Abstract
We have demonstrated that caspase-1 is a mediator of both cisplatin-induced acute kidney injury (AKI) and ischemic AKI. As caspase-1 is activated in the inflammasome, we investigated the inflammasome in cisplatin-induced and ischemic AKI. Mice were injected with cisplatin or subjected to bilateral renal pedicle clamping. Immunoblot analysis of whole kidney after cisplatin-induced AKI revealed: 1) an increase in apoptosis-associated Speck-like protein containing a caspase recruitment domain (ASC), the major protein that complexes with nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain containing proteins (NLRP) 1 or 3 to form the inflammasome; 2) an increase in caspase-1 activity, caspase-5, and NLRP1, components of the NLRP1 inflammasome; and 3) a trend toward increased NLRP3. To determine whether the NLRP3 inflammasome plays an injurious role in cisplatin-induced AKI, we studied NLRP knockout (NLRP3−/−) mice. In cisplatin-induced AKI, the blood urea nitrogen, serum creatinine, acute tubular necrosis score, and tubular apoptosis score were not significantly decreased in NALP3−/− mice compared with wild-type mice. We have previously demonstrated the injurious role of caspase-1 in ischemic AKI. NLRP3, but not ASC or NLRP1, is increased in ischemic AKI. NLRP3−/− mice with ischemic AKI had significantly lower blood urea nitrogen, serum creatinine, and acute tubular necrosis and apoptosis scores than the wild-type controls. The difference in protection against cisplatin-induced AKI compared with ischemic AKI in NLRP3−/− mice was not explained by the differences in proinflammatory cytokines interleukin (IL)-1β, IL-6, chemokine (C-X-C motif) ligand 1, or tumor necrosis factor α. NLRP3 inflammasome is a mediator of ischemic AKI but not cisplatin-induced AKI, and further investigation of the NLRP1 inflammasome in cisplatin-induced AKI should prove interesting.
Introduction
Cisplatin is one of the most widely used and most potent chemotherapeutic agents. The exposure of tubular cells to cisplatin activates complex signaling pathways that lead to tubular cell injury and death (Schrier, 2002; Safirstein, 2007) as well as a robust inflammatory response that further exacerbates renal tissue damage. The injurious role of inflammation in acute kidney injury (AKI) is increasingly appreciated with the involvement of leukocytes, adhesion molecules, and cytokines (Bonventre and Zuk, 2004; Friedewald and Rabb, 2004; Devarajan, 2006; Lee et al., 2011). The cellular inflammatory response in AKI is also evidenced by increased CD4 T cells (Faubel et al., 2005; Akcay et al., 2011), macrophages (Lu et al., 2008; He et al., 2009), and neutrophils (Melnikov et al., 2002; Faubel et al., 2007) in the kidney. The inflammasome is a protein scaffold or molecular complex that contains NLRP (nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain containing) proteins and an adaptor protein called ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) (Franchi et al., 2009; Schroder et al., 2010; Schroder and Tschopp, 2010). The most fully characterized inflammasome is the NLRP3 inflammasome, which contains the NLRP3 protein and the ASC protein (Schroder et al., 2010). Another less well-characterized inflammasome, the NLRP1 inflammsome, contains NLRP1 protein, ASC protein, and caspase-5 (Martinon et al., 2009). As inflammation plays a role in cisplatin-induced AKI and the inflammasome plays a crucial role in inflammation, we developed the hypothesis that the inflammasome is activated in AKI.
Our published data demonstrate that caspase-1 is a mediator of cisplatin-induced AKI (Faubel et al., 2004) and ischemic AKI (Melnikov et al., 2001). Caspase-1 is activated in the inflammasome. It is known that NLRPs recruit the adaptor apoptosis-associated Speck-like protein containing a caspase recruitment domain (ASC) that recruits caspase-1 resulting in secretion of “leaderless” proteins via direct or indirect physical interaction (Keller et al., 2008). Active caspase-1 in the inflammasome activates interleukin (IL)-1β and IL-18 and is a regulator of the “unconventional” protein secretion of “leaderless” proteins such as IL-1α (Guegan et al., 2002; Keller et al., 2008). IL-1α is a proinflammatory cytokine that is markedly increased in the kidney after cisplatin injection and is notably reduced in the caspase-1−/− mice (Faubel et al., 2007). IL-1β, IL-18, and caspase-1 are increased in cisplatin-induced AKI (Faubel et al., 2007) and ischemic AKI (Melnikov et al., 2001, 2002), and IL-1β and IL-18 are activated by caspase-1 in the inflammasome, so we developed the hypothesis that crucial inflammasome proteins such as NLRPs and ASC would be increased in AKI and that NLRP3 knockout would protect against cisplatin-induced and ischemic AKI.
Materials and Methods
Cisplatin-Induced AKI.
For all the mouse studies, 8- to 10-week-old male C57BL/6 mice weighing 20–25 g were used. All experiments were conducted adhering to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animal protocol was approved by the Animal Care and Use Committee of the University of Colorado at Denver. Mice were maintained on a standard diet, and water was freely available. Mice were housed five per cage under a 12-hour light/dark schedule for at least 1 week before cisplatin administration. Six hours before cisplatin administration, food and water were withheld. Cisplatin [cis-diamminedichloro-platinum (II)] (Sigma-Aldrich, Milwaukee, WI) was freshly prepared the day of administration in sterile normal saline at a concentration of 1 mg/ml. Mice were given 25 mg/kg body weight of cisplatin or vehicle (saline) intraperitoneally, after which the mice again had free access to food and water.
We have described this model of cisplatin-induced AKI in detail elsewhere (Faubel et al., 2007; Lu et al., 2008). Briefly, after the 25 mg/kg cisplatin injection, the blood urea nitrogen (BUN) and serum creatinine levels are normal on day 1 and slightly increased on day 2. On day 3 after the cisplatin injection, the renal dysfunction, renal neutrophil infiltration, renal tubular cell apoptosis, and acute tubular necrosis scores are severe.
Ischemia Protocol.
The operator was blinded to the treatment groups. The mice were anesthetized with i.p. Avertin (2,2,2-tribromoethanol; Sigma-Aldrich). A midline incision was made, and the renal pedicles were bilaterally clamped for 24 minutes with microaneurysm clamps. The time of ischemia was chosen to obtain a reversible model of ischemic AKI and avoid animal mortality. Serum creatinine levels reach a peak at 24–48 hours of reperfusion, then they gradually return to normal within 3–7 days. This model is well established in our laboratory (He et al., 2008). After clamp removal, the kidneys were observed for restoration of blood flow by the return to their original color. The abdomen was closed in two layers. The sham surgery consisted of the same surgical procedure except that clamps were not applied. During the first 24 hours of the reperfusion period, the animals were kept in an incubator at 29°C. The animals were sacrificed at 24 hours after ischemia for all the measurements performed in the study. Blood samples were obtained at sacrifice via cardiac puncture.
NLRP3−/− Mice.
NLRP knockout (NLRP3−/−) mice in the C57BL/6 background were generated by Millennium Pharmaceuticals (Cambridge, MA) and were obtained from Dr. Fayyaz Sutterwala (University of Iowa, Carver College of Medicine). The mice were genotyped as previously described elsewhere (Sutterwala et al., 2006). Age-, sex-, and weight-matched C57BL/6 mice obtained from The Jackson Laboratories (Bar Harbor, ME) were used as the controls.
Histologic Examination.
Kidneys fixed in paraformaldehyde (4%) and embedded in paraffin were sectioned at 4 μm and stained with periodic acid-Schiff (PAS) by standard methods. All histologic examinations were performed by a renal pathologist who had been blinded to the treatment. Histologic changes due to acute tubular necrosis (ATN score) were evaluated in the outer stripe of the outer medulla on PAS-stained tissue and were quantified by counting the percentage of tubules that displayed cell necrosis, loss of brush border, cast formation, and tubule dilatation as follows: 0 = none, 1 ≤10%, 2 = 10–25%, 3 = 26–45%, 4 = 46–75%, and 5 ≥75%. At least 10 fields (250×) were reviewed for each slide.
Morphologic criteria were used to count the apoptotic cells on PAS-stained tissue by a pathologist experienced in the evaluation of renal apoptosis. The morphologic characteristics included cellular rounding and shrinkage, nuclear chromatin compaction, and formation of apoptotic bodies (Gobe et al., 2000). Apoptotic tubular cells were quantitatively assessed per 10 high-power fields (400×) in the outer stripe of the outer medulla by the renal pathologist in a blinded fashion.
Neutrophil infiltration was quantitatively assessed on PAS-stained tissue by the renal pathologist counting the number of neutrophils per 10 high-power fields (400×), as we have previously described elsewhere (Faubel et al., 2004). At least 10 fields were counted in the outer stripe of the outer medulla for each slide.
RAW 264.7 Macrophages.
Mouse RAW 264.7 cells are a mouse monocyte/macrophage cell line from ascites fluid in a male mouse in which a tumor was induced by injection of Abelson leukemia virus (A-MuLV) (American Type Culture Collection, Manassas, VA). Cells are grown in Dulbecco’s modified Eagle’s high-glucose medium supplemented with Na2HCO3 (0.075%), l-glutamine, penicillin-streptomycin (1%), 0.5 mg/ml insulin, and 10% fetal bovine serum in a humidified atmosphere of 5% CO2 at 37°C. The cells are treated with 10 or 50 μM cisplatin for 6 or 12 hours.
Freshly Isolated Mouse Renal Proximal Tubules.
Proximal tubules were isolated from kidney cortex of male C57BL/6 mice using collagenase digestion and Percoll centrifugation, as we have described in detail elsewhere (Edelstein, 2000). We placed 6-ml aliquots of tubule suspension (approximately 1–2 mg/ml) in siliconized 25-ml Erlenmeyer flasks for a recovery period that included gassing with 95% O2/5% CO2 for 5 minutes on ice. We capped the flasks with rubber stoppers and kept them at room temperature for 5 minutes, then placed them in a shaking water bath at 37°C for 10 minutes. After a recovery period, the proximal tubular cells were incubated with vehicle (saline) or 10 or 50 µM cisplatin. The cisplatin was freshly prepared and dissolved in dimethylsulfoxide (final concentration 0.1%) at the time of the experiment.
The control proximal tubular cells were treated with the vehicle (0.1% dimethylsulfoxide). After adding the reagents, the flasks were regassed with 95% O2/5% CO2 for 5 minutes and then closed and kept in the shaking water bath for 25 minutes. At the end of the preincubation and experiment period, 1 ml of tubule suspension was sampled for measurement of lactate dehydrogenase and 5 ml was sampled for caspase activity, enzyme-linked immunosorbent assay (ELISA), or immunoblot analysis.
Caspase Assay.
The activity of caspases was determined on cytosolic extracts of whole kidneys or proximal tubules by the use of fluorescent substrates, as we have described in detail elsewhere (Faubel et al., 2004; Dursun et al., 2006). Ac-Typ-Glu-His-Asp-AMC (Ac-WEHD-AMC) was a susceptible substrate for caspase-5, and Ac-Tyr-Val-Ala-Asp-AMC (Ac-YVAD-AMC) was a susceptible substrate for caspase-1. The peptide cleavage was measured over 1 hour at 30°C using a Cytofluor 4000 series fluorescent plate reader (PerSeptive Biosystems, Framingham, MA) at an excitation wavelength of 380 nm and an emission wavelength of 460 nm. The caspase activity was expressed in nmol AMC released per minute of incubation time per milligram of lysate protein.
ELISA.
Mouse IL-1α, IL-1β, chemokine (C-X-C motif) ligand 1 (CXCL1) (also known as IL-8 or KC), and tumor necrosis factor-α (TNF-α) immunoassay kits were obtained from R&D Systems (Minneapolis, MN). We performed ELISA according to the manufacturer’s instructions.
Immunoblotting.
Cytosolic extracts of whole kidney or proximal tubular cells were immunoblotted as described in detail elsewhere (Dursun et al., 2006). The immunoblot analyses were performed with the following antibodies: 1) a purified rabbit polyclonal antibody raised against a peptide mapping at the C terminus of active caspase-1 p10 of mouse origin (1:200) (catalog no. sc-514; Santa Cruz Biotechnology, Santa Cruz, CA); 2) a rabbit polyclonal antibody to caspase-5 (1:500) (code no. JM-3029-100; MBL, Woburn, MA) that detects procaspase-5 (47 kDa) and cleaved caspase-5 (38 kDa); 3) a purified rabbit polyclonal antibody to ASC (1:200) (catalog no. sc-22514; Santa Cruz Biotechnology); 4) a polyclonal antibody to NLRP1 (1:200) (catalog no. 4990; Cell Signaling Technology, Danvers, MA); and 5) a polyclonal antibody to NLRP3 (1:1000) (catalog no. ab91525; Abcam, Cambridge, MA).
Statistical Analysis.
Non-normally distributed data were analyzed by the nonparametric unpaired Mann-Whitney test. Multiple group comparisons are performed using analysis of variance with posttest according to Newman-Keuls. P < 0.05 was considered statistically significant. Values are expressed as mean ± S.E.
Results
Increase in ASC in Cisplatin-Induced AKI.
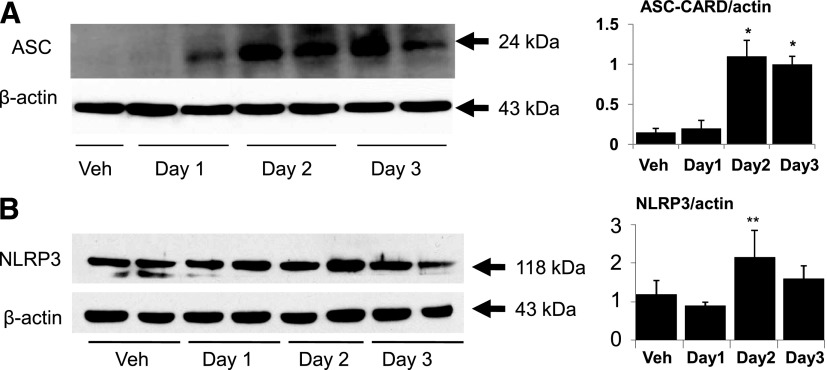
Immunoblot analysis of the whole kidney showed increases in ASC (Fig. 1A) on day 2 of cisplatin-induced AKI. NLRP3 protein was increased on day 2 of cisplatin-induced AKI, but the increase was not statistically significant (Fig. 1B). In the cisplatin-induced AKI model, we had previously demonstrated that there is increased caspase-1 activity and tubular apoptosis on day 2 and increased ATN, BUN, and creatinine activity on day 3 after cisplatin administration (Faubel et al., 2004). Thus, the increase in ASC and caspase-1 on day 2 preceded the development of AKI on day 3.
Fig. 1.
Renal ASC and NLRP3 in cisplatin-induced AKI. (A) Immunoblot analysis of whole kidney extracts found an increase in ASC (24 kDa) in cisplatin-induced AKI. (B) There was an increase in NLRP3 (118 kDa) in cisplatin-induced AKI that was not statistically significant. In densitometric analysis of immunoblots, data are presented as protein/actin ratios plotted on the y-axis. β-actin, used as a loading control, was not different between the groups. Representative immunoblots are derived from at least three separate experiments. *P < 0.05 vs. Veh (vehicle); **P = 0.1 vs. Veh.
Increase in Caspase-1, IL-1α, and IL-1β in Cisplatin-Treated Proximal Tubules.
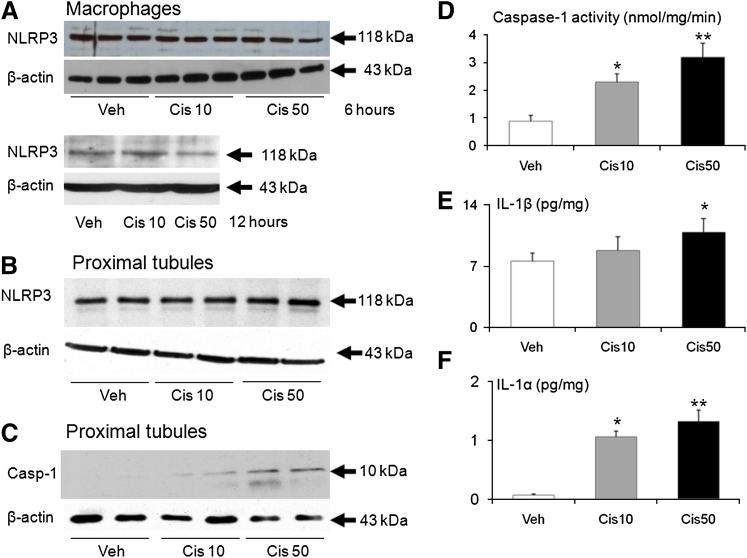
To determine the localization of NLRP3 and the effect of cisplatin-treatment on NLRP3 in macrophages and proximal tubules, we studied macrophages in culture and freshly isolated proximal tubules. The immunoblot analysis revealed that NLRP3 (118 kDa) was present in the isolated macrophages but was not increased with 10 or 50 µM cisplatin treatment of 6 or 12 hours (Fig. 2A). We further investigated the inflammasome in a model of freshly isolated proximal tubules treated with 10 or 50 µM cisplatin, which has been shown to induce necrosis and apoptosis (Dursun et al., 2006). On immunoblot analysis, NLRP3 (118 kDa) was present in the freshly isolated proximal tubules but was not increased by cisplatin treatment (Fig. 2B). A dose-dependent increase in active caspase-1 protein (10 kDa) (Fig. 2C) and an increase in caspase-1 activity (Fig. 2D) were seen in the proximal tubules treated with cisplatin.
Fig. 2.
NLRP3 in macrophages and NLRP3, caspase-1, IL-1β, and IL-1α in freshly isolated proximal tubules treated with cisplatin. NLRP3 was not increased in (A) macrophages treated with cisplatin 10 or 50 μM for 6 or 12 hours or (B) proximal tubules treated with cisplatin 10 or 50 μM for 25 minutes. (C) Caspase-1 protein, (D) caspase-1 activity, (E) IL-1β, and (F) IL-1α were increased in proximal tubules treated with cisplatin. β-actin, used as a loading control, was not different between the groups. Representative immunoblots are derived from at least three separate experiments. *P < 0.01 vs. Veh; **P < 0.05 vs. Cis 10.
Caspase-1 activates IL-1β and IL-1α in the inflammasome (Keller et al., 2008; Martinon et al., 2009). The IL-1α levels were statistically significantly increased in the 10 and 50 µM cisplatin-treated groups compared with the vehicle-treated controls (Fig. 2E). The IL-1β levels were increased in the 50 µM cisplatin-treated group compared with the 10 µM cisplatin and vehicle-treated groups (Fig. 2F).
NLRP3−/− Mice Not Protected against Cisplatin-Induced AKI.
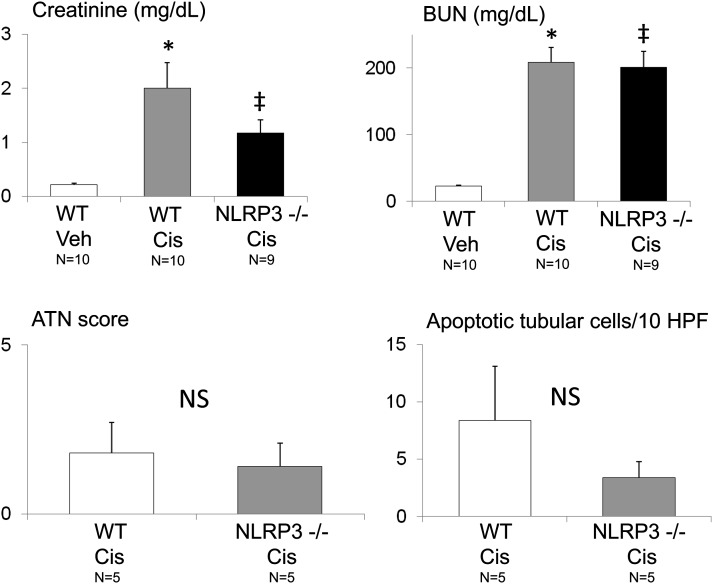
The decrease in serum creatinine in NLRP3−/− mice compared with wild-type (WT) mice with cisplatin-induced AKI did not reach statistical significance (Fig. 3). The BUN levels, ATN score, and apoptosis score were not statistically statistically different between the WT mice and the NLRP3−/− mice with cisplatin-induced AKI (Fig. 3).
Fig. 3.
Serum creatinine, BUN, ATN score, and apoptosis in NLRP3−/− mice in cisplatin-induced AKI. Creatinine and BUN were increased in cisplatin-induced AKI (Cis) compared with vehicle-treated (Veh) mice. The creatinine, BUN, ATN score, and number of apoptotic tubular cells were not statistically significantly decreased in NLRP3−/− mice with AKI compared with WT mice with AKI. *P < 0.01 vs. WT Veh; ‡P < 0.01 vs. WT Veh, not statistically significant (NS) vs. WT Cis. The number of animals per group is indicated on the figure. HPF, high-power field.
Increase in NLRP1 and Caspase-5 in Cisplatin-Induced AKI.
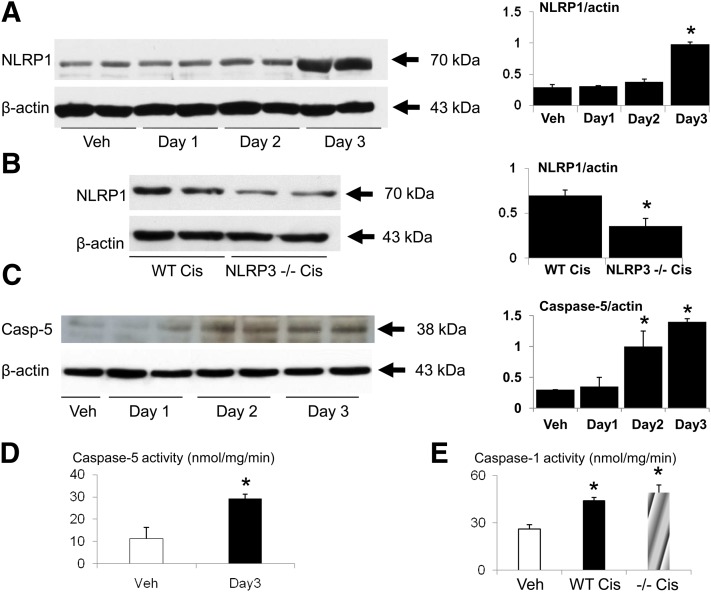
On immunoblot, there was a large increase in NLRP1 on day 3 of cisplatin-induced AKI (Fig. 4A). NLRP1 was decreased in NLRP3−/− mice on day 3 of cisplatin-induced AKI compared with WT mice (Fig. 4B). There was an increase in caspase-5 protein on days 2 and 3 of cisplatin-induced AKI (Fig. 4C). Caspase-5 activity was significantly increased on day 3 of cisplatin-induced AKI (Fig. 4D). Caspase-1 activity was increased in cisplatin-induced AKI in both WT and NLRP3−/− mice compared with vehicle-treated mice (Fig. 4E).
Fig. 4.
NLRP1 caspase-5 and caspase-1 activity in cisplatin-induced AKI. (A) NLRP1 protein was increased in the kidney on day 3 of cisplatin-induced AKI (Cis). *P < 0.01 vs. Veh, Day 1, Day 2. (B) NLRP1 protein was decreased in NLRP3−/− mice on day 3 of cisplatin-induced AKI compared with WT mice. *P < 0.05 vs. WT Cis. (C) Caspase-5 protein and (D) caspase-5 activity were increased in cisplatin-induced AKI. In densitometric analysis of the immunoblots, the data are presented as protein/actin ratios plotted on the y-axis. β-actin, used as a loading control, was not different between the groups. Representative immunoblots are derived from at least three separate experiments. *P < 0.05 vs. Veh (vehicle). (E) Caspase-1 activity was increased in cisplatin-induced AKI in both wild-type and NLRP3−/− mice compared with vehicle treated mice. n = 4 per group. *P < 0.01 vs. Veh.
NLRP3−/− Mice Protected against Ischemic AKI.
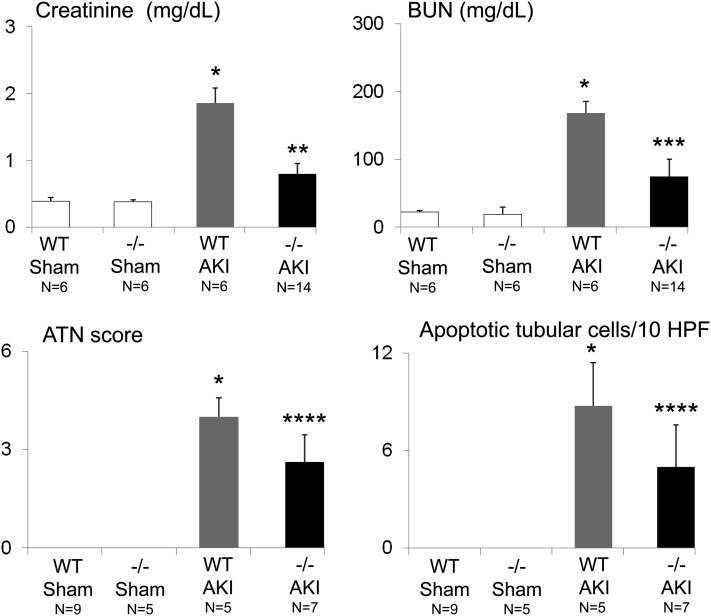
We have previously demonstrated the injurious role of caspase-1-mediated production of IL-18 in ischemic AKI (Melnikov et al., 2001, 2002). As caspase-1 and IL-18 are activated in the NLRP3 inflammasome, we determined whether NLRP3−/− mice are protected against ischemic AKI. BUN, serum creatinine, ATN score and apoptosis scores were significantly decreased in NLRP3−/− mice compared with WT mice with ischemic AKI (Fig. 5). Representative pictures of kidney histology are shown in Fig. 6, A–C.
Fig. 5.
Serum creatinine, BUN, ATN score, and apoptosis in NLRP3−/− mice in ischemic AKI. The creatinine, BUN, ATN score, and number of apoptotic tubular cells were increased in ischemic AKI (AKI) compared with sham-operated (sham) mice. The creatinine, BUN, ATN score, and number of apoptotic tubular cells were statistically significantly decreased in NLRP3−/− (−/−) compared with wild-type (WT) mice with ischemic AKI. *P < 0.01 vs. WT sham or −/− sham mice; **P < 0.001 vs. WT AKI; ***P < 0.01 vs. WT AKI; ****P < 0.05 vs. WT AKI. The number of animals per group is indicated on the figure. HPF, high-power field.
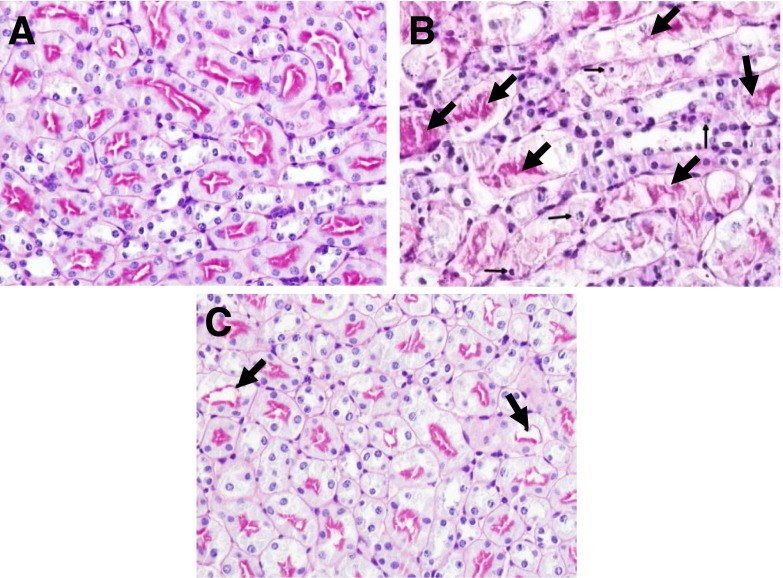
Fig. 6.
Representative pictures of kidney histology. PAS–stained section of (A) wild-type sham-operated, (B) wild-type ischemic AKI, and (C) NLRP3−/− mouse ischemic AKI are shown. In wild-type sham-operated (A) kidneys, the tubules have well-defined brush borders without necrosis or apoptosis. In wild-type ischemic AKI (B) there is acute tubular necrosis (large arrows) and tubular cell apoptosis (small arrows). In NLRP3−/− mouse ischemic AKI (C), acute tubular necrosis and tubular cell apoptosis is statistically significantly reduced.
Caspase-1, Neutrophil Infiltration, ASC, NLRP1, and NLRP3 in Ischemic AKI.
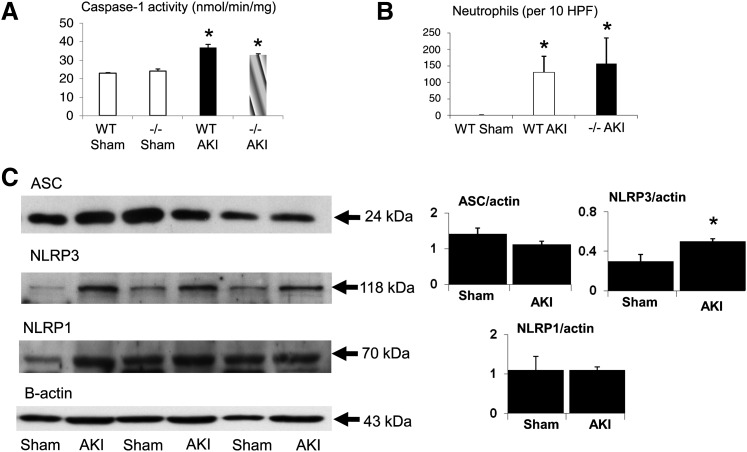
Caspase-1 activity was increased in ischemic AKI in both WT and NLRP3−/− mice compared with the sham-operated mice (Fig. 7A). Neutrophil infiltration in the kidney was increased in ischemic AKI in both WT and NLRP3−/− mice compared with the sham-operated mice (Fig. 7B). NLRP3, but not ASC or NLRP1, increased in ischemic AKI (Fig. 7C).
Fig. 7.
Caspase-1, neutrophil infiltration, ASC, NLRP1, and NLRP3 in ischemic AKI. (A) Caspase-1 activity was increased in ischemic AKI in both WT and NLRP3−/− (−/−) mice compared with sham-operated mice. n = 4 per group; *P < 0.05 vs. sham. (B) Neutrophil infiltration in the kidney was increased in ischemic AKI in both WT and NLRP3−/− (−/−) mice compared with sham-operated mice. n = 8 per group; *P < 0.05 vs. sham. (C) NLRP3, but not ASC or NLRP1, is increased in ischemic AKI. n = 4–6 per group. *P < 0.05 vs. sham. In densitometric analysis of immunoblots, data are presented as protein/β-actin ratios plotted on the y-axis. HPF, high-power field.
Cytokines in Cisplatin-Induced versus Ischemic AKI.
To determine the possible reason for the differences in protection against cisplatin-induced AKI versus ischemic AKI in the NLRP3−/− mice, the proinflammatory cytokines IL-1β, IL-6, CXCL1, and TNF-α were studied (Table 1). IL-1β was increased in ischemic AKI but not cisplatin-induced AKI. IL-6 and CXCL1 were increased in both cisplatin-induced and ischemic AKI. TNF-α was increased in cisplatin-induced AKI but not ischemic AKI. None of the cytokines except IL-6 were statistically significantly decreased in NLRP3−/− mice with either cisplatin-induced or ischemic AKI.
TABLE 1.
Cytokines in cisplatin-induced and ischemic acute kidney injury
| Cytokines | Vehicle WT | Cisplatin WT | Cisplatin NLRP3−/− | Sham WT | Ischemia WT | Ischemia NLRP3−/− |
|---|---|---|---|---|---|---|
| (n = 3–6) |
(n = 4–10) |
|||||
| pg/mg | ||||||
| IL-1β | 4.2 ± 1.1 | 6.2 ± 0.9 | 9.6 ± 1.3 | 4.2 ± 0.7 | 5.9 ± 0.3 | 11.4 ± 5.1 |
| IL-6 | 1.4 ± 0.4 | 4.0 ± 0.8a | 2.0 ± 0.4b | 1.9 ± 0.2 | 9.1 ± 0.2c | 22.4 ± 13c |
| CXCL1 | 2.4 ± 0.7 | 112.5 ± 7.2c | 98.0 ± 24.5c | 7.6 ± 0.8 | 116 ± 4.7c | 107.6 ± 25c |
| TNF-α | 0.3 ± 0.1 | 1.2 ± 0.1c | 1.2 ± 0.1c | 0.8 ± 0.2 | 0.2 ± 0.02 | 0.5 ± 0.05 |
P < 0.05 vs. vehicle/sham WT.
P < 0.05 vs. cisplatin WT.
P < 0.01 vs. vehicle/sham WT.
Discussion
Diseases associated with increased inflammasome activity include familial Mediterranean fever (FMF), adult onset Still disease, Behcet disease, vitiligo (mutation in NLRP1), gout, and pseudogout (Martinon at al., 2009; Lamkanfi and Dixit, 2012; Strowig et al., 2012). An interesting report that cisplatin treatment triggers FMF attacks (Toubi et al., 2003) stimulated our interest in the role of inflammasome in cisplatin-induced AKI. The link between cisplatin treatment and FMF relapse lies in increased production of IL-6, IL-1, IL-8, and TNF-α, which is common to both FMF and AKI. This is important clinically as cisplatin may aggravate the inflammatory response in patients who have diseases characterized by NLRP3 mutations or NLRP3 activation.
The role of inflammation in AKI, especially in ischemic AKI, has been described previously with involvement of leukocytes, adhesion molecules, and endothelial injury (Bonventre and Zuk, 2004; Friedewald and Rabb, 2004; Ramesh and Reeves, 2004; Devarajan, 2006). There is also increasing evidence that cisplatin-induced AKI is an inflammatory process (Faubel at al., 2004, 2007; Lu et al., 2008). Early inflammation is largely mediated by the innate immune system, which provides rapid nonadaptive responses against infections and injuries. The innate immune system uses an array of germline encoded pattern-recognition receptors such as Toll-like receptors and NOD-like receptors to detect pathogen-associated molecular patterns. Damaged cells, such as from trauma or ischemia, release endogenous danger signals or danger-associated molecular patterns that alert the innate immune system to imminent tissue damage (Iyer et al., 2009). NLRP1 and NLRP3 proteins act as pattern-recognition receptors to detect pathogen-associated and danger-associated molecular patterns (Schroder et al., 2010; Schroder and Tschopp, 2010).
Within the cytoplasm, NLRP proteins form a complex, the inflammasome, with ASC and inactive caspase-1. NLRP inflammasomes and caspase-1 are essential components of the inflammatory response. In our present study, there was an increase in crucial inflammasome proteins ASC and NLRP1 in the kidney and an increase in caspase-1 activity in the kidney in cisplatin-induced AKI. The increase in caspase-1 activity in cisplatin-induced AKI occurred in both WT and NLRP3−/− mice. The lack of a decrease in caspase-1 activity in NLRP3−/− mice was associated with the lack of protection against cisplatin-induced AKI.
To determine the localization of inflammasome activation by cisplatin, we examined macrophages, the major source of NLRP3 (Anders and Muruve, 2011). NLRP3 was present in macrophages in culture but was not increased by low-dose or high-dose cisplatin. In this regard, we have demonstrated that while macrophages increase on day 2 in cisplatin-induced AKI that macrophage depletion using liposomal encapsulated clodronate is not protective against cisplatin-induced AKI (Lu et al., 2008).
The lack of protection by macrophage depletion argues against the macrophage as a source of injurious NLRP3 inflammasome in cisplatin-induced AKI. NLRP proteins are expressed in CD4 T cells (Wilmanski et al., 2008). We and others have demonstrated that CD4 T cells increase early in cisplatin-induced AKI and that CD4 T-cell depletion markedly protects against cisplatin-induced AKI (Akcay et al., 2011). Thus, future investigation of CD4 T cells as a source of the NLRP1 inflammasome in cisplatin-induced AKI would be interesting.
Next, to further determine the localization of inflammasomes in the kidney, we examined proximal tubules, the major site of injury in cisplatin and ischemic AKI. There was an increase in caspase-1, IL-1α, and IL-1β in cisplatin-treated proximal tubules that was not associated with an increase in NLRP3, suggesting that activation of caspase-1, IL-1α, and IL-1β may be independent of the NLRP3 inflammasome in cisplatin-treated proximal tubules.
The role of the inflammasome is starting to emerge in kidney diseases (Anders and Muruve, 2011). A role for NLRP3 inflammasome was demonstrated in a unilateral ureteric obstruction model of chronic kidney disease (Vilaysane et al., 2010). NLRP3-deficiency protects mice against mortality, renal dysfunction, and neutrophil influx in a model of ischemic AKI (Iyer et al., 2009). In another study of ischemic AKI in mice, it was concluded that NLRP3 causes AKI by a direct effect on renal tubular epithelium and that the AKI was independent of inflammasome-induced proinflammatory cytokine production (Shigeoka et al., 2010).
Our study demonstrates for the first time an increase in ASC, the major component of the NLRP3 and NLRP1 inflammasome, and NLRP1 protein in the kidney in cisplatin-induced AKI. However, the lack of a statistically significant increase in NLRP3 in the kidney in cisplatin-induced AKI and in proximal tubules treated with cisplatin as well as the lack of protection against cisplatin-induced AKI in NLRP3−/− mice suggest that the NLRP3 inflammasome is not involved in cisplatin-induced AKI. The NLRP1 inflammasome consists of NLRP1 protein, ASC protein, and caspase-1 (Martinon et al., 2009). Both caspase-5 protein and activity and NLRP1-protein were increased in cisplatin-induced AKI, suggesting a role for the NLRP1 inflammasome in cisplatin-induced AKI. In future studies, when NLRP1−/− mice become available, further study of the role of the NLRP1 inflammsome in cisplatin-induced AKI will be of interest.
Two previous studies have reported protection against ischemic AKI in NLRP3−/− mice (Iyer et al., 2009; Shigeoka et al., 2010). There are important similarities and differences between our study and those previous reports. Our study confirms the impressive decrease in serum creatinine and ATN scores in ischemic AKI that were reported in the previous studies. In addition, we describe an impressive decrease in BUN, another marker of kidney function. Our model of ischemia differs from the two previous studies in that we use bilateral renal pedicle (artery and vein) clamping; the previous studies used a model that clamped the renal artery only. Their studies showed a decrease in IL-1β in the kidney in NLRP3−/− mice; our study found no decrease in IL-1β, IL-6, CXCL1, or TNF-α in NLRP3−/− mice. One of the previous studies found a decrease in caspase-1 in the kidney in NLRP3−/− mice (Shigeoka et al., 2010); our study found increased caspase-1 activity in ischemic AKI in both WT and NLRP3−/− mice, indicating that the protection against ischemic AKI in NLRP3−/− mice occurred despite the increase in caspase-1 activity.
Their study demonstrated a large decrease in terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) positive tubular cells in NLRP3−/− mice. Morphology, the gold standard for detection of apoptosis, and TUNEL staining fail to discriminate between proximal tubular apoptosis and necrosis, especially in vivo in the kidney; also, TUNEL staining grossly overestimates proximal tubular apoptosis in the kidney (Grasl-Kraupp et al., 1995; Andrade et al., 2000; Gobe et al., 2000). In our study, the morphologic criteria—such as cellular rounding and shrinkage, nuclear chromatin compaction, and formation of apoptotic bodies—were used to detect apoptosis and demonstrated a less impressive decrease in apoptotic tubular cells than had been previously reported using TUNEL staining in NLRP3−/− mice.
In summary, there is an increase in the inflammasome components ASC and caspase-1 in the kidney in mice with cisplatin-induced AKI and caspase-1, IL-1β, and IL-1α in proximal tubules treated with cisplatin. The increase in caspase-1 in kidney and proximal tubules was not associated with a statistically significant increase in NLRP3 protein. The NLRP3−/− mice were not protected against cisplatin-induced AKI, which suggests that the NLRP3 inflammsome is not a mediator of cisplatin-induced AKI. In contrast, the NLRP3−/− mice were protected against ischemic AKI, confirming the role of the NLRP3 inflammsome in ischemic AKI. The difference in protection against cisplatin-induced AKI versus ischemic AKI in NLRP3−/− mice is not explained by the differences in proinflammatory cytokines IL-1β, IL-6, CXCL1, or TNF-α. The NLRP1 and caspase-5 components of the NLRP1 inflammasome were increased in cisplatin-induced AKI. Thus, the role of other inflammasomes merits future study in cisplatin-induced AKI.
Abbreviations
- AKI
acute kidney injury
- AMC
7-amido-4-methyl coumarin
- ASC
apoptosis-associated Speck-like protein containing a caspase recruitment domain
- ATN
acute tubular necrosis
- BUN
blood urea nitrogen
- CXCL1
chemokine (C-X-C motif) ligand 1
- ELISA
enzyme-linked immunosorbent assay
- FMF
familial Mediterranean fever
- IL
interleukin
- NLRP
nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain containing proteins
- NLRP3−/−
NLRP knockout
- PAS
periodic acid-Schiff
- TNF-α
tumor necrosis factor-α
- TUNEL
terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling
- WT
wild-type
Authorship Contributions
Participated in research design: Edelstein, Jani, Keys.
Conducted experiments: Edelstein, Kim, Lee, Akcay, Nguyen, He, Ravichandran.
Performed data analysis: Edelstein, Ljubanovic.
Wrote or contributed to the writing of the manuscript: Edelstein, Kim, Lee, Keys, He, Ravichandran, Jani.
Footnotes
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants R01 DK056851, R01 DK056851S1, K08 DK069512]; and grants from Fatih University School of Medicine, Department of Nephrology, Ankara, Turkey (to A.A.); Pusan National University School of Medicine, Pusan, South Korea (to D.W.L.); and Gyeongsang National University School of Medicine, Gyeongsangnam-do, South Korea (to H.-J.K.).
References
- Akcay A, Nguyen Q, He Z, Turkmen K, Won Lee D, Hernando AA, Altmann C, Toker A, Pacic A, Ljubanovic DG, et al. (2011) IL-33 exacerbates acute kidney injury. J Am Soc Nephrol 22:2057–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders HJ, Muruve DA. (2011) The inflammasomes in kidney disease. J Am Soc Nephrol 22:1007–1018 [DOI] [PubMed] [Google Scholar]
- Andrade L, Vieira JM, Safirstein R. (2000) How cells die counts. Am J Kidney Dis 36:662–668 [DOI] [PubMed] [Google Scholar]
- Bonventre JV, Zuk A. (2004) Ischemic acute renal failure: an inflammatory disease? Kidney Int 66:480–485 [DOI] [PubMed] [Google Scholar]
- Devarajan P. (2006) Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17:1503–1520 [DOI] [PubMed] [Google Scholar]
- Dursun B, He Z, Somerset H, Oh DJ, Faubel S, Edelstein CL. (2006) Caspases and calpain are independent mediators of cisplatin-induced endothelial cell necrosis. Am J Physiol Renal Physiol 291:F578–F587 [DOI] [PubMed] [Google Scholar]
- Edelstein CL. (2000) Rat renal proximal tubules, hypoxia, ionomycin and calpain, in Calpain Methods and Protocols (Elce JS, ed) pp 225–238, Humana Press, Totowa, New Jersey: [DOI] [PubMed] [Google Scholar]
- Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, Oh DJ, Lu L, Klein CL, Dinarello CA, et al. (2007) Cisplatin-induced ARF is associated with an increase in the cytokines IL-1β, IL-18, IL-6 and neutrophil infiltration in the kidney. J Pharmacol Exp Ther 322:8–15 [DOI] [PubMed] [Google Scholar]
- Faubel SG, Ljubanovic D, Poole B, Dursun B, He Z, Cushing S, Somerset H, Gill RG, Edelstein CL. (2005) Peripheral CD4 T-cell depletion is not sufficient to prevent ischemic acute renal failure. Transplantation 80:643–649 [DOI] [PubMed] [Google Scholar]
- Faubel SG, Ljubanovic D, Reznikov LL, Somerset H, Dinarello CA, Edelstein CL. (2004) Caspase-1-deficient mice are protected against cisplatin-induced apoptosis and acute tubular necrosis. Kidney Int 66:2202–2213 [DOI] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. (2009) The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 10:241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald JJ, Rabb H. (2004) Inflammatory cells in ischemic acute renal failure. Kidney Int 66:486–491 [DOI] [PubMed] [Google Scholar]
- Gobé G, Zhang XJ, Willgoss DA, Schoch E, Hogg NA, Endre ZH. (2000) Relationship between expression of Bcl-2 genes and growth factors in ischemic acute renal failure in the rat. J Am Soc Nephrol 11:454–467 [DOI] [PubMed] [Google Scholar]
- Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R. (1995) In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology 21:1465–1468 [DOI] [PubMed] [Google Scholar]
- Guégan C, Vila M, Teismann P, Chen C, Onténiente B, Li M, Friedlander RM, Przedborski S. (2002) Instrumental activation of bid by caspase-1 in a transgenic mouse model of ALS. Mol Cell Neurosci 20:553–562 [DOI] [PubMed] [Google Scholar]
- He Z, Altmann C, Hoke TS, Ljubanovic D, Jani A, Dinarello CA, Faubel S, Edelstein CL. (2008) Interleukin-18 binding protein transgenic mice are protected against ischemic acute kidney injury. Am J Physiol Renal Physiol 295:F1414–F1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Dursun B, Oh DJ, Lu L, Faubel S, Edelstein CL. (2009) Macrophages are not the source of injurious interleukin-18 in ischemic acute kidney injury in mice. Am J Physiol Renal Physiol 296:F535–F542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, et al. (2009) Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci USA 106:20388–20393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Rüegg A, Werner S, Beer HD. (2008) Active caspase-1 is a regulator of unconventional protein secretion. Cell 132:818–831 [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. (2012) Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol 28:137–161 [DOI] [PubMed] [Google Scholar]
- Lee DW, Faubel S, Edelstein CL. (2011) Cytokines in acute kidney injury (AKI). Clin Nephrol 76:165–173 [DOI] [PubMed] [Google Scholar]
- Lu LH, Oh DJ, Dursun B, He Z, Hoke TS, Faubel S, Edelstein CL. (2008) Increased macrophage infiltration and fractalkine expression in cisplatin-induced acute renal failure in mice. J Pharmacol Exp Ther 324:111–117 [DOI] [PubMed] [Google Scholar]
- Martinon F, Mayor A, Tschopp J. (2009) The inflammasomes: guardians of the body. Annu Rev Immunol 27:229–265 [DOI] [PubMed] [Google Scholar]
- Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, Schrier RW, Edelstein CL. (2001) Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest 107:1145–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikov VY, Faubel SG, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL. (2002) Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest 110:1083–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh G, Reeves WB. (2004) Inflammatory cytokines in acute renal failure. Kidney Int Suppl S91:S56–S61 [DOI] [PubMed] [Google Scholar]
- Safirstein RL. (2007) Renal disease induced by anti-neoplastic agents, in Diseases of the Kidney and Urinary Tract (Schrier RW, ed) pp 1068–1081, Lippincott, Williams and Wilkins, Philadelphia [Google Scholar]
- Schrier RW. (2002) Cancer therapy and renal injury. J Clin Invest 110:743–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. (2010) The inflammasomes. Cell 140:821–832 [DOI] [PubMed] [Google Scholar]
- Schroder K, Zhou R, Tschopp J. (2010) The NLRP3 inflammasome: a sensor for metabolic danger? Science 327:296–300 [DOI] [PubMed] [Google Scholar]
- Shigeoka AA, Mueller JL, Kambo A, Mathison JC, King AJ, Hall WF, Correia JdaS, Ulevitch RJ, Hoffman HM, McKay DB. (2010) An inflammasome-independent role for epithelial-expressed Nlrp3 in renal ischemia-reperfusion injury. J Immunol 185:6277–6285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. (2012) Inflammasomes in health and disease. Nature 481:278–286 [DOI] [PubMed] [Google Scholar]
- Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galán JE, Askenase PW, et al. (2006) Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 24:317–327 [DOI] [PubMed] [Google Scholar]
- Toubi E, Gershoni-Baruch R, Kuten A. (2003) Cisplatin treatment triggers familial Mediterranean fever attacks. Tumori 89:80–81 [DOI] [PubMed] [Google Scholar]
- Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, Li Y, Clark SA, Tschopp J, Trpkov K, et al. (2010) The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol 21:1732–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmanski JM, Petnicki-Ocwieja T, Kobayashi KS. (2008) NLR proteins: integral members of innate immunity and mediators of inflammatory diseases. J Leukoc Biol 83:13–30 [DOI] [PMC free article] [PubMed] [Google Scholar]