Abstract
3,4-Methylenedioxymethamphetamine (MDMA) is a widely abused illicit drug that can cause severe and even fatal adverse effects. However, interest remains for its possible clinical applications in posttraumatic stress disorder and anxiety treatment. Preclinical studies to determine MDMA’s safety are needed. We evaluated MDMA’s pharmacokinetics and metabolism in male rats receiving 2.5, 5, and 10 mg/kg s.c. MDMA, and the associated pharmacodynamic consequences. Blood was collected via jugular catheter at 0, 0.5, 1, 2, 4, 6, 8, 16, and 24 hours, with simultaneous serotonin (5-HT) behavioral syndrome and core temperature monitoring. Plasma specimens were analyzed for MDMA and the metabolites (±)-3,4-dihydroxymethamphetamine (HHMA), (±)-4-hydroxy-3-methoxymethamphetamine (HMMA), and (±)-3,4-methylenedioxyamphetamine (MDA) by liquid chromatography–tandem mass spectrometry. After 2.5 mg/kg MDMA, mean MDMA Cmax was 164 ± 47.1 ng/ml, HHMA and HMMA were major metabolites, and <20% of MDMA was metabolized to MDA. After 5- and 10-mg/kg doses, MDMA areas under the curve (AUCs) were 3- and 10-fold greater than those after 2.5 mg/kg; HHMA and HMMA AUC values were relatively constant across doses; and MDA AUC values were greater than dose-proportional. Our data provide decisive in vivo evidence that MDMA and MDA display nonlinear accumulation via metabolic autoinhibition in the rat. Importantly, 5-HT syndrome severity correlated with MDMA concentrations (r = 0.8083; P < 0.0001) and core temperature correlated with MDA concentrations (r = 0.7595; P < 0.0001), suggesting that MDMA’s behavioral and hyperthermic effects may involve distinct mechanisms. Given key similarities between MDMA pharmacokinetics in rats and humans, data from rats can be useful when provided at clinically relevant doses.
Introduction
±3,4-Methylenedioxymethamphetamine (MDMA) was originally synthesized by Merck & Co. in 1912. The drug was largely forgotten until the late 1970s, when Shulgin and Nichols reported its unique “entactogen” properties, which include euphoria, friendliness, and increased emotional closeness with others (Shulgin and Nichols, 1978). Widespread MDMA misuse during the 1980s prompted placement of the drug into Schedule I control, banning its sale and use in the United States. Today, MDMA remains a popular illicit drug, with an estimated worldwide prevalence of 0.2%–0.6% of the population aged 15–64 (10.5–28 million users) (United Nations, 2012). MDMA abuse can be associated with severe or even fatal adverse effects in humans (Walubo and Seger, 1999; Logan, 2001), and drug administration to laboratory animals causes long-term deficits in brain serotonin (5-HT) neurons (Ricaurte et al., 2000). Despite the potential for such risks, there is increasing interest in MDMA as an adjunctive treatment of posttraumatic stress disorder (Mithoefer et al., 2011) and anxiety-related problems (www.clinicaltrials.gov). For these reasons, it is prudent for preclinical investigators to examine MDMA's effects at clinically relevant doses and relate these findings to adverse effects at higher doses to establish the margin of safety in animal models.
MDMA exerts its pharmacological effects by interacting with plasma membrane transporter proteins expressed on neurons, causing release of neurotransmitters by reverse transport (Baumann et al., 2007; Verrico et al., 2007). MDMA’s in vivo pharmacology in humans and animals is complicated by extensive hepatic metabolism via two pathways (Maurer et al., 2000; de la Torre et al., 2004). In the major pathway, MDMA is O-demethylenated by cytochrome P450 (CYP) CYP2D6, 1A2, 2B6, and 3A4 in humans and by CYP2D1 and 3A2 in rats to form (±)-3,4-dihydroxymethamphetamine (HHMA), which is O-methylated to generate (±)-4-hydroxy-3-methoxymethamphetamine (HMMA) by catechol-O-methyltransferase (COMT). In the minor pathway, MDMA is N-demethylated by CYP2B6, 1A2, and 2D6 in humans and CYP1A2 and 2D1 in rats to form (±)-3,4-methylenedioxyamphetamine (MDA). MDA is O-demethylenated by the same enzymes that act on MDMA, with subsequent metabolism by COMT. The hydroxylated metabolites of MDMA are excreted in urine as glucuronide and sulfate conjugates. The main differences between human and rat MDMA metabolism are the specific CYP isoforms involved, the shorter MDMA and metabolite half-lives in rats, the tendency for rats to form more MDA than humans, and the fact that glucuronide-conjugated metabolites predominate in rats (Maurer et al., 2000; de la Torre et al., 2004; Shima et al., 2008).
A clearer understanding of MDMA pharmacodynamics and pharmacokinetics in animals and humans is needed to extrapolate data between species. A wealth of pharmacodynamic data shows that neurochemical, endocrine, and behavioral effects of MDMA occur at similar doses in rats and humans (Schechter, 1988; Johanson et al., 2006; Baumann et al., 2007; Kolbrich et al., 2008a). However, few studies have examined MDMA pharmacokinetics in rats given doses of <10 mg/kg, comparable to those taken by humans (Chu et al., 1996; Starr et al., 2008; Baumann et al., 2009), and just one assessed the relationship between pharmacodynamics and pharmacokinetics (Chu et al., 1996). Most MDMA pharmacokinetic studies in rats investigated doses of 10 mg/kg or higher (Valtier et al., 2007; Meyer et al., 2008; Upreti and Eddington, 2008; Mueller et al., 2009). In two rat studies employing doses of <10 mg/kg, evidence for MDMA nonlinear accumulation was reported (Chu et al., 1996; Baumann et al., 2009), similar to the human situation (Mas et al., 1999; de la Torre et al., 2000; Kolbrich et al., 2008b). By contrast, Green et al. (2009) and Hirt et al. (2010) suggested linear MDMA kinetics in rat.
Given the discrepancies in data regarding the occurrence of nonlinear MDMA pharmacokinetics in rodent models, we sought to evaluate this phenomenon and examine its possible pharmacodynamic consequences. We report plasma concentrations of MDMA, HHMA, HMMA, and MDA in male rats receiving incremental s.c. MDMA (2.5, 5, and 10 mg/kg). Rats were fitted with indwelling catheters so repeated blood specimens could be collected, while we simultaneously measured 5-HT behavioral syndrome and core body temperature. Our findings reveal that increasing MDMA doses administered to rats produce greater than expected area-under-the curve (AUC) values for MDMA and MDA, while AUCs for HHMA and HMMA were lower than expected. These results provide compelling in vivo evidence that MDMA displays nonlinear accumulation in rats via inhibition of metabolism, similar to the effects in humans. 5-HT syndrome severity correlated with MDMA plasma concentrations, but core temperature correlated with MDA, showing distinct contributions of MDMA and its metabolite MDA to the overall pharmacology of administered MDMA.
Materials and Methods
Drugs and Reagents.
Racemic MDMA HCl was received from the National Institute on Drug Abuse, Drug Supply Program (Rockville, MD). MDMA solutions for injection into rats were prepared in sterile 0.9% NaCl (saline) immediately before administration. Reagents for liquid chromatography–tandem mass spectrometry (LC-MS/MS) assays were analytical-grade. Analyte standards at 1 mg/ml in methanol were purchased from Cerilliant (Round Rock, TX) and from Lipomed (Cambridge, MA).
Animals and Surgery.
Male Sprague-Dawley rats weighing 250–300 g were double-housed (lights on: 7:00 AM– 7:00 PM) under controlled temperature (22 ± 2°C) and humidity (45% ± 5%) with free access to food and water. All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996). Vivarium facilities were fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and study procedures were approved by the National Institute on Drug Abuse Intramural Research Program Animal Care and Use Committee. After 2 weeks of acclimation to the vivarium facilities, rats were anesthetized with pentobarbital sodium (60 mg/kg i.p.) and indwelling catheters made of Silastic tubing (Dow Corning, Midland, MI) were implanted into the jugular vein as described previously (Baumann et al., 2008). In brief, the proximal end of the catheter was inserted into the right jugular vein and advanced to the atrium, with the distal end exteriorized on the nape and plugged with a metal stylet. Rats were single-housed postoperatively and allowed 1 week to recover from surgery.
Blood Sampling Procedures.
On the morning of an experiment, rats were moved to the testing room in their home cages and given 1 hour to acclimate to surroundings. Feeding trays were removed, and wire lids were placed atop cages. Polyethylene extension tubes (30 cm) were filled with sterile saline, connected to i.v. catheters, and threaded outside the cages. Catheters were flushed with 0.3 ml of 48-IU/ml heparin saline to facilitate blood withdrawal. Groups of six or seven rats received 2.5, 5, or 10 mg/kg MDMA in a volume of 1 ml/kg by the s.c. route; injections were administered posterior to the shoulder blades on the back. Blood specimens (0.2 ml) were withdrawn before (t = 0) and at 0.5, 1, 2, 4, 6, 8, 16, and 24 hours after treatments. Blood was collected into 1-ml disposable tuberculin syringes, transferred to 1.5-ml plastic tubes on ice, and spun for 10 minutes at 1500 rpm; plasma was decanted and stored at −80°C. An equal volume of sterile saline was infused after each blood withdrawal to maintain volume and osmotic homeostasis.
5-HT Behavioral Syndrome and Core Temperature Evaluation.
The occurrence of the 5-HT behavioral syndrome and changes in core temperature were evaluated on the same schedule as specimen collection, with behaviors observed immediately before blood withdrawal and core temperatures measured after. Specific measured symptoms of 5-HT syndrome included flat body posture, forepaw treading, ambulation, and head weaving. Before each blood withdrawal, rats were observed for 1 minute, and the different elements were scored on a graded scale where 0 = absent, 1 = equivocal, 2 = present, and 3 = intense or continuous. Rats were given a single score at each time point that consisted of the summed scores for all behaviors (i.e., highest possible score of 12). Core temperatures were measured immediately after blood sampling by gently inserting a RET-2 temperature probe (Physitemp Instruments Inc., Clifton, NJ) into the colon.
MDMA and Metabolite Plasma Analysis.
MDMA, HHMA, HMMA, and MDA were measured concurrently in 0.1-ml plasma specimens with an LC-MS/MS procedure based on the method described by Mueller et al., (2007). Eleven calibrators were prepared in blank plasma to yield final concentrations of 5–1500 ng/ml. In addition, three quality control samples (QCs) at 15, 135, and 1350 ng/ml were prepared in blank plasma. Each calibrator, QC, and plasma specimen was spiked with 100 μl internal standard stock solution (50 ng/ml MDMA-d5, MDA-d5, and 4-hydroxymethamphetamine) and with 20 μl sodium metabisulfite (250 mM) and 10 μl EDTA (250 mM). To hydrolyze samples, 10 μl glucuronidase (Helix pomatia, Type HP-2) was added to each tube before incubation at 50°C for 90 minutes. Samples were cooled at room temperature and 20 μl 4-methylcatechol at 1 g/l was added. Plasma proteins were precipitated with 10 μl perchloric acid. After mixing and centrifugation at 15,000g at 4°C for 10 minutes, supernatants (125 μl) were transferred to autosampler vials and 5 μl was injected into the LC-MS/MS.
LC-MS/MS analysis was performed with a Shimadzu liquid chromatography system (Shimadzu Corporation, Columbia, MD) interfaced to a 3200 QTrap (AB Sciex, Foster City, CA) with a Turbo V electrospray source. The Shimadzu system consisted of LC-20AD XR pumps, DGU-20A3 degasser, SIL-20AD XR autosampler, and CTO-10AC column oven. Chromatographic separation was performed with a Synergi Polar-RP 100A, 100 × 2 mm, 4 μm, with a 4 × 2 mm identically packed guard column (Phenomenex, Torrance, CA), and gradient elution included mobile phase A (4 mM ammonium formate, pH 3.4, with 1% formic acid) and mobile phase B (acetonitrile) at a flow rate of 0.5 ml/min. The initial mixture (60A:40B) was maintained for 3.7 minutes; mobile phase B was increased to 90% at 5 minutes and held for 2.5 minutes. The mixture returned to initial conditions at 8 minutes, followed by 3 minutes of equilibration. The total run time was 11 minutes. Mass spectrometric data were acquired in positive electrospray ionization mode with the following source parameters: IonSpray voltage, 3000 V; temperature, 500°C; curtain gas, 25; ion source gas 1, 50; and ion source gas 2, 75. Data were recorded in multiple reaction monitoring mode. The following transitions were monitored (quantification transition in bold): 194.2 > 163.2 and 194.2 > 105.1 for MDMA; 180.1 > 132.9 and 180.1 > 105 for MDA; 182.1 > 151 and 182.1 > 122.9 for HHMA; 196.2 > 165.1 and 196.2 > 105.2 for HMMA; 199.1 > 165.1 and 199.1 > 107.2 for MDMA-d5; 185.1 > 110.1 and 185.1 > 138.2 for MDA-d5; and 166.1 > 107 and 166.1 > 135.1 for 4-hydroxymethamphetamine. The ratio of these transitions also was required to be within ±20% of the average ratio of calibrators.
Linearity range with 1/x weighting was from 5–1500 ng/ml. The lower limit of quantification was 5 ng/ml, and the limit of detection was 1 ng/ml for HMMA and HHMA and 2.5 ng/ml for MDMA and MDA. Assay accuracy at low, medium, and high QCs was 95.8%–107.1% (n = 10) and imprecision was 1.6%–10.2% (n = 10). Extraction efficiency was 89%–114% at three QC concentrations for all compounds (n = 5), and no matrix effect was observed for any compound in any QC (n = 5), except ion enhancement for low MDA QC (30.1%). Samples were stable after 72 hours on the autosampler, and there was no evidence of carryover in negative injections after a sample spiked at 3000 ng/ml.
Data Analysis and Statistics.
Pharmacokinetic and pharmacodynamic data were tabulated, analyzed, and graphically depicted using GraphPad Prism (version 5.02; GraphPad Software, Inc., La Jolla, CA). Plasma pharmacokinetic data were further analyzed using WinNonlin (version 5.2; Pharsight, Mountain View, CA) to determine noncompartmental pharmacokinetic constants including maximum concentration (Cmax), time of maximum concentration (Tmax), AUC, and elimination half-life (t1/2). At least three data points on the descending linear limb of the time-concentration curve were employed to calculate t1/2 values. To evaluate possible nonlinearity for plasma analyte concentrations, AUCs following 2.5 mg/kg were multiplied by 2 and 4 to calculate expected AUC values for 5- and 10-mg/kg doses, respectively. The expected values were compared with observed results at each MDMA dose. Expected versus observed AUC data were compared at each dose by unpaired t tests. Behavioral and temperature data were evaluated by two-way analysis of variance (treatment × time), followed by Bonferroni post hoc test. To examine potential relationships between pharmacokinetic and pharmacodynamic endpoints, Pearson’s correlation coefficients were calculated using data from individual rats treated with 10 mg/kg MDMA. Specifically, coefficients were generated for MDMA and MDA versus syndrome score and temperature. P < 0.05 was considered the minimal criterion for statistical significance.
Results
Pharmacokinetics of MDMA, MDA, HHMA, and HMMA.
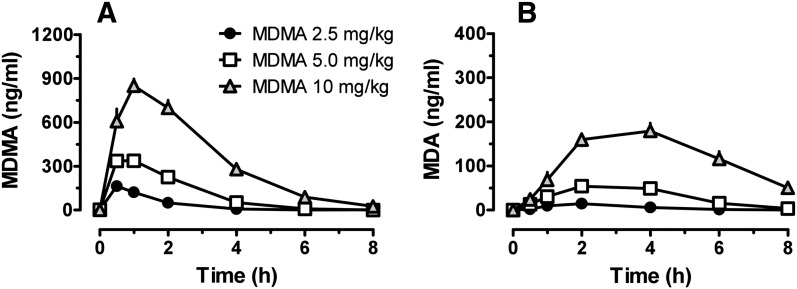
Figure 1 shows the time-concentration profiles for plasma MDMA and its N-demethylated metabolite MDA after s.c. administration of 2.5, 5, and 10 mg/kg MDMA. Pharmacokinetic constants derived from the MDMA curves are summarized in Table 1. MDMA Cmax was 164.1 ± 47.1 ng/ml after 2.5 mg/kg MDMA, 2-fold higher after 5 mg/kg MDMA, and 5-fold higher after 10 mg/kg MDMA. Tmax was 0.6 ± 0.2 hour after 2.5 mg/kg, 0.9 ± 0.6 hour after 5 mg/kg, and 1.1 ± 0.4 after 10 mg/kg. MDA plasma concentrations were much lower than those for MDMA after all administered doses, and MDA had a longer half-life than MDMA. MDA Cmax was 15.4 ± 3.7 ng/ml after 2.5 mg/kg MDMA, and increased 3.7- and 12.6-fold after 5 and 10 mg/kg MDMA, respectively. With increasing doses of MDMA, the ratio of MDA to MDMA rose markedly such that MDA AUC was 17% of MDMA AUC after 2.5 mg/kg, 30% after 5 mg/kg, and 40% after 10 mg/kg.
Fig. 1.
Time-concentration profiles for MDMA (A) and MDA (B) in groups of rats receiving s.c. injections of 2.5, 5, and 10 mg/kg MDMA. Rats received MDMA at time 0, and blood specimens were collected via indwelling jugular catheters at 0.5, 1, 2, 4, 6, 8, 16, and 24 hours after dosing. Analytes were assayed in plasma by LC-MS/MS. Data are mean ± S.E.M. for n = 6–7 rats/group.
TABLE 1.
Pharmacokinetic constants (mean ± S.D.) for plasma MDMA, MDA, HHMA, and HMMA after s.c. administration of 2.5, 5, and 10 mg/kg MDMA to rats
| Analyte | MDMA Dose | Cmax | Tmax | AUC | t1/2 |
|---|---|---|---|---|---|
| mg/kg | ng/ml | h | h⋅ng/ml | h | |
| MDMA | 2.5 (n = 7) | 164.1 ± 47.1 | 0.6 ± 0.2 | 272.1 ± 71.6 | 1.1 ± 0.9 |
| 5 (n = 6) | 370.8 ± 41 | 0.9 ± 0.6 | 879.1 ± 133.2 | 0.9 ± 0.1 | |
| 10 (n = 7) | 893.9 ± 90.7 | 1.1 ± 0.4 | 2879.9 ± 491.5 | 2 ± 0.6 | |
| MDA | 2.5 (n = 7) | 15.4 ± 3.7 | 1.9 ± 0.4 | 47.4 ± 23.5 | 1.2 ± 0.2 |
| 5 (n = 6) | 57.5 ± 11.9 | 3 ± 1.1 | 262.3 ± 83.3 | 1.7 ± 0.9 | |
| 10 (n = 7) | 193.7 ± 43.6 | 3.7 ± 0.8 | 1157.9 ± 244.2 | 1.8 ± 0.2 | |
| HHMA | 2.5 (n = 7) | 61.1 ± 11.2 | 3.7 ± 1.8 | 531.6 ± 97.3 | 4.1 ± 1.4 |
| 5 (n = 6) | 93.4 ± 29.9 | 4 ± 1.3 | 789.6 ± 193.7 | 3.7 ± 0.9 | |
| 10 (n = 7) | 102.7 ± 35.1 | 5.1 ± 2.5 | 969.9 ± 246.7 | 3.1 ± 0.9 | |
| HMMA | 2.5 (n = 7) | 159.1 ± 46.9 | 5.4 ± 1.0 | 1032.4 ± 160.5 | 2.7 ± 1 |
| 5 (n = 6) | 212 ± 74.6 | 5 ± 1.1 | 1442.4 ± 429.9 | 2.9 ± 0.3 | |
| 10 (n = 7) | 276 ± 100.6 | 7.1 ± 1.1 | 2132.5 ± 654.3 | 2.7 ± 0.5 |
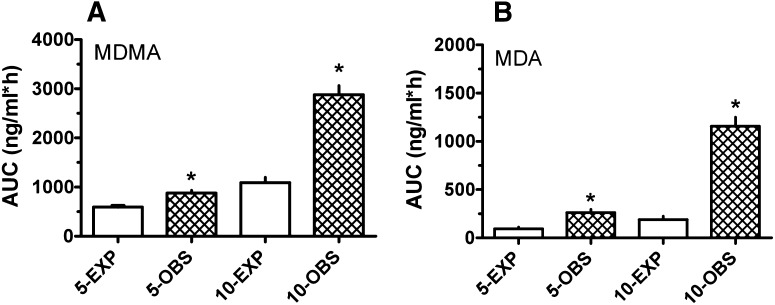
As depicted in Fig. 2, the observed AUC values for MDMA after 5 and 10 mg/kg were greater than dose-proportional, based on linear extrapolation from the 2.5-mg/kg dose. In the case of linear pharmacokinetics, AUC should increase 2-fold when the dose is increased from 2.5 to 5 mg/kg and 4-fold when the dose is increased from 2.5 to 10 mg/kg. However, we found that MDMA AUCs after 5 and 10 mg/kg were 3- and 10-fold greater than AUC after 2.5 mg/kg. The observed AUC values for MDMA were significantly greater than the expected AUC at 5 mg/kg (t = 4.507, df = 10; P < 0.01) and 10 mg/kg (t = 8.332, df = 12; P < 0.0001). The augmented AUCs, especially at 10 mg/kg, indicate that MDMA displays nonlinear kinetics at high doses in rats. MDMA t1/2 was about 1 hour after 2.5- and 5-mg/kg doses, but close to 2 hours after 10 mg/kg, demonstrating prolonged MDMA elimination times at high doses. A similar situation was observed for MDA AUC values, where observed AUC was significantly greater than expected after 5 mg/kg (t = 4.557, df = 11; P < 0.001) and 10 mg/kg (t = 9.789, df = 12; P < 0.0001).
Fig. 2.
Expected (EXP) versus observed (OBS) AUC values for MDMA (A) and MDA (B) at 5- and 10-mg/kg MDMA doses. Expected values were calculated by multiplying the observed AUC at 2.5 mg/kg by the proportionate increase in dose. Values are the mean ± S.E.M. for n = 6–7 rats/group. *P < 0.05.
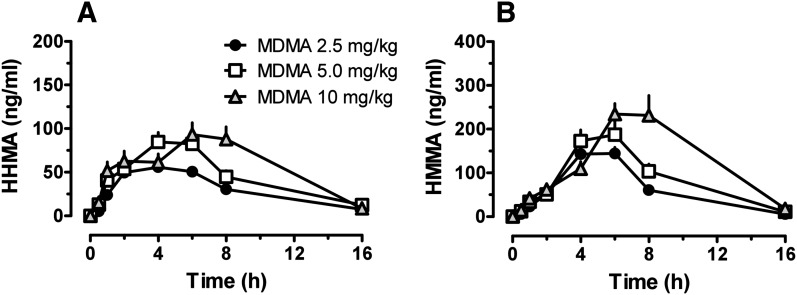
Figure 3 illustrates time-concentration profiles for plasma HHMA and HMMA in rats receiving 2.5, 5, and 10 mg/kg MDMA. Pharmacokinetic constants are summarized in Table 1. HHMA Cmax was 61.1 ± 11.2 ng/ml after 2.5 mg/kg MDMA, 93.4 ± 29.9 ng/ml after 5 mg/kg MDMA, and 102.7 ± 35.1 ng/ml after 10 mg/kg MDMA. HMMA Cmax values were 159.1 ± 46.9, 212 ± 74.6, and 276 ± 100.6 ng/ml after 2.5, 5, and 10 mg/kg MDMA, respectively. Cmax only increased 1.3- and 1.7-fold for HHMA and HMMA as the dose of MDMA increased 4-fold from 2.5 to 10 mg/kg. The concentration of both metabolites increased slowly over time, reaching Tmax at 4 hours for HHMA and 5 hours for HMMA. After the 2.5-mg/kg dose of MDMA, HHMA and HMMA AUC values were higher than those for MDMA. However, after the 10-mg/kg dose of MDMA, HHMA and HMMA AUCs were lower than MDMA AUC.
Fig. 3.
Time-concentration profiles for HHMA (A) and HMMA (B) in groups of rats receiving s.c. injections of 2.5, 5, and 10 mg/kg MDMA. Rats received MDMA at time 0, and blood specimens were collected via indwelling jugular catheters at 0.5, 1, 2, 4, 6, 8, 16, and 24 hours after dosing. Analytes were assayed in plasma by LC-MS/MS. Data are mean ± S.E.M. for n = 6–7 rats/group.
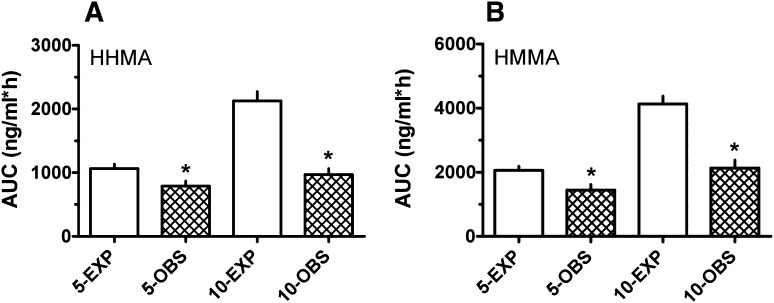
As depicted in Fig. 4, the observed AUC values for HHMA and HMMA were much lower than expected as the MDMA dose increased. More specifically, observed AUCs for these metabolites after 5 and 10 mg/kg MDMA were less than 2-fold above those after 2.5 mg/kg MDMA. The observed AUC for HHMA was significantly lower than expected at 5 mg/kg (t = 2.534, df = 11; P < 0.02) and 10 mg/kg (t = 6.642, df = 12; P < 0.0001), and similar data were found for HMMA. Collectively, the metabolite data indicate an impaired ability to form HHMA after high-dose MDMA, which could underlie the elevated MDMA concentrations and longer MDMA t1/2 seen at the 10-mg/kg drug dose.
Fig. 4.
Expected (EXP) versus observed (OBS) AUC values for HHMA (A) and HMMA (B) at 5- and 10-mg/kg MDMA doses. Expected values were calculated by multiplying the observed AUC at 2.5 mg/kg by the proportionate increase in dose. Values are the mean ± S.E.M. for n = 6–7 rats/group. *P < 0.05.
Pharmacodynamic Effects.
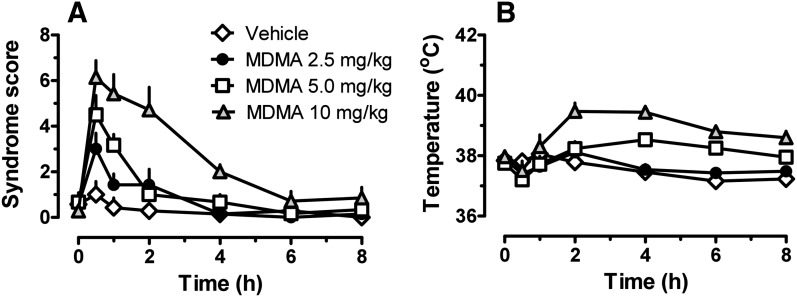
Figure 5 shows the time-effect curves for 5-HT behavioral syndrome and core body temperature for rats that received 2.5, 5, and 10 mg/kg MDMA. The occurrence of 5-HT syndrome was significantly affected by MDMA dose (F3,207 = 35.72; P < 0.0001), and there was significant dose × time interaction (F24,207 = 5.92; P < 0.0001). Syndrome scores were significantly greater than those of saline-treated controls at 0.5 h after 2.5 mg/kg MDMA (P < 0.05) and at 0.5 and 1 hour after 5 mg/kg MDMA (P < 0.05). The most robust effects were observed after 10 mg/kg, where 5-HT syndrome was significantly elevated above control levels for 4 hours postinjection (P < 0.05). It is noteworthy that the time course for behavioral activation seemed to track with plasma MDMA concentration (compare Figs. 1 and 5). Core temperature was significantly influenced by MDMA dose (F3,207 = 44.59; P < 0.0001), and there was a significant dose × time interaction (F24,207 = 4.602; P < 0.001). The 2.5-mg/kg MDMA dose did not affect temperature, but 5 mg/kg produced significant hyperthermia after 4 and 6 hours (P < 0.05). At the 10-mg/kg MDMA dose, core temperature was elevated above control levels at 2, 4, 6, and 8 hours postinjection. The time course of hyperthermic effects displayed a slow onset and appeared to track with plasma MDA concentrations (compare Figs. 1 and 5).
Fig. 5.
Time-effect curves for 5-HT syndrome (A) and core temperature (B) in rats receiving s.c. injections of 2.5, 5, and 10 mg/kg MDMA. Rats received MDMA at time 0; syndrome score and colonic temperature were monitored at 0.5, 1, 2, 4, 6, 8, 16, and 24 hours after dosing. Data are mean ± S.E.M. for n = 6–7 rats/group.
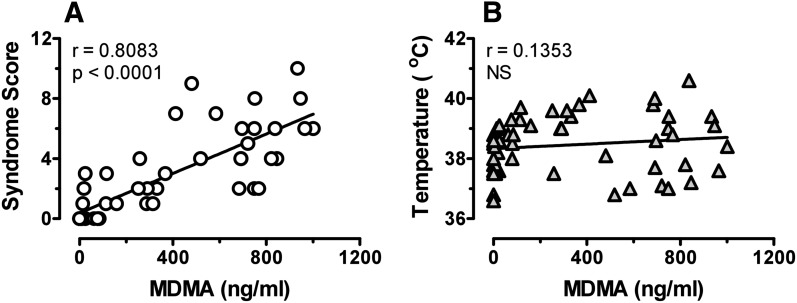
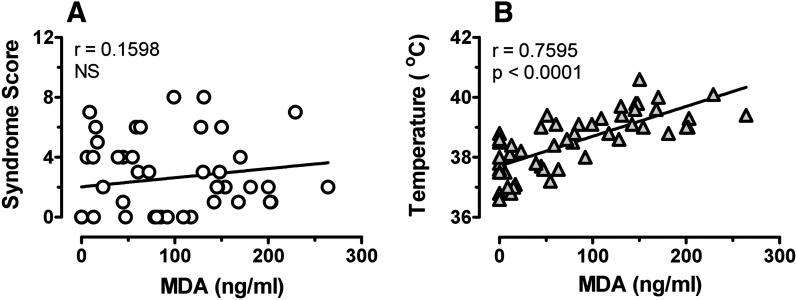
The design of our experiments (i.e., repeated blood sampling from freely behaving rats) allowed us to evaluate potential relationships between pharmacokinetic and pharmacodynamic endpoints for individual subjects. We chose to examine correlations between plasma concentrations of MDMA or MDA with behavior and temperature at the 10-mg/kg dose because this treatment produced robust pharmacodynamic effects in all subjects. Figure 6 shows that plasma MDMA was significantly correlated with 5-HT syndrome (Pearson’s r = 0.8083; P < 0.0001) but not temperature. By contrast, Fig. 7 reveals that plasma MDA was significantly correlated with temperature (r = 0.7595; P < 0.0001) but not 5-HT syndrome. These findings suggest that behavioral and hyperthermic effects of MDMA may involve distinct mechanisms.
Fig. 6.
Relationships between plasma MDMA concentration and 5-HT syndrome (A) or core temperature (B). Data from individual rats receiving 10 mg/kg MDMA were used to construct plots. Only data from time points postinjection were used (56 points/plot). Pearson’s correlation coefficient was calculated for each data set.
Fig. 7.
Relationships between plasma MDA concentration and 5-HT syndrome (A) or core temperature (B). Data from individual rats receiving 10 mg/kg MDMA were used to construct plots. Only data from time points postinjection were used (56 points/plot). Pearson’s correlation coefficient was calculated for each data set.
Discussion
We examined MDMA pharmacokinetics and metabolism in rats given increasing MDMA doses and assessed the relationship between pharmacokinetics and pharmacodynamic effects. Injections were administered s.c. because this route engenders the slowest possible MDMA kinetics in rats and is most similar to the po route in humans (Baumann et al., 2009). Additionally, the bulk of MDMA neurotoxicity studies in rats employed the s.c. route, making our findings directly comparable to the literature (Ricaurte et al., 2000). Few investigations in rats examined MDMA pharmacokinetics after doses similar to those taken by humans (i.e., 1–3 mg/kg), and only a handful of studies assessed the effects of dose on MDMA pharmacokinetic and pharmacodynamic parameters (Chu et al., 1996; Baumann et al., 2009).
There are discrepancies in whether MDMA follows nonlinear pharmacokinetics in rats, as documented in humans, monkeys, and mice (de la Torre et al., 2000; Kolbrich et al., 2008b; Mueller et al., 2008; Scheidweiler et al., 2011). Green et al. (2009, 2012) compared literature values for plasma MDMA concentrations following high-dose MDMA administration in rats (i.e., 5–20 mg/kg via i.p., s.c., or po routes) to those produced by low-dose administration in humans (i.e., 0.5–2 mg/kg po). From this comparison, the authors concluded that MDMA doses in rats must be 4-fold higher than human doses to achieve similar peak plasma MDMA concentrations and that MDMA pharmacokinetics are linear in rats. Because there are published findings contradicting both of these conclusions (Chu et al., 1996; Delaforge et al., 1999; Starr et al., 2008; Baumann et al., 2009), we sought to investigate these issues in more detail.
After s.c. administration of 2.5 mg/kg MDMA to rats, we found that MDMA Cmax was 164 ng/ml, a concentration similar to those reported for human males receiving 1.3–1.6 mg/kg po MDMA in laboratory settings (de la Torre et al., 2000; Kolbrich et al., 2008b). Starr et al. (2008) reported plasma MDMA Cmax of 300 ng/ml in male Long-Evans rats given i.p. injections of 3 mg/kg MDMA, and this concentration is in line with the findings reported here and elsewhere (Baumann et al., 2009). The similarities between MDMA Cmax in rats and humans support the shared sensitivity of both species to the same drug doses in vivo (Schechter, 1988; Johanson et al., 2006; Baumann et al., 2007; Kolbrich et al., 2008a). On the other hand, MDMA t1/2 in rat plasma after a low dose (2.5 mg/kg) is 1 hour, a much shorter interval than the 8–9 hours observed in humans receiving oral doses (Mas et al., 1999; de la Torre et al., 2000; Kolbrich et al., 2008b). Due to rapid MDMA clearance in rats, repeated s.c. injections of low-dose MDMA in this species might be an acceptable model for po doses administered to humans, but this hypothesis needs to be tested. In any case, the present findings do not support the notion that rats must receive 4-fold-higher doses of MDMA to achieve peak plasma concentrations similar to those observed in humans (Green et al., 2009).
Previous studies showed that HHMA and HMMA are formed in vivo after MDMA administration in both rats and humans (Lim et al., 1992; Segura et al., 2001; de la Torre et al., 2004; Valtier et al., 2007; Goni-Allo et al., 2008; Kolbrich et al., 2008b; Baumann et al., 2009; Mueller et al., 2009), but there is uncertainty about whether these hydroxylated metabolites represent major MDMA metabolites in rodents. Here we observed that AUCs for HHMA and HMMA were always greater than AUC for MDMA in rats given 2.5 mg/kg, confirming that O-demethylenation is the predominant pathway for biotransformation in rats given clinically relevant MDMA doses. Following 2.5 mg/kg MDMA, MDA Cmax was only 15 ng/ml and its AUC represented only 17% of MDMA AUC, documenting that N-demethylation is a secondary metabolic pathway in rats treated with low-dose MDMA. Nevertheless, humans typically convert <10% of MDMA to MDA (de la Torre et al., 2004), so our results support the contention that rats produce more MDA than humans, even at low MDMA doses. MDA is an important bioactive metabolite of MDMA that acts as a potent monoamine releaser and agonist at 5-HT2B receptors (Baumann and Rothman, 2009).
The first published report of nonlinear MDMA pharmacokinetics in rats appeared in 1996 (Chu et al., 1996). Chu et al. found that s.c. administration of 5–40 mg/kg (+)-MDMA produced elevations in plasma and brain MDMA concentrations that were far greater proportionally than increases in administered dose, suggesting nonlinear drug accumulation. No putative mechanism to explain the observed nonlinearity was proposed in that initial report. In our previous study in rats, we showed that MDMA AUC after 10 mg/kg was 10-fold greater than AUC after 2 mg/kg, whereas HMMA AUC did not differ. Those data provided circumstantial evidence for inhibition of MDMA metabolism, but the most direct O-demethylenated metabolite HHMA was not quantified in previous work. In the current experiments, we validated a method by LC-MS/MS (Mueller et al., 2007) to determine concentrations of MDMA, HHMA, HMMA, and MDA in small-volume plasma specimens from rats. Our data showed that AUC for HHMA and HMMA increased less than 2-fold as MDMA dose increased from 2.5 to 10 mg/kg. In the same rats, AUC for MDMA increased 10-fold. Collectively, these findings provide decisive in vivo evidence that MDMA displays nonlinear pharmacokinetics in the rat, most likely due to metabolic inhibition. If MDMA pharmacokinetics were linear, we should have observed AUC increases proportional to dose. We also observed evidence for robust nonlinear accumulation of MDA. As MDMA doses increased from 2.5 to 10 mg/kg, MDA AUC increased 24-fold. MDMA is O-demethylenated to form HHMA mainly by CYP2D6 in humans, and interaction of the drug with the enzyme causes irreversible inhibition of activity, thereby blocking the major pathway of MDMA biotransformation (Wu et al., 1997; de la Torre and Farre, 2004). At the molecular level, MDMA is capable of forming an inhibitory metabolite complex with human CYP2D6 and with the rat homolog CYP2D1 (Delaforge et al., 1999; Heydari et al., 2004), suggesting that a common mechanism may underlie sustained metabolic inhibition in humans and rats. In fact, it is well established that the methylenedioxy moiety found in the structure of MDMA and MDA can inhibit various enzymes, including rat CYP isoforms (Brady et al., 1986; Murray, 1997). It would be interesting to determine whether rats pretreated with high-dose MDMA have an impaired ability to O-demethylenate subsequent MDMA doses, as has been shown in humans (Farre et al., 2004).
In the present study, we evaluated two established MDMA pharmacodynamic measures, the 5-HT behavioral syndrome and core temperature, and the relationship between these pharmacodynamic effects and MDMA pharmacokinetics. Interestingly, neither of these endpoints was markedly altered by the 2.5-mg/kg dose of MDMA, a dose that generated clinically relevant amounts of MDMA in plasma. Previous studies showed that s.c. doses of 1–3 mg/kg MDMA induce large elevations in extracellular 5-HT in the brain (Baumann et al., 2008; Starr et al., 2008), yet these doses do not induce robust noncontingent behaviors or hyperthermia. By contrast, the 10-mg/kg dose of MDMA produced large and sustained increases in 5-HT syndrome and core temperature. We observed differential relationships between plasma MDMA, plasma MDA, and the pharmacodynamic endpoints after administration of high-dose MDMA. Specifically, plasma MDMA concentrations were positively correlated with 5-HT syndrome, whereas plasma MDA concentrations were positively correlated with core temperature. The correlative data indicate that the biologic mechanisms underlying these two responses are distinct. Rodsiri et al. (2011) reported that the change in locomotor activity and the magnitude of the hyperthermia appeared to be unrelated both to each other and to the magnitude of MDMA-induced 5-HT release. Previous studies suggested that MDMA hyperthermia may result from increased release of dopamine rather than 5-HT (Mechan et al., 2002). Our findings point to the nonlinear accumulation of MDA as a critical variable in mediating hyperthermia induced by high-dose MDMA administration. Future studies should be carried out to address this hypothesis in more detail.
To summarize, we demonstrated that s.c. administration of 2.5 mg/kg MDMA to rats produces peak plasma MDMA concentrations comparable to those observed in humans receiving recreational doses. After this low dose in the rat, HHMA and HMMA AUCs exceeded that of MDMA, implicating HHMA and HMMA as major MDMA metabolites under these conditions. After high-dose MDMA administration, MDMA AUC exceeds those of HHMA and HMMA because plasma MDMA concentrations increase nonlinearly in conjunction with an impaired ability to form HHMA due to metabolic inhibition. As MDMA dose increases above 2.5 mg/kg, a larger percentage of the administered dose is shunted to the N-demethylation pathway, resulting in greatly enhanced formation of MDA. MDMA pharmacokinetics correlated with 5-HT syndrome severity and MDA correlated with core temperature, suggesting that nonlinear accumulation of MDA could be a critical factor contributing to dangerous hyperthermia. Our findings indicate that MDMA studies in rats can generate clinically meaningful results if the dose and route of administration are carefully chosen.
Abbreviations
- 5-HT
serotonin
- AUC
area under the curve
- COMT
catechol-O-methyltransferase
- HHMA
(±)-3,4-dihydroxymethamphetamine
- HMMA
(±)-4-hydroxy-3-methoxymethamphetamine
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- MDA
(±)-3,4-methylenedioxyamphetamine
- MDMA
(±)-3,4-methylenedioxymethamphetamine
- QC
quality control
- t1/2
elimination half-life
Authorship Contributions
Participated in research design: Baumann, Scheidweiler, Rothman, Huestis.
Conducted experiments: Concheiro, Baumann, Scheidweiler, Marrone.
Contributed new reagents or analytic tools: Concheiro, Scheidweiler.
Performed data analysis: Concheiro, Baumann, Scheidweiler, Huestis.
Wrote or contributed to the writing of the manuscript: Concheiro, Bauman, Rothman, Scheidweiler, Huestis.
Footnotes
This work was supported by the Intramural Research Program of the National Institutes of Health National Institute on Drug Abuse.
References
- Baumann MH, Clark RD, Franken FH, Rutter JJ, Rothman RB. (2008) Tolerance to 3,4-methylenedioxymethamphetamine in rats exposed to single high-dose binges. Neuroscience 152:773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Rothman RB. (2009) Neural and cardiac toxicities associated with 3,4-methylenedioxymethamphetamine (MDMA). Int Rev Neurobiol 88:257–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Wang X, Rothman RB. (2007) 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology (Berl) 189:407–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Zolkowska D, Kim I, Scheidweiler KB, Rothman RB, Huestis MA. (2009) Effects of dose and route of administration on pharmacokinetics of (+ or -)-3,4-methylenedioxymethamphetamine in the rat. Drug Metab Dispos 37:2163–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JF, Di Stefano EW, Cho AK. (1986) Spectral and inhibitory interactions of (+/-)-3,4-methylenedioxyamphetamine (MDA) and (+/-)-3,4-methylenedioxymethamphetamine (MDMA) with rat hepatic microsomes. Life Sci 39:1457–1464 [DOI] [PubMed] [Google Scholar]
- Chu T, Kumagai Y, DiStefano EW, Cho AK. (1996) Disposition of methylenedioxymethamphetamine and three metabolites in the brains of different rat strains and their possible roles in acute serotonin depletion. Biochem Pharmacol 51:789–796 [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farré M. (2004) Neurotoxicity of MDMA (ecstasy): the limitations of scaling from animals to humans. Trends Pharmacol Sci 25:505–508 [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farré M, Ortuño J, Mas M, Brenneisen R, Roset PN, Segura J, Camí J. (2000) Non-linear pharmacokinetics of MDMA ('ecstasy') in humans. Br J Clin Pharmacol 49:104–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre R, Farré M, Roset PN, Pizarro N, Abanades S, Segura M, Segura J, Camí J. (2004) Human pharmacology of MDMA: pharmacokinetics, metabolism, and disposition. Ther Drug Monit 26:137–144 [DOI] [PubMed] [Google Scholar]
- Delaforge M, Jaouen M, Bouille G. (1999) Inhibitory metabolite complex formation of methylenedioxymethamphetamine with rat and human cytochrome P450. Particular involvement of CYP 2D. Environ Toxicol Pharmacol 7:153–158 [DOI] [PubMed] [Google Scholar]
- Farré M, de la Torre R, Mathúna BO, Roset PN, Peiró AM, Torrens M, Ortuño J, Pujadas M, Camí J. (2004) Repeated doses administration of MDMA in humans: pharmacological effects and pharmacokinetics. Psychopharmacology (Berl) 173:364–375 [DOI] [PubMed] [Google Scholar]
- Goni-Allo B, O Mathúna B, Segura M, Puerta E, Lasheras B, de la Torre R, Aguirre N. (2008) The relationship between core body temperature and 3,4-methylenedioxymethamphetamine metabolism in rats: implications for neurotoxicity. Psychopharmacology (Berl) 197:263–278 [DOI] [PubMed] [Google Scholar]
- Green AR, Gabrielsson J, Marsden CA, Fone KC. (2009) MDMA: on the translation from rodent to human dosing. Psychopharmacology (Berl) 204:375–378 [DOI] [PubMed] [Google Scholar]
- Green AR, King MV, Shortall SE, Fone KCF. (2012) Lost in translation: preclinical studies on 3,4-methylenedioxymethamphetamine provide information on mechanisms of action, but do not allow accurate prediction of adverse events in humans. Br J Pharmacol 166:1523–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydari A, Yeo KR, Lennard MS, Ellis SW, Tucker GT, Rostami-Hodjegan A. (2004) Mechanism-based inactivation of CYP2D6 by methylenedioxymethamphetamine. Drug Metab Dispos 32:1213–1217 [DOI] [PubMed] [Google Scholar]
- Hirt D, Fonsart J, Menet M-C, Debray M, Noble F, Declèves X, Scherrmann J-M. (2010) Population pharmacokinetics of 3,4-methylenedioxymethamphetamine and main metabolites in rats. Toxicol Sci 114:38–47 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC.
- Johanson CE, Kilbey M, Gatchalian K, Tancer M. (2006) Discriminative stimulus effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans trained to discriminate among d-amphetamine, meta-chlorophenylpiperazine and placebo. Drug Alcohol Depend 81:27–36 [DOI] [PubMed] [Google Scholar]
- Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. (2008a) Physiological and subjective responses to controlled oral 3,4-methylenedioxymethamphetamine administration. J Clin Psychopharmacol 28:432–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. (2008b) Plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine after controlled oral administration to young adults. Ther Drug Monit 30:320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HK, Zeng S, Chei DM, Foltz RL. (1992) Comparative investigation of disposition of 3,4-(methylenedioxy)methamphetamine (MDMA) in the rat and the mouse by a capillary gas chromatography-mass spectrometry assay based on perfluorotributylamine-enhanced ammonia positive ion chemical ionization. J Pharm Biomed Anal 10:657–665 [DOI] [PubMed] [Google Scholar]
- Logan BK. (2001) Amphetamines: an update on forensic issues. J Anal Toxicol 25:400–404 [DOI] [PubMed] [Google Scholar]
- Mas M, Farré M, de la Torre R, Roset PN, Ortuño J, Segura J, Camí J. (1999) Cardiovascular and neuroendocrine effects and pharmacokinetics of 3, 4-methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther 290:136–145 [PubMed] [Google Scholar]
- Maurer HH, Bickeboeller-Friedrich J, Kraemer T, Peters FT. (2000) Toxicokinetics and analytical toxicology of amphetamine-derived designer drugs (‘Ecstasy’). Toxicol Lett 112-113:133–142 [DOI] [PubMed] [Google Scholar]
- Mechan AO, Esteban B, O’Shea E, Elliott JM, Colado MI, Green AR. (2002) The pharmacology of the acute hyperthermic response that follows administration of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) to rats. Br J Pharmacol 135:170–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Piper BJ, Vancollie VE. (2008) Development and characterization of a novel animal model of intermittent MDMA (“Ecstasy”) exposure during adolescence. Ann N Y Acad Sci 1139:151–163 [DOI] [PubMed] [Google Scholar]
- Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Doblin R. (2011) The safety and efficacy of +/-3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. J Psychopharmacol 25:439–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Peters F, Maurer H, McCann U, Ricaurte GA. (2008) Non-linear pharmacokinetics of MDMA (“Ecstasy”) and its major metabolites in squirrel monkeys at plasma concentrations of MDMA that develop after typical psychoactive doses. J Pharmacol Exp Ther 327:38–44 [DOI] [PubMed] [Google Scholar]
- Mueller M, Peters FT, Ricaurte GA, Maurer HH. (2007) Validated liquid chromatographic-electrospray ionization mass spectrometric assay for simultaneous determination of 3,4-methylenedioxymethamphetamine and its metabolites 3,4-methylenedioxyamphetamine, 3,4-dihydroxymethamphetamine, and 4-hydroxy-3-methoxymethamphetamine in squirrel monkey plasma. J Chromatogr B Analyt Technol Biomed Life Sci 855:262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Yuan J, Felim A, Neudörffer A, Peters FT, Maurer HH, McCann UD, Largeron M, Ricaurte GA. (2009) Further studies on the role of metabolites in (+/-)-3,4-methylenedioxymethamphetamine-induced serotonergic neurotoxicity. Drug Metab Dispos 37:2079–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. (1997) Drug-mediated inactivation of cytochrome P450. Clin Exp Pharmacol Physiol 24:465–470 [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Yuan J, McCann UD. (2000) (+/-)3,4-Methylenedioxymethamphetamine (‘Ecstasy’)-induced serotonin neurotoxicity: studies in animals. Neuropsychobiology 42:5–10 [DOI] [PubMed] [Google Scholar]
- Rodsiri R, Spicer C, Green AR, Marsden CA, Fone KC. (2011) Acute concomitant effects of MDMA binge dosing on extracellular 5-HT, locomotion and body temperature and the long-term effect on novel object discrimination in rats. Psychopharmacology (Berl) 213:365–376 [DOI] [PubMed] [Google Scholar]
- Schechter MD. (1988) Serotonergic-dopaminergic mediation of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol Biochem Behav 31:817–824 [DOI] [PubMed] [Google Scholar]
- Scheidweiler KB, Ladenheim B, Barnes AJ, Cadet JL, Huestis MA. (2011) (±)-3,4-Methylenedioxymethamphetamine and metabolite disposition in plasma and striatum of wild-type and multidrug resistance protein 1a knock-out mice. J Anal Toxicol 35:470–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura M, Ortuño J, Farré M, McLure JA, Pujadas M, Pizarro N, Llebaria A, Joglar J, Roset PN, Segura J, et al. (2001) 3,4-Dihydroxymethamphetamine (HHMA). A major in vivo 3,4-methylenedioxymethamphetamine (MDMA) metabolite in humans. Chem Res Toxicol 14:1203–1208 [DOI] [PubMed] [Google Scholar]
- Shima N, Katagi M, Kamata H, Zaitsu K, Kamata T, Nishikawa M, Miki A, Tsuchihashi H, Sakuma T, Nemoto N. (2008) Urinary excretion of the main metabolites of 3,4-methylenedioxymethamphetamine (MDMA), including the sulfate and glucuronide of 4-hydroxy-3-methoxymethamphetamine (HMMA), in humans and rats. Xenobiotica 38:314–324 [DOI] [PubMed] [Google Scholar]
- Shulgin AT, Nichols DE (1978) Characterization of three new psychotomimetics, in The Psychopharmacology of Hallucinogens (Stillman RC, Willette RE eds) pp 74–83, Pergamon Press, New York. [Google Scholar]
- Starr MA, Page ME, Waterhouse BD. (2008) MDMA (3,4-methylenedioxymethamphetamine)-mediated distortion of somatosensory signal transmission and neurotransmitter efflux in the ventral posterior medial thalamus. J Pharmacol Exp Ther 327:20–31 [DOI] [PubMed] [Google Scholar]
- United Nations (2012) World Drug Report 2012, United Nations Publications, New York.
- Upreti VV, Eddington ND. (2008) Fluoxetine pretreatment effects pharmacokinetics of 3,4-methylenedioxymethamphetamine (MDMA, ECSTASY) in rat. J Pharm Sci 97:1593–1605 [DOI] [PubMed] [Google Scholar]
- Valtier S, Phelix CF, Cody JT. (2007) Analysis of MDMA and its metabolites in urine and plasma following a neurotoxic dose of MDMA. J Anal Toxicol 31:138–143 [DOI] [PubMed] [Google Scholar]
- Verrico CD, Miller GM, Madras BK. (2007) MDMA (Ecstasy) and human dopamine, norepinephrine, and serotonin transporters: implications for MDMA-induced neurotoxicity and treatment. Psychopharmacology (Berl) 189:489–503 [DOI] [PubMed] [Google Scholar]
- Walubo A, Seger D. (1999) Fatal multi-organ failure after suicidal overdose with MDMA, ‘ecstasy’: case report and review of the literature. Hum Exp Toxicol 18:119–125 [DOI] [PubMed] [Google Scholar]
- Wu D, Otton SV, Inaba T, Kalow W, Sellers EM. (1997) Interactions of amphetamine analogs with human liver CYP2D6. Biochem Pharmacol 53:1605–1612 [DOI] [PubMed] [Google Scholar]