Abstract
Impairment of drug disposition in the liver during inflammation has been attributed to downregulation of gene expression of drug-metabolizing enzymes (DMEs) and drug transporters. Inflammatory responses in the liver are primarily mediated by Toll-like receptors (TLRs). We have recently shown that activation of TLR2 or TLR4 by lipoteichoic acid (LTA) and lipopolysaccharide (LPS), respectively, leads to the downregulation of gene expression of DMEs/transporters. However, the molecular mechanism underlying this downregulation is not fully understood. The xenobiotic nuclear receptors, pregnane X receptor (PXR) and constitutive androstane receptor (CAR), regulate the expression of DMEs/transporter genes. Downregulation of DMEs/transporters by LTA or LPS was associated with reduced expression of PXR and CAR genes. To determine the role of CAR, we injected CAR+/+ and CAR−/− mice with LTA or LPS, which significantly downregulated (∼40%–60%) RNA levels of the DMEs, cytochrome P450 (Cyp)3a11, Cyp2a4, Cyp2b10, uridine diphosphate glucuronosyltransferase 1a1, amine N-sulfotransferase, and the transporter, multidrug resistance-associated protein 2, in CAR+/+ mice. Suppression of most of these genes was attenuated in LTA-treated CAR−/− mice. In contrast, LPS-mediated downregulation of these genes was not attenuated in CAR−/− mice. Induction of these genes by mouse CAR activator 1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene was sustained in LTA- but not in LPS-treated mice. Similar observations were obtained in humanized CAR mice. We have replicated these results in primary hepatocytes as well. Thus, LPS can downregulate DME/transporter genes in the absence of CAR, whereas the effect of LTA on these genes is attenuated in the absence of CAR, indicating the potential involvement of CAR in LTA-mediated downregulation of DME/transporter genes.
Introduction
Impairment of drug disposition during inflammation is attributed to downregulation of gene expression of phase I and phase II drug-metabolizing enzymes (DMEs) and transporters (Sewer et al., 1997; Renton and Nicholson, 2000). The modulation of DMEs and transporters at the transcriptional level is regulated by basal transcription factors as well as the xenobiotic nuclear receptors (NRs), pregnane X receptor (PXR) and constitutive androstane receptor (CAR). The NRs heterodimerize with retinoid X receptor α to bind to the promoter regions of the target genes (Mangelsdorf and Evans, 1995; Tien and Negishi, 2006; Kakizaki et al., 2008; Shen and Kong, 2009; Zordoky and El-Kadi, 2009). The molecular mechanism by which DMEs and transporters are downregulated during inflammation is not fully understood.
Toll-like receptors (TLRs) are the major mediators of inflammatory responses in the liver and recognize microbial components as well as endogenous ligands from damaged or stressed cells (Takeda and Akira, 2001; Ishii and Akira, 2004; Schwabe et al., 2006). TLRs are present on nonparenchymal cells, including Kupffer cells, as well as on hepatocytes in the liver (Liu et al., 2002; Matsumura et al., 2003). We have shown that activation of TLR4 by lipopolysaccharide (LPS) (Gram-negative bacterial component) downregulates the gene expression of select DMEs and transporters (Ghose et al., 2008). LPS-treated rodents are well-established models of inflammation, and LPS treatment induces proinflammatory cytokines, interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)α. These cytokines act on hepatocytes to reduce the expression of DME/transporter genes (Renton, 2004; Aitken et al., 2006). We have also shown that activation of TLR2 by lipoteichoic acid (LTA) (Gram-positive bacterial component) downregulates the expression of select DME and transporter genes (Ghose et al., 2009, 2011).
Several studies have shown that downregulation of PXR and CAR genes was associated with decreased DME and transporter gene expression (Beigneux et al., 2000, 2002; Ghose et al., 2004, 2008, 2009). We have also shown that there is preferential suppression of CAR and its target hepatic genes on administration of LTA (Ghose et al., 2009). Thus, it is likely that nuclear receptors play an important role in inflammation-mediated downregulation of gene expression of DMEs and transporters.
Studies in PXR−/− mice showed that PXR is not responsible for the regulation of cytochrome P450 (Cyp) genes during LPS-mediated inflammation (Hartmann et al., 2001; Richardson and Morgan, 2005). In contrast, PXR was shown to be involved in downregulation of Cyp3a11, multidrug resistance-associated protein (Mrp)2, and bile salt exporter pump in IL-6–treated mice (Teng and Piquette-Miller, 2005).
The goal of this work is to determine the role of CAR in downregulation of DMEs and transporters by comparing the effects of LTA or LPS in CAR+/+, CAR−/−, and CAR-activated mice (treated with mouse CAR activator, 1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene) [TCPOBOP]. We find that LTA administration led to significant downregulation of gene expression of key DMEs, Cyp3a11, Cyp2a4, Cyp2b10, uridine diphosphate glucuronosyltransferase (Ugt)1a1, amine N-sulfotransferase (Sultn), and the transporter, Mrp2, in CAR+/+ mice. Downregulation of Cyp3a11, Cyp2a4, Cyp2b10, and Ugt1a1 was completely attenuated, whereas the downregulation of Sultn and Mrp2 was attenuated at certain time points in LTA-treated CAR−/− mice. In contrast, LPS administration led to significant downregulation of Cyp3a11, Cyp2a4, Ugt1a1, and Mrp2 in CAR+/+ as well as CAR−/− mice. Activation of mouse CAR with TCPOBOP increased nuclear CAR protein levels and induced the expression of all the above-mentioned genes, as expected. Surprisingly, the induction of all these genes and increased CAR protein levels in the nucleus were still detected in the presence of LTA. In contrast, the induction of these genes by TCPOBOP was attenuated in the presence of LPS, along with attenuation of CAR protein levels in the nucleus. We have replicated the above results in primary hepatocytes as well. These results were also confirmed in humanized CAR (hCAR) mice treated with the universal CAR activator, phenobarbital (PB).
The outcome of these studies demonstrates that CAR is required for downregulation of hepatic DME/transporter genes by LTA, but LPS can downregulate these genes in the absence of CAR. Surprisingly, induction of CAR-mediated genes in TCPOBOP-treated mice was maintained in LTA, but not in LPS-injected mice. This was most likely due to the inability of LTA, in the presence of TCPOBOP, to downregulate gene, and consequently protein, expression of CAR. These results indicate that differential CAR expression may contribute to the regulation of hepatic DMEs and transporters upon infections by Gram-positive and Gram-negative bacterial components.
Materials and Methods
Highly purified lipoteichoic acid (Staphylococcus aureus) and lipopolysaccharide (Escherichia coli) were purchased from InvivoGen (San Diego, CA) and freshly diluted to the required concentration in 0.9% saline. The sequences of the primers and probes were obtained from the literature, as reported previously (Ghose et al., 2004, 2009, 2011). All oligonucleotides were purchased from Sigma Genosys (Houston, TX), and all real-time polymerase chain reaction (PCR) reagents were purchased from Applied Biosystems (Foster City, CA). Rabbit anti-CAR antibody (sc-8538), lactate dehydrogenase (LDH) (sc-33781), and lamin A/C (sc-20681) (Santa Cruz Biotechnology, Santa Cruz, CA) were used as per the manufacturer’s instructions. Cell culture media and media supplements were purchased from Gibco BRL (Gaithersburg, MD). Unless specified, all other materials were purchased from Sigma-Aldrich (St. Louis, MO).
Animals and Treatments.
Adult, male (∼8 weeks old, 20–25 g), CAR−/− mice (Wei et al., 2000) and the corresponding wild-type mice on a C57BL/6 background were maintained in a 12-hour dark/light cycle and in a temperature- and humidity-controlled environment. The mice had access to regular rodent chow and water ad libitum. The mice were i.p. injected with LTA (6 mg/kg b.wt.) or LPS (2 mg/kg b.wt.) and the vehicle, saline. Livers were harvested at various time points for RNA and protein analysis. To activate CAR, mice were i.p. injected with 3 mg/kg b.wt. TCPOBOP i.p. in corn oil for 3 days (Baskin-Bey et al., 2006) or PB (80 mg/kg/d, i.p. in saline) for 3 days prior to LTA or LPS treatment. The hCAR mice used were generated using the knock-in strategy, as described previously (Zhang et al., 2002). All the animal care and use protocols were approved by the Institutional Animal Care and Use Committee guidelines. All experiments were performed in triplicate and repeated three to four times.
Real-Time PCR.
Total RNA was isolated from mouse liver using TRIzol reagent (Sigma-Aldrich), according to the manufacturer’s protocol. cDNA was synthesized using the High Capacity Reverse Transcription Kit from Applied Biosystems. Real-time PCR was performed using an ABI PRISM 7300 Sequence Detection System instrument and software (Applied Biosystems), as described previously (Ghose et al., 2004). In short, each reaction mixture (total of 25 μl) contained 50–100 ng cDNA, 300 nM forward primer, 300 nM reverse primer, 200 nM fluorogenic probe, and 15 μl TaqMan Universal PCR Master Mix. We extrapolated the quantitative expression values from standard curves, and these values were normalized to cyclophilin.
Primary Hepatocyte Isolation and Treatment.
Primary mouse hepatocytes were isolated from CAR+/+ and CAR−/− mice using the two-step collagenase perfusion technique, as described previously (Li et al., 2002; Ghose et al., 2011). In short, after digestion, the liver was excised and then the hepatocytes were purified using a Percoll gradient (33%), washed, and screened for viability using trypan blue exclusion technique. Only isolations with viability of more than 90% were used for these studies. Cells were plated at a density of 500,000 cells per well in six-well Primaria plates (BD Biosciences, San Diego, CA) and allowed to attach for 4 hours. Cells were maintained for 48 hours with daily change of medium. The cells were incubated with serum-free Williams medium E 2 hours prior to treatment with 50 ng/ml LTA (8 hours) or 1 μg/ml LPS (16 hours). To activate CAR, the cells were treated with 250 nM TCPOBOP 24 hours or DMSO (0.025% v/v) prior to treatment with LTA or LPS. RNA was then isolated for real-time PCR analysis, as described above.
Immunoblotting.
Nuclear extracts were prepared, as described previously (Ghose et al., 2004), and the protein concentration was determined using the bicinchoninic acid assay according to the manufacturer’s protocol (Pierce, Rockford, IL). Equal amounts of protein (10 μg) were analyzed by SDS-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. The membranes were then probed with rabbit anti-CAR (1:500), anti-lamin A/C (1:500), or anti-LDH (1:500) antibody, followed by probing with a goat anti-rabbit IgG-alkaline phosphatase secondary antibody (1:2000). The membranes were then washed and incubated with Tropix CDP star nitroblock II ECL reagent as per the manufacturers’ instructions (Applied Biosystems). The membranes were analyzed using FlourChem FC imaging system (Alpha Innotech). The images were quantified by densitometer using AlphaEase software.
Statistical Analysis.
Treatment groups were compared using two-way analysis of variance, followed by a post hoc test (Tukey’s post hoc test) with P < 0.05. The results were presented as mean ± S.D.
Results
Effect of LTA Treatment on DME/Transporter Gene Expression in CAR+/+ and CAR−/− Mice.
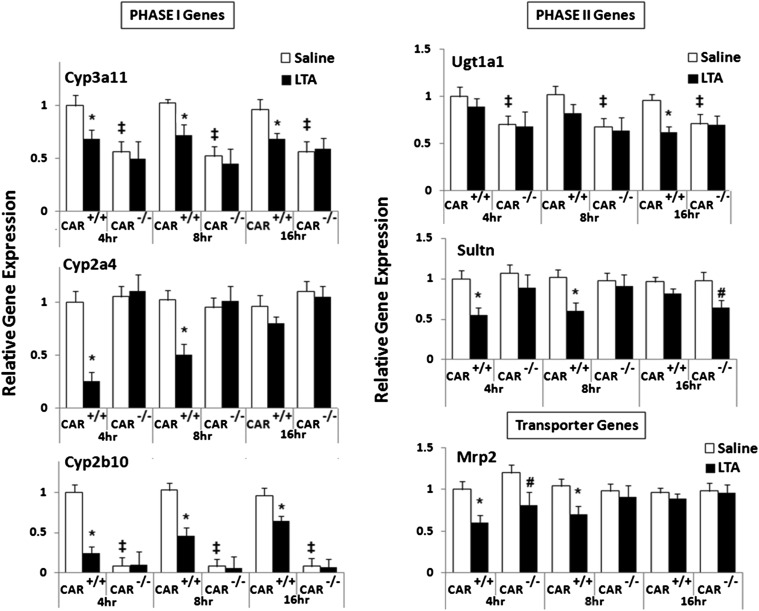
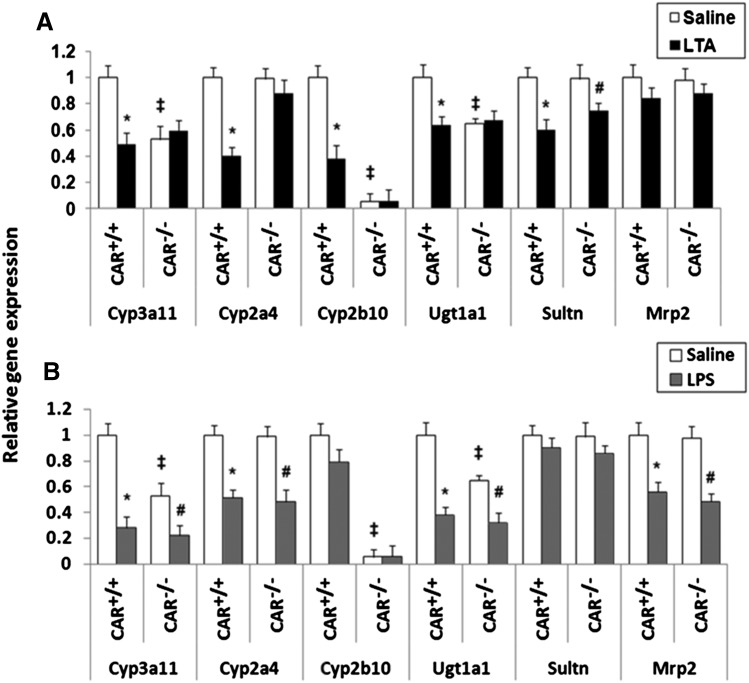
We have shown previously that LTA causes preferential suppression of CAR and its target genes (Ghose et al., 2009). To investigate the role of CAR in LTA-mediated downregulation of DMEs and transporters, CAR+/+ and CAR−/− mice were injected with LTA. RNA was isolated from the livers harvested at the indicated time points and analyzed by real-time PCR. RNA levels of the key phase I enzyme, Cyp3a11, were downregulated at 4–16 hours (∼30%–40%) in the CAR+/+ mice after LTA administration, whereas this downregulation was absent in CAR−/− mice. The basal expression of Cyp3a11 is significantly lower (∼50%–60%) in the CAR−/− mice. This observation is in agreement with other reports published previously (Assem et al., 2004). Cyp2a4 RNA levels were significantly downregulated in CAR+/+ mice at 4 and 8 hours (∼60%–80%), whereas this downregulation was attenuated in the CAR−/− mice. As seen previously (Ghose et al., 2009), the RNA levels of Cyp2b10 in CAR+/+ mice were downregulated at 4 hours (∼75%–80%) and remained reduced (∼40%) until 16 hours after LTA administration (Fig. 1). The basal expression of Cyp2b10 in the CAR−/− mice is too low to make any conclusive inference.
Fig. 1.
Regulation of DME and transporter mRNA levels in CAR+/+ and CAR−/− mice following LTA administration. CAR+/+ and CAR−/− were i.p. injected with saline or LTA (6 mg/kg), and livers were harvested at 4, 8, and 16 hours (n = 5–6 per group). RNA was isolated from the livers, and mRNA levels of phase I enzymes, phase II enzymes, and transporters were determined by real-time PCR analysis, as described earlier. All data are presented as ±S.D. and standardized for cyclophilin mRNA levels. Expression in saline-treated mice was set to 1; fold change after LTA treatment was compared with the saline-treated controls. * and #, Indicate significant difference (P < 0.05) between saline and LTA groups in CAR+/+ mice and CAR−/− mice, respectively, and ‡ indicates basal level differences between CAR+/+ and CAR−/− mice. The experiments were repeated at least thrice.
In the case of phase II enzymes, Ugt1a1 RNA levels were significantly (∼40%) reduced at 16 hours in CAR+/+ mice treated with LTA, whereas this reduction was attenuated in the CAR−/− mice. Ugt1a1 RNA levels remained unchanged at 4 and 8 hours in LTA-treated CAR+/+ and CAR−/− mice. Sultn RNA levels were downregulated in CAR+/+ mice at 4 and 8 hours (∼40%–55%), whereas no reduction was seen at 16 hours. The downregulation of Sultn in CAR−/− mice was not detected at 4 and 8 hours of LTA treatment, although there was downregulation of Sultn at 16 hours (∼60%). This suggests delayed action of LTA on Sultn in the absence of CAR, and/or the existence of alternate mechanisms.
RNA levels of the transporter, Mrp2, were downregulated (∼40%) by LTA at 4 hours in both CAR+/+ and CAR−/− mice, whereas at 8 hours, Mrp2 downregulation (∼35%) was detected only in the CAR+/+ mice. Mrp2 gene expression was unaffected at 16 hours in both CAR+/+ and CAR−/− mice treated with LTA. We also examined RNA levels of two other transporter genes, namely Mrp4 (unchanged in CAR+/+ and CAR−/− mice) and Mdr1b (upregulated in both CAR+/+ and CAR−/− mice) (data not shown). This finding indicates that basal expression of CAR is required for LTA to reduce DME and transporter genes.
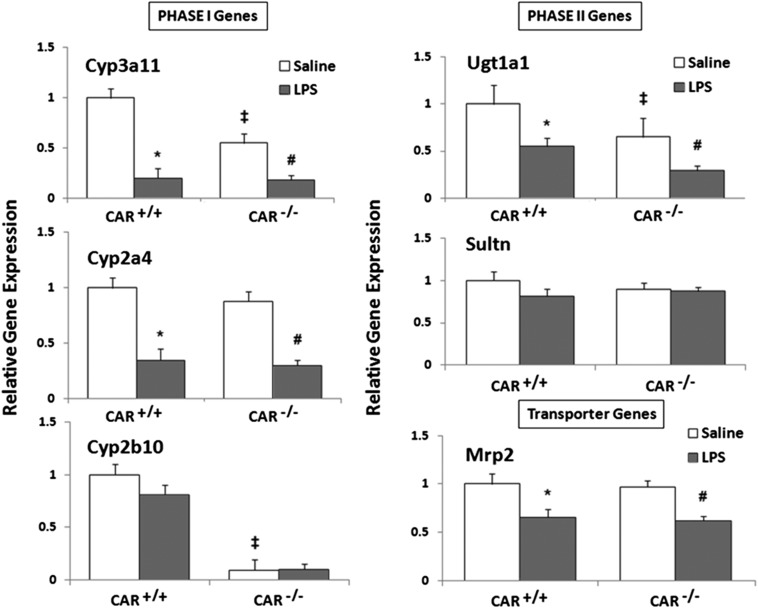
Effect of LPS Treatment on DME/Transporter Gene Expression in CAR+/+ and CAR−/− Mice.
LPS administration significantly downregulated RNA levels of the phase I enzymes (Cyp3a11, Cyp2a4), the phase II enzyme, Ugt1a1, and the transporter, Mrp2, in both CAR+/+ and CAR−/− mice at 16 hours (Fig. 2). Among all the genes studied, Cyp3a11 was the most affected (∼80% reduction), followed by Cyp2a4 (∼65% reduction), Ugt1a1 (∼50% reduction), and Mrp2 (∼40% reduction). Because LPS downregulates DME/transporter genes in the absence of CAR, these results indicate that CAR is not required for LPS-mediated downregulation of DME and transporter genes. Cyp2b10 and Sultn mRNA levels remained unchanged in both CAR+/+ and CAR−/− mice. We selected the 16-hour time point because we saw optimal downregulation of these DMEs and transporters at this time point (Ghose et al., 2004).
Fig. 2.
Regulation of DME and transporter mRNA levels in CAR+/+ and CAR−/− mice following LPS administration. CAR+/+ and CAR−/− mice were i.p. injected with saline or 2 mg/kg LPS, and livers were harvested at 16 hours (n = 5–6 per group). RNA was isolated from the livers, and mRNA levels of phase I enzymes, phase II enzymes, and transporters were determined by real-time PCR analysis, as described earlier. All data are presented as ±S.D. and standardized for cyclophilin mRNA levels. Expression in saline-treated mice was set to 1; fold change after LPS treatment was compared with the saline-treated controls. * and #, Indicate significant difference (P < 0.05) between saline and LPS groups in CAR+/+ mice and CAR−/− mice, respectively, and ‡ indicates basal level differences between CAR+/+ and CAR−/− mice. The experiments were repeated at least thrice.
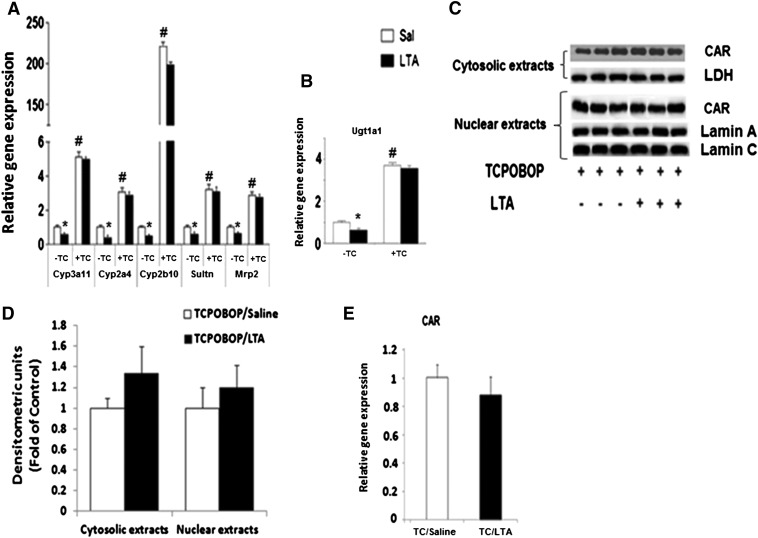
Effect of LTA or LPS Treatment on DME/Transporter Gene Expression in TCPOBOP-Treated Mice.
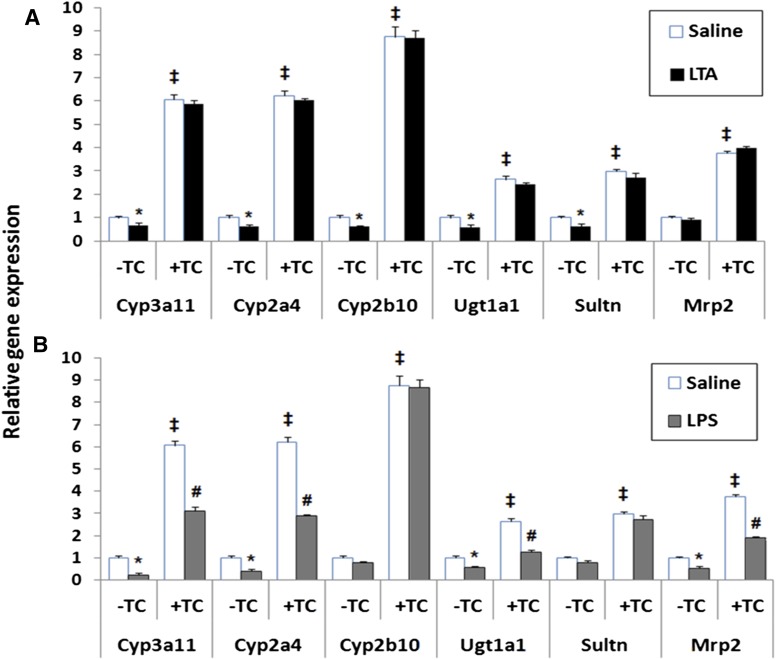
To further confirm the role of CAR in LTA-mediated downregulation of DME/transporter genes, we activated CAR using specific mouse CAR activator TCPOBOP (3 mg/kg/d in corn oil i.p.) 3 days prior to administration of LTA (Fig. 3). TCPOBOP treatment caused a significant induction of Cyp3a11, Cyp2a4, Cyp2b10, Ugt1a1, Sultn, and Mrp2 genes (Fig. 3, A and B). Because we saw maximum downregulation of DMEs and transporters by LTA at 4 hours, we treated these TCPOBOP-treated CAR+/+ mice with LTA for 4 hours. Surprisingly, this induction was still detected in the presence of LTA. LTA treatment at longer time points (8–16 hours) did not affect the induction of these DMEs/transporters by TCPOBOP (data not shown). Because LTA downregulated Ugt1a1 at 16 hours in CAR+/+ mice (Fig. 1), we analyzed mRNA levels of Ugt1a1 after 16-hour treatment with LTA in the TCPOBOP-treated mice (Fig. 3B). As seen with other DMEs, induction of Ugt1a1 RNA levels by TCPOBOP was not affected by LTA treatment. Similar results were observed by treatment of mice with the universal CAR activator, PB (data not shown).
Fig. 3.
Regulation of DME and transporter mRNA levels in TCPOBOP-pretreated mice following LTA administration. CAR+/+ mice were treated with the specific CAR activator, TCPOBOP (3 mg/kg/d), for 3 days prior to i.p. administration of saline or LTA (6 mg/kg). Livers were harvested after (A) 4 hours and (B) 16 hours, and mRNA levels were analyzed by real-time PCR, as described earlier. All data are presented as ±S.D. and standardized for cyclophilin mRNA levels. Expression in saline-treated mice was set to 1; fold change after LTA treatment was compared with the saline-treated controls. *Indicates significant difference (P < 0.05) between saline and LTA groups, and # indicates significant differences between saline samples of −TCPOBOP (−TC) and +TC groups. The experiments were repeated at least thrice. (C) Cytosolic and nuclear extracts were prepared from livers from CAR+/+ mice treated with TCPOBOP (3 mg/kg/d) for 3 days prior to saline and LTA (6 mg/kg) i.p. injections (4 hours), and CAR protein levels were measured by Western blotting. (D) The images were quantified by densitometer using AlphaEase software. The normalized values of fold difference, relative to the expression of LDH for cytosolic extracts and lamin A/C for nuclear extracts, which was set to 1, are presented as mean ± S.D. values. (E) Regulation of CAR mRNA levels by LTA in TCPOBOP-pretreated mice. C57BL/6 mice were pretreated for 3 days with 3 mg/kg TCPOBOP (i.p.) in corn oil prior to treatment with saline or LTA (6 mg/kg), and livers were harvested at 2 hours (n = 5 per group). All data are presented as ±S.D. and standardized for cyclophilin mRNA levels. Expression in TCPOBOP/saline-treated mice was set to 1; fold change after LTA treatment was compared with the saline-treated controls.
To understand the mechanism underlying the lack of effect of LTA on TCPOBOP-mediated induction of DME/transporter genes, we measured expression of CAR at the gene and protein levels in the presence of TCPOBOP. As observed in our previous publications (Ghose et al., 2009), we found that CAR gene expression and cytosolic protein levels were reduced by LTA in the absence of TCPOBOP (data not shown); nuclear levels of CAR were too low to obtain a conclusive inference.
TCPOBOP by itself caused increased accumulation of CAR in the nucleus with no effect on CAR gene expression, as expected. Interestingly, we did not observe any changes in CAR gene expression and nuclear/cytosolic levels by LTA in the presence of TCPOBOP (Fig. 3, C–E). Presence of high amount of CAR in TCPOBOP/LTA-treated mice may account for increased expression of CAR target genes in these mice compared with corn oil/LTA-treated controls.
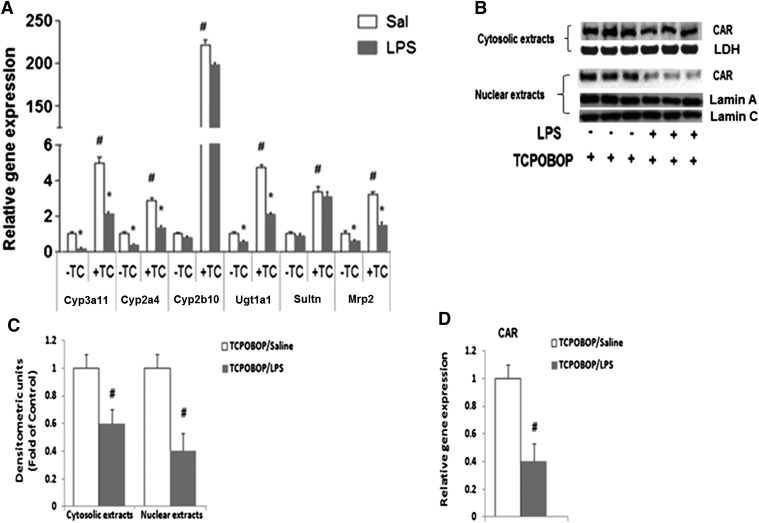
We find that LPS downregulated the DME and transporter genes to the same extent in mice pretreated with TCPOBOP or the vehicle control, corn oil (Fig. 4A). We also found that CAR gene expression as well as nuclear/cytosolic protein levels were downregulated by LPS to the same extent in CAR+/+ and TCPOBOP-treated mice (Fig. 4, B–D). As shown in our previous publications (Ghose et al., 2004), LPS downregulated CAR expression in the absence of TCPOBOP (data not shown).
Fig. 4.
Regulation of DME and transporter mRNA levels in TCPOBOP-pretreated mice following LPS administration. (A) CAR+/+ mice were treated with the specific CAR activator TCPOBOP 3 mg/kg/d for 3 days prior to LPS (2 mg/kg) i.p. administration. Livers were harvested after 16 hours, and mRNA levels were analyzed by real-time PCR, as described earlier. All data are presented as ±S.D. and standardized for cyclophilin mRNA levels. Expression in saline-treated mice was set to 1; fold change after LPS treatment was compared with the saline-treated controls. *Indicates significant difference (P < 0.05) between saline and LPS groups, and # indicates significant difference (P < 0.05) between saline samples of −TCPOBOP (TC) and +TC groups. The experiments were repeated at least thrice. (B) Cytosolic and nuclear extracts were prepared from livers of CAR+/+ mice treated with TCPOBOP 3 mg/kg/d for 3 days prior to saline, and LPS (2 mg/kg) i.p. injections (16 hours) and CAR protein levels were measured by Western blotting. (C) The images were quantified by densitometer using AlphaEase software. The normalized values of fold difference, relative to the expression of LDH for cytosolic extracts and lamin A/C for nuclear extracts, which was set to 1, are presented as mean ± S.D. values. #Indicates significant difference (P < 0.05) between saline and LPS treatment groups. (D) Regulation of CAR mRNA levels by LPS in TCPOBOP-pretreated mice. C57BL/6 mice were pretreated for 3 days with 3 mg/kg TCPOBOP (i.p.) in corn oil prior to treatment with saline or LPS (2 mg/kg), and livers were harvested at 16 hours (n = 5 per group). All data are presented as ±S.D. and standardized for cyclophilin mRNA levels. Expression in TCPOBOP/saline-treated mice was set to 1; fold change after LPS treatment was compared with the saline-treated controls. #Indicates significant difference (P < 0.05) between saline and LPS groups.
Effect of LTA or LPS Treatment on DME/Transporter Gene Expression in PB-Treated hCAR Mice.
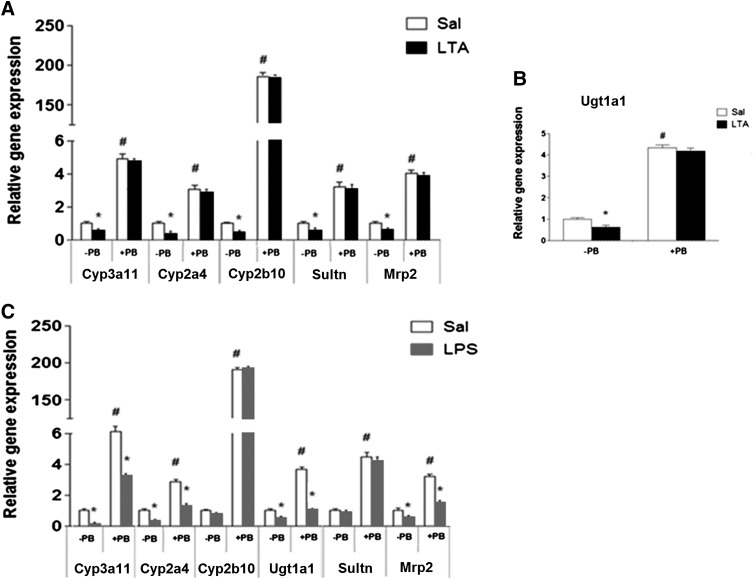
To test the relevance of these findings on human CAR, we injected hCAR mice with PB (80 mg/kg i.p.) for 3 days, prior to administration of LTA or LPS. LTA treatment led to downregulation of gene expression of Cyp3a11 (∼45%), Cyp2a4 (∼65%), Cyp2b10 (∼60%), Ugt1a1 (∼50%), Sultn (∼55%), and Mrp2 (∼50%) (Fig. 5, A and B). PB treatment significantly induced the expression of these genes, whereas LTA treatment did not attenuate this induction (Fig. 5, A and B). In contrast, LPS administration in hCAR mice led to downregulation of gene expression of Cyp3a11 (∼80%), Cyp2a4 (∼60%), Ugt1a1 (∼55%), and Mrp2 (∼45%) (Fig. 5C). Interestingly, LPS downregulated the gene expression of these genes to the same extent in mice pretreated with or without PB (Fig. 5C). These data are in agreement with the previous findings in LTA- or LPS-injected CAR+/+ mice pretreated with TCPOBOP.
Fig. 5.
Regulation of DME and transporter mRNA levels by LTA or LPS in hCAR mice pretreated with PB. hCAR mice were treated with the universal CAR activator PB (80 mg/kg/d) for 3 days prior to i.p. administration of (A) saline or LTA (6 mg/kg) for 4 hours and (B) 16 hours. (C) hCAR mice were treated with PB 80 mg/kg/d for 3 days prior to saline or LPS (2 mg/kg) treatment of 16 hours. Livers were harvested and mRNA levels were analyzed by real-time PCR, as described earlier. All data are presented as ±S.D. and standardized for cyclophilin mRNA levels. Expression in saline-treated mice was set to 1; fold change after LTA or LPS treatment was compared with the saline-treated controls. *Indicates significant difference (P < 0.05) between saline and LTA or LPS groups, and # indicates significant differences between saline samples of −PB and +PB groups. The experiments were repeated at least thrice.
Effect of LTA or LPS Treatment on DME/Transporter Gene Expression in Primary Hepatocytes from CAR+/+ and CAR−/− Mice.
The role of CAR was further examined in primary hepatocytes treated with saline or LTA (50 ng/ml) for 8 hours. RNA levels of Cyp3a11, Cyp2a4, Cyp2b10, and Ugt1a1 were significantly downregulated (∼40%–45%) in LTA-treated CAR+/+ hepatocytes, and this downregulation was attenuated in CAR−/− hepatocytes (Fig. 6A). Sultn gene expression was significantly downregulated (∼40%) in the CAR+/+ as well as in the CAR−/− hepatocytes; however, the extent of downregulation was lower in the CAR−/− hepatocytes (∼20%). We did not see any change in the Mrp2 expression either in the CAR+/+ or CAR−/− hepatocytes. As seen in vivo, there were significant differences in the basal levels of Cyp2b10, Cyp3a11, and Ugt1a1 expression between hepatocytes isolated from CAR+/+ and CAR−/− mice. The difference in the trends of Sultn gene expression and Mrp2 gene expression on treatment with LTA is most likely because of the difference in in vitro and in vivo conditions.
Fig. 6.
(A) Regulation of DME and transporter gene expression by LTA or LPS in primary hepatocytes from CAR+/+ and CAR−/− mice. Primary hepatocytes from CAR+/+ and CAR−/− mice were treated with (A) saline or LTA (50 ng/ml) for 8 hours, or (B) saline or LPS (1 μg/ml) for 16 hours. RNA was isolated, and real-time PCR was performed, as described earlier. n = 5–6 per group. All data are presented as ±S.D. and standardized for cyclophilin mRNA levels. *Indicates significant difference (P < 0.05) between saline and LTA/LPS of CAR+/+ groups; ‡ indicates significant differences (P < 0.05) between saline samples of CAR+/+ and CAR−/− groups; and # indicates significant difference (P < 0.05) between saline and LTA/LPS of CAR−/− group. The experiments were repeated at least thrice.
CAR+/+ and CAR−/− hepatocytes were treated with saline or LPS (1 μg/ml) for 16 hours. Cyp3a11 (∼70%), Cyp2a4 (∼50%), Ugt1a1 (∼60%), and Mrp2 (∼50%) were significantly downregulated in LPS-treated CAR+/+ as well as in CAR−/− hepatocytes (Fig. 6B).
Effect of LTA or LPS Treatment on DME/Transporter Gene Expression in Primary Hepatocytes Pretreated with TCPOBOP.
To activate CAR in vitro, CAR+/+ hepatocytes were treated with 250 nM TCPOBOP 24 hours prior to treatment with saline or LTA (50 ng/ml). TCPOBOP induced the expression of all the DMEs and transporters significantly. As seen in vivo, the downregulation of DMEs and transporters by LTA was attenuated in hepatocytes treated with TCPOBOP (Fig. 7A).
Fig. 7.
(A) Regulation of DME and transporter gene expression by LTA in TCPOBOP-pretreated primary hepatocytes. Primary hepatocytes from CAR+/+ were isolated and treated with specific CAR activator TCPOBOP (250 nM) for 24 h prior to treatment with (A) saline or LTA (50 ng/ml) for 8 hours, or (B) saline or LPS (1 μg/ml) for 16 hours. RNA was isolated, and real-time PCR was performed, as described earlier. n = 5 per group. All data are presented as ±S.D. and standardized for cyclophilin mRNA levels. *Indicates significant difference (P < 0.05) between saline and LTA/LPS of −TCPOBOP (TC) groups; ‡ indicates significant differences (P < 0.05) between saline samples of −TC and +TC groups; and # indicates significant difference (P < 0.05) between saline and LTA/LPS of +TC group. The experiments were repeated at least thrice.
CAR+/+ hepatocytes were pretreated with 250 nM TCPOBOP 24 hours prior to treatment with LPS (1 μg/ml). LPS administration led to downregulation of Cyp3a11 (∼75%), Cyp2a4 (∼50%), Ugt1a1 (∼50%), and Mrp2 (∼50%). As expected, LPS caused no change in the gene expression of Cyp2b10 or Sultn in hepatocytes treated with TCPOBOP (Fig. 7B). Our in vitro findings concur with our in vivo results, suggesting that downregulation of DME/transporter genes by LPS is associated with downregulation of gene expression of CAR; however, the DME/transporter genes are downregulated by LPS even in the absence of CAR, suggesting the presence of alternative mechanisms. We also performed these experiments in PB-treated primary mouse hepatocytes from hCAR mice and found same results (data not shown).
Discussion
Infection and inflammation can alter the expression and activities of several of phase I and phase II DMEs and transporters (Cheng and Morgan, 2001; Hartmann et al., 2001). Downregulation of hepatic DME/transporter genes during inflammation and infection is associated with reduced gene expression of NRs, PXR and CAR. Although, studies have shown that PXR has no role in LPS-mediated regulation of hepatic genes, the role of CAR is not known. In this study, we found that basal levels of CAR are required for LTA to downregulate the expression of DME/transporter genes. In contrast, LPS can downregulate these genes even in the absence of CAR in CAR−/− mice.
Our results indicate that both LTA and LPS downregulate CAR expression; however, they differentially regulate DME and transporter genes. In addition, the timeline of downregulation of CAR gene expression is different for LTA and LPS, which can partially account for the differential effects on CAR target genes. LTA downregulates CAR gene expression (∼65%) at 2–4 hours, whereas LPS downregulates CAR gene expression (∼70%) at 16 hours (Ghose et al., 2008, 2009). LTA administration led to downregulation of Cyp3a11, Cyp2a4, Cyp2b10, Ugt1a1, Sultn, and Mrp2 genes in CAR+/+ mice at time points ranging from 4 to 16 hours, as we have seen previously (Ghose et al., 2009). The reason for discord in the time course of suppression could be the mechanism of downregulation is different for different genes. Furthermore, LTA may affect corepressor expression and recruitment differently, which can lead to differences in the time course of downregulation of DMEs and transporters. LTA-mediated downregulation of most of these DME and transporter genes was attenuated in the CAR−/− mice, suggesting that CAR plays an important role in LTA-mediated downregulation of these genes.
LPS administration led to downregulation of Cyp3a11, Cyp2a4, Ugt1a1, and Mrp2 genes in both CAR+/+ and CAR−/− mice, suggesting that LPS can downregulate DME/transporter genes in the absence of CAR. It is possible that this LPS-mediated downregulation may occur through PXR in the absence of CAR. However, we did not find any difference in the expression of PXR between CAR+/+ and CAR−/− mice treated with saline or LPS (data not shown). This indicates that downregulation of DME/transporter genes by LPS in CAR−/− mice is not due to overexpression of PXR. Experiments in PXR/CAR double-knockout mice will further explain the roles of PXR and CAR in LTA- or LPS-mediated DME and transporter downregulation.
Treatment of CAR+/+ mice with specific mouse CAR activator TCPOBOP results in induction of gene expression of all the DME/transporters mentioned above. Immunoblotting of nuclear extracts from TCPOBOP-pretreated mice revealed that induction of CAR protein levels in the nucleus remained unchanged by LTA treatment (Fig. 3, C and D). Furthermore, CAR gene expression in TCPOBOP/LTA-treated mice was significantly higher than corn oil/LTA-injected mice (Fig. 3E).
Because LTA administration in TCPOBOP-treated mice did not change gene expression of DMEs and transporters, we administered 12 mg/kg dose of LTA in TCPOBOP-pretreated mice (double the original dose). However, gene expression of DMEs and transporters still remained unchanged (data not shown). We did not find any downregulation of DME/transporter genes at different time points (2, 4, 8, 16, 24, and 48 hours) in TCPOBOP/LTA-treated mice (data not shown). To rule out a ligand-dependent effect, we tried another CAR activator PB (80 mg/kg/d i.p. for 3 days) and observed the same results (data not shown).
The mechanism of how LTA downregulates CAR and its target genes is unclear. We find that activation of CAR with TCPOBOP leads to accumulation of CAR in the nucleus, and LTA treatment does not affect the concentration of nuclear CAR protein levels. Cytosolic CAR protein and CAR gene expression were also unaffected by LTA in the presence of TCPOBOP. Because LTA treatment of CAR−/− mice did not cause downregulation of DME/transporter genes, we anticipated that induction of CAR by TCPOBOP would facilitate the downregulation of these genes by LTA. However, in TCPOBOP-treated mice, LTA did not affect DME and transporter gene expression most likely due to its lack of effect on CAR gene and protein expression. It is possible that the induction of DME and transporter genes on administration of TCPOBOP led to enhanced clearance of LTA. Previous studies have shown that LTA is cleared mainly by the liver and kidneys (Hyzy et al., 1992). Thus, the induction of DME/transporter genes may lead to faster clearance of LTA in TCPOBOP-pretreated CAR+/+ mice, leading to diminished effects of LTA on DME/transporter genes.
Activation of TLR2 by its ligands leads to upregulation of its expression. LTA induced TLR2 expression by ∼50-fold in the liver (Ghose et al., 2009) and ∼5-fold in odontoblasts (Durand et al., 2006). Another TLR2 ligand, i.e., porin of Shigella dysenteriae, also induced the expression of TLR2 in hemopoietic cells (Ray et al., 2003). It is possible that induction of TLR2 expression is required for mediating the effects of TLR2 ligands. We find that TLR2 RNA levels were significantly induced by LTA treatment of CAR+/+ and CAR−/− mice (∼40- to 45-fold; Supplemental Fig. 1). Interestingly, TCPOBOP pretreatment attenuated the induction of TLR2 by LTA. We did not find any induction of TLR2 RNA levels in TCPOBOP/saline-treated mice (Supplemental Fig. 1). Lower TLR2 expression can cause reduced binding of LTA, which can contribute toward lower in vivo concentration of LTA, thus leading to lack of its effect on DME/transporter genes.
LPS administration in CAR−/− mice led to downregulation of DME and transporter genes (Fig. 2); hence, we expected that LPS-mediated downregulation of the genes would be independent of CAR. As expected, LPS treatment led to downregulation of DMEs and transporters even in mice pretreated with TCPOBOP (Fig. 4A). Interestingly, TCPOBOP-induced CAR accumulation in the nucleus was significantly attenuated by LPS. Cytosolic CAR protein was also reduced by LPS in TCPOBOP-treated mice. This is most likely due to the reduction in CAR gene expression by LPS in the presence of TCPOBOP (Fig. 4, B–D). The effect of LPS on CAR nuclear protein levels is contrary to the effects of LTA in TCPOBOP-pretreated mice, and this is reflected in the different effects of LPS and LTA on DME/transporter genes.
Proinflammatory cytokines have been implicated in the downregulation of hepatic genes during inflammation (Barker et al., 1992; Muntane-Relat et al., 1995). Our results show that the induction of cytokines in the liver by LPS and LTA occurred to different extents. LTA administration led to ∼80-, 35-, and 65-fold induction in IL-6, IL-1β, and TNFα, respectively, whereas LPS administration led to ∼15-, 210-, and 160-fold inductions (Ghose et al., 2008, 2009). The differential regulation of DME/transporter genes by LTA and LPS may be caused by the differences in induction levels of the cytokines.
LTA treatment induces the proinflammatory cytokines, IL-1β, IL-6, and TNFα, at 2 hours, which coincides with the downregulation of CAR and its target genes (Ghose et al., 2009). In CAR−/− mice, these cytokines are induced at comparable levels by LTA at 2 hours (Supplemental Fig. 2), but DME/transporter genes are not downregulated by LTA, probably because of the absence of CAR. IL-6 and IL-1β RNA levels are induced similarly in TCPOBOP-pretreated mice on treatment with LTA; however, the extent of TNFα induction is significantly lower in comparison with CAR+/+ mice (Supplemental Fig. 2). It is possible that lower expression of TNFα in TCPOBOP/LTA-treated mice accounts for lack of effect of LTA on DME/transporter genes in these mice.
Our study is the first of its kind to determine the role of CAR in regulating DMEs and transporters during inflammation. The downregulation of DMEs and transporters during inflammation is a complex process, and the molecular mechanism is not fully understood. The effect on hepatic DME/transporter genes depends on the type of inflammatory stimuli, and our results show that there are distinct mechanistic differences between TLR2- and TLR4-induced bacterial inflammation. This indicates that patients exposed to Gram-positive and Gram-negative infections may have differences in drug disposition due to mechanistic differences in the regulation of DME/transporter genes. Targeting CAR may have therapeutic implications in countering the deleterious effects of Gram-positive bacterial infections on hepatic detoxification processes.
Supplementary Material
Abbreviations
- CAR
constitutive androstane receptor
- Cyp
cytochrome P450
- DME
drug-metabolizing enzyme
- hCAR
humanized CAR
- IL
interleukin
- LPS
lipopolysaccharide
- LTA
lipoteichoic acid
- Mrp
multidrug resistance-associated protein
- NR
nuclear receptor
- PB
phenobarbital
- PCR
polymerase chain reaction
- PXR
pregnane X receptor
- Sultn
amine N-sulfotransferase
- TCPOBOP
1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- Ugt
uridine diphosphate glucuronosyltransferase
Authorship Contributions
Participated in research design: Ghose, Shah.
Conducted experiments: Shah, Guo.
Contributed new reagents or analytic tools: Moore.
Performed data analysis: Shah, Ghose.
Wrote or contributed to the writing of the manuscript: Shah, Ghose.
Footnotes
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health [Grant K01DK076057-02] (to R.G.)
 This article has supplemental materials available at dmd.aspetjournals.org.
This article has supplemental materials available at dmd.aspetjournals.org.
References
- Aitken AE, Richardson TA, Morgan ET. (2006) Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu Rev Pharmacol Toxicol 46:123–149 [DOI] [PubMed] [Google Scholar]
- Assem M, Schuetz EG, Leggas M, Sun D, Yasuda K, Reid G, Zelcer N, Adachi M, Strom S, Evans RM, et al. (2004) Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J Biol Chem 279:22250–22257 [DOI] [PubMed] [Google Scholar]
- Barker CW, Fagan JB, Pasco DS. (1992) Interleukin-1 beta suppresses the induction of P4501A1 and P4501A2 mRNAs in isolated hepatocytes. J Biol Chem 267:8050–8055 [PubMed] [Google Scholar]
- Baskin-Bey ES, Huang W, Ishimura N, Isomoto H, Bronk SF, Braley K, Craig RW, Moore DD, Gores GJ. (2006) Constitutive androstane receptor (CAR) ligand, TCPOBOP, attenuates Fas-induced murine liver injury by altering Bcl-2 proteins. Hepatology 44:252–262 [DOI] [PubMed] [Google Scholar]
- Beigneux AP, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. (2000) The acute phase response is associated with retinoid X receptor repression in rodent liver. J Biol Chem 275:16390–16399 [DOI] [PubMed] [Google Scholar]
- Beigneux AP, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. (2002) Reduction in cytochrome P-450 enzyme expression is associated with repression of CAR (constitutive androstane receptor) and PXR (pregnane X receptor) in mouse liver during the acute phase response. Biochem Biophys Res Commun 293:145–149 [DOI] [PubMed] [Google Scholar]
- Cheng PY, Morgan ET. (2001) Hepatic cytochrome P450 regulation in disease states. Curr Drug Metab 2:165–183 [DOI] [PubMed] [Google Scholar]
- Durand SH, Flacher V, Roméas A, Carrouel F, Colomb E, Vincent C, Magloire H, Couble ML, Bleicher F, Staquet MJ, et al. (2006) Lipoteichoic acid increases TLR and functional chemokine expression while reducing dentin formation in in vitro differentiated human odontoblasts. J Immunol 176:2880–2887 [DOI] [PubMed] [Google Scholar]
- Ghose R, Guo T, Haque N. (2009) Regulation of gene expression of hepatic drug metabolizing enzymes and transporters by the Toll-like receptor 2 ligand, lipoteichoic acid. Arch Biochem Biophys 481:123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose R, Guo T, Vallejo JG, Gandhi A. (2011) Differential role of Toll-interleukin 1 receptor domain-containing adaptor protein in Toll-like receptor 2-mediated regulation of gene expression of hepatic cytokines and drug-metabolizing enzymes. Drug Metab Dispos 39:874–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose R, White D, Guo T, Vallejo J, Karpen SJ. (2008) Regulation of hepatic drug-metabolizing enzyme genes by Toll-like receptor 4 signaling is independent of Toll-interleukin 1 receptor domain-containing adaptor protein. Drug Metab Dispos 36:95–101 [DOI] [PubMed] [Google Scholar]
- Ghose R, Zimmerman TL, Thevananther S, Karpen SJ. (2004) Endotoxin leads to rapid subcellular re-localization of hepatic RXRalpha: a novel mechanism for reduced hepatic gene expression in inflammation. Nucl Recept 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann G, Kim H, Piquette-Miller M. (2001) Regulation of the hepatic multidrug resistance gene expression by endotoxin and inflammatory cytokines in mice. Int Immunopharmacol 1:189–199 [DOI] [PubMed] [Google Scholar]
- Hyzy J, Sciotti V, Albini B, Stinson M. (1992) Deposition of circulating streptococcal lipoteichoic acid in mouse tissues. Microb Pathog 13:123–132 [DOI] [PubMed] [Google Scholar]
- Ishii KJ, Akira S. (2004) Toll-like receptors and sepsis. Curr Infect Dis Rep 6:361–366 [DOI] [PubMed] [Google Scholar]
- Kakizaki S, Yamazaki Y, Takizawa D, Negishi M. (2008) New insights on the xenobiotic-sensing nuclear receptors in liver diseases—CAR and PXR—. Curr Drug Metab 9:614–621 [DOI] [PubMed] [Google Scholar]
- Li D, Zimmerman TL, Thevananther S, Lee HY, Kurie JM, Karpen SJ. (2002) Interleukin-1 beta-mediated suppression of RXR:RAR transactivation of the Ntcp promoter is JNK-dependent. J Biol Chem 277:31416–31422 [DOI] [PubMed] [Google Scholar]
- Liu S, Gallo DJ, Green AM, Williams DL, Gong X, Shapiro RA, Gambotto AA, Humphris EL, Vodovotz Y, Billiar TR. (2002) Role of Toll-like receptors in changes in gene expression and NF-kappa B activation in mouse hepatocytes stimulated with lipopolysaccharide. Infect Immun 70:3433–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Evans RM. (1995) The RXR heterodimers and orphan receptors. Cell 83:841–850 [DOI] [PubMed] [Google Scholar]
- Matsumura T, Degawa T, Takii T, Hayashi H, Okamoto T, Inoue J, Onozaki K. (2003) TRAF6-NF-kappaB pathway is essential for interleukin-1-induced TLR2 expression and its functional response to TLR2 ligand in murine hepatocytes. Immunology 109:127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntané-Relat J, Ourlin JC, Domergue J, Maurel P. (1995) Differential effects of cytokines on the inducible expression of CYP1A1, CYP1A2, and CYP3A4 in human hepatocytes in primary culture. Hepatology 22:1143–1153 [PubMed] [Google Scholar]
- Ray A, Chatterjee NS, Bhattacharya SK, Biswas T. (2003) Porin of Shigella dysenteriae enhances mRNA levels for Toll-like receptor 2 and MyD88, up-regulates CD80 of murine macrophage, and induces the release of interleukin-12. FEMS Immunol Med Microbiol 39:213-219. [DOI] [PubMed] [Google Scholar]
- Renton KW. (2004) Cytochrome P450 regulation and drug biotransformation during inflammation and infection. Curr Drug Metab 5:235–243 [DOI] [PubMed] [Google Scholar]
- Renton KW, Nicholson TE. (2000) Hepatic and central nervous system cytochrome P450 are down-regulated during lipopolysaccharide-evoked localized inflammation in brain. J Pharmacol Exp Ther 294:524–530 [PubMed] [Google Scholar]
- Richardson TA, Morgan ET. (2005) Hepatic cytochrome P450 gene regulation during endotoxin-induced inflammation in nuclear receptor knockout mice. J Pharmacol Exp Ther 314:703–709 [DOI] [PubMed] [Google Scholar]
- Schwabe RF, Seki E, Brenner DA. (2006) Toll-like receptor signaling in the liver. Gastroenterology 130:1886–1900 [DOI] [PubMed] [Google Scholar]
- Sewer MB, Koop DR, Morgan ET. (1997) Differential inductive and suppressive effects of endotoxin and particulate irritants on hepatic and renal cytochrome P-450 expression. J Pharmacol Exp Ther 280:1445–1454 [PubMed] [Google Scholar]
- Shen G, Kong AN. (2009) Nrf2 plays an important role in coordinated regulation of Phase II drug metabolism enzymes and Phase III drug transporters. Biopharm Drug Dispos 30:345–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Akira S. (2001) Regulation of innate immune responses by Toll-like receptors. Jpn J Infect Dis 54:209–219 [PubMed] [Google Scholar]
- Teng S, Piquette-Miller M. (2005) The involvement of the pregnane X receptor in hepatic gene regulation during inflammation in mice. J Pharmacol Exp Ther 312:841–848 [DOI] [PubMed] [Google Scholar]
- Tien ES, Negishi M. (2006) Nuclear receptors CAR and PXR in the regulation of hepatic metabolism. Xenobiotica 36:1152–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. (2000) The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature 407:920–923 [DOI] [PubMed] [Google Scholar]
- Zhang J, Huang W, Chua SS, Wei P, Moore DD. (2002) Modulation of acetaminophen-induced hepatotoxicity by the xenobiotic receptor CAR. Science 298:422–424 [DOI] [PubMed] [Google Scholar]
- Zordoky BN, El-Kadi AO. (2009) Role of NF-kappaB in the regulation of cytochrome P450 enzymes. Curr Drug Metab 10:164–178 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.