly assess fatality, secondary to respiratory arrest, after the administration of GHB 1500 mg/kg. In this experiment, the prevention of the normal compensatory increase in tidal volume with ethanol administration translated into an increased risk of death from respiratory depression. l-Lactate treatment decreased the fatality rate, at a dose determined in our previous study to result in clinically relevant increases in plasma lactate concentrations of approximately 1.5 mM, which result in no adverse effects (Morse et al., 2012). Due to results indicating complete prevention of respiratory depression with pretreatment of SCH50911, treatment with this inhibitor was also evaluated. SCH50911 pretreatment completely prevented fatality in GHB-ethanol intoxication, and was effective at a surprisingly low dose.
In summary, ethanol potentiates the sedative and respiratory effects of GHB, and inhibition of the compensatory increase in tidal volume with ethanol coadministration leads to an increased risk of death due to respiratory depression in GHB overdose. GABAB receptors appear to be involved in both sedation and respiratory depression in GHB-ethanol intoxication, whereas current data support little involvement of GABAA receptors in GHB-ethanol toxicodynamics. Increasing GHB clearance via MCT inhibition with l-lactate results in improvement in toxicodynamic measures with GHB-ethanol administration, including the prevention of death from respiratory arrest. Therefore, MCT inhibition represents a potential clinical therapeutic strategy for the treatment of GHB overdose in the presence of ethanol. GABAB receptor inhibition may also be effective in GHB-ethanol intoxication, pending approval for use of GABAB receptor antagonists in the clinic.
Acknowledgments
The authors thank Joel Uhlander for technical assistance in preliminary studies. The authors also thank Dr. Melanie A. Felmlee for providing control data for sedation studies.
Abbreviations
- ABEC
area below the effect curve
- AUC
area under the plasma concentration-time curve
- CL
clearance
- CL/F
oral clearance
- CLm
metabolic clearance
- CLR
renal clearance
- Emax
maximum pharmacodynamic effect
- GHB
γ-hydroxybutyrate
- LRR
loss-of-righting reflex
- MCT
monocarboxylate transporter
- RRR
return-to-righting reflex
- SCH50911
(2S)-(+)-5,5-dimethyl-2-morpholineacetic acid
- SGS742
3-aminopropyl-n-butyl-phosphinic acid
Authorship Contributions
Participated in research design: Morse, Morris.
Conducted experiments: Morse.
Contributed new reagents or analytic tools: Morse, Morris.
Performed data analysis: Morse.
Wrote or contributed to the writing of the manuscript: Morse, Morris.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant R01-DA023223]; and by a fellowship from Pfizer Global Research and Development.
A portion of this work was previously presented as an abstract as the following meeting: Morse B, Uhlander J, and Morris M (2011) Respiratory depression in γ-hydroxybutyrate overdose: interaction with ethanol and treatment using monocarboxylate transporter inhibition. AAPS Annual Meeting and Exposition; 2011 Oct 23–27; Washington, DC.
References
- Adinoff B, Bone GH, Linnoila M. (1988) Acute ethanol poisoning and the ethanol withdrawal syndrome. Med Toxicol 3:172–196 [DOI] [PubMed] [Google Scholar]
- Altose MD, Hudgel DW. (1986) The pharmacology of respiratory depressants and stimulants. Clin Chest Med 7:481–494 [PubMed] [Google Scholar]
- Arena C, Fung HL. (1980) Absorption of sodium gamma-hydroxybutyrate and its prodrug gamma-butyrolactone: relationship between in vitro transport and in vivo absorption. J Pharm Sci 69:356–358 [DOI] [PubMed] [Google Scholar]
- Beleslin DB, Djokanović N, Jovanović Mićić D, Samardzić R. (1997) Opposite effects of GABAA and NMDA receptor antagonists on ethanol-induced behavioral sleep in rats. Alcohol 14:167–173 [DOI] [PubMed] [Google Scholar]
- Boje KM, Fung HL. (1989) Characterization of the pharmacokinetic interaction between nifedipine and ethanol in the rat. J Pharmacol Exp Ther 249:567–571 [PubMed] [Google Scholar]
- Bolser DC, Blythin DJ, Chapman RW, Egan RW, Hey JA, Rizzo C, Kuo SC, Kreutner W. (1995) The pharmacology of SCH 50911: a novel, orally-active GABA-beta receptor antagonist. J Pharmacol Exp Ther 274:1393–1398 [PubMed] [Google Scholar]
- Caldicott DG, Chow FY, Burns BJ, Felgate PD, Byard RW. (2004) Fatalities associated with the use of gamma-hydroxybutyrate and its analogues in Australasia. Med J Aust 181:310–313 [DOI] [PubMed] [Google Scholar]
- Carai MA, Colombo G, Brunetti G, Melis S, Serra S, Vacca G, Mastinu S, Pistuddi AM, Solinas C, Cignarella G, et al. (2001) Role of GABA(B) receptors in the sedative/hypnotic effect of gamma-hydroxybutyric acid. Eur J Pharmacol 428:315–321 [DOI] [PubMed] [Google Scholar]
- Carai MA, Colombo G, Gessa GL. (2005) Resuscitative effect of a gamma-aminobutyric acid B receptor antagonist on gamma-hydroxybutyric acid mortality in mice. Ann Emerg Med 45:614–619 [DOI] [PubMed] [Google Scholar]
- Chin RL, Sporer KA, Cullison B, Dyer JE, Wu TD. (1998) Clinical course of gamma-hydroxybutyrate overdose. Ann Emerg Med 31:716–722 [PubMed] [Google Scholar]
- Cook CD, Biddlestone L, Coop A, Beardsley PM. (2006) Effects of combining ethanol (EtOH) with gamma-hydroxybutyrate (GHB) on the discriminative stimulus, locomotor, and motor-impairing functions of GHB in mice. Psychopharmacology (Berl) 185:112–122 [DOI] [PubMed] [Google Scholar]
- Couper FJ, Thatcher JE, Logan BK. (2004) Suspected GHB overdoses in the emergency department. J Anal Toxicol 28:481–484 [DOI] [PubMed] [Google Scholar]
- Felmlee MA, Wang Q, Cui D, Roiko SA, Morris ME. (2010a) Mechanistic toxicokinetic model for gamma-hydroxybutyric acid: inhibition of active renal reabsorption as a potential therapeutic strategy. AAPS J 12:407–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee MA, Roiko SA, Morse BL, Morris ME. (2010b) Concentration-effect relationships for the drug of abuse gamma-hydroxybutyric acid. J Pharmacol Exp Ther 333:764–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froestl W, Gallagher M, Jenkins H, Madrid A, Melcher T, Teichman S, Mondadori CG, Pearlman R. (2004) SGS742: the first GABA(B) receptor antagonist in clinical trials. Biochem Pharmacol 68:1479–1487 [DOI] [PubMed] [Google Scholar]
- Fung HL, Tsou PS, Bulitta JB, Tran DC, Page NA, Soda D, Mi Fung S. (2008) Pharmacokinetics of 1,4-butanediol in rats: bioactivation to gamma-hydroxybutyric acid, interaction with ethanol, and oral bioavailability. AAPS J 10:56–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galicia M, Nogue S, Miró O. (2011) Liquid ecstasy intoxication: clinical features of 505 consecutive emergency department patients. Emerg Med J 28:462–466 [DOI] [PubMed] [Google Scholar]
- Gonçalves P, Araújo JR, Martel F. (2011) Characterization of butyrate uptake by nontransformed intestinal epithelial cell lines. J Membr Biol 240:35–46 [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Cryan JF, Wellendorph P, Mombereau C, Sansig G, Klebs K, Schmutz M, Froestl W, van der Putten H, Mosbacher J, et al. (2003) Specific gamma-hydroxybutyrate-binding sites but loss of pharmacological effects of gamma-hydroxybutyrate in GABA(B)(1)-deficient mice. Eur J Neurosci 18:2722–2730 [DOI] [PubMed] [Google Scholar]
- Lam WK, Felmlee MA, Morris ME. (2010) Monocarboxylate transporter-mediated transport of gamma-hydroxybutyric acid in human intestinal Caco-2 cells. Drug Metab Dispos 38:441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettieri JT, Fung HL. (1979) Dose-dependent pharmacokinetics and hypnotic effects of sodium gamma-hydroxybutyrate in the rat. J Pharmacol Exp Ther 208:7–11 [PubMed] [Google Scholar]
- Liljequist S, Engel J. (1982) Effects of GABAergic agonists and antagonists on various ethanol-induced behavioral changes. Psychopharmacology (Berl) 78:71–75 [DOI] [PubMed] [Google Scholar]
- Mason PE, Kerns WP., 2nd (2002) Gamma hydroxybutyric acid (GHB) intoxication. Acad Emerg Med 9:730–739 [DOI] [PubMed] [Google Scholar]
- McCabe ER, Layne EC, Sayler DF, Slusher N, Bessman SP. (1971) ynergy of ethanol and a natural soporific—gamma hydroxybutyrate. Science 171:404–406 [DOI] [PubMed] [Google Scholar]
- Morris ME, Hu K, Wang Q. (2005) Renal clearance of gamma-hydroxybutyric acid in rats: increasing renal elimination as a detoxification strategy. J Pharmacol Exp Ther 313:1194–1202 [DOI] [PubMed] [Google Scholar]
- Morris ME, Morse BL, Baciewicz GJ, Tessena MM, Acquisto NM, Hutchinson DJ, DiCenzo R. (2011) Monocarboxylate transporter inhibition with osmotic diuresis increases γ-hydroxybutyrate renal elimination in humans: a proof-of-concept study. J Clin Toxicol 1:105 [DOI] [PMC free article] [PubMed]
- Morse BL, Vijay N, Morris ME. (2012) γ-Hydroxybutyrate (GHB)-induced respiratory depression: combined receptor-transporter inhibition therapy for treatment in GHB overdose. Mol Pharmacol 82:226–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration-Center for Behavioral Health Statistics and Quality (2011) Drug Abuse Warning Network 2008: National Estimates of Drug-Related Emergency Department Visits: HHS Publication No. SMA 11-4618. Rockville, MD [Google Scholar]
- Thai D, Dyer JE, Benowitz NL, Haller CA. (2006) Gamma-hydroxybutyrate and ethanol effects and interactions in humans. J Clin Psychopharmacol 26:524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sassenbroeck DK, De Paepe P, Belpaire FM, Buylaert WA. (2003) Characterization of the pharmacokinetic and pharmacodynamic interaction between gamma-hydroxybutyrate and ethanol in the rat. Toxicol Sci 73:270–278 [DOI] [PubMed] [Google Scholar]
- Wang Q, Darling IM, Morris ME. (2006) Transport of gamma-hydroxybutyrate in rat kidney membrane vesicles: Role of monocarboxylate transporters. J Pharmacol Exp Ther 318:751–761 [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang X, Morris ME. (2008a) Effects of l-lactate and d-mannitol on gamma-hydroxybutyrate toxicokinetics and toxicodynamics in rats. Drug Metab Dispos 36:2244–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang Q, Morris ME. (2008b) Pharmacokinetic interaction between the flavonoid luteolin and gamma-hydroxybutyrate in rats: potential involvement of monocarboxylate transporters. AAPS J 10:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvosec DL, Smith SW, Porrata T, Strobl AQ, Dyer JE. (2011) Case series of 226 γ-hydroxybutyrate-associated deaths: lethal toxicity and trauma. Am J Emerg Med 29:319–332 [DOI] [PubMed] [Google Scholar]