Abstract
AIDS-related B-cell non-Hodgkin's lymphoma (AIDS-NHL) is a significant cause of morbidity and mortality among individuals infected with human immunodeficiency virus type 1 (HIV-1). AIDS-NHL is clinically and histologically heterogeneous, but common features include an aggressive clinical course and frequent extranodal presentation. HIV-1 infection of nonimmune cells that interact with malignant B cells at extranodal sites may influence both the development and the clinical presentation of disease. Our previous studies have shown that coculture of B-lymphoma (BL) cells with HIV-1-infected endothelial cells (EC) leads to contact activation of EC and firm BL-cell adhesion. The key event promoting EC-BL-cell adhesion was HIV-1 upregulation of endothelial CD40, which allowed induction of vascular cell adhesion molecule 1 (VCAM-1) in a CD40-dependent manner. The present study was designed to identify the HIV-1 protein(s) that influence EC-BL-cell adhesion. When HIV-1 proteins were individually expressed in EC by using recombinant adenoviruses, cultured BL cells adhered exclusively to Vpu-transduced EC. As with HIV-infected EC, adhesive properties were linked to the capacity of Vpu to upregulate CD40, which in turn allowed efficient expression of VCAM-1. When EC were infected with an HIV-1 pseudotype lacking the Vpu gene, CD40 upregulation and BL-cell adhesive properties were lost, indicating an essential role for Vpu in EC-BL-cell interactions. Thus, these data reveal a novel function for HIV-1 Vpu and further suggest a role for Vpu in the development of AIDS-NHL at EC-rich extranodal sites.
Patients infected with human immunodeficiency virus type 1 (HIV-1) are at significantly greater risk for developing AIDS-related non-Hodgkin's lymphoma (AIDS-NHL) than the general population (19). The incidence of AIDS-NHL is ∼100-fold higher than NHL arising in the immunocompetent population, and as many as 10% of NHL cases in the United States and Europe are AIDS related (10). AIDS-NHLs are primarily high-grade B-cell lymphomas whose clinical spectrum encompasses systemic lymphoma, primary central nervous system lymphoma and, less frequently, primary effusion lymphoma and plasmablastic lymphoma of the oral cavity (11). Pathologically, these tumors are characterized by a diffuse growth pattern, high-grade morphology and a B-cell origin. Clinical features of AIDS, including polyclonal B-cell stimulation, reduced immune surveillance, and dysregulated cytokine profiles, all contribute to tumor characteristics, either via predisposing the patient to malignant conversion or exacerbating tumor development (5, 18, 22, 29). Although malignant B cells are not themselves HIV infected, HIV-1 may also contribute more directly to the development and clinical presentation of AIDS-NHL via infection of nonmalignant bystander cells that interact with lymphoma cells.
Approximately 80% of all AIDS-NHLs are systemic, high-grade B-cell lymphomas falling into one of three histopathologic categories: Burkitt's lymphoma, diffuse large-cell lymphoma with immunoblastic features (also called immunoblastic lymphoma), and diffuse large-cell lymphoma with centroblastic features (also called large cell lymphoma) (11). Despite this heterogeneity, a common feature of the systemic lymphomas is localization to extranodal tissue sites, including the meninges, bone marrow, gastrointestinal tract, liver, and kidney (26, 30, 57). Interestingly, HIV-1 infection of the endothelium has been documented at the majority of these extranodal sites (see reference 12 for a comprehensive review). Although HIV-1 infection of endothelial cells (EC) is less efficient than infection of CD4+ leukocytes and may not play a significant role in the progression of immunodeficiency per se, endothelial infection could contribute to HIV-1 pathogenesis in more subtle ways. For example, HIV-1 infection of vascular and stromal EC could create a microenvironment conducive to AIDS-NHL attachment and growth. In support of this hypothesis, we have previously demonstrated the ex vivo outgrowth of malignant B cells from bone marrow stroma obtained from AIDS-NHL patients (41). Importantly, B-lymphoma (BL) cells adhered specifically to HIV-infected stromal microvascular EC (MVEC), and infection was absolutely required to sustain lymphoma cell proliferation and survival. In the same study, MVEC isolated from brain tissue developed a phenotype supportive of the attachment and growth of heterologous Burkitt's lymphoma cells after their in vitro infection with HIV-1. The lymphoma support phenotype was linked to an HIV-1-induced increase in expression of the tumor necrosis factor receptor (TNF-R) family molecule CD40. Ligation of CD40 led to preferential induction of vascular cell adhesion molecule 1 (VCAM-1) on HIV-1-infected/CD40high MVEC, which in turn facilitated increased lymphoma cell attachment via the VCAM-1 binding partner, VLA-4. In vivo, the initial induction of CD40, as well as post-CD40-ligation signals, may contribute to VCAM-1 induction and increased BL-cell attachment to HIV-1-infected endothelium. Direct cell-cell contact, allowing close proximity to EC-produced cytokines, could in turn promote lymphoma cell proliferation and the outgrowth of malignant foci at extranodal sites of endothelial infection.
The accessory and regulatory proteins of HIV-1 modulate diverse aspects of host cell function in order to facilitate the viral life cycle (see reference 21 for a recent review) and could conceivably contribute to the lymphoma support phenotype induced in HIV-1-infected MVEC. To identify HIV-1 gene(s) that influence BL attachment to EC, viral regulatory (Rev and Tat) accessory (Nef, Vif, Vpr, and Vpu) proteins were individually expressed in both large vessel (umbilical vein) and microvascular (dermal) EC by using an adenovirus expression system. When transduced EC were screened for their ability to support the attachment of cocultured CD40L+/VLA4+ BL cells, only Vpu-expressing EC supported firm BL-cell adhesion. To verify a role for CD40 in the adhesion process, the ability of Vpu to induce expression of CD40 and prime cells to respond to a CD40-triggered VCAM-1 induction stimulus was confirmed in the adenovirus system. An absolute requirement for Vpu in the context of HIV-1 infection was confirmed by the loss of the adhesive phenotype in EC infected with an HIV-1 mutant lacking the Vpu gene. Vpu is an HIV-1 accessory protein known to enhance virion release from HIV-infected cells (48), possibly by virtue of its ion channel activity (17), and to mediate the selective degradation of CD4 in the endoplasmic reticulum (7, 55). This latter function requires interaction of Vpu with the human β-transducin repeat-containing protein (βTrCP) (35). Unlike other βTrCP substrates, however, Vpu is not degraded by the SCF-βTrCP E3 ubiquitin ligase complex, allowing it to perturb the physiological functioning of this proteolytic pathway. Although the consequences of this transdominant effect of Vpu have yet to be fully realized, accumulation of the SCF-βTrCP substrates IκB, β-catenin, and ATF4 have all been documented in Vpu-expressing cells (3, 6). Our current data suggest an as-yet-unrecognized function of Vpu: modulation of host cell levels of the TNF-R family molecule CD40. In addition, to uncovering a novel Vpu function, our data predict a role of clinical importance for the Vpu gene, namely, regulation of the characteristic extranodal localization of malignant B cells in AIDS-NHL.
MATERIALS AND METHODS
Cell lines and cell culture.
Human umbilical vein endothelial cells (HUVEC) were obtained from BioWhittaker, Inc. (San Diego, Calif.). HUVEC monolayers were cultured in an endothelial growth medium (EGM; Cambrex, Walkersville, Md.) supplemented with 10% human AB serum (Sigma, St. Louis, Mo.) and a penicillin (100 U/ml)-streptomycin (100 μg/ml)-glutamine (2 mM) solution (PSG) on 35-mm or 60-mm Primaria tissue culture dishes (Becton Dickinson, Bedford, Mass.) and used for experiments at passages 4 to 6. Dermal MVEC (DMVEC) are an immortalized cell line not restricted by tissue culture passage that were derived as previously described (40) and cultured as described for HUVEC. BL cells are an Epstein-Barr virus-negative, CD40 ligand-positive (CD40L+), VLA-4-positive Burkitt's lymphoma line that was originally derived from an HIV-1-positive patient (41). BL cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (HyClone, St. Louis, Mo.) and PSG. Human embryonic 293 and 293T kidney cells (American Type Culture Collection, Manassas, Va.) and H-MAGI cells (National Institutes of Health [NIH] AIDS Research and Reference Reagent Program, Rockville, Md.) were grown in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum and PSG.
Antibodies and cytokines.
The anti-CD40 monoclonal antibody (MAb; Clone 5C3) used for flow cytometry was from Pharmingen (BD Biosciences, San Diego, Calif.). The anti-CD40 MAb used for CD40 ligation was a low-endotoxin, sodium azide-free (NA/LE) functional grade preparation of clone 5C3 from Pharmingen. Primary antibodies used for Western blot analysis included a rabbit anti-human CD40 antibody (Research Diagnostics, Inc., Flanders, N.J.), an anti-Tat monoclonal antibody (Advanced Biotechnologies, Inc., Columbia, Md.), and an anti-paxillin MAb (Upstate Biotechnology, Lake Placid, N.Y.). Second conjugate reagents included peroxidase-labeled anti-mouse and anti-rabbit antibodies (Amersham Pharmacia Biotech, Piscataway, N.J.). The anti-VCAM-1 MAb (clone 51-10C9) used for flow cytometry was from Pharmingen. For flow cytometry experiments, a mouse immunoglobulin G1 (IgG1) antibody (Clone MOPC-31C) from Pharmingen was used as an isotype control, and a Cy5-conjugated goat anti-mouse antibody (Amersham Pharmacia Biotech) was used as the fluorescent secondary reagent. Anti-HIV-1 p24 antibodies were obtained from Dako (Carpinteria, Calif.). Recombinant human TNF alpha (TNF-α) was purchased from R&D Systems, Inc. (Minneapolis, Minn.).
Adenoviral construction and infection.
Recombinant adenovirus vectors were constructed as previously described (24, 49). Each of the accessory (vpu, vpr, vif, and nef) and regulatory (tat and rev) genes of HIV-1 was amplified by PCR from proviral plasmid pNL4-3 (NIH AIDS Research and Reference Reagent Program) with the gene-specific forward and reverse primers. Each PCR product was cloned into an adenovirus shuttle vector (pΔE1sp1Btet/EF1EGFPTKpolyA) derived from plasmid pΔE1sp1Btet with the addition of EF1-EGFP and herpes simplex virus thymidine kinase poly(A). The recombinant adenoviruses were produced by cotransfection of 293 cells with the shuttle plasmid containing the various HIV-1 genes and pJM17 (Microbix, Toronto, Ontario, Canada), which has an adenovirus genome with E1A deleted. Titers of the recombinant adenoviruses in 293 cells were determined by limiting dilution. All adenovirus infections were performed by coinfection of an adenovirus transactivator-expressing virus (Ad/trans) at an equivalent, preoptimized multiplicity of infection (MOI). Specifically, EC monolayers were infected with adenoviruses expressing the different HIV-1 proteins (Ad/Vpu, Ad/Tat, Ad/Vpr, Ad/Vif, Ad/Rev, or Ad/Nef) and Ad/trans, each at an MOI of 100 unless specified otherwise. Infections were performed in the presence of Polybrene (2 μg/ml; of hexadimethrine bromide; Sigma) for 6 h, followed by rinsing and incubation in normal medium for 24 to 48 h. Control monolayers were mock infected or infected with Ad/trans alone at an MOI of 200. The functional integrity of each adenovirus-expressed HIV-1 protein was confirmed by demonstrating the following functions in cells infected with the respective recombinant adenoviruses: the capacity of Ad/Vpu and Ad/Nef to downregulate CD4 (43, 55), Ad/Vpr to accumulate cells in the G2/M phase of the cell cycle (1), Ad/Vif to degrade the editing enzyme APOBEC3G (CEM-15) (36), Ad/Rev to rescue HIV-1 p24 production from a Rev-deleted provirus (44), and Ad/Tat to transactivate the HIV-1 long terminal repeat (LTR) in CD4-LTR/β-galactosidase-expressing MAGI cells (54), where methods and results pertaining to these experiments are described).
HIV-1/VSV-G pseudotype virus construction and infection.
Recombinant HIV-1/VSV-G (HIV-G) pseudotype viruses were constructed as described previously (2). Proviral plasmids (pBru3oriΔenv, pME351Δvpu, pME341Δvpr, pME342Δvif, pMAVIIΔrev, and pSFiev+Δnef) used to construct HIV-G wild-type and deletion viruses were obtained through the AIDS Research and Reference Reagent Program. Briefly, cell-free viral stock was obtained from cotransfection of 293T cells with various proviral plasmids, pL-VSV-G plasmid and EGFP plasmid (transfection control). The virus stocks were harvested and concentrated, and titers were determined by using a standard H-MAGI cell assay as described previously (54). Pseudotype viral infection was performed by exposing EC to the titered viral inoculae at an MOI of 1 with Polybrene (2 μg/ml; Sigma) for 2 h in a minimal volume of medium to promote adsorption, followed by an overnight incubation under normal culture conditions. The following day, EC monolayers were rinsed in HBSS and recultured in EGM. HIV-G infection was confirmed by the detection of HIV-1 p24 gag protein expression by immunofluorescent staining with an anti-p24 antibody (1:100).
Western blot analysis.
For the determination of total CD40 levels, HUVEC grown in 60-mm Primaria tissue culture dishes were mock infected, infected with the appropriate adenovirus (Ad/Vpu and Ad/trans), or stimulated with TNF-α as described elsewhere. Monolayers were harvested at 48 h postinfection (p.i.), lysed in 500 μl of 2× sodium dodecyl sulfate (SDS) lysis buffer (2× SDS loading dye, 1 M dithiothreitol, and 2% bromophenol blue), removed, and boiled for 5 min. Then, 30 μl of total cell lysate from each sample was electrophoresed by SDS-polyacrylamide gel electrophoresis on 12% polyacrylamide gels and blotted onto nitrocellulose membranes (Schleicher & Schuell, Keene, N.H.). Membranes were blocked with 1% nonfat milk in TTBS (100 mM Tris-Cl [pH 7.5], 150 mM NaCl, 0.1% Tween 20) for 1 h, followed by incubation with rabbit anti-human CD40 antibody (1:250 dilution in TTBS with 1% nonfat milk) for 2 h at room temperature. After incubation, membranes were washed and treated with a secondary peroxidase-labeled anti-rabbit IgG (1:1,500 in TTBS with 1% milk) for 1 h. For determination of Tat levels in Ad/Tat- and HIV-G-infected cells and conditioned supernatants, the protocol was performed essentially as described above, but membranes were incubated with a mouse anti-Tat monoclonal antibody (1:100) and a peroxidase-labeled anti-mouse IgG (1:1,500) secondary antibody. An enhanced chemiluminescence system was used to visualize the protein of interest and was developed by using chemiluminescence and autoradiography. The amount of reactivity in each lane was quantified by densitometry. A mouse anti-paxillin MAb (1:1,000; Upstate Biotechnology, Lake Placid, N.Y.) was used as a loading control.
RT-PCR for CD40 transcripts.
Total RNA was isolated from Ad/Vpu-infected DMVEC at 48 h p.i. by using a Qiagen RNeasy Total RNA kit according to the manufacturer's method and reverse transcribed to generate cDNA by using Superscript II reverse transcriptase (RT; Invitrogen). As a positive control for CD40 transcript amplification, RNA extracted from HIV-infected brain EC was used. Additional controls included cells infected with Ad/Tat and Ad/trans, cells infected with Ad/trans alone, mock-infected cells, and cells stimulated with 10 ng of TNF-α/ml. Next, 2 μl of each cDNA was used as a template in the PCR with CD40-specific forward and reverse primers (forward, 5′-ATGATGCCCTGCTTCCTT-3′; reverse, 5′-CCTTCTGCCGCTTCTTCCT-3′) and AmpliTaq DNA polymerase (Applied Biosystems). PCR was performed at 94°C for 5 min, followed by 35 cycles of 94°C for 60 s, 55°C for 60 s, and 72°C for 90 s, followed by 72°C for 10 min and then 4°C indefinitely. Expression of the cellular HPRT gene was analyzed to control for equivalent cDNA levels. The HPRT primers were as follows: forward, 5′-CCTGCTGGATTACATCAAAGCACTG-3′; and reverse, 5′-TCCAACACTTCGTGGGGTCCT-3′. The HPRT conditions were as follows: 95°C for 5 min, followed by 35 cycles of 94°C for 60 s, 59°C for 60 s, and 72°C for 90 s, followed by 72°C for 10 min and 4°C indefinitely. Portions (10 μl) of each PCR product (CD40, 369 bp; HPRT, 300 bp) were visualized on ethidium bromide-stained agarose gels. Amplification reactions were also performed on samples generated in the absence of RT to control for the amplification of genomic DNA.
Flow cytometric analysis.
HUVEC were mock infected, infected with adenoviruses or HIV-1/VSV-G pseudotype viruses, or stimulated with TNF-α (10 ng/μl), and analyzed for cell surface expression of CD40 or VCAM-1 by using flow cytometry as previously described (41). For analysis of VCAM-1 expression, EC were additionally subjected to a CD40 ligation protocol (see below). Cells were washed twice with Ca2+/Mg2+-free Dulbecco phosphate-buffered saline (PBS) and stained indirectly with a mouse anti-human CD40 MAb, an anti-VCAM-1 MAb, or an isotype-matched mouse IgG1 antibody (all at 1:100). This step was followed by incubation with Cy5-conjugated goat anti-mouse immunoglobulin secondary antibody (1:300) and fixation with 2% paraformaldehyde. Cells were detached nonenzymatically and analyzed on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, Calif.) equipped with CellQuest software (Becton Dickinson). A minimum of 10,000 cells was analyzed for each sample.
CD40 ligation.
EC were mock infected or infected with recombinant adenoviruses for 6 h and then recultured with EGM for an additional 24 h. To cross-link surface CD40, EC were incubated with a NA/LE anti-CD40 monoclonal antibody used at 5 μg/ml for a further 24 h. CD40 ligation of EC was performed prior to (i) flow cytometric analysis of VCAM-1 expression (described above) or (ii) coculture with BL cells to evaluate EC-BL-cell adhesion (described below).
BL-cell adhesion assay.
HUVEC or DMVEC were plated on 35-mm Primaria dishes and allowed to grow to 80% confluence. Monolayers were infected with various adenoviruses or VSV-G pseudotype viruses as described elsewhere. CD40L+ BL cells (105 cells/dish) were added to infected or mock-infected EC monolayers at day 2 p.i. for a further 24 h to allow for contact-activation of EC and subsequent EC-BL-cell adhesion. Nonadherent and loosely adherent BL cells were subsequently removed by extensive washes with Hanks balanced salt solution (HBSS). After being washed, cultured cells were recultured in normal medium and immediately examined under a light microscope. Random ×10-magnified fields across each monolayer were counted to enumerate the numbers of BL cells remaining tightly adherent to the EC monolayer. Representative cell fields were photographed to record the extent of BL-cell adhesion. To demonstrate a specific requirement for CD40 engagement, an NA/LE CD40 MAb was added to adenovirus-infected EC at day 2 p.i. as a VCAM-1 induction stimulus. For these assays, BL cells were preloaded with the cell-permeant fluorescent dye Calcein-AM (5 μl/μl cell suspension; Molecular Probes) to facilitate quantitation of adherent cells. Calcein-AM-loaded BL cells were incubated with CD40-cross-linked EC for 4 h to allow adhesion, followed by rinsing with HBSS to remove loosely adherent and nonadherent cells. Adherent fluorescent BL cells were visualized under a fluorescence microscope with a ×10 objective lens, and random fields across the monolayer were recorded for counting with a digital camera. Calcein-AM-loaded BL cells were readily distinguishable from green fluorescent protein (GFP)-expressing EC on the basis of morphology and the intensity of the fluorescent dye.
CD40/CD40L blocking assay.
A CD40 fusion protein (CD40.Ig) that specifically binds CD40L and prevents CD40-CD40L binding was used to demonstrate an essential role for CD40-CD40L interactions in Vpu-mediated EC-BL-cell adhesion. The CD40.Ig fusion protein and a control human IgG1κ myeloma protein were generously provided by David Parker (Oregon Health and Science University). CD40L+ BL cells were cultured for 4 h in medium containing CD40.Ig fusion protein or control IgG1κ protein (both at 50 μg/ml) and then cocultured with DMVEC in 35-mm dishes (5 × 104 cells/dish). DMVEC were infected with Ad/Vpu and Ad/trans 24 h prior to BL-cell coculture. As additional controls, BL cells incubated without any protein were added to DMVEC infected with Ad/Vpu and Ad/trans, DMVEC infected with Ad/trans alone, mock-infected DMVEC, or DMVEC treated with TNF-α (10 ng/ml). After 24 h of coculture to allow for CD40-CD40L signaling, CD40-stimulated adhesion molecule induction, and BL-cell adhesion, nonadherent BL cells were removed by thorough rinsing with HBSS. Random ×10-magnified fields across each monolayer were counted to enumerate the number of BL cells remaining tightly adherent to the EC monolayer. Representative cell fields were photographed to record the extent of BL-cell adhesion.
RESULTS
HIV-1 Vpu-expressing endothelial cells support BL-cell adhesion.
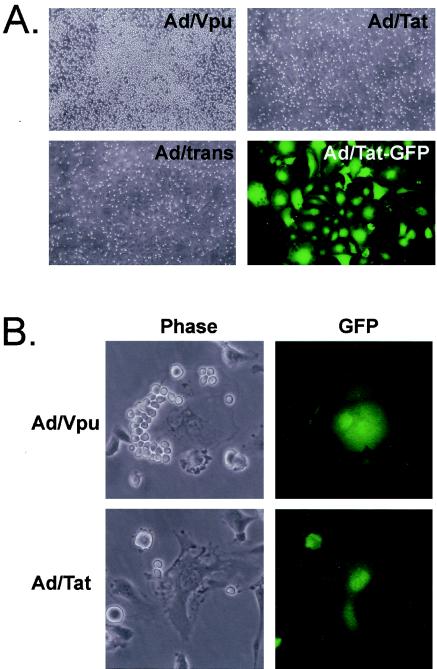
When BL-cell lines are cocultured with HIV-1-infected and uninfected EC, HIV-1-infected EC support significantly increased levels of BL-cell adhesion (41). To identify HIV-1 genes that influence EC-BL-cell adhesion, HIV-1 regulatory (tat and rev) and accessory (nef, vif, vpr, and vpu) genes were expressed in microvessel (DMVEC) and large-vessel (HUVEC) EC by using recombinant adenoviruses. Coexpression of GFP was used to verify successful transduction, which was always >80% of virus-exposed EC. BL cells were cocultured with adenovirus-transduced EC for 48 h, followed by stringent washing to remove nonadherent and loosely adherent cells. When cultures were evaluated by light microscopy, BL-cell adhesion was observed exclusively to Vpu-expressing EC monolayers. BL-cell adhesion to EC transduced with all other adenoviruses was minimal and comparable to mock-infected EC and EC infected with the adenovirus transactivator (Ad/trans) alone. Figure 1A illustrates BL-cell adhesion to a HUVEC monolayer infected with Ad/Vpu compared to a control monolayer infected with Ad/trans alone. BL-cell adhesion to HUVEC infected with Ad/Tat is shown as an example of the amount of residual BL-cell adhesion to all other adenovirus-transduced monolayers. Also shown is extensive GFP expression in a duplicate Ad/Tat-infected monolayer to verify efficient transduction. Tat expression in Ad/Tat-infected EC was further confirmed by Western blotting and immunofluorescence (data not shown). Similar adhesion results were observed when BL cells were cocultured with DMVEC infected with the recombinant adenovirus panel. Figure 1B illustrates representative high-power phase and fluorescence fields from Vpu- and Tat-transduced DMVEC cultures to illustrate significant BL-cell adhesion to a Vpu+ GFP+ cell compared to negligible adhesion to a Tat+ GFP+ cell. Thus, visual examinations of EC-BL-cell cocultures revealed that HIV-1 Vpu selectively induces an adhesive phenotype in both large-vessel and microvessel EC.
FIG. 1.
Endothelial cells expressing HIV-1 Vpu support the adhesion of BL cells. (A) HUVEC infected with recombinant adenoviruses expressing Vpu (Ad/Vpu), Tat (Ad/Tat), or transactivator alone (Ad/trans) as a control were cocultured with BL cells for 48 h, followed by a rinsing step to remove nonadherent BL cells. BL cells adhered exclusively to Ad/Vpu-infected monolayers. Magnification, ×100. The infection efficiency was monitored by GFP expression. As an example, the far left panel reveals GFP in >80% of the Ad/Tat-infected HUVEC (Ad/Tat-GFP) Magnification, ×200. (B) Ad/Vpu- or Ad/Tat-infected DMVEC cocultured with BL cells as described for panel A were photographed at high power under phase and fluorescent filters. Adhesion to Ad/Vpu+ GFP+ cells but not Ad/Tat+ GFP+ cells is shown. Magnification, ×400.
HIV-1 Vpu induces CD40 expression on endothelial cells.
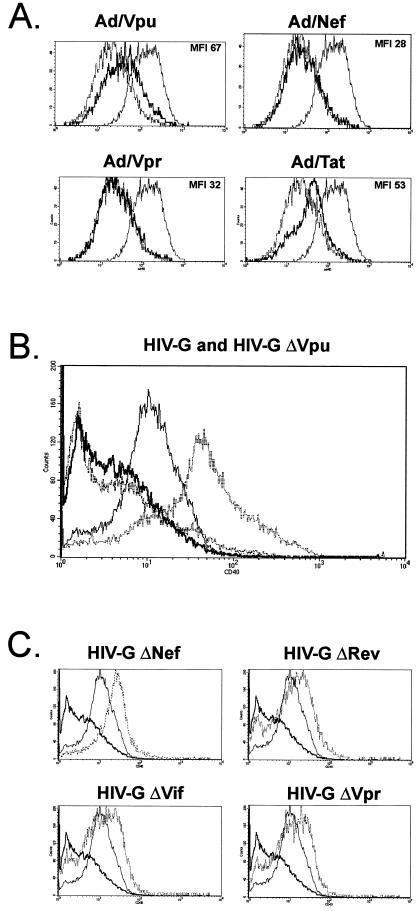
Previous studies by our group have identified HIV-1 upregulation of the cytokine receptor CD40 as a key event leading to enhanced EC-BL-cell adhesion. When CD40high EC are cocultured with CD40L+ BL cells, or a CD40-ligating antibody, cross-linking of CD40 leads to preferential upregulation of the adhesion molecule VCAM-1, which in turn allows tight adhesion of BL cells through VCAM-1-VLA-4 interactions (41). To determine whether the adhesive properties of Vpu-expressing EC could be correlated with the induction of CD40, HUVEC monolayers were mock infected or infected with Ad/Vpu for 48 h, nonenzymatically detached, and evaluated for CD40 expression by flow cytometry (Fig. 2A). HUVEC infected with Ad/Nef, Ad/Rev, Ad/Tat, Ad/Vif, and Ad/Vpr were similarly tested. Uninfected, TNF-α-stimulated HUVEC and HUVEC infected with Ad/trans alone were included as positive and negative controls, respectively. As expected, TNF-α-treated HUVEC expressed high levels of CD40 (mean fluorescence intensity [MFI] 88), whereas HUVEC infected with Ad/trans alone expressed basal CD40 levels equivalent to those seen in mock-infected cells (MFI 38). After HIV-1 gene transduction, increased levels of CD40 levels were seen on cells infected with Ad/Vpu (MFI 67) and, to a lesser extent, Ad/Tat (MFI 54). CD40 levels on cells expressing Vpr, Nef, Rev, and Vif (Fig. 2A and not shown) were similar to basal expression levels (all with MFI < 35).
FIG. 2.
HIV-1 Vpu upregulates surface CD40 on endothelial cells. (A) HUVEC were infected with recombinant adenoviruses for 48 h, and quantitative CD40 expression was determined by flow cytometry. HUVEC infected with Ad/trans alone or stimulated with TNF-α (10 ng/ml) served as negative or positive controls for CD40 expression, respectively. For each panel, the profile obtained with the respective recombinant adenovirus is shown as a bold solid line. Superimposed are the baseline expression levels obtained with the Ad/trans control (dashed line) and the elevated expression levels induced by TNF-α-treatment (thin solid line). The MFI value for each profile is given in parentheses. An increase in CD40 expression was detected on cells infected with Ad/Vpu (MFI 67) relative to cells infected with Ad/trans alone (MFI 37). No change was seen in cells infected with Ad/Nef (MFI 28), Ad/Vpr (MFI 32), Ad/Rev (not shown), or Ad/Vif (not shown). A moderate increase in CD40 was detected in cells infected with Ad/Tat (MFI 53). CD40 expression was efficiently induced by TNF-α (MFI 88). (B) HUVEC were infected with an HIV-1 pseudotyped virus (HIV-G) (thin solid line), as well as with an HIV-Gmutant lacking Vpu (ΔVpu) (bold solid line), and the expression of surface CD40 was evaluated by flow cytometry. Mock-infected (bold hatched line) and TNF-α-stimulated (thin hatched line) HUVEC served as negative and positive controls for CD40 expression, respectively. Constitutive CD40 levels on mock-infected EC were low (MFI 2.7) but were significantly increased by infection with HIV-G (MFI 10.4). Infection of EC with the ΔVpu mutant did not lead to CD40 induction (MFI 2.8). CD40 expression was efficiently induced by TNF-α (MFI 32.5). (C) Expression of CD40 on HUVEC infected with HIV-G mutants (hatched lines) lacking HIV-1 Nef (ΔNef), Rev (ΔRev), Vif (ΔVif), or Vpr (ΔVpr) was similar to that of the wild-type HIV-G (thin solid line). The decrease in CD40 seen with ΔVpu HIV-G (bold line) is superimposed on each image for reference.
The unique ability of the HIV-1 accessory protein Vpu to both induce CD40 and promote EC-BL-cell adhesion (Fig. 1) suggested that Vpu was the viral protein mediating the CD40-dependent adhesion of BL cells to HIV-infected EC. Functions attributed to Vpu to date suggest that this viral protein has a diverse capacity to modulate host cell proteins and physiologic processes (reviewed in reference 8). Our finding that Vpu increases CD40 expression and promotes EC-BL-cell adhesion identifies a potential novel and important function for Vpu. We thus chose to study Vpu induction of CD40, and its consequences for CD40-mediated EC-BL-cell interactions, in more detail.
HIV-1 Vpu is essential for induction of CD40 on HIV-1-infected endothelial cells.
The experiments described above involved individual expression of HIV genes in EC with adenovirus vectors. Before further investigation of Vpu as a modulator of the EC adhesive phenotype, we sought to confirm an essential role for Vpu induction of endothelial CD40 in the context of other HIV-1 proteins. Thus, HIV-induced CD40 expression was evaluated by using an HIV-1 mutant that lacks Vpu. HUVEC were infected with an HIV-1/VSV-G-pseudotyped virus (HIV-G) or with a variant of HIV-G with vpu deleted (Δvpu HIV-G). CD40 surface expression was then determined by flow cytometry as before. As controls, Δnef, Δvpr, Δvif, and Δrev HIV-G mutants were tested in parallel. TNF-α-stimulated cells were included as positive controls for CD40 induction, and mock-infected cells were used as a measure of basal CD40 expression levels. Figure 2B illustrates that infection of EC with the wild-type HIV-G significantly increased CD40 levels compared to the low constitutive levels expressed on mock-infected EC (MFI increase from 2.7 to 10.4). Importantly, CD40 levels on cells infected with the Δvpu HIV-G were the same as on mock-infected cells (MFI 2.8), indicating that Vpu was absolutely required for CD40 induction. CD40 levels on cells infected with the Δnef, Δvpr, Δvif, and Δrev HIV-G mutants that retained expression of Vpu were similar to those seen with the wild type (Fig. 2C), suggesting that none of these viral proteins was absolutely required for CD40 induction. These experiments confirm an essential role for Vpu in the HIV-1-mediated upregulation of surface CD40 expression.
Because a functional Δtat HIV-G pseudotype could not be made, parallel experiments in HIV-G-infected cells that lacked Tat were not performed. It is worth noting, however, that Tat expressed by the Δvpu HIV-G-infected EC did not reconstitute CD40 levels on these infected cells. The discrepancy between the ability of Tat to induce CD40 when expressed in EC from an adenovirus vector, but not rescue CD40 expression on Δvpu HIV-G-infected cells, is interesting. Note that whereas Western blot analysis of EC infected with Ad/Tat or HIV-G for 48 h showed similar levels of Tat expression in cell lysates, Tat protein could only be detected in the supernatants of EC infected with Ad/Tat (data not shown). Since extracellular Tat is known to activate several cell types, including endothelial cells (reviewed in reference 45), it is likely that Tat secreted in the context of the adenovirus expression system contributes to CD40 expression, either directly or via inducing inflammatory cytokines. Thus, the potential for Tat to contribute to CD40 induction in vivo in the context of replication competent virus should not be ruled out. For the purposes of our in vitro study, however, the adenovirus system used allows elucidation of Vpu induction of CD40 independent of any contribution from Tat.
Adhesion of BL cells to Vpu-positive EC occurs via a CD40-dependent pathway.
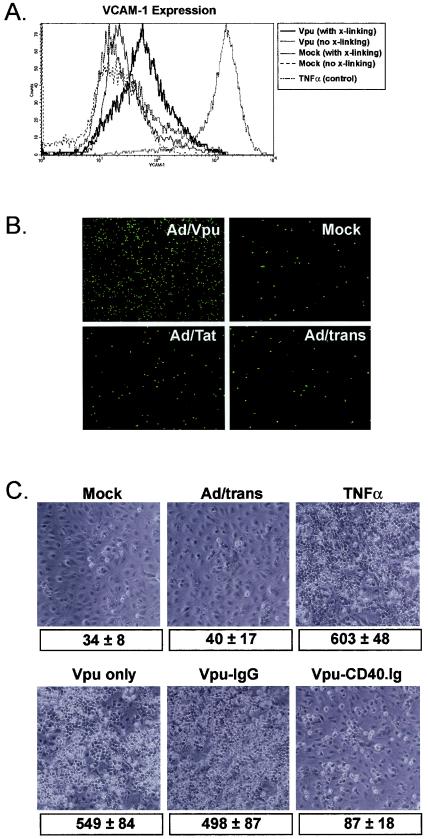
Lymphocyte-EC contact interactions induce endothelial adhesion molecule expression, and members of the TNF-R:TNF family, including CD40:CD40L, play an important role in this contact-mediated activation (51). Since adenovirus-mediated expression of Vpu in EC induced both BL-cell adhesion and expression of CD40, we next investigated a direct role for CD40 in inducing the adhesive phenotype. Because the BL-cell line used in these studies is CD40L, it can contact activate CD40-positive cells independent of the need for an additional CD40 ligation stimulus (Fig. 1) (41). However, to confirm a specific requirement for CD40 in Vpu-mediated adhesion, Vpu-expressing EC were left unstimulated or were stimulated with a cross-linking CD40 MAb for 48 h prior to the addition of BL cells for a short (4-h) period to allow adhesion. As controls, mock-infected or Ad/trans-infected EC were similarly treated. The efficacy of the CD40-ligation stimulus was confirmed by flow cytometric evaluation of the B-cell adhesion molecule VCAM-1 on control- and Vpu-expressing EC. As illustrated in Fig. 3A, Vpu expression alone had no effect on constitutive VCAM-1 levels; expression was equivalent to the low constitutive levels seen in mock-infected cells. After CD40 ligation, however, VCAM-1 expression was significantly elevated on Ad/Vpu-infected cells. As expected, mock-infected cells expressing basal CD40 levels were refractory to CD40 ligation. These experiments confirm the utility of the cross-linking protocol, as well as the ability of CD40 ligation to induce VCAM-1 expression on EC (27).
FIG. 3.
BL-cell adhesion to Vpu-expressing EC is CD40 dependent. (A) To verify the ability of CD40 cross-linking to induce VCAM-1 expression on Vpu-expressing EC, mock-infected EC, and Ad/Vpu-infected EC were either not treated (no cross [x]-linking) or were treated witha CD40 MAb to cross-link CD40 (with cross-linking). Mock-infected MVEC did not express VCAM-1 constitutively and were unresponsive to CD40 cross-linking. In contrast, whereas Ad/Vpu-infected EC only expressed only basal levels of VCAM-1, CD40 cross-linking effectively stimulated VCAM-1 expression. VCAM-1 expression nonspecifically induced by TNF-α stimulation was measured as a positive control. (B) EC infected with Ad/Vpu were treated with a CD40 cross-linking MAb to induce VCAM-1 (as shown in panel A). As controls, mock-infected EC or EC infected with Ad/Tat or Ad/trans alone were similarly treated. CD40-activated cells were cocultured with VLA4+ BL cells preloaded with a green fluorescent vital dye (Calcein-AM) for 4 h, and cells remaining tightly adherent to the monolayer after a stringent rinsing were counted by using a fluorescence microscope. Although all monolayers were treated with the CD40-specific VCAM-1 induction stimulus, only Vpu-expressing cells were responsive and were able to support BL-cell adhesion. Magnification, ×90. Table 1 gives actual cell counts for a representative experiment. (C) Ad/Vpu-infected EC were cocultured with CD40L+ BL cells in the presence of a CD40.Ig fusion protein (Vpu-CD40.Ig) to block CD40-CD40L interactions. Ad/Vpu-infected EC were also cocultured with BL cells without fusion protein (Vpu only) or with a control non-blocking IgG protein (Vpu-IgG). Additional controls included coculture of untreated BL cells with mock-infected EC (Mock), EC infected with Ad/trans alone (Ad/trans), or EC stimulated with TNF-α. After 24 h of coculture, cells remaining tightly adherent to the monolayer after a stringent rinsing were counted under a phase microscope. Magnification, ×180. The values shown underneath each image are the corresponding adherent BL-cell counts (average ± the SD for five random fields) from a typical experiment.
For experiments to evaluate adhesion to CD40-activated EC, BL cells preloaded with a fluorescent dye were cocultured with CD40-activated EC for 4 h, followed by stringent rinsing to remove nonadherent or loosely adherent BL cells. In the absence of CD40 cross-linking, BL-cell adhesion to all EC was negligible, regardless of the adenovirus infection status (data not shown). Adhesion to mock- and Ad/trans-infected EC stimulated with the α-CD40 MAb was similarly low, but a significant number of BL cells remained adherent to Vpu-expressing monolayers after CD40 ligation. Figure 3B illustrates the appearance of a typical experiment with DMVEC as the EC source. Table 1 records actual cell counts from two representative experiments where values represent the number of adherent BL cells in a ×10-magnified field. The average ± the standard deviation (SD) of four randomly selected fields is shown. Collectively, these data suggest that Vpu induction of CD40 primes EC to respond to a CD40 activation signal (in this case cross-linking by α-CD40 MAb), with the consequence being increased expression of adhesion molecules such as VCAM-1 and promotion of EC-BL-cell adhesion.
TABLE 1.
BL-cell adhesion to CD40-activated EC expressing HIV-1 Vpu
| EC groupa | Mean BL-cell adhesionb ± SD
|
|
|---|---|---|
| Expt 1 | Expt 2 | |
| Ad/Vpu-infected EC | 722 ± 33 | 844 ± 100 |
| Mock-infected EC | 38 ± 6 | 56 ± 8 |
| Ad/Tat-infected EC | 78 ± 11 | 144 ± 16 |
| Ad/trans-infected EC | 42 ± 7 | 96 ± 32 |
DMVEC were infected with recombinant adenoviruses expressing HIV-1 Vpu (Ad/Vpu), activated with a CD40 MAb, and cocultured with BL cells as described in Materials and Methods. Control EC were mock infected or infected with Ad/Tat or the adenovirus transactivator alone (Ad/trans).
Determined as the number of BL cells adhering to DMVEC monolayers. Counts from two independent experiments (mean ± the SD of four randomly selected × 10 fields) are shown.
Although the aforementioned data shows that Vpu-expressing EC respond preferentially to CD40 activation signals, it does not conclusively establish the importance of the CD40-CD40L interaction for Vpu-mediated EC-BL-cell adhesion. To directly address this issue, a CD40.Ig fusion protein was used to inhibit CD40-CD40L interactions between Vpu-expressing CD40+ EC and CD40L+ BL cells, and the effect on EC-BL-cell adhesion was then evaluated (Fig. 3C). For these experiments, BL cells were cocultured with EC for 24 h to allow CD40 cross-linking by BL cells and CD40 induction of adhesion molecules, but a CD40.Ig fusion protein was included to prevent CD40:CD40L binding. This protocol was previously used to successfully inhibit CD40:CD40L interactions between HIV-1-infected EC and BL cells (41) As predicted, minimal BL-cell binding to mock- or Ad/trans-infected EC was observed, whereas binding to Vpu-expressing EC was equivalent to that of TNF-α-activated EC. Importantly, whereas a control IgG fusion protein had no effect on BL-cell adhesion to Vpu+EC, the CD40.Ig protein significantly inhibited binding. Figure 3C illustrates the degree of adhesion recorded for each condition in a representative experiment. Cell counts (average ± the SD of five randomly selected fields) are given below each corresponding image. Thus, functional expression of CD40 and specific CD40 triggering are absolutely required for the enhanced binding of BL cells to Vpu-expressing EC.
HIV-1 Vpu is essential for induction of BL-cell adhesive properties in HIV-1-infected endothelial cells.
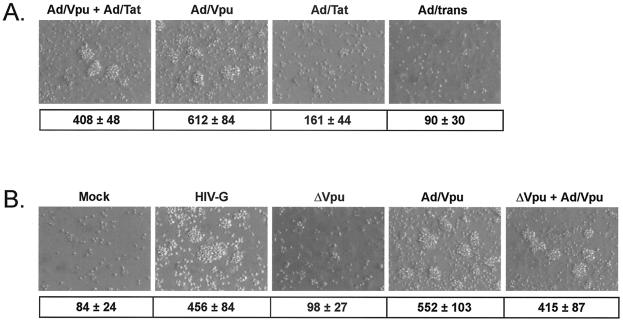
Vpu was the only HIV-1 protein tested with the capacity to confer BL-cell adhesive properties on EC when expressed alone, but both Tat and Vpu were able to influence CD40 expression. To examine whether coexpression of Tat and Vpu could influence EC-BL-cell adhesion, HUVEC were infected with Ad/Tat or Ad/Vpu, each at an MOI of 100, or were coinfected with Ad/Tat and Ad/Vpu, each at an MOI of 50. At day 2 p.i., CD40L+BL cells were cocultured with EC for an additional 24 h to allow contact activation of EC and subsequent EC-BL-cell adhesion. Adherent cells remaining after stringent rinsing were photographed and counted. No enhancement of adhesion was seen in the presence of both Tat and Vpu compared to Vpu alone. In fact, coinfection with Ad/Tat and Ad/Vpu led to reduced adhesion levels that were probably attributable to an MOI-dependent reduction of the percentage of Vpu-expressing cells. Figure 4A illustrates the degree of adhesion recorded for each treatment in a typical experiment. The numbers of adherent BL cells (average ± the SD of four randomly selected fields) counted in a representative experiment are given below each corresponding image. These data indicate that the expression of Vpu is sufficient to induce an adhesive phenotype that is not complemented synergistically or additively by coexpression of Tat.
FIG. 4.
HIV-1 Vpu is necessary and sufficient to support BL-cell adhesion to HIV-infected EC, and Tat coexpression does not influence Vpu activity. (A) EC were coinfected with Ad/Tat and Ad/Vpu, and BL-cell adhesion to coinfected monolayers was evaluated by examination of rinsed monolayers after a period of BL-cell-mediated contact activation. As controls, EC singly infected with Ad/Vpu or Ad/Tat or infected with Ad/trans only were similarly tested. Tat alone did not influence contact-activated BL-cell adhesion, and coexpression of Tat in Vpu-expressing cultures did not lead to increased levels of BL-cell adhesion compared to EC expressing Vpu only. Magnification, ×90. The values shown underneath each image are the corresponding adherent BL-cell counts (average ± the SD for four random fields) from a typical experiment. (B) EC were infected with an HIV-1 pseudotype virus (HIV-G), as well as with an HIV-G mutant lacking Vpu (ΔVpu), and BL-cell adhesion to HIV-G-infected monolayers was evaluated as for panel A. To restore Vpu expression, ΔVpu-infected EC were coinfected with Ad/Vpu. As controls for basal and Vpu-induced adhesion, respectively, EC were mock infected or infected with Ad/Vpu. Infection with HIV-G wild-type (HIV-G) induced adhesion to levels seen with Ad/Vpu infection (Ad/Vpu). Loss of Vpu from HIV-G-infected cells (via infection with the HIV-G ΔVpu mutant) led to a loss of BL-cell adhesion that could be rescued when Vpu was supplied via adenovirus infection (ΔVpu + Ad/Vpu). The values shown underneath each image are the corresponding adherent BL-cell counts (average ± the SD for four random fields) from a typical experiment.
To confirm that Vpu is essential for inducing an adhesive phenotype when expressed from an HIV-1 provirus, BL-cell adhesion experiments were repeated with HUVEC that were infected with an HIV-1 pseudotype virus lacking Vpu (Δvpu HIV-G) and compared to HUVEC infected with the wild-type HIV-G. To rescue the Vpu-induced phenotype, HUVEC infected with Δvpu HIV-G were coinfected with the recombinant adenovirus expressing Vpu (Ad/Vpu). For reference, control monolayers were mock infected or infected with Ad/Vpu. At day 2 p.i., CD40L+BL cells were cocultured with EC for an additional 24 h to allow contact activation of EC and subsequent EC-BL-cell adhesion. Adherent cells remaining after a stringent rinsing were photographed and counted. As illustrated in Fig. 4B, infection with the HIV-G wild-type virus induced adhesive properties in EC that were similar to those seen with Ad/Vpu. Importantly, when the Δvpu mutant was used, the adhesion phenotype was lost but could be rescued by adenovirus-mediated expression of Vpu. The numbers of adherent BL cells (average ± the SD of four randomly selected fields) counted in a representative experiment are displayed below each corresponding image. These data clearly show that the loss of Vpu is sufficient to abolish the adhesive properties of HIV-1-infected cells even in the presence of other viral proteins. The inability of Tat expressed by the Δvpu virus to reconstitute adhesion correlates with the inability of adenovirus-expressed Tat to support adhesion (Fig. 1) or to complement Vpu in coinfection experiments (Fig. 4A). Thus, Vpu appears essential for inducing BL-cell adhesion when expressed at levels seen in HIV-infected cells and in the under presence of other viral proteins. Collectively, these data confirm a role for Vpu as the HIV-1 protein that is essential and sufficient for influencing the adhesive properties of HIV-infected EC.
Total CD40 levels are increased in Ad/Vpu-infected HUVEC.
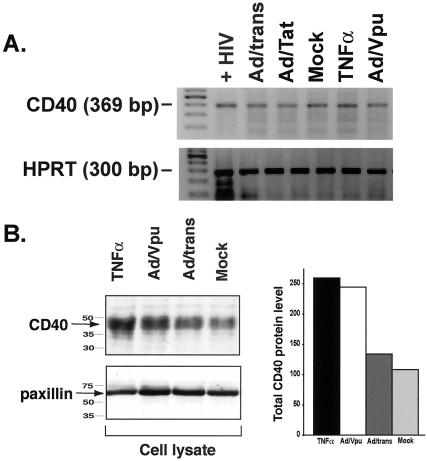
Flow cytometry data revealed that Vpu expression in EC resulted in increased expression of CD40 at the cell surface. To determine whether Vpu was increasing CD40 levels at the transcriptional level, we performed CD40-specific RT-PCR on EC to compare levels of CD40 transcript in control EC (either mock infected or infected with Ad/trans alone) or EC infected with Ad/Vpu and Ad/trans for 48 h. As illustrated in Fig. 5A, the constitutive levels of CD40 message expressed in EC were unchanged by adenovirus infection and expression of Vpu. Thus, Vpu must act via a posttranscriptional mechanism to upregulate surface CD40, perhaps by increasing total CD40 protein levels and/or by redirecting CD40 protein to the cell surface. To determine whether total CD40 levels in Vpu-expressing cell were increased, we performed a Western blot analysis of whole-cell lysates from Ad/Vpu-infected HUVEC compared the results to those obtained with mock-infected HUVEC or HUVEC infected with Ad/trans alone for 48 h. Lysates from TNF-α-treated HUVEC and were used as a positive control for CD40 induction. As illustrated in Fig. 5B, the total levels of CD40, as measured by densitometry in Vpu-expressing cells (CD40 level of 244.6), were increased twofold relative to mock-infected cells (CD40 level of 108.8) or cells expressing transactivator alone (CD40 level of 134). Although the exact mechanism has yet to be determined, HIV-1 Vpu may increase total CD40 protein levels by promoting production or inhibiting degradation. This influence on CD40 may occur directly or via intermediate protein(s) that regulates cellular CD40 levels.
FIG. 5.
HIV-Vpu increases CD40 protein levels in endothelial cells via a posttranscriptional mechanism. (A) RT-PCR for CD40-specific transcripts reveals no induction of CD40 message in EC infected with Ad/Vpu (Ad/Vpu) relative to mock-infected EC (Mock), EC infected with Ad/trans alone (Ad/trans), or EC infected with Ad/Tat (Ad/Tat). A slight increase in CD40 RNA was observed in cytokine (TNF-α)-stimulated EC. EC infected with HIV (+HIV) were included as a positive control for CD40 amplification. Amplified PCR products at the expected molecular weights for CD40 (369 bp; top panel) and HPRT (300 bp; bottom panel) were visualized by ethidium bromide staining. Primer sequences and PCR conditions are described in Materials and Methods. (B) Total CD40 protein levels were determined by Western blot analysis of whole-cell lysates. The levels of the cellular protein paxillin in each lysate were measured as a loading control. CD40 levels in Ad/Vpu-infected cells (Ad/Vpu) were twice that seen in mock-infected (Mock) or Ad/trans-infected (Ad/trans) control cells and were comparable to levels induced by stimulation with TNF-α. CD40 levels were quantitatively measured by densitometry and are graphically represented. Both experiments represent cells at 48 h p.i. Similar results were detected at 24 h p.i.
In summary, all of the results presented above are consistent with a primary role for HIV-1 Vpu in mediating EC-BL-cell adhesion. This is a novel function for Vpu that appears to be directly related to the ability of Vpu to upregulate the cell surface expression of the cytokine receptor CD40. In addition to revealing yet another protein whose cell fate is modulated by the action of Vpu, these data suggest a role for Vpu in regulating an AIDS-related clinical syndrome, the development of AIDS-associated lymphoma. In the context of AIDS-NHL, Vpu modulation of endothelial CD40 could prime EC to respond to CD40:CD40L activation pathways, and the subsequent adhesive properties would promote the attachment of lymphoma cells at extranodal tissue sites.
DISCUSSION
After HIV-1 infection, EC acquire a phenotype supportive of B-cell adhesion that may have important implications for the distinctive development of B-cell AIDS-NHL at extranodal sites such as the liver, brain, and bone marrow (41). We report here that the HIV-1 Vpu protein is able to recapitulate the adhesive phenotype of HIV-infected EC and that Vpu-mediated upregulation of the cytokine receptor CD40 is integral in this process. Since the CD40L is expressed by normal and neoplastic B cells, (15, 20, 56), B cells in vivo have the capacity to activate CD40-linked EC signaling pathways via a contact-dependent receptor-ligand interaction. Indeed, in our coculture system, this mechanism of CD40 ligation was shown to be effective for both Vpu-expressing EC as well as HIV-infected EC. In vivo, CD40L+ T cells or monocytes in the microenvironment, or soluble CD40L (sCD40L), could similarly deliver activation signals to CD40+ EC. Note that CD40L is overexpressed on CD4+ T cells in HIV-1-infected patients and is thought to contribute to cell trafficking disturbances (47). In addition, a recent study has documented increased sCD40L levels in HIV-1 patients, providing an additional mechanism for CD40 activation in the context of HIV (46). In vitro, adhesion experiments performed after CD40 MAb cross-linking mimic the CD40L-activated condition. Regardless of the CD40L source, our data suggest that HIV upregulation of CD40 is an essential initial event defining the EC activation status and that Vpu is the HIV-1 protein primarily responsible for EC priming.
Expression of HIV-1 proteins via recombinant adenoviruses was initially used to identify Vpu as the HIV gene responsible for EC-BL-cell adhesion via a CD40-mediated pathway. Although this system evaluates expression of viral proteins in isolation, it is important to note that adenovirus-expressed Vpu faithfully retained two previously established functions of Vpu, i.e., downregulation of CD4 and MHC I molecules from the cell surface (28, 55). In addition, infection of EC with an HIV-1 mutant lacking Vpu (Δvpu HIV-G) established that Vpu is absolutely required for CD40 expression and induction of the adhesive phenotype, even in the context of other HIV-1 proteins. Although adenovirus-expressed Tat upregulated CD40 expression, Ad/Tat-infected EC did not support the adhesion of cocultured B cells. In addition, coexpression of Tat and Vpu in EC did not enhance the degree of adhesion seen with Vpu expression alone. These findings suggested that Tat probably does not play an essential role in CD40-activated BL-cell adhesion. This conclusion was further supported by the fact that the presence of Tat in EC infected with the Δvpu HIV-G mutant virus was not sufficient to compensate for the loss of Vpu, either for CD40 expression or BL-cell adhesion. Note, however, that under conditions of active HIV replication, extracellular Tat produced by infected cells may influence endothelial cell adhesive properties via Vpu-independent pathways (16).
Our study identifies Vpu as an HIV-1 protein responsible for conferring endothelial cell adhesive properties on HIV-infected EC via upregulation of surface CD40. CD40 is a member of the TNF-R superfamily that is expressed on immune cells and several types of nonimmune cells, including vascular endothelium. Under physiological conditions, CD40 is expressed at low levels on EC but is significantly elevated in areas of inflammation (27) and angiogenesis (14) and after infection with endothelium-tropic viruses such as Kaposi's sarcoma-associated herpesvirus (42), HIV-1 (41), and cytomegalovirus (34). Ligation of CD40 leads to receptor oligomerization and initiation of a signaling cascade that elicits several cell type-dependent downstream events. In EC, CD40 ligation induces the production of inflammatory chemokines and cytokines, growth factors, metalloproteinases, and adhesion molecules (25, 32, 33, 37, 38, 50). In vivo, receptor oligomerization is accomplished via binding of the trimeric ligand CD40L (CD154) expressed on activated immune cells (monocytes, T cells, B cells, or platelets) or the soluble isoform (sCD40L) that circulates in serum as a biologically active protein. Evidence from a number of biological systems suggests that the interaction between CD40L+ leukocytes and CD40-bearing endothelia plays an important role in inflammation and disease (4). For example, coculture of intestinal MVEC with CD40L+ platelets induces expression of adhesion molecules (ICAM-1 and VCAM-1) and soluble factors (interleukin-8 and RANTES) in a CD40-dependent fashion (13). These events facilitate increased MVEC-T-cell adhesion and define a role for activated platelets in inflammatory bowel disease. A similar role for CD40/CD40L in endothelial-macrophage inflammatory reactions in atherosclerosis has been proposed (31, 33). Recently, contact-mediated activation of EC by T cells via CD40-CD40L interactions was shown to induce EC production of cytokines and chemokines (39). We have defined an important role for CD40:CD40L in the pathogenesis of AIDS-NHL, where HIV-1 infection of EC plays a direct role (33). Specifically, HIV-1-infected EC upregulate CD40 at the cell surface, thereby priming the infected cell to respond to CD40L-mediated activation. Contact activation by CD40L+ BL cells leads to upregulation of VCAM-1 and firm EC-BL-cell adhesion via VCAM-1:VLA-4. Our finding here that HIV-1 Vpu is the viral protein responsible for initiating this cellular cascade may help to elucidate the viral and cellular mechanisms that promote homing and development of AIDS-NHL at extranodal sites. Although EC were the target cell type investigated here, Vpu dysregulation of CD40-transduced signals could occur in other HIV-permissive cells. Due to the actions of Vpu, CD40-mediated events would be focally restricted to sites of HIV-1 infection and allow CD40-CD40L interactions to constitute an important intermediary between diverse virus-mediated inflammatory and immune processes.
Exactly how HIV-1 Vpu intersects CD40 induction and signaling pathways has yet to be defined. CD40 expression on EC is induced by a variety of inflammatory mediators (25, 27), as well as by viral infections other than HIV infection (34). Studies on CD40 signal transduction have revealed that multiple mediators and pathways are involved, including activation of protein tyrosine kinases, phosphatidylinositol 3-kinase, and phospholipase Cγ2 (see reference 51 and references therein). Members of the TNF-R-associated factor family that associate with the cytoplasmic domain of CD40 and link CD40 engagement to activation of different kinases and transcription factors (see reference 9 for a recent review) may also constitute candidates for Vpu interaction.
HIV-1 Vpu is not incorporated into the viral particle and exerts its effects within the infected cell. Vpu degrades nascent CD4 in the endoplasmic reticulum (55) and augments virion release from the plasma membrane probably through an ion channel activity (48). In addition, Vpu interferes with the synthesis and surface expression of MHC class I molecules (28) and protein transport to the plasma membrane (53). CD4 degradation is mediated through a physical interaction between the cytoplasmic domains of CD4 and Vpu. Phosphorylation of two serine residues (Ser52 and Ser56) in the conserved DSGXXS phosphorylation motif of Vpu are critical for CD4 degradation (7) and are required for recruitment of human WD40 βTrCP (35). βTrCP is a key component of the SkpI-Cdc53-F-box protein (SCF) E3 ubiquitin ligase complex that selects cellular proteins, including CD4, for polyubiquitination and targeting to the proteasome for degradation (6, 23). The ability of Vpu to act as an adapter molecule linking CD4 to the ubiquitin-proteasome pathway suggests that Vpu may similarly interact with CD40 and/or CD40-associated cellular proteins. In contrast to CD4, however, the consequence of the CD40-Vpu interaction would be stabilization, accumulation, and/or redistribution of the CD40 protein. Indeed, the ability of Vpu to act as a transdominant-negative inhibitor of βTrCP (6) suggests that Vpu may impair CD40 degradation pathways, thus increasing overall CD40 levels in HIV-infected cells. In this regard, a recent report (3) has demonstrated that Vpu, through its ability to function as a competitive inhibitor of βTrCP, allows the accumulation of certain βTrCP substrates, specifically β-catenin, ATF4, and IκB-α, in the host cell cytoplasm. Vpu interaction with βTrCP, as well as with a related protein βTrCP2, was required for this effect and depended on the presence of the intact DSGXXS phosphorylation motif in the Vpu cytoplasmic domain. Preliminary results in our laboratory (unpublished data) obtained with Vpu truncation mutants (kindly provided by Edward Stephens, University of Kansas Medical Center) have shown that a Vpu construct containing amino acids 1 through 60 was the minimal construct that was able to induce CD40 expression in transfected EC. This construct contains the N-terminal hydrophobic membrane anchor (residues 1 to 27), the first alpha helix (residues 32 to 45), and the conserved random coil region (residues 51 to 57), which includes the DSGXXS motif. This protein lacks 21 C-terminal residues, including the second alpha helix (residues 57 to 69), suggesting that this region is dispensable for CD40 induction. Although these data do not directly demonstrate that an intact DSGXXS phosphorylation motif is required for Vpu induction of CD40, it is in keeping with a model whereby Vpu sequestration of βTrCP allows accumulation of CD40 in the cytoplasm of Vpu-expressing cells. If CD40 is a normal substrate of βTrCP (or βTrCP2), then the Vpu-βTrCP interaction may prevent CD40 degradation. Alternately, the Vpu-βTrCP interaction could, in a manner analogous to Vpu degradation of CD4, promote degradation of a second protein whose function (in the absence of Vpu) is to downmodulate CD40. Either way, the consequence would be an increase in CD40 protein levels in the Vpu-expressing cell, as was shown by Western blot in our system. The exact mechanism of Vpu regulation of CD40 fate is currently under scrutiny. Note that the positive effect of Vpu on HIV-1 particle release was recently linked to its ability to overcome a host antiviral factor that restricts virion assembly (52). We speculate that Vpu is similarly able to override the cellular regulation imposed on CD40 expression, with consequences of clinical significance for the host.
In summary, the results presented herein demonstrate a novel function for HIV-1 Vpu: induction of the cytokine receptor CD40. Two key features of Vpu biology—the property of accumulation in HIV-1-infected cells and the capacity to form stable complexes with βTrCP, a key component of the E3 ubiquitin-ligase complex—make it an ideal candidate for dysregulation of CD40 levels in HIV-1-infected cells. Increased adhesion of malignant B cells to Vpu-expressing cells after appropriate CD40L-mediated activation suggests an important clinical manifestation of this Vpu function in the setting of AIDS-NHL.
Acknowledgments
This study was supported by the NIH grant R01HL61928 (A.V.M.) and the N. L. Tartar Trust Research Fellowship (W.W.H.).
We thank Scott Wong, David Parker, and Gary Thomas for invaluable advice and critical discussions. We thank Andrew Townsend for technical assistance.
REFERENCES
- 1.Bartz, S. R., M. E. Rogel, and M. Emerman. 1996. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J. Virol. 70:2324-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartz, S. R., and M. A. Vodicka. 1997. Production of high-titer human immunodeficiency virus type 1 pseudotyped with vesicular stomatitis virus glycoprotein. Methods 12:337-342. [DOI] [PubMed] [Google Scholar]
- 3.Besnard-Guerin, C., N. Belaidouni, I. Lassot, E. Segeral, A. Jobart, C. Marchal, and R. Benarous. 2004. HIV-1 Vpu sequesters βTrCP in the cytoplasm and provokes the accumulation of β-catenin and others SCF-βTrCP substrates. J. Biol. Chem. 279:788-795. [DOI] [PubMed] [Google Scholar]
- 4.Biancone, L., V. Cantaluppi, and G. Camussi. 1999. CD40-CD154 interaction in experimental and human disease. Int. J. Mol. Med. 3:343-353. [DOI] [PubMed] [Google Scholar]
- 5.Biggar, R. J., and C. S. Rabkin. 1996. The epidemiology of AIDS-related neoplasms. Hematol. Oncol. Clin. N. Am. 10:997-1010. [DOI] [PubMed] [Google Scholar]
- 6.Bour, S., C. Perrin, H. Akari, and K. Strebel. 2001. The human immunodeficiency virus type 1 Vpu protein inhibits NF-κB activation by interfering with βTrCP-mediated degradation of IκB. J. Biol. Chem. 276:15920-15928. [DOI] [PubMed] [Google Scholar]
- 7.Bour, S., U. Schubert, and K. Strebel. 1995. The human immunodeficiency virus type 1 Vpu protein specifically binds to the cytoplasmic domain of CD4: implications for the mechanism of degradation. J. Virol. 69:1510-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bour, S., and K. Strebel. 2003. The HIV-1 Vpu protein: a multifunctional enhancer of viral particle release. Microbes Infect. 5:1029-1039. [DOI] [PubMed] [Google Scholar]
- 9.Bradley, J. R., and J. S. Pober. 2001. Tumor necrosis factor receptor-associated factors (TRAFs). Oncogene 20:6482-6491. [DOI] [PubMed] [Google Scholar]
- 10.Carbone, A. 2002. AIDS-related non-Hodgkin's lymphomas: from pathology and molecular pathogenesis to treatment. Hum. Pathol. 33:392-404. [DOI] [PubMed] [Google Scholar]
- 11.Carbone, A. 2003. Emerging pathways in the development of AIDS-related lymphomas. Lancet Oncol. 4:22-29. [DOI] [PubMed] [Google Scholar]
- 12.Chi, D., J. Henry, J. Kelley, R. Thorpe, J. K. Smith, and G. Krishnaswamy. 2000. The effects of HIV infection on endothelial function. Endothelium 7:223-242. [DOI] [PubMed] [Google Scholar]
- 13.Danese, S., C. de la Motte, A. Sturm, J. D. Vogel, G. A. West, S. A. Strong, J. A. Katz, and C. Fiocchi. 2003. Platelets trigger a CD40-dependent inflammatory response in the microvasculature of inflammatory bowel disease patients. Gastroenterology 124:1249-1264. [DOI] [PubMed] [Google Scholar]
- 14.Deregibus, M. C., S. Buttiglieri, S. Russo, B. Bussolati, and G. Camussi. 2003. CD40-dependent activation of phosphatidylinositol 3-kinase/Akt pathway mediates endothelial cell survival and in vitro angiogenesis. J. Biol. Chem. 278:18008-18014. [DOI] [PubMed] [Google Scholar]
- 15.Desai-Mehta, A., L. Lu, R. Ramsey-Goldman, and S. K. Datta. 1996. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J. Clin. Investig. 97:2063-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhawan, S., R. K. Puri, A. Kumar, H. Duplan, J. M. Masson, and B. B. Aggarwal. 1997. Human immunodeficiency virus-1-tat protein induces the cell surface expression of endothelial leukocyte adhesion molecule-1, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 in human endothelial cells. Blood 90:1535-1544. [PubMed] [Google Scholar]
- 17.Ewart, G. D., T. Sutherland, P. W. Gage, and G. B. Cox. 1996. The Vpu protein of human immunodeficiency virus type 1 forms cation-selective ion channels. J. Virol. 70:7108-7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaidano, G., and R. Dalla-Favera. 1992. Biologic aspects of human immunodeficiency virus-related lymphoma. Curr. Opin. Oncol. 4:900-906. [DOI] [PubMed] [Google Scholar]
- 19.Goedert, J. J., T. R. Cote, P. Virgo, S. M. Scoppa, D. W. Kingma, M. H. Gail, E. S. Jaffe, and R. J. Biggar. 1998. Spectrum of AIDS-associated malignant disorders. Lancet 351:1833-1839. [DOI] [PubMed] [Google Scholar]
- 20.Grammer, A. C., M. C. Bergman, Y. Miura, K. Fujita, L. S. Davis, and P. E. Lipsky. 1995. The CD40 ligand expressed by human B cells costimulates B-cell responses. J. Immunol. 154:4996-5010. [PubMed] [Google Scholar]
- 21.Greene, W. C., and B. M. Peterlin. 2002. Charting HIV's remarkable voyage through the cell: basic science as a passport to future therapy. Nat. Med. 8:673-680. [DOI] [PubMed] [Google Scholar]
- 22.Grulich, A. E., X. Wan, M. G. Law, S. T. Milliken, C. R. Lewis, R. J. Garsia, J. Gold, R. J. Finlayson, D. A. Cooper, and J. M. Kaldor. 2000. B-cell stimulation and prolonged immune deficiency are risk factors for non-Hodgkin's lymphoma in people with AIDS. AIDS 14:133-140. [DOI] [PubMed] [Google Scholar]
- 23.Hart, M., J. P. Concordet, I. Lassot, I. Albert, R. del los Santos, H. Durand, C. Perret, B. Rubinfeld, F. Margottin, R. Benarous, and P. Polakis. 1999. The F-box protein βTrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr. Biol. 9:207-210. [DOI] [PubMed] [Google Scholar]
- 24.Hitt, M., Bett, A., Prevec, L., and Graham, F. 1994. Construction and propagation of human adenovirus vector, p. 479-490. In J. Celis (ed.), Cell biology. Academic Press, Inc., New York, N.Y.
- 25.Hollenbaugh, D., N. Mischel-Petty, C. P. Edwards, J. C. Simon, R. W. Denfeld, P. A. Kiener, and A. Aruffo. 1995. Expression of functional CD40 by vascular endothelial cells. J. Exp. Med. 182:33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan, L. D., D. I. Abrams, E. Feigal, M. McGrath, J. Kahn, P. Neville, J. Ziegler, and P. A. Volberding. 1989. AIDS-associated non-Hodgkin's lymphoma in San Francisco. JAMA 261:719-724. [PubMed] [Google Scholar]
- 27.Karmann, K., C. C. Hughes, J. Schechner, W. C. Fanslow, and J. S. Pober. 1995. CD40 on human endothelial cells: inducibility by cytokines and functional regulation of adhesion molecule expression. Proc. Natl. Acad. Sci. USA 92:4342-4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerkau, T., I. Bacik, J. R. Bennink, J. W. Yewdell, T. Hunig, A. Schimpl, and U. Schubert. 1997. The human immunodeficiency virus type 1 (HIV-1) Vpu protein interferes with an early step in the biosynthesis of major histocompatibility complex (MHC) class I molecules. J. Exp. Med. 185:1295-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knowles, D. M. 1997. Molecular pathology of acquired immunodeficiency syndrome-related non-Hodgkin's lymphoma. Semin. Diagn. Pathol. 14:67-82. [PubMed] [Google Scholar]
- 30.Levine, A. M. 1992. Acquired immunodeficiency syndrome-related lymphoma. Blood 80:8-20. [PubMed] [Google Scholar]
- 31.Lutgens, E., and M. J. Daemen. 2002. CD40-CD40L interactions in atherosclerosis. Trends Cardiovasc. Med. 12:27-32. [DOI] [PubMed] [Google Scholar]
- 32.Mach, F., U. Schonbeck, R. P. Fabunmi, C. Murphy, E. Atkinson, J. Y. Bonnefoy, P. Graber, and P. Libby. 1999. T lymphocytes induce endothelial cell matrix metalloproteinase expression by a CD40L-dependent mechanism: implications for tubule formation. Am. J. Pathol. 154:229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mach, F., U. Schonbeck, G. K. Sukhova, T. Bourcier, J. Y. Bonnefoy, J. S. Pober, and P. Libby. 1997. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proc. Natl. Acad. Sci. USA 94:1931-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maisch, T., B. Kropff, C. Sinzger, and M. Mach. 2002. Upregulation of CD40 expression on endothelial cells infected with human cytomegalovirus. J. Virol. 76:12803-12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Margottin, F., S. P. Bour, H. Durand, L. Selig, S. Benichou, V. Richard, D. Thomas, K. Strebel, and R. Benarous. 1998. A novel human WD protein, h-βTrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell 1:565-574. [DOI] [PubMed] [Google Scholar]
- 36.Marin, M., K. M. Rose, S. L. Kozak, and D. Kabat. 2003. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat. Med. 9:1398-1403. [DOI] [PubMed] [Google Scholar]
- 37.Melter, M., M. E. Reinders, M. Sho, S. Pal, C. Geehan, M. D. Denton, D. Mukhopadhyay, and D. M. Briscoe. 2000. Ligation of CD40 induces the expression of vascular endothelial growth factor by endothelial cells and monocytes and promotes angiogenesis in vivo. Blood 96:3801-3808. [PubMed] [Google Scholar]
- 38.Miller, D. L., R. Yaron, and M. J. Yellin. 1998. CD40L-CD40 interactions regulate endothelial cell surface tissue factor and thrombomodulin expression. J. Leukoc. Biol. 63:373-379. [DOI] [PubMed] [Google Scholar]
- 39.Monaco, C., E. Andreakos, S. Young, M. Feldmann, and E. Paleolog. 2002. T cell-mediated signaling to vascular endothelium: induction of cytokines, chemokines, and tissue factor. J. Leukoc. Biol. 71:659-668. [PubMed] [Google Scholar]
- 40.Moses, A. V., K. N. Fish, R. Ruhl, P. P. Smith, J. G. Strussenberg, L. Zhu, B. Chandran, and J. A. Nelson. 1999. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J. Virol. 73:6892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moses, A. V., S. E. Williams, J. G. Strussenberg, M. L. Heneveld, R. A. Ruhl, A. C. Bakke, G. C. Bagby, and J. A. Nelson. 1997. HIV-1 induction of CD40 on endothelial cells promotes the outgrowth of AIDS-associated B-cell lymphomas. Nat. Med. 3:1242-1249. [DOI] [PubMed] [Google Scholar]
- 42.Pammer, J., A. Plettenberg, W. Weninger, B. Diller, M. Mildner, A. Uthman, W. Issing, M. Sturzl, and E. Tschachler. 1996. CD40 antigen is expressed by endothelial cells and tumor cells in Kaposi's sarcoma. Am. J. Pathol. 148:1387-1396. [PMC free article] [PubMed] [Google Scholar]
- 43.Piguet, V., Y. L. Chen, A. Mangasarian, M. Foti, J. L. Carpentier, and D. Trono. 1998. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the mu chain of adaptor complexes. EMBO J. 17:2472-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pomerantz, R. J., T. Seshamma, and D. Trono. 1992. Efficient replication of human immunodeficiency virus type 1 requires a threshold level of Rev: potential implications for latency. J. Virol. 66:1809-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rusnati, M., and M. Presta. 2002. HIV-1 Tat protein and endothelium: from protein/cell interaction to AIDS-associated pathologies. Angiogenesis 5:141-151. [DOI] [PubMed] [Google Scholar]
- 46.Sipsas, N. V., P. P. Sfikakis, A. Kontos, and T. Kordossis. 2002. Levels of soluble CD40 ligand (CD154) in serum are increased in human immunodeficiency virus type 1-infected patients and correlate with CD4+ T-cell counts. Clin. Diagn. Lab. Immunol. 9:558-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sousa, A. E., A. F. Chaves, M. Doroana, F. Antunes, and R. M. Victorino. 1999. Early reduction of the overexpression of CD40L, OX40, and Fas on T cells in HIV-1 infection during triple anti-retroviral therapy: possible implications for lymphocyte traffic and functional recovery. Clin. Exp. Immunol. 116:307-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strebel, K., T. Klimkait, F. Maldarelli, and M. A. Martin. 1989. Molecular and biochemical analyses of human immunodeficiency virus type 1 Vpu protein. J. Virol. 63:3784-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Streblow, D. N., C. Soderberg-Naucler, J. Vieira, P. Smith, E. Wakabayashi, F. Ruchti, K. Mattison, Y. Altschuler, and J. A. Nelson. 1999. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell 99:511-520. [DOI] [PubMed] [Google Scholar]
- 50.Thienel, U., J. Loike, and M. J. Yellin. 1999. CD154 (CD40L) induces human endothelial cell chemokine production and migration of leukocyte subsets. Cell. Immunol. 198:87-95. [DOI] [PubMed] [Google Scholar]
- 51.van Kooten, C., and J. Banchereau. 2000. CD40-CD40 ligand. J. Leukoc. Biol. 67:2-17. [DOI] [PubMed] [Google Scholar]
- 52.Varthakavi, V., R. M. Smith, S. P. Bour, K. Strebel, and P. Spearman. 2003. Viral protein U counteracts a human host cell restriction that inhibits HIV-1 particle production. Proc. Natl. Acad. Sci. USA 100:15154-15159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vincent, M. J., and M. Abdul Jabbar. 1995. The human immunodeficiency virus type 1 Vpu protein: a potential regulator of proteolysis and protein transport in the mammalian secretory pathway. Virology 213:639-649. [DOI] [PubMed] [Google Scholar]
- 54.Vodicka, M. A., W. C. Goh, L. I. Wu, M. E. Rogel, S. R. Bartz, V. L. Schweickart, C. J. Raport, and M. Emerman. 1997. Indicator cell lines for detection of primary strains of human and simian immunodeficiency viruses. Virology 233:193-198. [DOI] [PubMed] [Google Scholar]
- 55.Willey, R. L., F. Maldarelli, M. A. Martin, and K. Strebel. 1992. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J. Virol. 66:7193-7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wykes, M., J. Poudrier, R. Lindstedt, and D. Gray. 1998. Regulation of cytoplasmic, surface and soluble forms of CD40 ligand in mouse B cells. Eur. J. Immunol. 28:548-559. [DOI] [PubMed] [Google Scholar]
- 57.Ziegler, J. L., J. A. Beckstead, P. A. Volberding, D. I. Abrams, A. M. Levine, R. J. Lukes, P. S. Gill, R. L. Burkes, P. R. Meyer, C. E. Metroka, et al. 1984. Non-Hodgkin's lymphoma in 90 homosexual men. Relation to generalized lymphadenopathy and the acquired immunodeficiency syndrome. N. Engl. J. Med. 311:565-570. [DOI] [PubMed] [Google Scholar]