Abstract
Botulinum neurotoxins (BoNTs) are well recognized to cause potent, selective, and long-lasting neuroparalytic actions by blocking cholinergic neurotransmission to muscles and glands. There is evidence that BoNT isoforms can also inhibit neurotransmission in the brain. In this study, we examined whether locally delivered BoNT/A and BoNT/B can attenuate kindling measures in amygdala-kindled rats. Male rats were implanted with a combination infusion cannula–stimulating electrode assembly into the right basolateral amygdala. Fully kindled animals received a single infusion of vehicle or BoNT/A or BoNT/B at doses of 1, 3.2, or 10 ng over a 20-minute period by convection-enhanced delivery. Electrographic (EEG) and behavioral kindling measures were determined at selected times during the 3- to 64-day period after the infusion. BoNT/B produced a dose-dependent elevation in after-discharge threshold and duration and a reduction in the seizure stage and duration of behavioral seizures that lasted for up to 50 days after infusion. BoNT/A had similar effects on EEG measures; behavioral seizure measures were also reduced, but the effect did not reach statistical significance. The effects of both toxins on EEG and behavioral measures progressively resolved during the latter half of the observation period. Animals gained weight normally, maintained normal body temperature, and did not show altered behavior. This study demonstrates for the first time that locally delivered BoNTs can produce prolonged inhibition of brain excitability, indicating that they could be useful for the treatment of brain disorders, including epilepsy, that would benefit from long-lasting suppression of neurotransmission within a circumscribed brain region.
Introduction
Focal drug delivery is an experimental epilepsy treatment approach that avoids the systemic toxicities of oral antiepileptic medications, as well as untoward effects caused by drug actions on brain regions that are not involved in seizure generation and propagation (Nilsen and Cock, 2004). Focally administered small-molecule antiepileptic drugs (such as diazepam and carbamazepine) and inhibitory substances (such as adenosine, GABA, and muscimol) have been demonstrated to prevent seizure generation and inhibit ongoing seizures in animal models (Eder et al., 1997; Stein et al., 2000; Anschel et al., 2004; Fisher and Chen, 2006; Ludvig et al., 2010). However, these agents have a short duration of action because they diffuse from the site of delivery or are biologically inactivated. For such agents, clinical application would require continuous delivery and a mechanism to ensure an adequate supply of the therapeutic agent. To overcome these limitations, we have sought to identify agents that provide prolonged seizure protection after local delivery. We previously demonstrated that local infusion of peptide N-type calcium channel toxins can confer protection against amygdala-kindled seizures in rats that persists for up to 1 week (Gasior et al., 2007).
Local delivery of botulinum neurotoxins could provide an alternative approach for long-term inhibition of seizure generation (Costantin et al., 2005; Antonucci et al., 2009). Botulinum neurotoxins, a group of homologous dichain proteins from Clostridium botulinum, are well recognized to target peripheral cholinergic nerve terminals, causing a sustained block of acetylcholine release that leads to neuromuscular or autonomic paralysis. However, there is evidence that the toxins can also affect chemical neurotransmission in the brain (Bozzi et al., 2006; Caleo et al., 2009). The seven botulinum neurotoxin (BoNT) serotypes are indicated with letters from A to G, each of which is composed of a disulfide-linked, ∼100-kDa heavy chain and ∼50-kDa light chain. The heavy chain mediates toxin binding, and the light chain acts as an endopeptidase that specifically cleaves proteins of the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex, leading to an irreversible inhibition of neurotransmitter release. The SNARE protein sites targeted by different BoNTs vary. Specifically, BoNT/A cleaves synaptosomal-associated protein 25 (SNAP-25 protein), whereas BoNT/B cleaves vesicle-associated membrane protein 2 (synaptobrevin 2) (Simpson, 2004). Intraventricular injection of BoNT/A and BoNT/B in mice causes behavioral alterations and, at high doses, lethality (Luvisetto et al., 2003, 2004). Although these actions were initially attributed to effects on central cholinergic synapses, it is now recognized that BoNTs can inhibit the release of neurotransmitters other than acetylcholine, including the major excitatory neurotransmitter glutamate (Bigalke et al., 1981; Ashton and Dolly, 1988; Habermann et al., 1988; Foran et al., 2003; Costantin et al., 2005). Recently, it was found that BoNT/A and BoNT/E inhibit the release of glutamate more effectively than they inhibit the release of GABA (Verderio et al., 2004, 2007). Inasmuch as seizures result from pathologically excessive glutamate-mediated excitation relative to GABA-mediated inhibition, these properties might confer antiseizure activity. Indeed, it has been found that intrahippocampal injection of BoNT/E in mice inhibits electrographic seizure activity induced by intrahippocampal kainic acid and behavioral seizures induced by systemic kainic acid (Costantin et al., 2005) and reduces the frequency of spontaneous recurrent seizures produced by intrahippocampal kainate in a model of mesial temporal lobe epilepsy (Antonucci et al., 2009).
To be practically useful in the treatment of epilepsy, it is desirable for a locally administered treatment to have a long duration of action. Recovery of function from neuromuscular paralysis by botulinum neurotoxins varies according to the serotype and the target tissue, but for BoNT/A, the duration of action is typically 1 to 3 months (Rossetto et al., 2001). BoNT/B had a shorter duration in some (Sloop et al., 1997; Blitzer, 2005) but not all (Pappert et al., 2008; Guidubaldi et al., 2011) studies, whereas recovery from paralysis by BoNT/E was more rapid (Eleopra et al., 1998). The duration of the functional central nervous system (CNS) actions of these toxins is not known, although in one study cleaved SNAP-25 was evident for a prolonged period after intrahippocampal injection of BoNT/A (Antonucci et al., 2008). In the present study, we sought to assess the potential of locally delivered botulinum neurotoxins to confer seizure protection in the amygdala kindling model, an experimental paradigm that allows assessment of the duration of toxin action for weeks to months. We administered the toxins using convection-enhanced delivery (CED), which provides a larger and more homogeneous distribution than conventional bolus injection and does not damage the surrounding tissue (Gasior et al., 2007; Rogawski, 2009).
Materials and Methods
Animals.
Experimentally naive male Sprague-Dawley rats, weighing 225 to 250 g at the beginning of the study, were obtained from Taconic Farms (Germantown, NY). Rats were housed individually under a controlled environment (temperature, 24 ± 2°C; humidity, 45 ± 5%; 12-hour light-dark cycle with lights on between 6:00 AM and 6:00 PM). Each rat had free access to tap water and a nutritionally balanced rodent diet supplemented regularly with fresh fruit and sweetened gelatin dessert. Experiments were conducted between 9:00 AM and 4:00 PM.
All experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee of the National Institute of Neurological Disorders and Stroke, National Institutes of Health (Bethesda, MD), in strict compliance with the Guide for the Care and Use of Laboratory Animals (National Research Council in 1996, National Academy Press, Washington, DC; http://www.nap.edu/readingroom/books/labrats/). The animal facilities were fully accredited by the American Association for the Accreditation of Laboratory Animal Care.
Neurotoxins.
Botulinum neurotoxins types A and B) were obtained from List Biologic Laboratories, Inc. (Campbell, CA). Each neurotoxin (Mr 150,000) was dissolved in 0.1% (w/v) solution of bovine serum in 0.1 M phosphate-buffered saline (Sigma-Aldrich, St. Louis, MO). The neurotoxins were administered at the following three doses: 1.0 (≈6.7 fmol), 3.2 (≈21.4 fmol), and 10 ng (≈67 fmol). Each neurotoxin was injected only once. Separate groups of rats were used to test each dose of the two neurotoxins. Control rats were injected with vehicle.
Surgery and Cannula-Electrode Assembly.
Each rat was chronically implanted with a custom-made guide cannula-bipolar stimulating electrode assembly (Plastics One, Roanoke, VA) during an aseptic surgical procedure with the rats under general anesthesia induced by a mixture of ketamine (60–75 mg/kg) and medetomidine (0.25–0.5 mg/kg). The cannula-electrode assembly consisted of a 26-gauge, 6.5-mm-long stainless steel guide cannula with two 0.23-mm-diameter stainless steel electrode wires attached diametrically at the periphery (Gasior et al., 2007). The electrode wires were polyamide-insulated, with the exception of the 0.5-mm most distal extent; the tips of the wires were separated by 0.5 mm and projected 3.5 mm past the end of the guide cannula.
The cannula-electrode was fixed to a threaded central plastic pedestal. The electrode wires passed to a second threaded pedestal with pin connectors to allow a connection with the kindling stimulator. The bipolar electrodes were used for recording and stimulating. Between infusions, the guide cannula was plugged with a dummy cannula assembly consisting of a solid wire of the same length (i.e., 6.5 mm) as the guide cannula and a threaded plastic cap. The electrode tip was implanted into the basolateral nucleus of the right amygdala at stereotaxic coordinates, measured from the bregma: anteroposteriorly, −2.8; mediolaterally, 5.0; and dorsoventrally, −8.7 (Paxinos and Watson, 1998). Dental acrylic cement (Lang Dental, Wheeling, IL) and stabilizing stainless steel screws (Plastics One) were used to secure the cannula-electrode assembly to the skull. Ketoprofen was given subcutaneously after surgery followed by atipamezole (1 mg/kg) to reverse anesthesia. At least 10 days were allowed for recovery after the surgery. The position of the cannula-electrode assembly tip was histologically verified in randomly selected rats at the end of the study.
Kindling.
Rats were stimulated individually within a 29-cm-diameter Plexiglas cylinder. Each rat was connected to a custom-made stimulator (National Institutes of Health Research Services Branch, Bethesda, MD) via a swivel attachment to allow free movement within the chamber. The stimulator was set to deliver 1-millisecond, bipolar, square current pulses at 60 Hz for 1 second at variable current intensities. Depth electroencephalogram (EEG) signals were recorded via the stimulating electrode (except during the stimulation interval) with a Grass CP511 AC EEG preamplifier (Astro-Med, West Warwick, RI) and stored in digital form using Axotape 9 (Axon Instruments, Foster City, CA).
Kindled seizure activity was assessed using four dependent measures as follows: after-discharge threshold, after-discharge duration, severity of behavioral seizures, and duration of behavioral seizure. After-discharge threshold refers to the lowest stimulating current intensity (in milliamperes) that induces an after-discharge consisting of a train of EEG spikes at 1 Hz or greater lasting for at least 5 seconds, with an amplitude at least twice the baseline amplitude. After-discharge duration is the total duration of the after-discharge (in seconds). Severity of behavioral seizures was scored according to Racine (1972), with the following designations: stage 0, no apparent change in behavior; stage 1, facial twitching; stage 2, head nodding associated with more severe facial twitching; stage 3, forelimb clonus; stage 4, rearing; and stage 5, rearing and loss of balance. Duration of behavioral seizure (in seconds) reflects the duration of limbic seizures (stage 1–2) or motor seizures (stage 3–5). Behavioral changes such as immobility with occasional facial twitches that often occurred after the end of motor seizures were not considered in the duration determination.
After the rats recovered from the surgery, kindling began and consisted of three phases: 1) prekindling determination of the after-discharge threshold, 2) kindling development, and 3) postkindling redetermination of the after-discharge threshold (Pinel et al., 1976; Freeman and Jarvis, 1981). On day 1, after-discharge threshold was determined by delivering a series of stimulations of increasing intensities (starting at 50 μA and increasing in 25% increments every 3–5 minutes) until after-discharge was triggered. Rats were excluded from the study if a current of 466 μA intensity failed to produce after-discharge on the first day of kindling. During the second phase of kindling, each rat was stimulated daily at a current intensity of 125% of its individual after-discharge threshold value determined on day 1. Daily stimulations continued until the rat exhibited stage 5 seizures during 5 consecutive days or during 8 days of the last 10 stimulation days. Rats meeting this criterion were considered “kindled.” Rats that failed to meet the kindling criterion within 30 stimulations were excluded from the study. During the third phase of kindling, after-discharge threshold was redetermined in the same way as during the first phase of kindling. Threshold was redetermined on several consecutive days until after-discharge threshold and the behavioral seizure score produced by stimulation at the after-discharge threshold were stable and reproducible. Rats that did not show stable responses to the electrical stimulation at their respective after-discharge thresholds for several consecutive days were excluded from further testing.
CED of BoNT/A and BoNT/B.
The CED system consisted of a programmable infusion pump (model KDS200; KD Scientific Inc., Holliston, MA), a gas-tight 50-µl Hamilton syringe with a 22-gauge needle (Hamilton Company, Reno, NV), a counter-weighted swivel to allow free movement of the rat (Instech Laboratories Inc., Plymouth Meeting, PA), and a 33-gauge infusion cannula (Plastics One). All components were connected with thick-wall polyethylene 50 tubing (Plastics One). While gently restraining the rat, the infusion cannula was slowly inserted into the brain through the guide cannula. The tip of the infusion cannula extended to a depth of 0.5 mm above the tips of the stimulating electrode wires and was maintained at the appropriate depth by a plastic stop at the top of the cannula. After inserting the infusion cannula, the rat was released and placed in a plastic cylinder for the entire infusion. All infusions were performed in conscious and unrestrained animals. After infusion cannula insertion, the brain tissue was allowed to seal around the cannula for a few minutes before initiation of the infusion. Infusions were delivered at a constant rate of 0.25 µl/min, with a total volume of 5 µl, which was previously determined to be optimal for CED of large molecules into the rat brain (Bobo et al., 1994; Chen, et al., 1999). At the end of the infusion, the cannula was left in place for a few minutes to minimize infusate backflow and ensure better distribution of the infusate. The general behavioral state of each rat was assessed after the infusion.
The effects of the neurotoxins on seizure sensitivity in fully kindled rats were assessed, as described earlier, by measuring after-discharge threshold and the other three parameters of the amygdala-kindled seizures 3, 7, 10, 15, 21, 35, 50, and 64 days after the infusion. Weights and rectal body temperatures were recorded before each electrical stimulation to assess the general health of the animals.
Histologic Analysis.
After completion of testing, selected animals were perfused transcardially with 4% paraformaldehyde, and the brains were removed for sectioning and for cresyl violet and silver staining to assess cannula placement and evidence of neuronal damage.
Statistical Analysis.
Once the stability of the kindling parameters was established in each individual rat, two to four measures collected on separate days preceding the infusion were averaged for that rat and constituted its baseline values, against which the effects of treatment (neurotoxin or vehicle) were later statistically compared. Group means are plotted in Figs. 2 and 3. For statistical analysis, the percent change from baseline was calculated using the equation 100 × (B − A)/A, where A and B are values before and after treatment, respectively. Area-under-the-curve (AUC) values were calculated from the percent change values at time points 3 to 64 days following CED using the trapezoidal method. Statistical analyses were performed by one-way (within a group) and two-way (between groups) repeated measures analysis of variance (ANOVA) after transformation of the percentage change in data using arcsine-root transformation. When appropriate, post hoc analysis was performed using Dunnett’s test or Tukey’s test. Kindling measures are group means ± S.E.M. Group differences were considered statistically significant at P < 0.05. All calculations were made using SigmaStat (SPSS, Chicago, IL) and GraphPad Prism (GraphPad Software, La Jolla, CA).
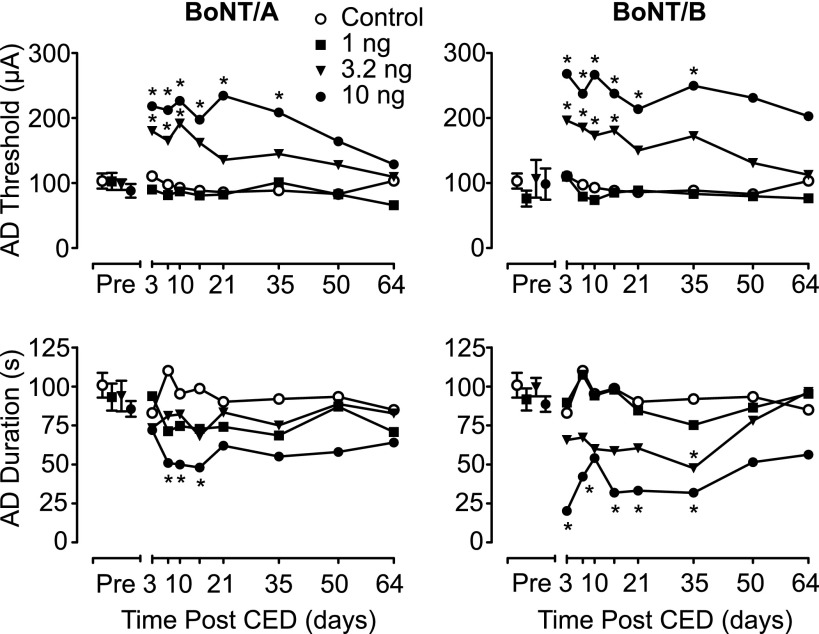
Fig. 2.
Mean after-discharge (AD) threshold and duration values (in microamperes and seconds, respectively) 2–4 days before the CED infusion (Pre) and 3–64 days after the CED infusion of BoNT/A (left) and BoNT/B (right). Data from the same control group (treated with vehicle) are shown for comparison with BoNT/A and BoNT/B. Each toxin was administered once in a dose of 1, 3.2, or 10 ng. The active treatment groups consisted of seven rats per dose; vehicle was administered in eight control rats. Each data point represents the mean of values from the seven or eight rats in each group. *Significantly different from vehicle value at that time point (Tukey test) after the detection of a statistically significant main effect by a two-way repeated measures ANOVA. Results of the ANOVA and post hoc analyses are presented in Table 1.
Fig. 3.
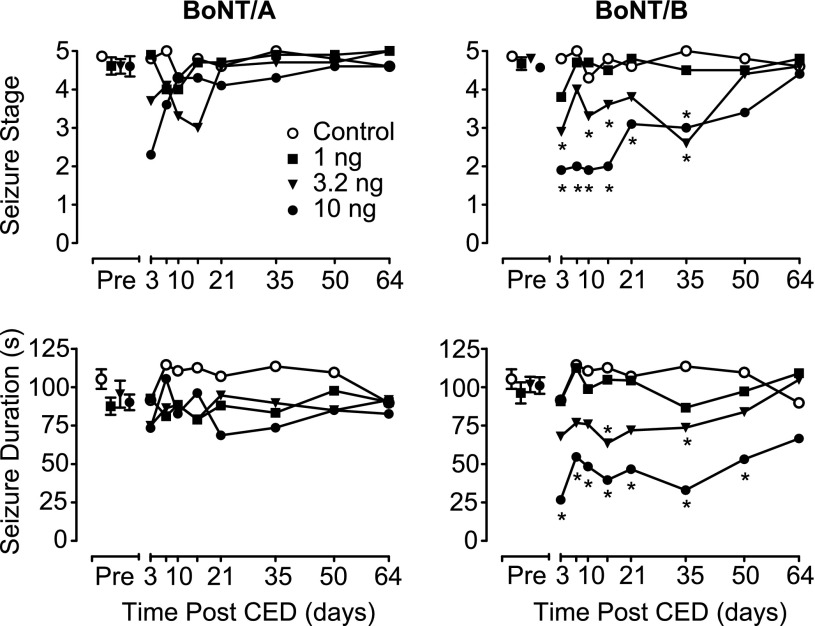
Mean behavioral seizure stage and duration (in seconds) values 2–4 days before the CED infusion (Pre) and 3–64 days after the CED infusion of BoNT/A (left) and BoNT/B (right). See legend to Fig. 1 for details.
Results
Development of Kindling and Response to Stimulation at After-Discharge Threshold in Fully Kindled Rats.
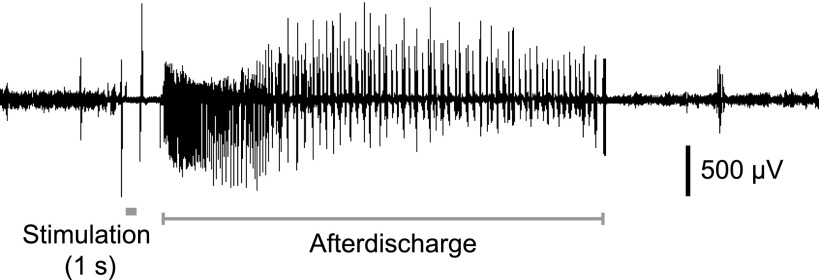
A total of 50 rats successfully completed the kindling procedure and were used in the experiments reported here. In these animals, the kindling criterion was reached in 16.5 ± 1.0 (mean ± S.E.M.) daily stimulations. All animals exhibited stable thresholds to elicit stage 5 seizures during the redetermination period. The mean after-discharge threshold and duration during the redetermination period were 96.8 ± 6.4 μA and 93.7 ± 2.7 seconds, respectively. A sample after-discharge recording is shown in Fig. 1. The mean behavioral stage and behavioral seizure duration at threshold were 4.7 ± 0.1 and 97.0 ± 2.4 seconds, respectively.
Fig. 1.
A representative EEG recoding in a fully kindled rat showing baseline EEG activity and response to stimulation. The signal is blanked during stimulation. The after-discharge continues for 39.3 seconds and is followed by the return to EEG activity comparable to the prestimulation period.
The fully kindled rats were randomly assigned to seven treatment groups (seven or eight rats per group) to receive vehicle or one of three doses of each of the two neurotoxins (BoNT/A and BoNT/B) at doses of 1, 3.2, or 10 ng. After randomization, baseline EEG and behavioral seizure parameters were not statistically different between the treatment groups (e.g., F6,43 = 0.323, P = 0.916 for after-discharge thresholds).
Effects of BoNT/A and BoNT/B on Kindling Measures.
Animals receiving CED infusions of vehicle or a 1-ng dose of either BoNT/A or BoNT/B exhibited stable kindling measures (after-discharge threshold, after-discharge duration, seizure stage, and behavioral seizure duration) when tested between 3 and 64 days after the infusion (Figs. 2 and 3). In contrast, a single 20-minute CED infusion of BoNT/A or BoNT/B at doses of 3.2 or 10 ng was associated with statistically significant changes in kindling parameters (Table 1). Specifically, CED of BoNT/A and BoNT/B at a dose of 10 ng resulted in a statistically significant elevation in after-discharge threshold with an accompanying decrease in after-discharge duration at time points from 3 to 35 days after infusion. The magnitude of the effect produced by BoNT/A and BoNT/B was comparable. Administration of the intermediate dose (3.2 ng) of BoNT/B and BoNT/A also resulted in statistically significant elevations in after-discharge threshold; however, only BoNT/B at that dose had a statistically significant effect on the after-discharge duration. In contrast to BoNT/A, CED of BoNT/B attenuated the stage of behavioral seizures, as well as their duration. In addition, this effect was dose- and time-dependent and temporally correlated with the changes produced by BoNT/B on after-discharge threshold and after-discharge duration. Table 1 summarizes the results of the statistical analyses for each dose of the two toxins tested. It should be noted that all changes produced by the toxins dissipated by 50 days after the CED infusion. Specifically, when kindling measures were determined 64 days after CED infusion, they did not differ significantly (P > 0.05) from the kindling parameters before the infusion.
TABLE 1.
Summary of the outcomes of two-way repeated measures analysis of variance and post hoc statistical analyses (Tukey’s test) for BoNT/A and BoNT/B effects on kindling parameters in the experiments of Figs. 2 and 3
Numerical values represent P values.
| BoNT/A |
BoNT/B |
|||||||
|---|---|---|---|---|---|---|---|---|
| Treatment Effect (F3,175) | Post Hoc Analysis at Dose |
Treatment Effect (F3,175) | Post Hoc Analysis at Dose |
|||||
| 1.0 ng/inj | 3.2 ng/inj | 10 ng/inj | 1.0 ng/inj | 3.2 ng/inj | 10 ng/inj | |||
| After-discharge threshold | 0.005 | NS | <0.05 | <0.05 | 0.009 | NS | <0.05 | <0.05 |
| After-discharge duration | 0.008 | NS | NS | <0.05 | <0.001 | NS | <0.05 | <0.05 |
| Seizure stage | NS (0.469) | <0.001 | NS | <0.05 | <0.05 | |||
| Duration of behavioral seizure | NS (0.725) | <0.001 | NS | <0.05 | <0.05 | |||
inj, injection; NS, statistically insignificant (P > 0.05).
Figure 4 presents an AUC analysis derived from the time course data in Figs. 2 and 3 that shows more clearly the dose-response relationships. Statistical analysis of the AUC values using one-way ANOVA confirmed the statistical analysis of the time course data presented in Table 1.
Fig. 4.
AUC analysis demonstrating dose-response relationships for the effects of BoNT/A and BoNT/B on EEG and behavioral seizure measures from the experiments of Figs. 1 and 2. The graphs represent: after-discharge threshold (upper left), after-discharge duration (upper right), behavioral seizure stage (lower left), and duration of behavioral seizures (lower right). AUC was calculated for each rat separately for the period of 3–64 days after CED infusion and is expressed as the percent change relative to the animal’s individual baseline value before CED. Each data point represents the mean ± S.E.M. of the AUC values for all animals receiving a specific toxin and dose. Solid circles (●) and squares (▪) indicate BoNT/A and BoNT/B, respectively; open squares (□) represents vehicle-treated control group. *Significantly different from vehicle value (Dunnett’s test) following the detection of a statistically significant main effect by a one-way ANOVA.
Behavior and Health Assessment.
No apparent differences were noted in the behavior of animals that received BoNT treatment in comparison with controls. General animal health during the course of the study was further assessed by measuring body weight and rectal temperature before each stimulation. As shown in Fig. 5, over the course of the study, animals treated with the neurotoxins gained weight normally and exhibited an age-dependent fall in body temperature that did not deviate statistically from controls. Regardless of the treatment received, all rats gained weight at a comparable rate (BoNT/A, F3,175 = 0.820, P = 0.495; BoNT/B, F3,175 = 1.352, P = 0.280). Likewise, age-related decreases in body temperature were comparable across treatment groups (BoNT/A, F3,175 = 1.171, P = 0.341; BoNT/B, F3,175 = 0.691, P = 0.566).
Fig. 5.
Mean weight (in g) and body temperature (°C) values 2–4 days before the CED infusion (Pre) and 3–64 days after the CED infusion of BoNT/A (left) and BoNT/B (right). See legend to Fig. 1 for details.
Histopathology.
Localization of the infusion cannula–stimulating electrode assembly was verified in the amygdala in all animals selected for histological analysis. In confirmation of a previous study (Gasior et al., 2007), minimal damage to the amygdala and surrounding brain structures along the path of the assembly occurred. No inflammation or neuronal damage was detected in the surrounding tissue.
Discussion
This study demonstrates for the first time that local infusion of BoNTs can have persistent actions in the CNS, lasting more than 1 month. With the highest dose of BoNT/B tested, the effect on behavioral seizures persisted for 50 days. At all doses, recovery began at approximately 1 month and progressed during subsequent weeks. BoNT/B treatment was associated with an increase in the threshold and duration of after-discharges, representing electrographic seizure activity and also with an inhibition of behavioral seizures. In a separate group of animals, BoNT/A caused similar statistically significant effects on electrographic seizures. There was also a reduction in behavioral seizure measures with this toxin, but the effect did not reach statistical significance. The dissociation between the electrographic and behavioral effects in the BoNT/A group could be due to a slightly reduced potency of the toxin preparation used in the study and does not necessarily imply that BoNT/B is intrinsically more active; however, there is evidence that BoNT/B spreads more effectively within tissues than BoNT/A (Flynn and Clark, 2003). Expression of the after-discharge is related to local synchronous neuronal activity, whereas the behavioral seizure requires the spread of electrical activity from the discharge zone to distant sites. If BoNT/B is able to reach a larger brain volume, this could explain its ability to inhibit not only the local electrographic discharge but also the resultant behavioral seizures that occur on spread of the discharge.
The mechanisms accounting for the remarkably persistent neuroparalytic actions of BoNTs, which are at the basis of their diverse therapeutic utilities, have been of considerable interest. Recent studies indicate that a key factor in the case of the BoNT/A is resistance to proteasomal degradation of the light-chain protease responsible for SNAP-25 cleavage (Tsai et al., 2010; Wang et al., 2011). A comparable understanding of the persistence of BoNT/B is not yet available. Some investigators have concluded that the neuromuscular paralysis resulting from BoNT/B injection is not as complete or long-lasting as that resulting from BoNT/A (Sloop et al., 1997). Moreover, in a study with cerebellar granule cells in culture, Foran et al. (2003) found that BoNT/A produces a more potent and prolonged blockade of evoked glutamate release than does BoNT/B. In the CNS in vivo, it appears that BoNT/B produces at least as robust and long-lasting inhibition of excitability as BoNT/A.
Only limited information is available on the actions of BoNTs in the CNS. There is emerging evidence that BoNT/A injected into skeletal muscle may to some extent be transported in a retrograde fashion into the CNS and directly affect the functional activity of brain or spinal cord circuits (Wiegand et al., 1976; Caleo et al., 2009). Moreover, exposure of noncholinergic neurons in dissociated cultures (Keller et al., 1999; Foran et al., 2003) or organotypic slice cultures (Capogna et al., 1997) to BoNT/A or BoNT/B leads to inhibition of glutamate release. For botulinum neurotoxins A and E that act on SNAP-25, there is evidence that glutamate release is blocked more effectively than GABA release, although prolonged exposure to high toxin concentrations does inhibit GABA release (Verderio et al., 2004, 2007). The observation that BoNTs inhibit the release of glutamate suggests that the neurotoxins could have antiseizure actions, and there are now at least two reports that provide experimental support for the concept. Thus, Costantin et al. (2005) demonstrated that intrahippocampal bolus injection of BoNT/E in rats delays the onset and reduces the frequency of the electrographic seizure discharges induced by injection of kainic acid at a nearby site 2 days later. These investigators also reported inhibition of behavioral seizure activity with systemic injection of kainic acid 1 day after intrahippocampal BoNT/E injection (Costantin et al., 2005). It has also been reported that intrahippocampal BoNT/E inhibits kindled seizures evoked 2 to 5 days later (Costantin et al., 2005; Antonucci et al., 2009). To our knowledge, the duration of action of BoNTs in the CNS has not been previously characterized. Our results indicate that BoNT/A and BoNT/B can produce prolonged inhibition of seizure activity that is comparable in duration to their neuroparalytic actions in peripheral tissues. The ability of BoNTs to cause long-lasting inhibition CNS excitability suggests that they may be useful in the treatment of brain disorders where suppression of activity in a circumscribed brain region is desired. Such a “chemical neurosurgical” approach may be preferred to traditional neurosurgery in that the chemical approach is fully reversible, and catheter delivery may allow targeting of structures that are inaccessible with conventional neurosurgical approaches.
The clinical application of local BoNT infusion in the treatment of brain disorders, including epilepsy, requires that BoNT can be safely administered. CED has an advantage over other brain delivery methods in that the distribution of the therapeutic agent within a brain volume can be restricted (Rogawski, 2009). At the same time, CED permits uniform delivery within the target zone; however, leakage can occur. Intracerebroventricular delivery of BoNT/A and BoNT/B in mice at doses comparably less by brain weight than those administered in the present study are associated with failure to thrive and lethality (Luvisetto et al., 2003). Minor behavioral impairment is first evident 2 hours after injection, followed by progressive systemic and neurologic impairment and death within 1–3 days. In rats, we found that intracerebroventricular BoNT/B at doses comparable to the highest doses tested in the present study is associated with weight loss on the third day after injection (D. Zolkowska, D. Ancona, and M. A. Rogawski, unpublished data). Therefore, leakage of BoNT into the cerebrospinal fluid could be catastrophic in a clinical application. In the present study, when BoNT/A and BoNT/B were infused locally into the amygdala by CED, no effects on behavior were observed and the animals gained weight normally. We previously provided evidence that another class of nondiffusable toxins delivered by CED can remain restricted to the infusion zone; however, at supratherapeutic doses, neurobehavioral effects suggesting leakage into the cerebrospinal fluid did occur (Gasior et al., 2007). In the present study, we confirmed that potentially toxic peptides can be safely delivered into the brain by CED; however, approaches to mitigate the risks of toxin leakage must be developed before clinical application can be contemplated.
Acknowledgments
The authors thank Cha-Min Tang and Mitchell F. Brin for discussions.
Abbreviations
- ANOVA
analysis of variance
- AUC
area under the curve
- BoNT/A
botulinum neurotoxin type A
- BoNT/B
botulinum neurotoxin type B
- CED
convection-enhanced delivery
- CNS
central nervous system
- EEG
electroencephalogram
- SNAP-25
synaptosomal-associated protein 25
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
Authorship Contributions
Participated in research design: Gasior, Rogawski.
Conducted experiments: Gasior, Tang.
Performed data analysis: Gasior, Rogawski.
Wrote or contributed to the writing of the manuscript: Gasior, Rogawski.
Footnotes
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants NS002877, NS072094, NS079202]; and the Intramural Research Program of the National Institutes of Health [National Institute of Neurological Disorders and Stroke].
References
- Anschel DJ, Ortega EL, Kraus AC, Fisher RS. (2004) Focally injected adenosine prevents seizures in the rat. Exp Neurol 190:544–547 [DOI] [PubMed] [Google Scholar]
- Antonucci F, Bozzi Y, Caleo M. (2009) Intrahippocampal infusion of botulinum neurotoxin E (BoNT/E) reduces spontaneous recurrent seizures in a mouse model of mesial temporal lobe epilepsy. Epilepsia 50:963–966 [DOI] [PubMed] [Google Scholar]
- Antonucci F, Rossi C, Gianfranceschi L, Rossetto O, Caleo M. (2008) Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci 28:3689–3696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton AC, Dolly JO. (1988) Characterization of the inhibitory action of botulinum neurotoxin type A on the release of several transmitters from rat cerebrocortical synaptosomes. J Neurochem 50:1808–1816 [DOI] [PubMed] [Google Scholar]
- Bigalke H, Heller I, Bizzini B, Habermann E. (1981) Tetanus toxin and botulinum A toxin inhibit release and uptake of various transmitters, as studied with particulate preparations from rat brain and spinal cord. Naunyn Schmiedebergs Arch Pharmacol 316:244–251 [DOI] [PubMed] [Google Scholar]
- Blitzer A. (2005) Botulinum toxin A and B: a comparative dosing study for spasmodic dysphonia. Otolaryngol Head Neck Surg 133:836–838 [DOI] [PubMed] [Google Scholar]
- Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. (1994) Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA 91:2076–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzi Y, Costantin L, Antonucci F, Caleo M. (2006) Action of botulinum neurotoxins in the central nervous system: antiepileptic effects. Neurotox Res 9:197–203 [DOI] [PubMed] [Google Scholar]
- Caleo M, Antonucci F, Restani L, Mazzocchio R. (2009) A reappraisal of the central effects of botulinum neurotoxin type A: by what mechanism? J Neurochem 109:15–24 [DOI] [PubMed] [Google Scholar]
- Capogna M, McKinney RA, O’Connor V, Gähwiler BH, Thompson SM. (1997) Ca2+ or Sr2+ partially rescues synaptic transmission in hippocampal cultures treated with botulinum toxin A and C, but not tetanus toxin. J Neurosci 17:7190–7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MY, Lonser RR, Morrison PF, Governale LS, Oldfield EH. (1999) Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J Neurosurg 90:315–320 [DOI] [PubMed] [Google Scholar]
- Costantin L, Bozzi Y, Richichi C, Viegi A, Antonucci F, Funicello M, Gobbi M, Mennini T, Rossetto O, Montecucco C,, et al. (2005) Antiepileptic effects of botulinum neurotoxin E. J Neurosci 25:1943–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder HG, Stein A, Fisher RS. (1997) Interictal and ictal activity in the rat cobalt/pilocarpine model of epilepsy decreased by local perfusion of diazepam. Epilepsy Res 29:17–24 [DOI] [PubMed] [Google Scholar]
- Eleopra R, Tugnoli V, Rossetto O, De Grandis D, Montecucco C. (1998) Different time courses of recovery after poisoning with botulinum neurotoxin serotypes A and E in humans. Neurosci Lett 256:135–138 [DOI] [PubMed] [Google Scholar]
- Fisher RS, Chen DK. (2006) New routes for delivery of anti-epileptic medications. Acta Neurol Taiwan 15:225–231 [PubMed] [Google Scholar]
- Flynn TC, Clark RE., II (2003) Botulinum toxin type B (MYOBLOC) versus botulinum toxin type A (BOTOX) frontalis study: rate of onset and radius of diffusion. Dermatol Surg 29:519–522, discussion 522 [DOI] [PubMed] [Google Scholar]
- Foran PG, Mohammed N, Lisk GO, Nagwaney S, Lawrence GW, Johnson E, Smith L, Aoki KR, Dolly JO. (2003) Evaluation of the therapeutic usefulness of botulinum neurotoxin B, C1, E, and F compared with the long lasting type A: basis for distinct durations of inhibition of exocytosis in central neurons. J Biol Chem 278:1363–1371 [DOI] [PubMed] [Google Scholar]
- Freeman FG, Jarvis MF. (1981) The effect of interstimulation interval on the assessment and stability of kindled seizure thresholds. Brain Res Bull 7:629–633 [DOI] [PubMed] [Google Scholar]
- Gasior M, White NA, Rogawski MA. (2007) Prolonged attenuation of amygdala-kindled seizure measures in rats by convection-enhanced delivery of the N-type calcium channel antagonists ω-conotoxin GVIA and ω-conotoxin MVIIA. J Pharmacol Exp Ther 323:458–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidubaldi A, Fasano A, Ialongo T, Piano C, Pompili M, Mascianà R, Siciliani L, Sabatelli M, Bentivoglio AR. (2011) Botulinum toxin A versus B in sialorrhea: a prospective, randomized, double-blind, crossover pilot study in patients with amyotrophic lateral sclerosis or Parkinson’s disease. Mov Disord 26:313–319 [DOI] [PubMed] [Google Scholar]
- Habermann E, Müller H, Hudel M. (1988) Tetanus toxin and botulinum A and C neurotoxins inhibit noradrenaline release from cultured mouse brain. J Neurochem 51:522–527 [DOI] [PubMed] [Google Scholar]
- Keller JE, Neale EA, Oyler G, Adler M. (1999) Persistence of botulinum neurotoxin action in cultured spinal cord cells. FEBS Lett 456:137–142 [DOI] [PubMed] [Google Scholar]
- Ludvig N, Medveczky G, French JA, Carlson C, Devinsky O, Kuzniecky RI. (2010) Evolution and prospects for intracranial pharmacotherapy for refractory epilepsies: the subdural hybrid neuroprosthesis. Epilepsy Res Treat 2010:725696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luvisetto S, Marinelli S, Rossetto O, Montecucco C, Pavone F. (2004) Central injection of botulinum neurotoxins: behavioural effects in mice. Behav Pharmacol 15:233–240 [PubMed] [Google Scholar]
- Luvisetto S, Rossetto O, Montecucco C, Pavone F. (2003) Toxicity of botulinum neurotoxins in central nervous system of mice. Toxicon 41:475–481 [DOI] [PubMed] [Google Scholar]
- Nilsen KE, Cock HR. (2004) Focal treatment for refractory epilepsy: hope for the future? Brain Res Brain Res Rev 44:141–153 [DOI] [PubMed] [Google Scholar]
- Pappert EJ, Germanson T, Myobloc/Neurobloc European Cervical Dystonia Study Group (2008) Botulinum toxin type B vs. type A in toxin-naïve patients with cervical dystonia: Randomized, double-blind, noninferiority trial. Mov Disord 23:510–517 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (1998) The Rat Brain in Stereotaxic Coordinates, 4th ed Academic Press, Sydney [Google Scholar]
- Pinel JP, Skelton R, Mucha RF. (1976) Kindling-related changes in afterdischarge “thresholds”. Epilepsia 17:197–206 [DOI] [PubMed] [Google Scholar]
- Racine RJ. (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294 [DOI] [PubMed] [Google Scholar]
- Rogawski MA. (2009) Convection-enhanced delivery in the treatment of epilepsy. Neurotherapeutics 6:344–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetto O, Seveso M, Caccin P, Schiavo G, Montecucco C. (2001) Tetanus and botulinum neurotoxins: turning bad guys into good by research. Toxicon 39:27–41 [DOI] [PubMed] [Google Scholar]
- Simpson LL. (2004) Identification of the major steps in botulinum toxin action. Annu Rev Pharmacol Toxicol 44:167–193 [DOI] [PubMed] [Google Scholar]
- Sloop RR, Cole BA, Escutin RO. (1997) Human response to botulinum toxin injection: type B compared with type A. Neurology 49:189–194 [DOI] [PubMed] [Google Scholar]
- Stein AG, Eder HG, Blum DE, Drachev A, Fisher RS. (2000) An automated drug delivery system for focal epilepsy. Epilepsy Res 39:103–114 [DOI] [PubMed] [Google Scholar]
- Tsai YC, Maditz R, Kuo CL, Fishman PS, Shoemaker CB, Oyler GA, Weissman AM. (2010) Targeting botulinum neurotoxin persistence by the ubiquitin-proteasome system. Proc Natl Acad Sci USA 107:16554–16559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderio C, Grumelli C, Raiteri L, Coco S, Paluzzi S, Caccin P, Rossetto O, Bonanno G, Montecucco C, Matteoli M. (2007) Traffic of botulinum toxins A and E in excitatory and inhibitory neurons. Traffic 8:142–153 [DOI] [PubMed] [Google Scholar]
- Verderio C, Pozzi D, Pravettoni E, Inverardi F, Schenk U, Coco S, Proux-Gillardeaux V, Galli T, Rossetto O, Frassoni C, et al. (2004) SNAP-25 modulation of calcium dynamics underlies differences in GABAergic and glutamatergic responsiveness to depolarization. Neuron 41:599–610 [DOI] [PubMed] [Google Scholar]
- Wang J, Zurawski TH, Meng J, Lawrence G, Olango WM, Finn DP, Wheeler L, Dolly JO. (2011) A dileucine in the protease of botulinum toxin A underlies its long-lived neuroparalysis: transfer of longevity to a novel potential therapeutic. J Biol Chem 286:6375–6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand H, Erdmann G, Wellhöner HH. (1976) 125I-labelled botulinum A neurotoxin: pharmacokinetics in cats after intramuscular injection. Naunyn Schmiedebergs Arch Pharmacol 292:161–165 [DOI] [PubMed] [Google Scholar]